Tree Species Identity Drives Fungal, but Not Bacterial, Soil Community Shifts in Tropical Monoculture Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Focal Species
2.2. Soil Sampling and Soil Chemistry
2.3. DNA Extraction and Metabarcoding
2.4. Bioinformatics
3. Results
3.1. Tree Communities and Soil Chemistry
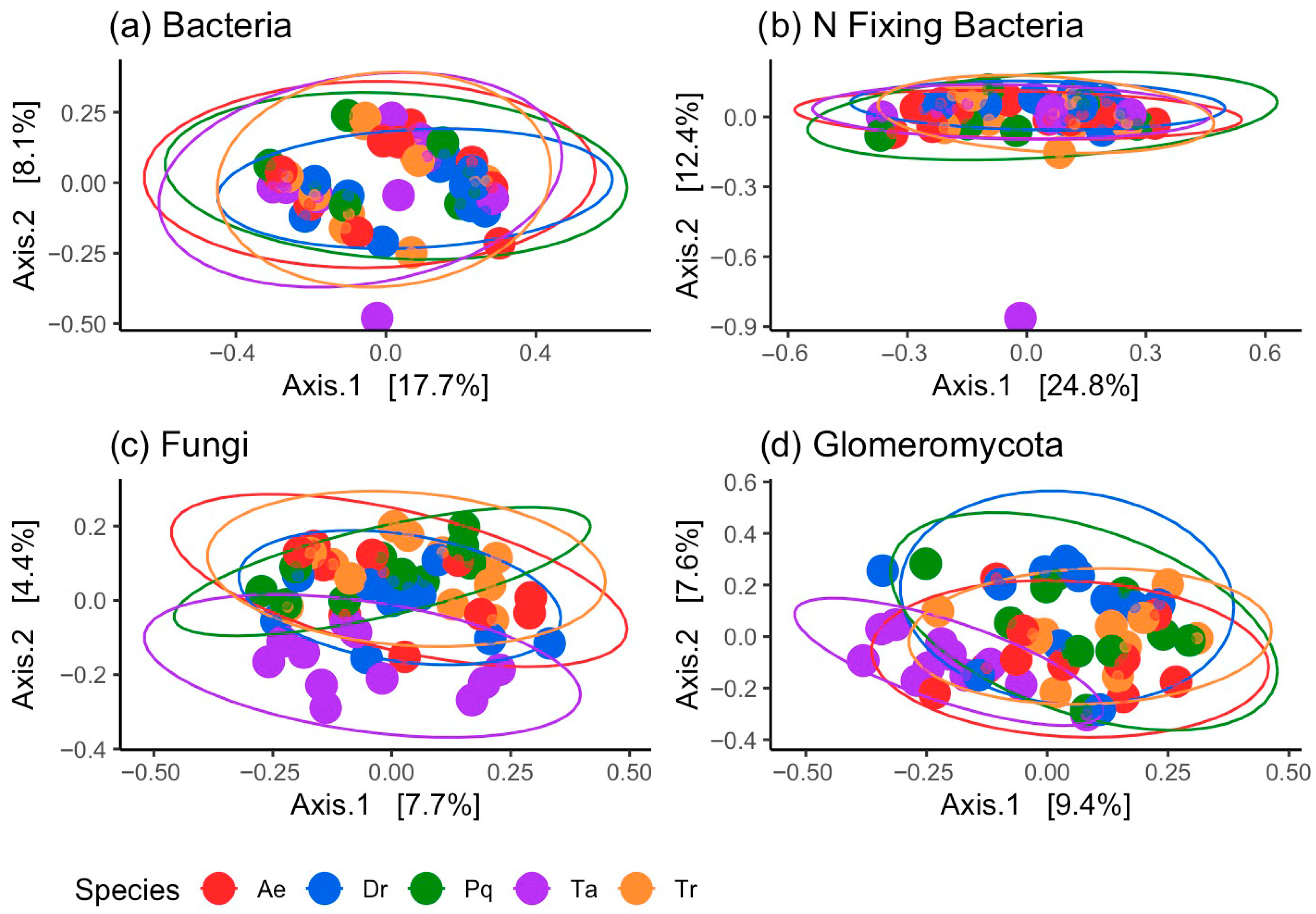
3.2. Microbial Diversity and Community Composition
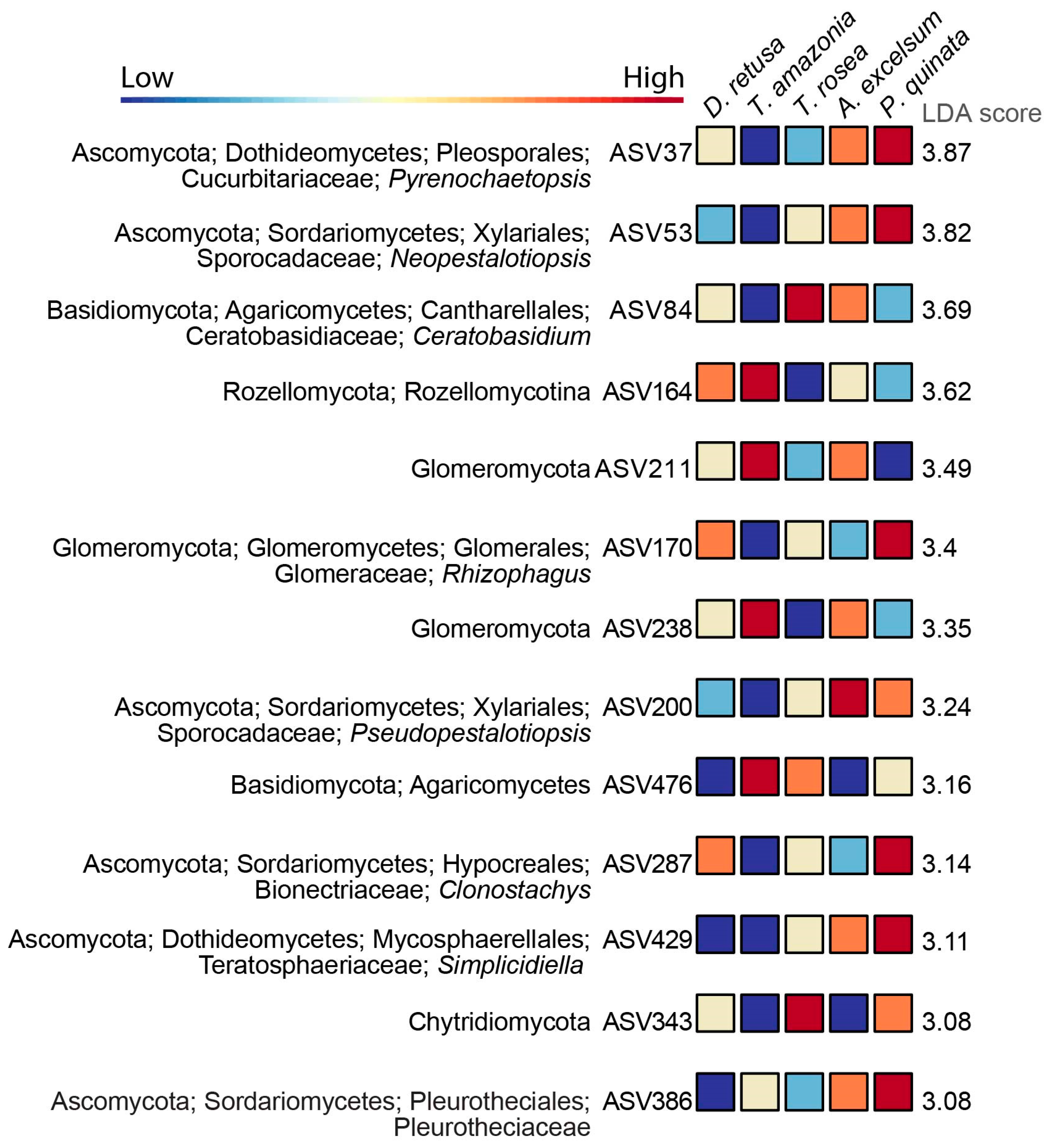
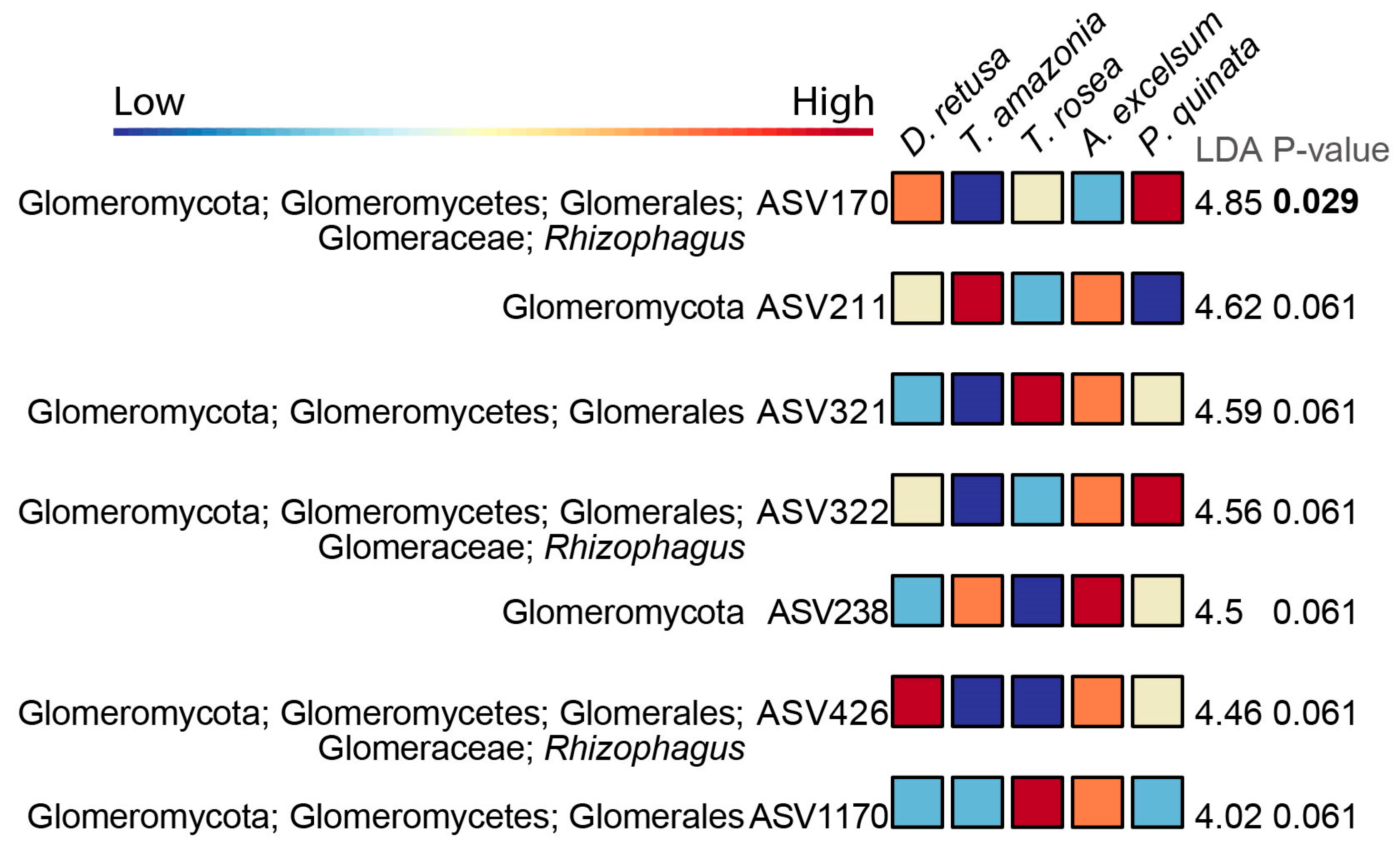
3.3. Differential Abundance of Microbial Taxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPBES. The IPBES Assessment Report on Land Degradation and Restoration; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018; p. 744. [Google Scholar] [CrossRef]
- DeFries, R.S.; Rudel, T.; Uriarte, M.; Hansen, M. Deforestation driven by urban population growth and agricultural trade in the twenty-first century. Nat. Geosci. 2010, 3, 178–181. [Google Scholar] [CrossRef]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-use change affects phosphorus fractions in highly weathered tropical soils. CATENA 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Qu, X.; Li, X.; Bardgett, R.D.; Kuzyakov, Y.; Revillini, D.; Sonne, C.; Xia, C.; Ruan, H.; Liu, Y.; Cao, F.; et al. Deforestation impacts soil biodiversity and ecosystem services worldwide. Proc. Natl. Acad. Sci. USA 2024, 121, e2318475121. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Ambiente. Estrategia Nacional Forestal, 2050; Ministerio de Ambiente: Panama City, Panama, 2019. Available online: https://dcc.miambiente.gob.pa/wp-content/uploads/2021/07/Estrategia_nacional_de_cambio_climatico.pdf (accessed on 10 July 2025).
