Compensatory Regulation and Temporal Dynamics of Photosynthetic Limitations in Ginkgo Biloba Under Combined Drought–Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Synchronous Measurement of Gas Exchange and Chlorophyll Fluorescence
2.3. Estimation of Mesophyll Conductance
2.4. Estimation of the Maximum Carboxylation Rate
2.5. Photosynthetic Limitation Analysis
2.6. Path Analysis Using Structural Equation Modeling
2.7. Statistical Analysis
3. Results
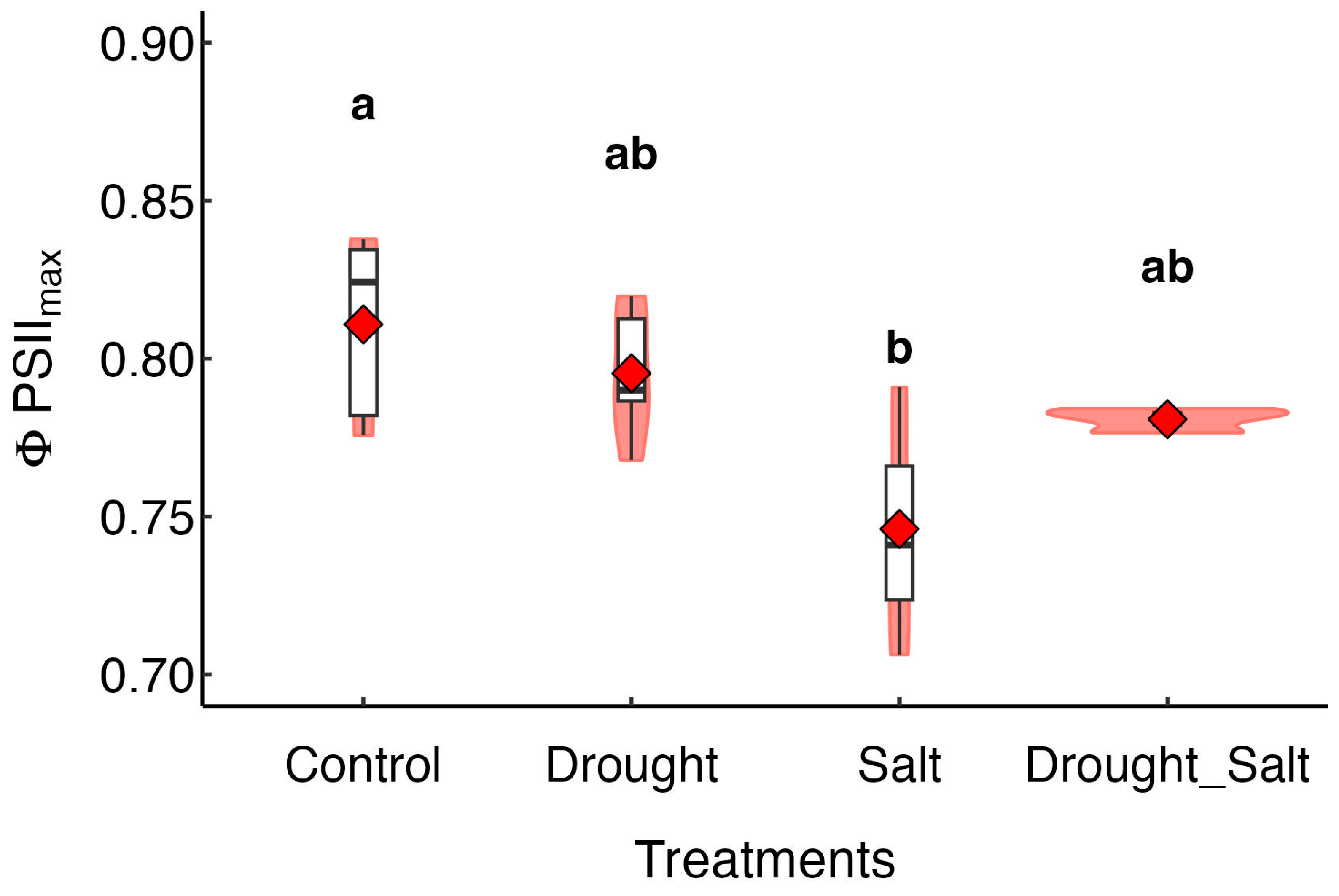
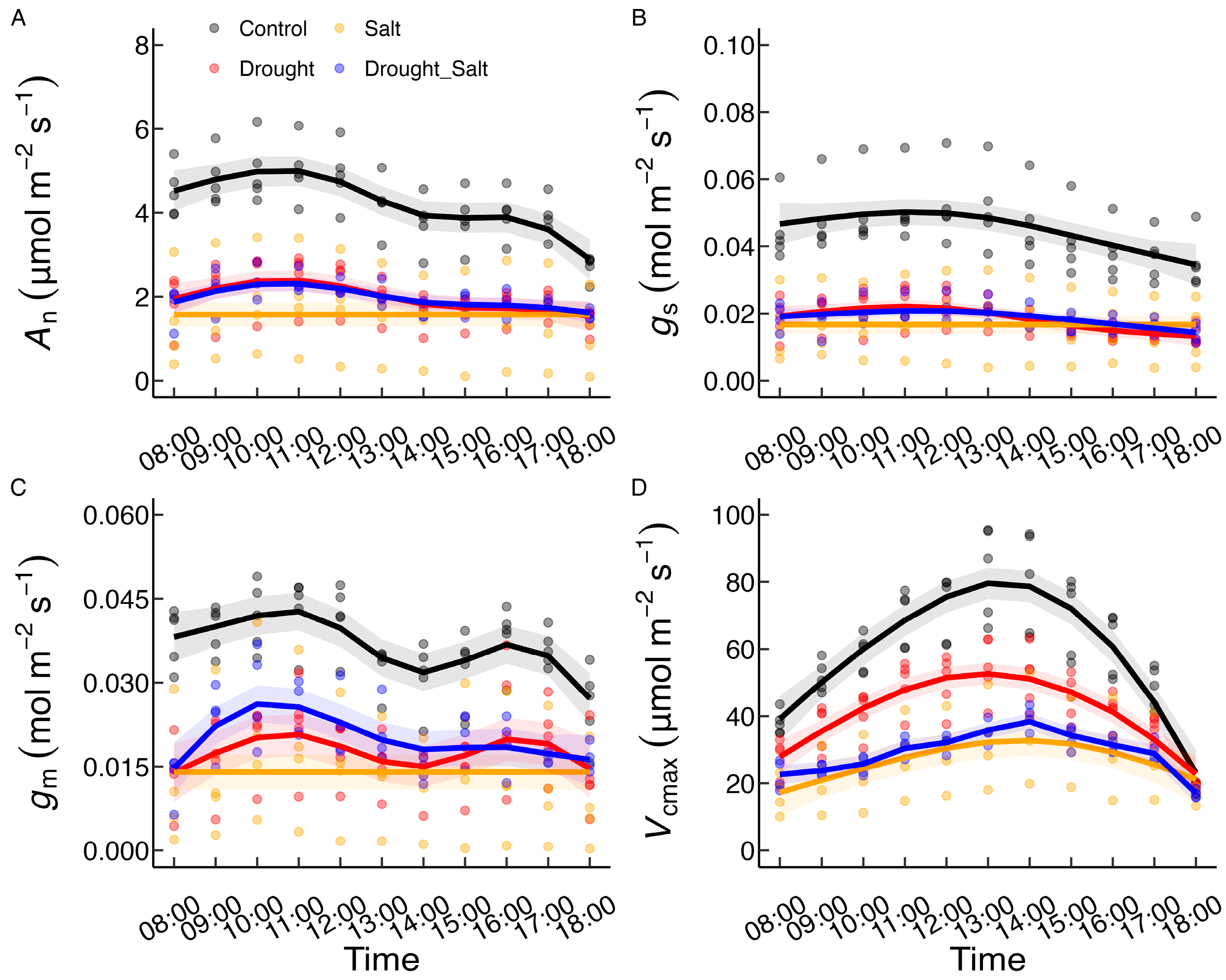
3.1. Diurnal Variations in Photosynthetic Characteristics Under Different Treatments
3.2. The Relationships Among Various Photosynthetic Characteristics Under Different Treatments
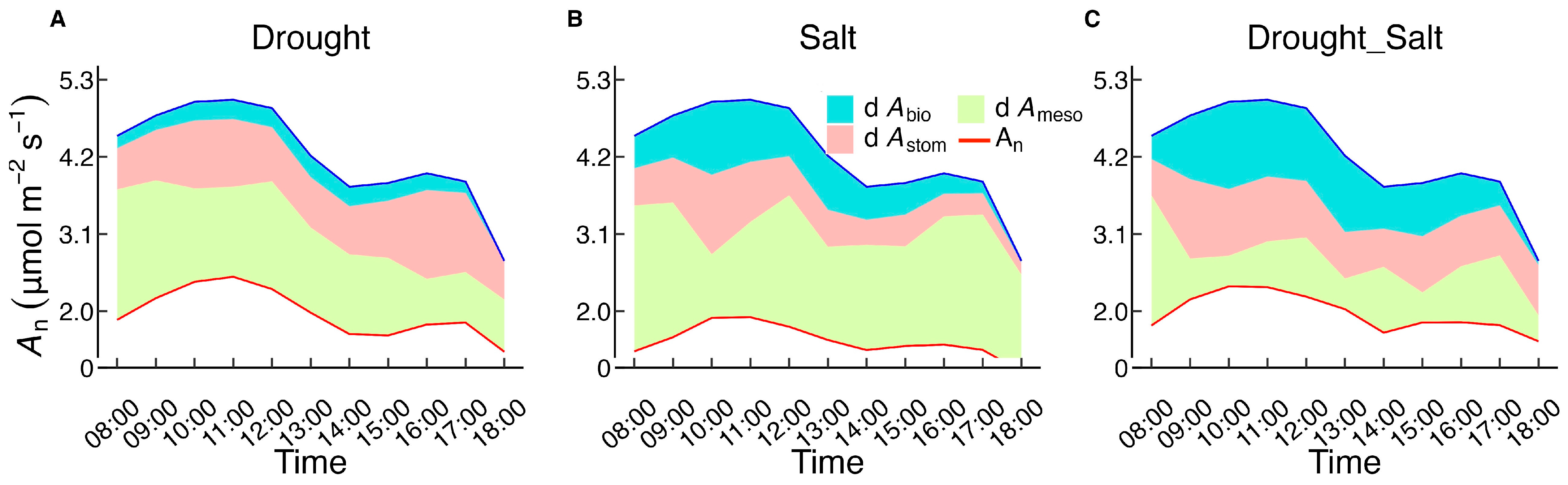
3.3. Diurnal Variations in Photosynthetic Limiting Factors of G. Biloba Under Different Treatments
3.4. Path Analysis Showing Differential Regulation of Photosynthesis by Conductance and Biochemical Factors in Stressed G. Biloba
4. Discussion
4.1. Stress-Induced Shifts in Photosynthetic Dynamics
4.2. Synergistic Alleviate of Mesophyll Limitation Under Combined Drought_salt Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Luächli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Zhang, W.F.; Xiong, D.L.; Flexas, J.; Zhang, Y.L. Mesophyll conductance and its limiting factors in plant leaves. Chin. J. Plant Ecol. 2017, 41, 914–924. