Abstract
Maturity estimation before seed collection is necessary in reducing the costs of seed collection; it allows vigorous seeds to be collected, ensuring that maximum germination will be reached and producing quality planting stock. In addition to this, appropriate temperature, seed size, pH, light, and stress conditions also influence germination. Cones of Cedrus deodara were collected at different intervals to estimate the maturity of the cones. A seed germination test was conducted in the laboratory under constant temperature, seed size, pH, light conditions, and water and salinity stress conditions. Significant (p < 0.05) variations in cones, such as seed morphological characteristics, germination, and related parameters, of C. deodara at different maturity periods were observed. The morphological traits of cones, such as seed weight, seed length, seed width, and seed germination, increased with increasing maturity, while the cone weight, moisture contents, specific gravity, and seed moisture decreased with increasing maturity. A constant temperature of 15 °C to 20 °C (98.0% to 92.0%) and the use of large-sized seeds (99.0%) led to maximum germination. Lower concentrations of Polyethylene glycol (98.0%) and NaCl (78.0%) contributed to maximum seed germination. The germination of C. deodara is temperature-dependent and seed size, light, and high water and salinity stress significantly influence seed germination.
1. Introduction
The Garhwal Himalayan forests, a part of the Western Himalayas, vary in altitude and are distributed with broad-leaved dominant species in the sub-montane region and evergreen broad-leaved species and conifers in the montane Himalaya, near the timberline [1]. Cedrus deodara G. Don is an evergreen monoecious tree. It is one of the most useful conifer species in the family Pinaceae and is an important timber-yielding species in the Western Himalayas. C. deodara is commonly known as Deodar, or Himalayan Cedar [2]. It is distributed from Afghanistan to the North-western Himalayas in the Garhwal and Kumaon regions, with an altitude range of 1200 to 3000 m [3]. C. deodara trees reach a height of up to 65 m, with a diameter at breast height of more than 2 m. A mass seed year in C. deodara occurs after 4–5 years [4]. The natural populations of C. deodara in the North-western Himalayas have been recorded to ascertain multiple sexual morphs [5] and a high outcrossing level of 0.962 [6], suggesting that the species is self-incompatible; pollination success in terms of seed formation and production in the natural population is thus a product of random mating, which ultimately produces vigorous seeds and progeny (this is a good sign for the survival and growth of progeny in their natural habitat). C. deodara grows pure forests in the Western Himalayas and has great potential for producing high biomass and aiding in carbon sequestration [7]. It is an important timber tree that is extensively used for building houses, doors, windows, railway sleepers, and carriages. Essential cedar oils extracted from its wood are used as medicines in the treatments of different diseases like skin diseases, stones, asthma, ulcers, joint disorders, and brain and immunological disorders in India, Pakistan, Korea, China, etc. [8,9]. C. deodara leaves are also used in the treatment of tuberculosis [10]. They have also been experimentally examined for their potential to exhibit different pharmacological actions, such as anti-diabetes and anti-proliferation behaviours, and in the treatment of herpes simplex virus type-1 [11,12,13].
Information about the time of seed maturity for forest tree species is necessary for the efficient collection of healthy and viable germinable seeds. The optimal time for harvesting mature seeds is directly influenced by the physical and physiological changes that occur during the maturation of the seeds [14]. Seed maturity is significant; it is the most imperative factor that determines changes in colour, seed size, quality, seed moisture, and seed vigour [15,16,17]. The physiological and functional changes that occur during seed maturation are changes that start from the time of anthesis and continue until the seeds are ready for harvest [18]. Finch-Savage and Bassel [19] studied the physiological maturity of seeds, revealing that seeds collected at maturity give the maximum germination under stressful conditions and remain viable in long-term dry storage [20]. The size and quality of a seed are primarily determined by its maturity, with optimal results achieved when seeds are harvested at their physiologically mature stage. This approach is highly recommended for studies on the impact of climatic variations in forest species, as highlighted by Geetha et al. [21].
C. deodara is a valuable tree for afforestation in the Western Himalayan region. The need to collect plentiful healthy seeds during good seed years and store them in favourable conditions fulfils the requirements of nursery growers for the development of desired planting stock. The collection of healthy mature cones before natural bursting is a challenging task because mature cones are delicate and burst naturally to disperse seeds over the forest floor. After the natural bursting of the cones, seed collection takes time, and is more laborious and economically infeasible. A usable maturity index to inform the collection of cones for growing seedlings in nurseries is lacking for the Garhwal Himalayas. It is important to develop a maturity index for C. deodara, as it will aid in the collection of cones before natural bursting, allowing practitioners the degraded hilly area of the Garhwal Himalaya to obtain a good quantity and quality of seeds for mass propagation and storage for future afforestation.
Considering all these facts, a study was proposed with the hypothesis that the estimation of cone collection time concerning cone maturity is crucial for the collection of viable seeds, thereby producing high-quality planting materials for large-scale afforestation in the Garhwal Himalayas. For the production of high-quality C. deodara seedlings, proper seed germination and seedling growth in the nursery are essential; this is influenced by temperature and seed size, light, and water and salinity stress. Therefore, the present study was aimed to established the answers to the following questions: (1) What is the best time to collect C. deodara cones in the Garhwal Himalayas in support of mass multiplication in the nursery, enabling the rehabilitation and reforestation of degraded forest lands? (2) Do temperature and seed size influence the seed germination and seedling growth of C. deodara? (3) To what extent do light availability, water stress, and salinity conditions affect the germination and radicle growth of C. deodara seeds?
2. Materials and Methods
2.1. Study Site
The experiment was conducted at the College of Forestry, V.C.S.G., Uttarakhand University of Horticulture and Forestry, located at Ranichauri, Tehri Garhwal; the college is situated between 30°15′ N latitude and 78°30′ E longitude in Northwest Uttarakhand at an altitude ranging from 1700 to 1850 m above mean sea level. The climate in the study area is predominantly humid, marked by chilling winters. Typically, January and February experience the coldest temperatures, while May and June are characterized by the highest temperatures. Throughout the summer months, the average annual maximum and minimum temperatures fluctuate between 8.9 °C and 23.5 °C, and in winter, it ranges from 0.6 °C to 14.1 °C. The mean annual rainfall in the region is recorded to be 1200–1500 mm, with the majority occurring from June to September.