- Pawson, S.M.; Brin, A.; Brockerhoff, E.G.; Lamb, D.; Payn, T.W.; Paquette, A.; Parrotta, J.A. Plantation forests, climate change and biodiversity. Biodivers. Conserv. 2013, 22, 1203–1227. [Google Scholar] [CrossRef]
- Cook-Patton, S.C.; Leavitt, S.M.; Gibbs, D.; Harris, N.L.; Lister, K.; Anderson-Teixeira, K.J.; Briggs, R.D.; Chazdon, R.L.; Crowther, T.W.; Ellis, P.W.; et al. Mapping carbon accumulation potential from global natural forest regrowth. Nature 2020, 585, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Turnbull, J.W. Plantation Forestry in the Tropics: The Role, Silviculture, and Use of Planted Forests for Industrial, Social, Environmental, and Agroforestry Purposes; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Hall, J.S.; Ashton, M.S.; Garen, E.J.; Jose, S. The ecology and ecosystem services of native trees: Implications for reforestation and land restoration in Mesoamerica. For. Ecol. Manag. 2011, 261, 1553–1557. [Google Scholar] [CrossRef]
- Hauff, R.D. A Case Study Assessment of Agroforestry. J. Sustain. For. 1998, 8, 39–51. [Google Scholar] [CrossRef]
- Sinacore, K.; García, E.H.; Howard, T.; van Breugel, M.; Lopez, O.R.; Finkral, A.J.; Hall, J.S. Towards effective reforestation: Growth and commercial value of four commonly planted tropical timber species on infertile soils in Panama. New For. 2023, 54, 125–142. [Google Scholar] [CrossRef]
- Shackleton, C.M.; Garekae, H.; Sardeshpande, M.; Sinasson Sanni, G.; Twine, W.C. Non-timber forest products as poverty traps: Fact or fiction? For. Policy Econ. 2024, 158, 103114. [Google Scholar] [CrossRef]
- Wishnie, M.H.; Dent, D.H.; Mariscal, E.; Deago, J.; Cedeño, N.; Ibarra, D.; Condit, R.; Ashton, P.M.S. Initial performance and reforestation potential of 24 tropical tree species planted across a precipitation gradient in the Republic of Panama. For. Ecol. Manag. 2007, 243, 39–49. [Google Scholar] [CrossRef]
- Wheeler, C.E.; Omeja, P.A.; Chapman, C.A.; Glipin, M.; Tumwesigye, C.; Lewis, S.L. Carbon sequestration and biodiversity following 18years of active tropical forest restoration. For. Ecol. Manag. 2016, 373, 44–55. [Google Scholar] [CrossRef]
- Ugalde, L.; Gómez, M. Perspectivas Economicas y Ambientales de las Plantaciones de Teca Bajo Manejo Sostenible, en Panamá; CATIE: Cartago, Costa Rica, 2006. [Google Scholar]
- Ministerio de Ambiente & GIZ. Programa Nacional de Restauración Forestal; Ministerio de Ambiente: Panama City, Panama, 2020. Available online: https://miambiente.gob.pa/conozca-mas-del-plan-nacional-de-restauracion-forestal-en-nuestra-web/ (accessed on 10 July 2025).