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Xu, J.Y. The Photosynthetic Characteristics, Ion Homeostasis and the Correlation Between Them in GLYCINE Soja Under Salt Stress. Ph.D. Dissertation, Northeast Normal University, Changchun, China, 2016. [Google Scholar]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Han, J.M.; Lei, Z.Y.; Zhang, Y.J.; Yi, X.P.; Zhang, W.F.; Zhang, Y.L. Drought-introduced variability of mesophyll conductance in Gossypium and its relationship with leaf anatomy. Physiol. Plant. 2018, 166, 873–887. [Google Scholar] [CrossRef]
- Cheng, T.T.; Zhang, G.Z.; Zhang, S.Y.; Ai, Z.; Zhang, Y.T. Photosynthesis Diurnal Variation of Xanthoceras sorbifolia Bunge under Different Soil Water Conditions. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1828–1835. [Google Scholar]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Pons, T.L.; Welschen, R.A.M. Midday depression of net photosynthesis in the tropical rainforest tree Eperua grandiflora: Contributions of stomatal and internal conductances, respiration and Rubisco functioning. Tree Physiol. 2003, 23, 937–947. [Google Scholar] [CrossRef]
- Zhao, Y.P. Study on Photosynthetic Diurnal Variation of Salix Matsudana Under Salt Stress. Ph.D. Dissertation, Shanxi Academy of Forestry and Grassland Sciences, Taiyuan, China, 2023. [Google Scholar]
- Zhou, D.F.; Han, D.H.; Meng, H.J.; Zhao, T.; Guo, H. Study on Summer Diurnal Variation of Photosynthesis of Zhenzhuyouxing Apricot in Sandy Wasteland of Middle Heihe River. J. Northeast Agric. Sci. 2019, 47, 128–130. [Google Scholar]
- Wang, L.; Cui, J.W.; Jin, B.; Zhao, J.G.; Xu, H.M.; Lu, Z.G.; Li, W.; Li, X.; Li, L.; Liang, E.; et al. Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. USA 2020, 117, 2201–2210. [Google Scholar] [CrossRef]