2.2. Estimation of Cone Maturity
Twenty phenotypically superior trees in four sub-compartments (2a, 2b, 12m and 13) (five phenotypically superior trees in each sub-compartment) were selected in the Dandachali forests (Lohital Beat of Tehri Forest Division, compartment no. 2, 12, 13) at the time of flowering in October 2019, based on the tree comparison method. The criteria for the selection of phenotypically superior trees are based on bole height, the age of the tree, cone production, and tree health. To determine the best time for the collection of cones, a total of nine collections were made from 5 August to 28 October 2021 (at maturity) in different time intervals. A total of one hundred cones (five cones per tree) were harvested and collected from the selected 20 phenotypically superior trees at each time of collection; collection was conducted manually, by climbing the trees (Table 1). After each harvest, the cones were brought to the laboratory and stored in cotton bags at room temperature (14 °C), where they were subjected to various physical studies to determine their maturity. Fifty cones were put in the laboratory for their natural bursting, and the other fifty cones were subjected to observation to determine their morphological characteristics, including cone colour, length, width, weight, and specific gravity. The colour of the cones was judged and verified by ten different people by ocular estimation [22], and colour was also matched using the Munsell colour chart. Cone length was measured with the help of a meter scale, and the diameters (at the mid portion of the cone) for 50 cones (10 cones from each of the 5 replicates) were measured with the help of a digital Vernier calliper (Mitutoyo Absolute) in cm. The cone weight of 50 cones was separately weighed with the help of an electronic weighing machine (Contech make). The density was assessed using a floating test, also known as the water-displacement method, as outlined by Barnet in [23]. For each estimation of density, fifty cones were individually employed. The density (D) was calculated using the following formula:
This method involves determining the weight of the cone and measuring the volume of water displaced by the cone when it is submerged.
The fresh weight of fifty cones was taken; then, the cones were dried in the oven at 70 °C for 24 h or till the weight became constant. Then, the moisture content was determined by the formula presented by Schubert and Adams [24]:
The scale length, width, and scale thickness of each cone (10 cones from each of the 5 replicates) were measured with the help of a digital Vernier calliper (Mitutoyo Absolute) in cm. The number of viable seeds and non-viable seeds was counted manually. The lengths of the seeds with wings (20 seeds with 5 replicates) were measured with the help of a meter scale in cm. The lengths, widths, and seed thickness of the seeds without wings (20 seeds with 5 replicates) were measured with the help of a screw gauge in cm. Seed weight (100 seeds with 8 replicates) was estimated with the help of an electronic weighing machine (Contech make) in gm. The fresh weight of 20 seeds from the 5 replicates was estimated, and the seeds were kept in an oven at 70 °C for 24 h. The moisture percentage was calculated as per the formula prescribed above.
2.3. Effects of Temperature, Seed Size, Light Conditions, and Water and Salinity Stress on Seed Germination
The seeds from the cones collected at maturity (28 October 2021) were used to examine the effects of different temperatures, seed sizes, light conditions, and water and salinity stresses on seed germination, and the methods followed for determining the different parameters are described under the following sub-headings.
- Temperature:
One hundred seeds (twenty seeds from each of the five replicates) were kept in 9 cm glass Petri dishes, each with two layers of Whatman no 1 filter paper in a germinator (Colton) at different pre-fixed constant temperature regimes, i.e.,15 °C, 20 °C, 25 °C, and 30 °C.
- Seed size:
Seeds were categorised as small (<1.4 cm), medium (1.4–1.7 cm), and large (>1.7 cm) to record the effect of seed size on germination. Twenty seeds from the five replicates from each category were kept in 9 cm dia. glass Petri dishes with two layers of Whatman No. 1 filter paper, used to record the germination at a pre-fixed temperature of 15 °C.
- Light conditions:
To record the effects of different lights, i.e., white, blue, yellow, green, and dark conditions, twenty seeds in 5 replicates were selected randomly, and their germination was recorded. The Petri dishes were covered with different light coloured sheets (cellophane papers), i.e., white, blue, yellow, and green, and kept at pre-fixed 15 °C temperatures. Fixed tube lights in the germinator provided 24 h of light. For the dark condition, the Petri dishes were covered with silver foil.
- Water stress:
To record the effect of different Polyethylene glycol (PEG) solutions, 20 seeds from each of the 5 replicates were selected randomly for each PEG solution (0.05 MPA, 0.1 MPA, 0.15 MPA, 0.2 MPA, and 0.4 MPA). The seeds were put on top of two layers of Whatman No. 1 filter paper in Petri dishes (9 cm); each concentration of PEG solution was provided for its effect on seed germination at a pre-fixed temperature of 15 °C.
- Salt stress:
To record the effect of different NaCl solutions, 20 seeds from each of the 5 replicates were selected randomly for each NaCl solution (0.12 M, 0.20 M, 0.24 M, and 0.60 M). The seeds were placed on top of two layers of Whatman filter paper in the Petri dishes (9 cm) and kept at a pre-fixed temperature of 15 °C; each concentration of NaCl solution was provided for its effect on seed germination.
2.4. Data Processing
After the germination time was complete, i.e., at 21 days, and following a further week until no germination continued to occur in any of the treatments (temperature, seed size, water stress, salinity stress, and light conditions), the following parameters were measured: (i) The germination percentage was calculated as per the standard formula:
Germination percentage (%) = Total number of germination seeds/Total number of seeds sown × 100.
(ii) The mean germination time (MGT) was calculated by the formula given by Ellis and Roberts [25]. (iii) The germination index (GI) was calculated as per the formula given by Kendrick and Frankland [26]. (iv) The length of the radicle (10 seeds from each of the 5 replicates) was measured at the end of the germination test for each treatment.
The mean and standard deviation of the cone and seed morphologies and germination were calculated using Excel 2007.Arcsine transformation was applied for normality in data, and analysis of variance was computed by using JMP Pro 14.0 software; Duncan’s multiple range test (DMRT) was applied for the estimation of variation among the various treatments. Accordingly, the morphological traits of the cones and the seeds were determined at different time intervals in each treatment (temperature, seed size, light conditions, water stress, and salinity stress) to determine the effects of the applied conditions on seed germination and the related parameters of C. deodara.
3. Results
3.1. Experiment I
Significant (p < 0.05) variations were recorded for cone characteristics (i.e., cone colour, length, diameter, weight, density, moisture content, number of viable seeds per cone, number of non-viable seeds, scale length, scale width, and scale thickness) between different cone collection times. The cone colour of C. deodara changes towards maturity and changes to various shades of green and yellowish brown with the advancement of maturity. The data recorded on cone length (27.91%), cone diameter (28.7%), number of viable seeds per cone (44.13%), scale length (28.52%), scale width (11.23%), and cone scale thickness (24.07%) showed an increasing trend from first collection towards maturity. The cone weight (55.18%), cone density (82.3%), cone moisture percentage (59.7%), and the number of non-viable seeds (56.97%) decreased as the cones proceeded toward maturity (Figure 1 and Figure 2).