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Holste, E.K.; Holl, K.D.; Zahawi, R.A.; Kobe, R.K. Reduced aboveground tree growth associated with higher arbuscular mycorrhizal fungal diversity in tropical forest restoration. Ecol. Evol. 2016, 6, 7253–7262. [Google Scholar] [CrossRef]
- Hutchison, C.; Gravel, D.; Guichard, F.; Potvin, C. Effect of diversity on growth, mortality, and loss of resilience to extreme climate events in a tropical planted forest experiment. Sci. Rep. 2018, 8, 15443. [Google Scholar] [CrossRef]
- Mayoral, C.; van Breugel, M.; Cerezo, A.; Hall, J.S. Survival and growth of five Neotropical timber species in monocultures and mixtures. For. Ecol. Manag. 2017, 403, 1–11. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Barberán, A.; McGuire, K.L.; Wolf, J.A.; Jones, F.A.; Wright, S.J.; Turner, B.L.; Essene, A.; Hubbell, S.P.; Faircloth, B.C.; Fierer, N. Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol. Lett. 2015, 18, 1397–1405. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Barron, A.R.; Purves, D.W.; Hedin, L.O. Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia 2011, 165, 511–520. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Kelly, C.K. Pathogen mortality of tropical tree seedlings: Experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 1984, 61, 211–217. [Google Scholar] [CrossRef]
- Spear, E.R.; Broders, K.D. Host-generalist fungal pathogens of seedlings may maintain forest diversity via host-specific impacts and differential susceptibility among tree species. New Phytol. 2021, 231, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.A.; Hall, J.S.; Turner, B.L.; Hedin, L.O.; LaHaela Walter, J.K.; Sheldon, P.; van Breugel, M. Phosphatase activity and nitrogen fixation reflect species differences, not nutrient trading or nutrient balance, across tropical rainforest trees. Ecol. Lett. 2018, 21, 1486–1495. [Google Scholar] [CrossRef]
- Sinacore, K.; Asbjornsen, H.; Hernandez-Santana, V.; Hall, J.S. Drought differentially affects growth, transpiration, and water use efficiency of mixed and monospecific planted forests. Forests 2019, 10, 153. [Google Scholar] [CrossRef]
- Condit, R.; Engelbrecht, B.M.J.; Pino, D.; Pérez, R.; Turner, B.L. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl. Acad. Sci. USA 2013, 110, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.A.; Aranda, J.; Marshall, J.D.; Winter, K. Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol. 2007, 173, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Potvin, C.; Mancilla, L.; Buchmann, N.; Monteza, J.; Moore, T.; Murphy, M.; Oelmann, Y.; Scherer-Lorenzen, M.; Turner, B.L.; Wilcke, W.; et al. An ecosystem approach to biodiversity effects: Carbon pools in a tropical tree plantation. For. Ecol. Manag. 2011, 261, 1614–1624. [Google Scholar] [CrossRef]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determining Cation Exchange Capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. Next Gener. Seq. Data Anal. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2023, 52, D791–D797. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecol. Package 2.6—6.1; 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 19 August 2025). [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Mayoral, C.; van Breugel, M.; Turner, B.L.; Asner, G.P.; Vaughn, N.R.; Hall, J.S. Effect of microsite quality and species composition on tree growth: A semi-empirical modeling approach. For. Ecol. Manag. 2019, 432, 534–545. [Google Scholar] [CrossRef]
- Saltonstall, K.; van Breugel, M.; Navia, W.; Castillo, H.; Hall, J.S. Soil microbial communities in dry and moist tropical forests exhibit distinct shifts in community composition but not diversity with succession. Microbiol. Spectr. 2025, 13, e01931-24. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Kiers, E.T.; Lovelock, C.E.; Krueger, E.L.; Herre, E.A. Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: Implications for tropical forest diversity. Ecol. Lett. 2000, 3, 106–113. [Google Scholar] [CrossRef]