- Roig-Oliver, M.; Bresta, P.; Nadal, M.; Liakopoulos, G.; Nikolopoulos, D.; Karabourniotis, G.; Bota, J.; Flexas, J. Cell wall composition and thickness affect mesophyll conductance to CO2 diffusion in Helianthus annuus under water deprivation. J. Exp. Bot. 2020, 71, 7198–7209. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, K.; Yang, X.; Su, X.; Ding, P.; Zhu, Y.; Cao, F.; Han, J. Leaf nitrogen allocation to non-photosynthetic apparatus reduces mesophyll conductance under combined drought-salt stress in Ginkgo biloba. Front. Plant Sci. 2025, 16, 1557412. [Google Scholar] [CrossRef]
- Ni, J.; Hao, J.; Jiang, Z.; Zhan, X.; Dong, L.; Yang, X.; Sun, Z.; Xu, W.; Wang, Z.; Xu, M. NaCl Induces flavonoid biosynthesis through a putative novel pathway in post-harvest Ginkgo leaves. Front. Plant Sci. 2017, 8, 920. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, F.; Yang, H.; Yue, L.; Hu, F.; Wang, J.; Luo, Y.; Cao, F. Effect of varying NaCl doses on flavonoid production in suspension cells of Ginkgo biloba: Relationship to chlorophyll fluorescence, ion homeostasis, antioxidant system and ultrastructure. Acta Physiol. Plant. 2014, 36, 3173–3187. [Google Scholar] [CrossRef]
- Shi, W.Y.; Du, Y.T.; Ma, J.; Min, D.H.; Jin, L.G.; Chen, J.; Chen, M.; Zhou, Y.B.; Ma, Y.Z.; Xu, Z.S.; et al. The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int. J. Mol. Sci. 2018, 19, 4087. [Google Scholar] [CrossRef] [PubMed]
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T.D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by the analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageoriou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. [Google Scholar]