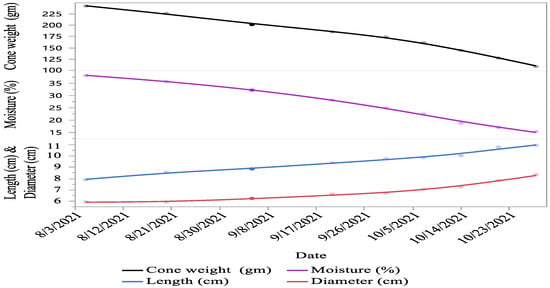
Figure 1.
Cone morphology of C. deodara at different collection times as the cones progressed towards maturity.
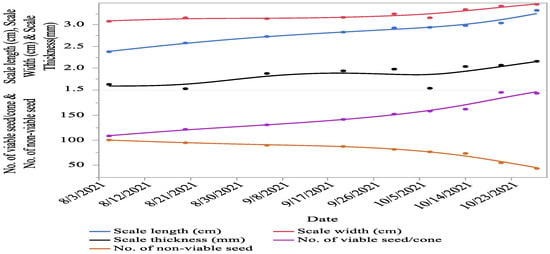
Figure 2.
Cone and seed morphology of C. deodara at different collection times as the cones progressed towards maturity.
Significant (p < 0.01) variations were recorded for seed characteristics, i.e., seed length with wings, seed length, seed width, seed thickness, seed weight, and moisture percentage between different cone collection times. The seed length with wings (20.12%), seed length (36.42%), seed width (60.66%), seed thickness (16.79%), seed weight (61.21%), germination (87.75%), GI (92.56%), and radicle length (94.92%) increased from first collection as the cones progressed towards maturity. However, the seed moisture contents (52.22%) and MGT (31.63%) decreased between the first collection and maturity (Figure 3 and Figure 4).
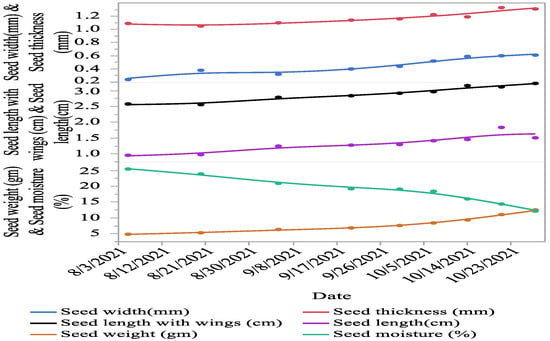
Figure 3.
Seed morphology of C. deodara at different times of collection as the seeds progressed towards maturity.
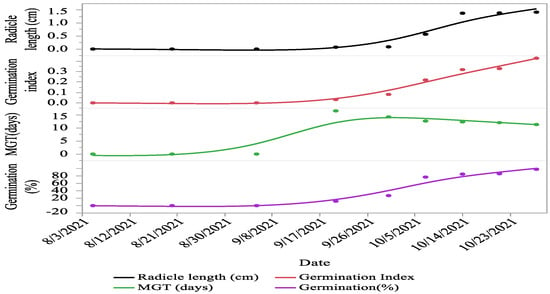
Figure 4.
Seed germination, MGT, GI, and radicle growth of C. deodara at different times of collection as the seeds progressed towards maturity.
The regression equation and coefficient of determination showed a negative significant relationship between cone weight, cone moisture percent, and seed moisture contents with seed germination percentage, while the seed weight showed a positive relationship with the seed germination of C. deodara (Figure 5).
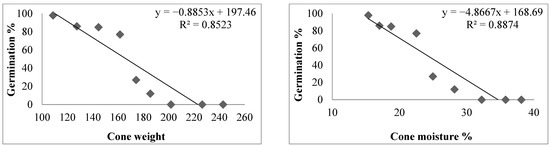
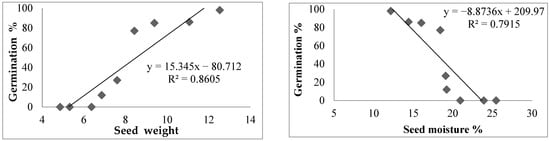
Figure 5.
The regression equation and coefficient of determination for germination percentage and cone weight, cone moisture percent, and seed weight of C. deodara.
3.2. Experiment II
The germination percentage, mean germination time, germination index, and radicle length showed significant (p < 0.01) variations among different temperatures. The highest germination percentage, GI, and radicle length (98.0, 0.30, and 1.38 cm, respectively) were recorded at a constant temperature of 15 °C; the lowest germination percentage, GI, and radicle length (72.0%, 18.0, and 0.82 cm, respectively) were recorded at a constant temperature of 30 °C. The maximum (16.12 days) MGT was recorded at a constant temperature of 30 °C, and the minimum (13.59 days) MGT was recorded at a constant temperature of 15 °C (Table 1).


Table 1.
Effect of temperature, seed size, pH, and light conditions on seed germination, MGT, GI, and radicle growth of C. deodara (mean values followed by the same letter within the column are not significant (p < 0.05) among the temperature, seed size, pH, and light condition treatments).
Table 1.
Effect of temperature, seed size, pH, and light conditions on seed germination, MGT, GI, and radicle growth of C. deodara (mean values followed by the same letter within the column are not significant (p < 0.05) among the temperature, seed size, pH, and light condition treatments).
| Temperature | Germination (%) | MGT (Days) | GI | Radicle Length (cm) |
|---|---|---|---|---|
| 15 °C | 98.0 a | 13.59 b | 0.30 a | 1.38 a |
| 20 °C | 92.07 a | 15.18 a | 0.23 b | 1.24 ab |
| 25 °C | 83.0 b | 15.91 a | 0.20 bc | 1.20 b |
| 30 °C | 72.0 c | 16.12 a | 0.18 c | 0.82 c |
| Seed size | ||||
| Small | 79.0 b | 15.21 b | 0.22 c | 0.26 c |
| Medium | 98.0 a | 13.62 b | 0.26 b | 0.51 b |
| Large | 99.0 a | 13.22 b | 0.29 a | 0.70 a |
| Light conditions | ||||
| Dark | 60.0 c | 14.75 a | 0.16 d | 0.13 c |
| Green | 84.0 b | 13.14 b | 0.23 c | 0.24 bc |
| White | 85.0 b | 13.04 b | 0.24 c | 0.36 b |
| Yellow | 93.0 a | 10.95 c | 0.28 b | 0.69 a |
| Blue | 97.0 a | 10.11 c | 0.35 a | 0.72 a |
The germination percentage, mean germination time, GI, and radicle length showed significant (p < 0.01) variations among different seed sizes. The maximum germination percentage (99.0), GI (0.29), and radicle length (0.70 cm) were recorded in large-size seeds, and the minimum germination percentage (79.0), GI (0.22), and radicle length (0.26 cm) were recorded in small-size seeds. The maximum MGT (15.21 days) was recorded in small-sized seeds, and the minimum MGT (13.22 days) was recorded in large-sized seeds (Table 1).