- Sheldrake, M.; Rosenstock, N.P.; Mangan, S.; Revillini, D.; Sayer, E.J.; Olsson, P.A.; Verbruggen, E.; Tanner, E.V.J.; Turner, B.L.; Wright, S.J. Responses of arbuscular mycorrhizal fungi to long-term inorganic and organic nutrient addition in a lowland tropical forest. ISME J. 2018, 12, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, M.; Hall, J.S.; Sinacore, K. Nutrient Strategies Used by Native Tree Species in Low-Fertility Soils [Poster]; BCI100 Symposium: Smithsonian Tropical Research Institute: Gamboa, Panamá, 2024. [Google Scholar]
- Sinacore, K.; Hall, J.S.; Potvin, C.; Royo, A.A.; Ducey, M.J.; Ashton, M.S. Unearthing the hidden world of roots: Root biomass and architecture differ among species within the same guild. PLoS ONE 2017, 12, e0185934. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Gaby, J.C.; Buckley, D.H. A Comprehensive Evaluation of PCR Primers to Amplify the nifH Gene of Nitrogenase. PLoS ONE 2012, 7, e42149. [Google Scholar] [CrossRef]
- Stefani, F.; Bencherif, K.; Sabourin, S.; Hadj-Sahraoui, A.L.; Banchini, C.; Séguin, S.; Dalpé, Y. Taxonomic assignment of arbuscular mycorrhizal fungi in an 18S metagenomic dataset: A case study with saltcedar (Tamarix aphylla). Mycorrhiza 2020, 30, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- McGuire, K.L.; Fierer, N.; Bateman, C.; Treseder, K.K.; Turner, B.L. Fungal community composition in Neotropical rain forests: The influence of tree diversity and precipitation. Microb. Ecol. 2012, 63, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Rivest, M.; Whalen, J.K.; Rivest, D. Tree diversity is not always a strong driver of soil microbial diversity: A 7-yr-old diversity experiment with trees. Ecosphere 2019, 10, e02685. [Google Scholar] [CrossRef]
| Crown Phenology a | Nutrient Cycling b | P Affinity c | Water Use Efficiency d | Aboveground Biomass (kg/tree) e | Mortality (%) f | |
|---|---|---|---|---|---|---|
| Anacardium excelsum | E | - | 0.90 | Low | 9.6 | 3.6 ± 2.4 |
| Dalbergia retusa | D (briefly) | N | - | High | 9.4 | 1.2 ± 1.1 |
| Pachira quinata | D | - | 1.01 | Low | 3.1 | 5.9 ± 6.6 |
| Tabebuia rosea | D | - | 1.62 | - | 2.8 | 4.0 ± 2.4 |
| Terminalia amazonia | E | - | −1.19 | Low | 29.1 | 1.6 ± 2.8 |
| Tree Species | N | pH (in H2O) | Resin P (mg/kg) | K (mg/L) | NO3 (mg/g) | NH4 (mg/g) | TOC (mg/L) | TN (mg/L) |
|---|---|---|---|---|---|---|---|---|
| A. excelsum | 11 | 5.6 ± 0.1 | 0.5 ±0.1 | 1.3 ± 0.2 | 10.0 ± 5.7 | 29.6 ± 2.5 | 11.9 ± 1.8 | 1.4 ± 0.2 |
| D. retusa | 12 | 5.6 ± 0.1 | 0.5 ±0.1 | 1.1 ± 0.2 | 10.8 ± 2.7 | 29.1 ± 2.9 | 11.5 ± 0.9 | 1.4 ± 0.1 |
| P. quinata | 10 | 5.4 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.1 | 11.6 ± 2.0 | 30.6 ± 2.2 | 14.3 ± 1.1 | 1.7 ± 0.1 |
| T. amazonia | 11 | 5.4 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 14.7 ± 8.1 | 26.3 ± 2.1 | 13.0 ± 1.1 | 1.4 ± 0.1 |
| T. rosea | 11 | 5.5 ± 0.1 | 0.5 ± 0.1 | 1.4 ± 0.2 | 6.8 ± 1.4 | 23.6 ± 1.2 | 14.5 ± 1.0 | 1.6 ± 0.1 |
| Overall Means | 55 | 5.5 ± 0.2 | 0.5 ± 0.3 | 1.1 ± 0.5 | 10.8 ± 15.4 | 27.8 ± 7.7 | 13.0 ± 4.10 | 1.5 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltonstall, K.; Spear, E.R.; Glodowska, M.A.; Hall, J.S. Tree Species Identity Drives Fungal, but Not Bacterial, Soil Community Shifts in Tropical Monoculture Plantations. Forests 2025, 16, 1366. https://doi.org/10.3390/f16091366
Saltonstall K, Spear ER, Glodowska MA, Hall JS. Tree Species Identity Drives Fungal, but Not Bacterial, Soil Community Shifts in Tropical Monoculture Plantations. Forests. 2025; 16(9):1366. https://doi.org/10.3390/f16091366
Chicago/Turabian StyleSaltonstall, Kristin, Erin R. Spear, Martyna A. Glodowska, and Jefferson S. Hall. 2025. "Tree Species Identity Drives Fungal, but Not Bacterial, Soil Community Shifts in Tropical Monoculture Plantations" Forests 16, no. 9: 1366. https://doi.org/10.3390/f16091366
APA StyleSaltonstall, K., Spear, E. R., Glodowska, M. A., & Hall, J. S. (2025). Tree Species Identity Drives Fungal, but Not Bacterial, Soil Community Shifts in Tropical Monoculture Plantations. Forests, 16(9), 1366. https://doi.org/10.3390/f16091366