- von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO: Collingwood, Australia, 2000. [Google Scholar]
- Walker, B.; Ariza, L.S.; Kaines, S.; Badger, M.R.; Cousins, A.B. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: Comparisons to Nicotiana tabacum. Plant Cell Environ. 2013, 36, 2108–2119. [Google Scholar] [CrossRef]
- Deans, R.M.; Brodribb, T.J.; Busch, F.A.; Farquhar, G.D. Plant water-use strategy mediates stomatal effects on the light induction of photosynthesis. New Phytol. 2019, 222, 382–395. [Google Scholar] [CrossRef]
- Deans, R.M.; Farquhar, G.D.; Busch, F.A. Estimating stomatal and biochemical limitations during photosynthetic induction. Plant Cell Environ. 2019, 42, 3227–3240. [Google Scholar] [CrossRef]
- Liu, T.; Barbour, M.M.; Yu, D.; Rao, S.; Song, X. Mesophyll conductance exerts a significant limitation on photosynthesis during light induction. New Phytol. 2022, 233, 360–372. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Nogues, S.; Alegre, L. Diurnal variations of photosynthesis and dew absorption by leaves in two evergreen shrubs growing in Mediterranean field conditions. New Phytol. 1999, 144, 109–119. [Google Scholar] [CrossRef]
- Han, J.M.; Flexas, J.; Xiong, D.L.; Galmes, J.; Zhang, Y. Regulation of photosynthesis and water-use efficiency in pima and upland cotton species subjected to drought and recovery. Photosynthetica 2024, 62, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, W.T.; Sun, Y.R.; Xu, Y.N.; Li, L.; Miao, G.; Tcherkez, G.; Gong, X.Y. The response of mesophyll conductance to short-term CO2 variation is related to stomatal conductance. Plant Cell Environ. 