The data on germination percentage, MGT, GI, and radicle length varied significantly (p < 0.01) between the different light conditions. The maximum germination percentage (97.0), GI (0.35), and radicle length (0.72 cm) were recorded in blue light, and the minimum germination percentage (60.0), GI (0.16), and radicle length (0.13 cm) were recorded in dark light. The maximum (14.75 days) value of MGT was recorded in the dark conditions, and the minimum (10.11 days) MGT was recorded in blue light (Table 1).
The data concerning the germination percentage, MGT, GI, and radicle length on different PEG concentrations showed significant variation (p < 0.01) for different water stresses. The highest (98.0, 0.41, and 1.42 ± 0.16 cm) germination percentage, GI, and radicle length were recorded with the −0.05 MPa PEG solution; the lowest (68.0, 0.17, and 0.19 cm) germination percentage, GI, and radicle length were recorded with the −0.4 MPa PEG solution. The maximum (16.21 days) MGT was recorded with the −0.4 MPa PEG solution, and the minimum (11.63 days) MGT was recorded with the −0.05 MPa PEG solution (Table 2).

Table 2.
Effect of water stress condition and salinity stress on seed germination, MGT, GI, and radicle length of C. deodara (mean values followed by the same letter within the column are not significant (p < 0.05) among the water stress and salinity stress condition treatments).
The relevant data on germination percentage, MGT, GI, and radicle length exhibited significant variation (p < 0.01) for different salinity stresses. The maximum germination, GI, and radicle length (79.0, 0.20, and 0.68 cm, respectively) were recorded with the 0.12 M NaCl solutions, and the minimum (38.0, 0.0, and 0.16 cm) germination, GI, and radicle length was recorded with the 0.6 M NaCl solution, respectively. The maximum MGT (16.58 days) was recorded with the 0.6 M NaCl solution, and the minimum MGT (10.66 days) was recorded with the 0.12 M NaCl solution (Table 2).
4. Discussion
The collection of cones should be timed to coincide with the maturity of the seeds, and accurate knowledge of the specific stage and timing of cone and seed maturity is crucial. Harvesting immature seeds can lead to a range of issues, such as low germination, diminished seed vigour, resulting in smaller seedlings, and heightened susceptibility to diseases, decreased storage potential, and an elevated occurrence of abnormal seedlings. Furthermore, extracting immature seeds is a waste of time, money, and resources, and it is a more challenging process compared to harvesting mature seeds. On the other hand, delayed cone collection results in seed loss, because the cones start to open on the tree and disperse their seeds on the ground, thus making seed collection difficult. Accurate information surrounding seed maturity ensures that seed harvesting is conducted precisely the time at which the seeds are ripe and fully mature. Harvesting at this optimal moment maximizes the quality and viability of the seeds, contributing to successful germination, robust seedling development, and overall improved performance in the subsequent stages of plant growth. Further emphasizes the significance of meticulous timing in the harvesting process to achieve the best possible outcomes for seed quality.
The various indicators of maturation encompass visual, physical, biochemical, and climatic changes. These indicators can be employed effectively to discern the appropriate timing for seed harvest, helping to avoid the collection of immature seeds. As noted by Tanaka [27], careful consideration of these maturation indicators is essential in ensuring the quality and viability of the harvested seeds. In the present study, the results revealed that the cone colour of C. deodara changes towards maturity. At first collection, the cones were light green, which changed to various shades of green and yellowish brown with the advancement of maturity; this shows that harvesting should be performed when the cones become yellowish brown in colour. Mughal and Thapliyal [28] also reported a change in the colour of C. deodara cones from green to brown towards maturity, positing this change in colour as a useful index of ripeness. Singh et al. [29] documented that the colour of Pinus halepensis cones progressed through stages of light green and brown, before culminating in a glossy red–brown hue at full maturity, undergoing a noticeable transformation. Negi and Todaria [30] also found colour changes to be the best indicator of maturity for five speciesof the Garhwal Himalayas: Acer oblongum, Kydia calycina, Terminalia chebula, T. tomentosa, and T. belerica. Similar results were also observed in Myrica esculenta by Shah et al. [31], Quercus leucotrichophora by Tewari et al. [32], Acacia nilotica by Lavania et al. [33], and Pinus wallichiana by Bhat et al. [34]. Therefore, colour changes can be used to determine the complete maturity stage of cones, and a ten-day delay in the collection may cause problems in cone collections as the seeds would have been fallen to the forest floor. To obtain abundant seeds of good quality, it is recommended that the harvesting should be performed at the proper time during maturity.
The morphometric characteristics of the cones showed an increasing trend toward maturity, which is due to physiological development and maturation with age. Similar results were also observed by Lavania et al. [33] in Acacia nilotica, Rezaei and Arman [35] in Albizia julibrissin, and Singh et al. [36] in Albizia lebbek. The cone weight, density, and moisture percentage of C. deodara revealed that all the parameters decrease towards maturity. Cone weight, density, and moisture percentage are intricately linked to the ripening of seeds. The decline in these parameters, namely cone weight, moisture percent, and density, serves as a reliable indicator of seed maturity. This decrease is attributed to the loss of moisture due to desiccation and the allocation of nutrients to the seeds, marking the progression of the maturation phase. As the cones undergo desiccation, moisture is progressively lost, leading to a decrease in weight. This change in weight serves as a valuable indicator of the maturation process and monitoring it, is crucial for determining the optimal time for seed harvesting [37]. Mughal and Thapliyal [28] observed a decrease in the cone weight of C. deodara as it approached maturity. Similar kinds of results were also reported in other species by Joshi [38] for Dalbergia sissoo, Singh [39] for Abies pindrow, Singh et al. [40] for Pinus halepensis, and Singh and Kacharia [41] for Pinus kesiya. The morphological characteristics of cones significantly influenced the number of scales per cone, viable seeds and non-viable seeds per cone, scale length, width, thickness, and germination of C. deodara. Bhat et al. [34] reported similar findings in Pinus wallichiana, where the length and diameter of the cones were found to be directly associated with the number of scales and viable seeds per cone, and were inversely related to non-viable seed quantities.
Seed traits, including length, width, thickness, weight, and moisture percent, displayed variations based on the time of collection. The seed length, seed width, seed thickness, and seed weight increases, while the moisture percentage decreases as the seeds reach maturity. Differences in seed size may arise from both internal factors (maternal, hereditary, and age) and external factors (environmental conditions) during seed development [42,43]. Variation in seed size is attributed to the disruption of nutrient flow, the hardening of the seed coat, impeding water imbibition, and gaseous exchange [44]. The accumulations of specific proteins and oligosaccharides during seed maturation are linked with the acquisition of desiccation tolerance, enabling seeds to endure maturation drying and a spontaneous reduction in seed moisture, concluding the seed development [45]. Similar trends were observed in Pinus halepensis, where seed weight increased and moisture percent decreased as cones matured [40]. These findings are aligned with those presented in previous studies on C. deodara by Mughal [46] and on Pinus roxburghii by Mukherjee [47].
Different cone collection times significantly influenced seed germination, MGT, GI, and radicle length of C. deodara. Maximum seed germination, GI, and radicle length and minimum MGT were related to increasing seed maturity. The maximum germinability with vigour index and maximum seed weight indicated that physiological maturity (seed weight, germination, and vigour) was reached by C. deodara seeds in the month of October; this provides a signpost for collecting high-quality seeds which will provide higher germination and vigour, enabling high-quality seedling production. Similar results were recorded by Lavania et al. [33] in Acacia nilotica, Singh et al. [40] in Pinus halepensis, Singh [39] in Abies pindrow, Singh and Kachari [41] in Pinus kesiya, and Uniyal and Nautiyal [48] in Ougenia dalbergioides. The collective evidence from these studies supports the notion that seed germination tends to improve as the seeds progress toward maturity during their development.
Different temperatures also harmed early seedling growth, as the radicle length decreased in the seeds when germinated under high temperatures. Each tree species has a specific optimal temperature range for germination. The optimal temperature represents the point at which the maximum number of seeds for a particular species, such as C. deodara, will germinate in the shortest amount of time. For C. deodara, among different constant temperatures applied during the experiments revealed that the constant temperatures of 15 °C and 20 °C were found to provide the most favourable conditions for germination. Maintaining and understanding these optimal temperature conditions is crucial in ensuring successful and efficient seed germination, contributing to the overall success of tree propagation and establishment. Seeds subjected to temperatures of 15 °C and 20 °C gave the maximum germination percentage, germination index, and radicle growth, with the minimum MGT. Seeds subjected to a temperature of 30 °C gave minimum germination percentage, germination index, and radicle growth, alongside the maximum MGT. The optimum temperature is the point at which the germination rate is the highest. However, beyond this optimal temperature, the germination rate was found to start to decline by up to 8 percent with a 5 °C increase in temperature in the present study. If the temperature rises in the natural conditions of the forest in the future, as is expected in the current scenario of climate change, the natural regeneration of C. deodara may also be affected: the seed germination of C. deodara is temperature-dependent, and maximum germination was recorded at a lower temperature as compared to higher temperatures. Gao et al. [49] studied Pinus yunnanensis and found that seeds germinate to a high percentage (approximately 90.0%) at temperatures of 15 °C or 20 °C, with or without light, whereas higher temperatures of 25 °C or 30 °C were found to impede radicle protrusion and result in a sharply decreasing germination percentage (within 5%). Topwal et al. [50] also reported that seed germination of C. deodara reached a maximum at 15 °C. Singh and Bhatt [51] obtained optimum seed germination in Dalbergia sissoo at 25 °C. Singh et al. [52] tested germination at seven temperature regimes; the optimum regime was found to be 25 °C. Yadav et al. [53], during their course of study on two species of Bauhinia, achieved faster and more uniform germination with a 25 °C regime. Kumar et al. [54] reported that an incubation temperature of 15 °C showed better results in comparison with 25 °C in Pinus gerardiana.
The germination of seeds, MGT, GI, and radicle length of C. deodara significantly varied under different seed sizes. The present study revealed that the larger seeds produce better and uniform germination, MGT, GI, and radicle growth, while small-sized seeds have lower germination, GI, radicle length, and a higher mean germination time. Seed germination is a complex process, influenced by numerous internal and external factors, such as the level of starch, carbohydrates, fats, and other food reserves, and it is notably impacted by seed size, which is significantly correlated with seed food reserves [55]. This parameter plays a crucial role in shaping the germination, emergence, seedling establishment, growth rate, plant size, and biomass of nursery seedlings, ultimately influencing the trajectory of the future crop, i.e., survivorship, competitive ability, and the reproductive ability of adult plants [56,57,58]. Germination efficiency is linked to the seed’s ability to utilize reserves effectively, emphasizing the importance of the efficient utilization of stored energy in the seeds for germination traits [45,59]. A similar study shows that seeds with large sizes have higher seed characterization and biochemical constituents in the Calophyllumino phylum, which in turn was reflected in the germination parameters [60]. Yadav [53], in his study on two species of Bauhinia, showed that the germination and germination index are higher in large-sized seeds, whereas MGT is lower.
Light is not a requirement for germination in most plants. However, seeds of some species achieve maximum germination under dark conditions, and some other seeds achieve maximum germination in continuous-light conditions. The overgrowth of plants and the opening and closing of stomata are suppressed by blue light and strongly help in the transportation and conservation of water in plants. Blue light is important for use alone during the early phases of tree growth, such as the start of the radicle and seedling growth. Darkness is a significant requirement, as it permits the tree crop to rest and activate the flowering response [61]. Day-neutral tree crops bloom with no start for the duration of darkness, but they usually perform best with longer light durations. After germination, light becomes crucial for each plant species because the initial sprout will not survive if it cannot reach to a light source [61]. Light conditions vary significantly (p < 0.01), influencing the seed germination, MGT, GI, and radicle length of C. deodara. We found that the maximum germination was recorded for the seeds under blue light conditions, along with the minimum mean germination time (MGT). The maximum germination index and radicle length were recorded in the blue light condition. The light and dark conditions did not influence the seed germination of Pinus pinea [62].