2024, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhao, P.; Wu, K.; Yang, H.; Yang, Q.; Fan, M.; Long, G. Reduced and deep application of controlled-release urea maintained yield and improved nitrogen-use efficiency. Field Crops Res. 2023, 295, 108876. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signaling: The coordination of responses to stress in plants. J. Exp. Bot. 2002, 53, 1143–1154. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Sarwas, M.K.S.; Ullah, I.; Rahman, M.U.; Ashraf, M.Y.; Zafar, Y. Glycine betaine accumulation and its relation to yield and yield components in cotton genotypes grown under water deficit conditions. Pak. J. Bot. 2006, 38, 1449–1456. [Google Scholar]
- Chen, T.H.H.; Murata, N. Glycine betaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2010, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jarin, A.; Ghosh, U.K.; Hossain, M.S.; Mahmud, A.; Khan, M.A.R. Glycine betaine in plant responses and tolerance to abiotic stresses Afsana. Disc. Agric. 2024, 2, 127. [Google Scholar] [CrossRef]
- Hussain, T.; Asrar, H.; Zhang, W.; Liu, X. The combination of salt and drought benefits selective ion absorption and nutrient use efficiency of halophyte Panicum antidotale. Front. Plant Sci. 2023, 14, 1091292. [Google Scholar] [CrossRef]
- Hussain, T.; Koyro, H.W.; Zhang, W.; Liu, X.; Gul, B.; Liu, X. Low salinity improves photosynthetic performance in Panicum antidotale under drought stress. Front. Plant Sci. 2000, 11, 481. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Tansley review Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants: Stress combination in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]