In terrestrial environments, water stress is the most important limiting factor controlling primary production. The limited water supply creates water stress on plant roots and reduces the rate of transpiration in plants. Water stress results in the disruption of crops, consequently affecting biomass production in the world and resulting in a dearth [63]. Seed germination and seedling growth of C. deodara were significantly decreased with increasing water stress. The decrease in seed germination and growth of seedlings under increasing water stress took the form of hydraulic signalling (i.e., decreased root growth, water uptake, water potential, turgidity, and leaf enlargement) [64]. The water stress condition also inhibits the water potential and transpiration rate of the plant, and it is evident in cell turgor and the relative water content, which damages the plant cells [65]. The highest water stress conditions significantly varied (p < 0.01), which influenced the seed germination of C. deodara. MGT, GI, and radicle length are also influenced by the higher concentrations of water stress conditions. We found that the maximum germination and minimum MGT with the −0.05 MPa PEG solution. The maximum germination index and maximum radicle length were recorded with the −0.05 MPa PEG solution. Gao et al. [49] reported that the water potential effect depended on temperature and provenance in Pinus yunnanensis. The results indicate that a lower water potential (−0.2 MPa) also reduces germination. Several researchers reported that seed germination was reduced when seeds were subjected to increasing water stress: Singh et al. [29] in Holoptelia integrifolia, Topacoglu [66] in Pinus nigra, Sah et al. [67] in Pinus species, Wafa et al. [68] in Ephedra alata, and Yigit et al. [69] in Pinus species. Salinity reduces moisture accessibility, which induces osmotic stress, nutrient inequity, and ionic toxicity [70,71].
5. Conclusions
The maturity index estimation is the most important index for the collection of high-quality seeds, ensuring that seed collection remains low-cost, time-efficient, and economically viable. Mature seeds germinate fast and uniformly, producing vigorous seedlings. The cones of C. deodara trees should be collected in the second fortnight of October, when the cones turn yellowish brown in colour. Optimum germination was recorded at constant temperatures of 15 °C and 20 °C. Large-sized seeds showed better germination, GI, and radicle length in comparison with smaller seeds. Light conditions were not found to be a limiting factor for germination, but they might be important in the radicle protrusion of C. deodara seeds. Higher water stress and salinity stress conditions were found to significantly influence the seed germination of C. deodara. The seed germination of C. deodara is influenced by temperature, and larger seeds should be segregated to obtain maximum germination. Water and salinity stresses are the most prominent factors that inhibit the germination and growth of C. deodara. The results of the present study indicate that the seed germination of C. deodara is negatively influenced by higher temperature, higher water stress, and higher salt stress conditions; such conditions in natural populations may create problems for the natural regeneration of this potential timber-yielding species, with changing climatic conditions expected in the future for the Himalayan region. Sustainable regeneration by artificial means using large seeds is recommended for management deodar forest by the forest departments and nursery growers who are seeking to produce high-quality planting materials for their different afforestation activities in the mountainous region of the natural habitat of C. deodara. The inferences of this study are based on data collected for only one year in the Garhwal Himalayas; therefore, a long-term study should be conducted to further examine the effect of seed size, water stress, salinity stress, and suitable time for seed collection on tree growth. Such future studies of this precious timber-yielding tree species should be conducted in their natural growing region in the North-western Himalayas.
Author Contributions
G.P. wrote the original manuscript; V.P.K. read, reviewed and edited the manuscript; B.S. read and edited the manuscript and acted as a corresponding author; R.S.B. helped in conducting the laboratory analyses; I.S. helped in conducting seed collection; D.R. read and edited the manuscript; M.K.R. helped in conducting the analyses. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data cannot be shared openly but are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rawat, V.S.; Chandra, J. Vegetational diversity analysis across different habitats in Garhwal Himalaya. J. Bot. 2014, 2014, 538242. [Google Scholar] [CrossRef]
- Sinha, D. A review on phytochemical, ethnobotanical, pharmacological, and antimicrobial, importance of Cedrus deodara (Roxb. Ex D. Don) G. Don. Int. J. Green Pharm. 2019, 13, 1. [Google Scholar] [CrossRef]
- Troup, R.S. Silviculture of Indian Tree (Volumes 1–3); Oxford University Press: Oxford, UK, 1921. [Google Scholar]
- Sofi, P.A.; Malik, A.R.; Butola, J.S.; Bhat, G.M.; Dhanai, C.S. Standardization of Seed Storage Conditions for Cedrus deodara (Roxb.) G. Don. Indian For. 2016, 142, 390–393. [Google Scholar]
- Khanduri, V.P.; Sukumaran, A.; Sharma, C.M. Gender plasticity uncovers multiple sexual morphs in natural populations of Cedrus deodara (Roxb.) G. Don. Ecol. Process. 2021, 10, 35. [Google Scholar] [CrossRef]
- Khanduri, V.P.; Sharma, C.M.; Riyal, M.K.; Sukumaran, A. racial hybridization and inbreeding depression in Cedrus deodara (Roxb.) G. Don. Kastamonu Univ. J. For. Fac. 2022, 22, 47–55. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Kumar, M.; Todaria, N.P.; Bhat, J.A.; Kumar, A.; Pandey, R. Contribution of Cedrus deodara forests for climate mitigation along altitudinal gradient in Garhwal Himalaya, India. Mitig. Adapt. Strateg. Glob. Change 2021, 26, 5. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, R. Himalayan (Himachal region) cedar wood (Cedrus deodara: Pinaceae) essential oil, its processing, ingredients and uses: A review. J. Pharmacogn. Phytochem. 2019, 8, 2228–2238. [Google Scholar]
- Bisht, A.; Jain, S.; Misra, A.; Dwivedi, J.; Paliwal, S.; Sharma, S. Cedrus deodara (Roxb. ex D. Don) G.Don: A review of traditional use, phytochemical composition, and pharmacology. J. Ethnopharmacol. 2021, 279, 114361. [Google Scholar] [CrossRef]
- Narayan, S.; Thakur, C.P.; Bahadur, S.; Thakur, M.; Pandey, S.N.; Thakur, A.K.; Mitra, D.K.; Mukherjee, P.K. Cedrus deodara: In vitro: Antileishmanial efficacy & immunomodulatory activity. Indian J. Med. Res. 2017, 146, 780–787. [Google Scholar]
- Antoine M, S.; Lampronti, I.; Borgatti, M.; Finotti, A.; Harb, F.; Safi, S.; Gambari, R. In vitro evaluation of the antiproliferative activities of the wood essential oils of three Cedrus species against K562 human chronic myelogenous leukaemia cells. Nat. Prod. Res. 2012, 26, 2227–2231. [Google Scholar]
- Saab, A.M.; Gambari, R.; Sacchetti, G.; Guerrini, A.; Lampronti, I.; Tacchini, M.; El Samrani, A.; Medawar, S.; Makhlouf, H.; Tannoury, M. Phytochemical and pharmacological properties of essential oils from Cedrus species. Nat. Prod. Res. 2018, 32, 1415–1427. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Shamim, A.; Mazumder, A. Isolation, structural elucidation and in vitro antioxidant activity of compounds from chloroform extract of Cedrus deodara (Roxb.) Loud. Nat. Prod. Res. 2015, 29, 268–273. [Google Scholar] [CrossRef]
- Bhatt, J.; Ram, J. Seed maturity indices in Carpinus viminea (Himalayan hornbeam) along altitudinal gradients in relation to climate change. Int. J. Recent Sci. Res. 2015, 6, 40–50. [Google Scholar]
- Schmidt, L. Guide to the Handling of Tropical and Subtropical Forest Seed; Danida Forest Seed Centre: Humlebaek, Denmark, 2000; p. 511. [Google Scholar]
- Mittal, A.; Tewari, A.; Singh, N. Indicator of seed maturation in Cornus macrophylla wall. In Kumaun Himalayan India. J. Environ. Biosci. 2017, 31, 69–73. [Google Scholar]
- Ellis, R.H. Temporal patterns of seed quality development, decline, and timing of maximum quality during seed development and maturation. Seed Sci. Res. 2019, 29, 135–142. [Google Scholar] [CrossRef]
- Marcos Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds. Plant Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Geetha, V.V.; Bhaskaran, M.; Balamurugan, P. Physiological maturity studies in Mustard. Am. Int. J. Res. Form. Appl. Nat. Sci. 2013, 4, 43–46. [Google Scholar]
- William, R.L. A Guide to Forest Seed Handling with Special Reference to the Tropics; FAO: Rome, Italy, 1985. [Google Scholar]
- Barnet, J.P. Maturation of tree seeds. In Proceedings of the a Symposium on Flowering and Seed Development in Trees; South Forestry Experimentation Station, Mississippi State University, IUFRO: Poplarville, MS, USA, 1979; pp. 272–280. [Google Scholar]
- Schubert, G.H.; Adams, R.S. Reforestation Practice for Conifers in California; State of California Resources, Agency Department of Conservation, Division of Forestry: Sacramento, CA, USA, 1971. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kendrick, R.E.; Frankland, B. Photocontrol of germination in Amaranthus caudatus. Planta 1969, 85, 326–329. [Google Scholar] [CrossRef]
- Tanaka, Y. Assuring seed quality for seedling production: Cone collection and seed processing, testing, storage, and stratification. In Forestry Nursery Manual: Production of Bare-Root Seedlings; Springer: Dordrecht, The Netherlands, 1984; pp. 27–39. [Google Scholar]
- Mughal, A.H.; Thapliyal, R.C. Provenance variation in cone and seed characteristics of Cedrus deodara (D.DON) G.DON in Jammu and Kashmir. For. Stud. China 2012, 14, 193–199. [Google Scholar] [CrossRef]
- Singh, B.; Uniyal, A.K.; Todaria, N.P. Effect of water, salinity stress with varying temperature regimes on seed germination and early seedling growth of Holoptelia integrifolia (Roxb.) Planchon. J. Plant Biol. 2007, 34, 87. [Google Scholar]
- Negi, A.K.; Todaria, N.P. Improvement of germination of some Himalayan tree seeds by temperature treatment. Seed Sci. Technol. 1993, 21, 675–678. [Google Scholar]
- Shah, S.; Tewari, A.; Tewari, B.; Singh, R.P. Seed maturity indicators in Myrica esculenta, Buch-Ham.Ex. D. Don.: A multipurpose tree species of subtropical-temperate Himalayan region. New For. 2010, 40, 9–18. [Google Scholar] [CrossRef]
- Tewari, A.; Mittal, A.; Singh, N. Seed maturation timing in Quercus leucotrichophora, A. campus along an altitudinal gradient in Uttarakhand Himalaya. Environ. Conserv. J. 2017, 18, 53–59. [Google Scholar] [CrossRef]
- Lavania, P.; Singh, R.P.; Tiwari, A.; Sharma, H.K. Studies on seed maturity indices of Acacia nilotica in Uttar Pradesh. J. Pharmacogn. Phytochem. 2018, 7, 192–195. [Google Scholar]
- Bhat, G.M.; Mughal, A.H.; Malik, A.R.; Khan, P.A.; Sofi, P.A.; Islam, M.A. Cone and seed maturity indices in Pinus wallichiana under temperate conditions of Kashmir Himalayas, India. J. Appl. Nat. Sci. 2017, 9, 1987–1993. [Google Scholar] [CrossRef]
- Rezaei, A.; Arman, Z. Effects of pod maturity phases on physical dormancy induction in silk tree (Albizia julibrissin Durazz) seeds. Silva World 2023, 2, 21–25. [Google Scholar] [CrossRef]
- Singh, B.; Bahtt, S.; Uniyal, P.; Rawat, J.M.S. Effect of seed maturity on germination and seedling growth of Albizia lebbek (L.) Wild. Natl. Inst. Ecol. 2008, 19, 15–19. [Google Scholar]
- Bhat, G.M. Altitudinal Variation in Phenology, Seedling Characteristics and Natural Regeneration of Pinus wallichiana AB, Jackson. Ph.D. Thesis, Sher-e-Kashmir University, Jammu and Kashmir, India, 2013. [Google Scholar]
- Joshi, M. Effect of Nursery Management Practices on the Stock Quality and Out Planting Performance of Some Populus ciliata Clones. Ph. D. Thesis, Dr. Y. S. Parmar University of Horticulture and Forestry, Solan, India, 2000; p. 167. [Google Scholar]
- Singh, O. Seed maturity indices in silver fir (Abies pindrow Spach). Indian For. 1998, 124, 243–246. [Google Scholar]