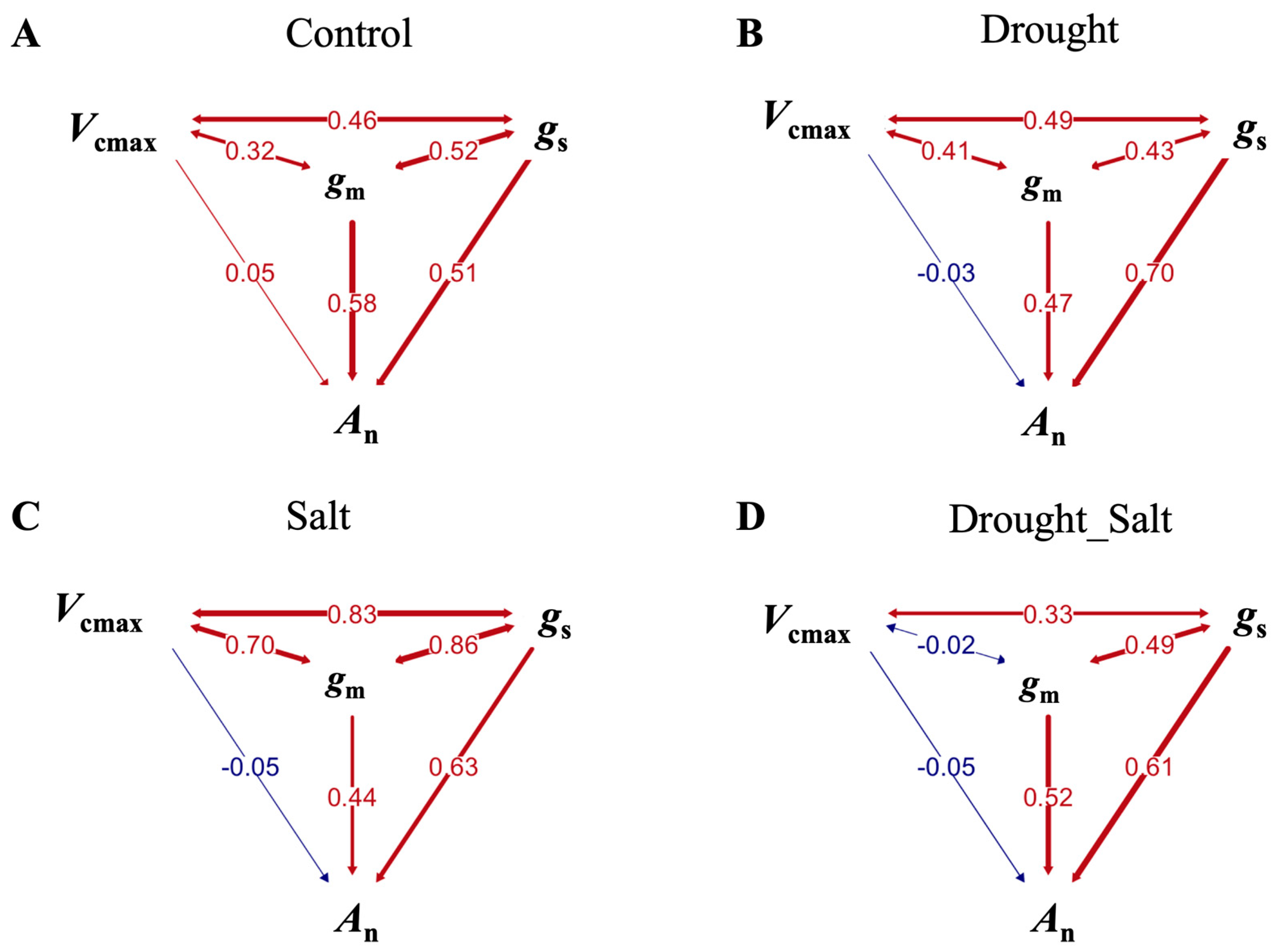
| Treatments | Path | Direct Effect (Standardized β ± Bootstrap Standard Error) | Indirect Effect (Standardized β ± Bootstrap Standard Error) | Significance | Total Effect (Standardized β ± Bootstrap Standard Error) (Significance) |
|---|---|---|---|---|---|
| Control | gs→An | 0.51 ± 0.05 | / | *** | 0.82 ± 0.09 (***) |
| gs→gm→An | / | 0.30 ± 0.08 | *** | ||
| gs→Vcmax→An | / | 0.02 ± 0.02 | ns | ||
| gm→An | 0.58 ± 0.04 | / | *** | 0.86 ± 0.08 (***) | |
| gm→gs→An | / | 0.26 ± 0.08 | ** | ||
| gm→Vcmax→An | / | 0.02 ± 0.02 | ns | ||
| Vcmax→An | 0.05 ± 0.05 | / | ns | 0.46 ± 0.14 (**) | |
| Vcmax→gs→An | / | 0.23 ± 0.07 | ** | ||
| Vcmax→gm→An | / | 0.18 ± 0.08 | * | ||
| Drought | gs→An | 0.70 ± 0.04 | / | *** | 0.89 ± 0.06 (***) |
| gs→gm→An | / | 0.20 ± 0.08 | * | ||
| gs→Vcmax→An | / | –0.01 ± 0.01 | ns | ||
| gm→An | 0.47 ± 0.04 | / | *** | 0.75 ± 0.11 (***) | |
| gm→gs→An | / | 0.29 ± 0.09 | ** | ||
| gm→Vcmax→An | / | –0.01 ± 0.01 | ns | ||
| Vcmax→An | –0.03 ± 0.03 | / | ns | 0.50 ± 0.12 (***) | |
| Vcmax→gs→An | / | 0.34 ± 0.09 | *** | ||
| Vcmax→gm→An | / | 0.19 ± 0.06 | ** | ||
| Salt | gs→An | 0.63 ± 0.09 | / | *** | 0.96 ± 0.07 (***) |
| gs→gm→An | / | 0.37 ± 0.10 | *** | ||
| gs→Vcmax→An | / | –0.04 ± 0.04 | ns | ||
| gm→An | 0.44 ± 0.78 | / | *** | 0.94 ± 0.09 (***) | |
| gm→gs→An | / | 0.53 ± 0.12 | *** | ||
| gm→Vcmax→An | / | –0.03 ± 0.03 | ns | ||
| Vcmax→An | –0.05 ± 0.04 | / | ns | 0.76 ± 0.13 (***) | |
| Vcmax→gs→An | / | 0.51 ± 0.11 | *** | ||
| Vcmax→gm→An | / | 0.30 ± 0.08 | *** | ||