- Singh, A.; Husain, M.; Mir, N.A.; Wani, A.A.; Bhat, G.M.; Mugloo, J.A. Influence of cone collection date on cone, seed and germination characteristics in Allepo Pine (Pinus halepensis still.) in Kashmir Valley, India. Int. J. Pure App. Biosci. 2017, 5, 1050–1057. [Google Scholar]
- Singh, O.; Kachari, J. Seed maturity indices in Khasi pine (Pinus kesiya). Indian For. 2006, 132, 1689–1691. [Google Scholar]
- Bladé, C.; Vallejo, V.R. Seed mass effects on performance of Pinus halepensis Mill. Seedlings sown after fire. For. Ecol. Manag. 2008, 255, 2362–2372. [Google Scholar] [CrossRef]
- Callejas-Díaz, M.; Chambel, M.R.; San-Martín-Lorén, J.; Gea-Izquierdo, G.; Santos-Del-Blanco, L.; Postma, E.; Climent, J.M. The role of maternal age, growth, and environment in shaping offspring performance in an aerial conifer seed bank. Am. J. Bot. 2022, 109, 366–376. [Google Scholar] [CrossRef]
- Kelly, K.M.; Staden, J.V.; Bell, W.E. Seed coat structure and dormancy. Plant Growth Regul. 1992, 11, 213–222. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mughal, A.H. Variation in Cone, Seed and Seedling Characteristics of Cedrus deodara. Ph.D Thesis, Forest Research Institute, Deemed University, Dehradun, India, 2002; p. 230. [Google Scholar]
- Mukherjee, S. Study on Provenance Variation in Cone Seed Seedling Characteristics of Pinus roxburghii Sarg. Ph.D. Thesis, FRI Deemed University, Dehra Dun, India, 2005. [Google Scholar]
- Uniyal, R.C.; Nautiyal, A.R. Seed germination and seedling extension growth in Ougeinia dalbergioides Benth. Under water and salinity stress. New For. 1998, 16, 265–272. [Google Scholar] [CrossRef]
- Gao, C.; Liu, F.; Zhang, C.; Feng, D.; Li, K.; Cui, K. Germination responses to water potential and temperature variation among provenances of Pinus yunnanensis. Flora 2021, 276, 151786. [Google Scholar] [CrossRef]
- Topwal, D.; Khanduri, V.P.; Singh, B.; Bali, R.S.; Rawat, D. Provenance variation in cone morphology, seed traits, and seed germination of Cedrus deodara from Uttarakhand Himalaya. Forestist 2024, 74, 62–73. [Google Scholar] [CrossRef]
- Singh, B.; Bhatt, B.P. Germination behaviour of Dalbergia sissoo as affected by seed source and temperature. Indian J. For. 2008, 31, 565–570. [Google Scholar] [CrossRef]
- Singh, B.; Bhatt, B.P.; Prasad, P. Effect of seed source and temperature on seed germination of Celtis australis L.: A promising agroforestry tree-crop of Central Himalaya, India. For. Trees Livelihoods 2004, 14, 53–60. [Google Scholar] [CrossRef]
- Yadav, N.; Khanduri, V.P.; Singh, B.; Dhanai, C.S.; Riyal, M.K.; Rawat, D.; Ahmad, T.; Kumar, M. Effect of temperature seed size sowing depth position on seed germination seedling growth of Bauhinia retusa Roxb Bauhinia variegata L. Forests 2023, 14, 1664. [Google Scholar] [CrossRef]
- Kumar, R.; Shamet, G.S.; Mehta, H.; Alam, N.M.; Tomar, J.M.S.; Chaturvedi, O.P.; Khajuria, N. Influence of gibberellic acid and temperature on seed germination in Chilgoza pine (Pinus gerardiana Wall.). Indian J. Plant Physiol. 2014, 19, 363–367. [Google Scholar] [CrossRef]
- Okonwu, K.; Ifenuaguta, A.U.; Ogazie, C.A.; Agogbua, J.U. Legume seed sizes and their consequential growth performance. Res. J. Seed Sci. 2022, 15, 1–8. [Google Scholar] [CrossRef]
- Ambika, S.; Manonmani, V.; Somasundaram, G. Review on the effect of seed size on seedling vigour and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Shahi, C.; Vibhuti, K.B.; Bargali, S.S. How seed size and water stress effect the seed germination and seedling growth in wheat varieties. Curr. Agric. Res. J. 2015, 3, 60–68. [Google Scholar] [CrossRef]
- Pandey, R.; Bargali, S.S.; Bargali, K. Does seed size affect water stress tolerance in Quercus leucotrichophora A. Camus Germination Early Seedling Growth Stage? Biodivers. Int. J. 2017, 1, 00005. [Google Scholar]
- Sikder, S.; Hasan, M.A.; Hossain, M.S. Germination characteristics and mobilization of seed reserves in maize varieties as influenced by temperature regimes. J. Agric. Rural. Dev. 2009, 7, 51–56. [Google Scholar] [CrossRef]
- Ajeesh, R.; Jijeesh, C.M.; Vidyasagaran, K.; Vikas, K. Impact of seed weight on germination parameters of Calophyllum inophyllum L.: A potential biodiesel tree species of coastal region. Bioscan 2014, 9, 1087–1091. [Google Scholar]
- Watkins, D. The Best Plant Light Spectrum for Growing Flowering Plants. Home Guides. SF Gate. 2018. Available online: http://homeguides.sfgate.com/plant-light-spectrum-growing-floweringplants-72801.html (accessed on 16 August 2025).
- Ganatsas, P.P.; Tsakaldimi, M.N. Effect of light conditions and salinity on germination behaviour and early growth of umbrella pine (Pinus pinea L.) seed. J. Hortic. Sci. Biotechnol. 2007, 82, 605–610. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plant scope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 90. [Google Scholar] [CrossRef]
- Novak, V.; Lipiec, J. Water extraction by roots under environmental stresses. In Pollution and Water Resources, Columbia University Seminar Proceedings: Impact of Anthropogenic Activity and Climate Changes on the Environment of Central Europe and USA; Columbia University Press: New York, NY, USA, 2012; p. 17. [Google Scholar]
- Rodriguez-Iturbe, I.; Porporato, A.; Laio, F.; Ridolfi, L. Plants in water-controlled ecosystems: Active role in hydrologic processes and response to water stress: Scope and general outline. Adv. Water Resour. 2001, 24, 695–705. [Google Scholar] [CrossRef]
- Topacoglu, O.; Sevik, H.; Akkuzu, E. Effects of water stress on germination of Pinus nigra Arnold. Seeds. Pak. J. Bot 2016, 48, 447–453. [Google Scholar]
- Sah, V.K.; Chaturvedi, O.P.; Saxena, A.K. Effects of water stress. pH, temperature, and light on seed germination of four Pine species. Proc. Indian Nat. Sci. Avad. 1989, B55, 73–74. [Google Scholar]
- Wafa’a, A.; Al-Qarawi, A.A.; Alsubiee, M.S. Effect of water stress by Polyethylene Glycol 8000 and Sodium Chloride on germination of Ephedra alata Decne seeds. Saudi J. Biol. Sci. 2010, 17, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species. In Water Stress in Plants; Intech: Houston, TX, USA, 2016; p. 43. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).