| Drought_Salt | gs→An | 0.61 ± 0.05 | / | *** | 0.84 ± 0.09 (***) |
| gs→gm→An | / | 0.25 ± 0.09 | ** | ||
| gs→Vcmax→An | / | –0.02 ± 0.02 | ns | ||
| gm→An | 0.52 ± 0.06 | / | *** | 0.81 ± 0.11 (***) | |
| gm→gs→An | / | 0.29 ± 0.10 | ** | ||
| gm→Vcmax→An | / | 0.00 ± 0.01 | ns | ||
| Vcmax→An | –0.05 ± 0.04 | / | ns | 0.14 ± 0.16 (na) | |
| Vcmax→gs→An | / | 0.20 ± 0.09 | * | ||
| Vcmax→gm→An | / | –0.01 ± 0.08 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wu, Y.; Liang, S.; Li, L.; Zhu, Y.; Ding, P.; Liu, C.; Tang, S.; Han, J. Compensatory Regulation and Temporal Dynamics of Photosynthetic Limitations in Ginkgo Biloba Under Combined Drought–Salt Stress. Forests 2025, 16, 1334. https://doi.org/10.3390/f16081334
Meng Y, Wu Y, Liang S, Li L, Zhu Y, Ding P, Liu C, Tang S, Han J. Compensatory Regulation and Temporal Dynamics of Photosynthetic Limitations in Ginkgo Biloba Under Combined Drought–Salt Stress. Forests. 2025; 16(8):1334. https://doi.org/10.3390/f16081334
Chicago/Turabian StyleMeng, Yuxuan, Yang Wu, Shengjie Liang, Lehao Li, Ying Zhu, Peng Ding, Chenhang Liu, Sunjie Tang, and Jimei Han. 2025. "Compensatory Regulation and Temporal Dynamics of Photosynthetic Limitations in Ginkgo Biloba Under Combined Drought–Salt Stress" Forests 16, no. 8: 1334. https://doi.org/10.3390/f16081334
APA StyleMeng, Y., Wu, Y., Liang, S., Li, L., Zhu, Y., Ding, P., Liu, C., Tang, S., & Han, J. (2025). Compensatory Regulation and Temporal Dynamics of Photosynthetic Limitations in Ginkgo Biloba Under Combined Drought–Salt Stress. Forests, 16(8), 1334. https://doi.org/10.3390/f16081334






