Climate–Growth Sensitivity Reveals Species-Specific Adaptation Strategies of Montane Conifers to Warming in the Wuyi Mountains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
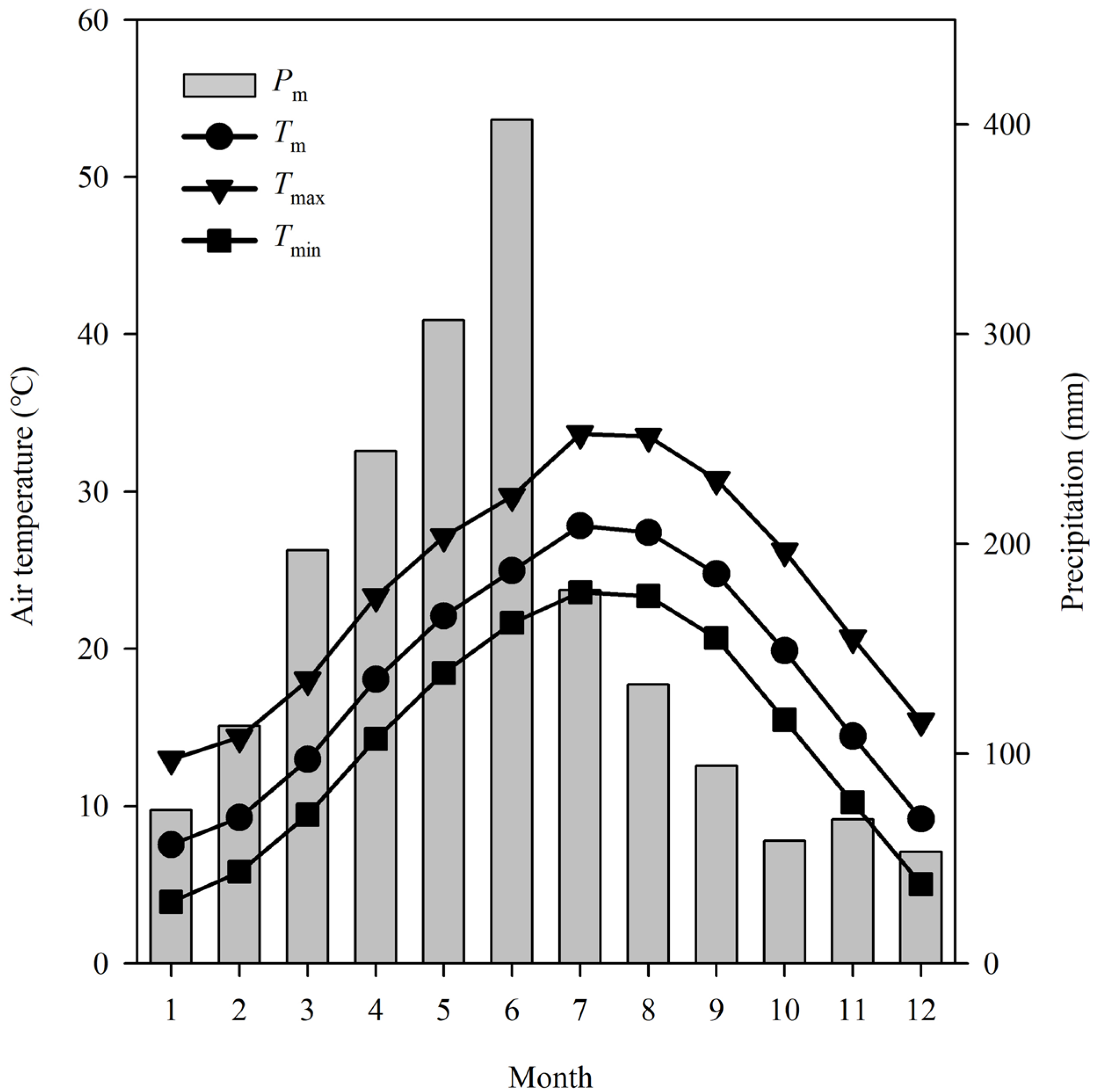
2.2. Regional Climate Data
2.3. Tree-Ring Sampling and Chronology Development
2.4. Data and Statistical Analysis
3. Results
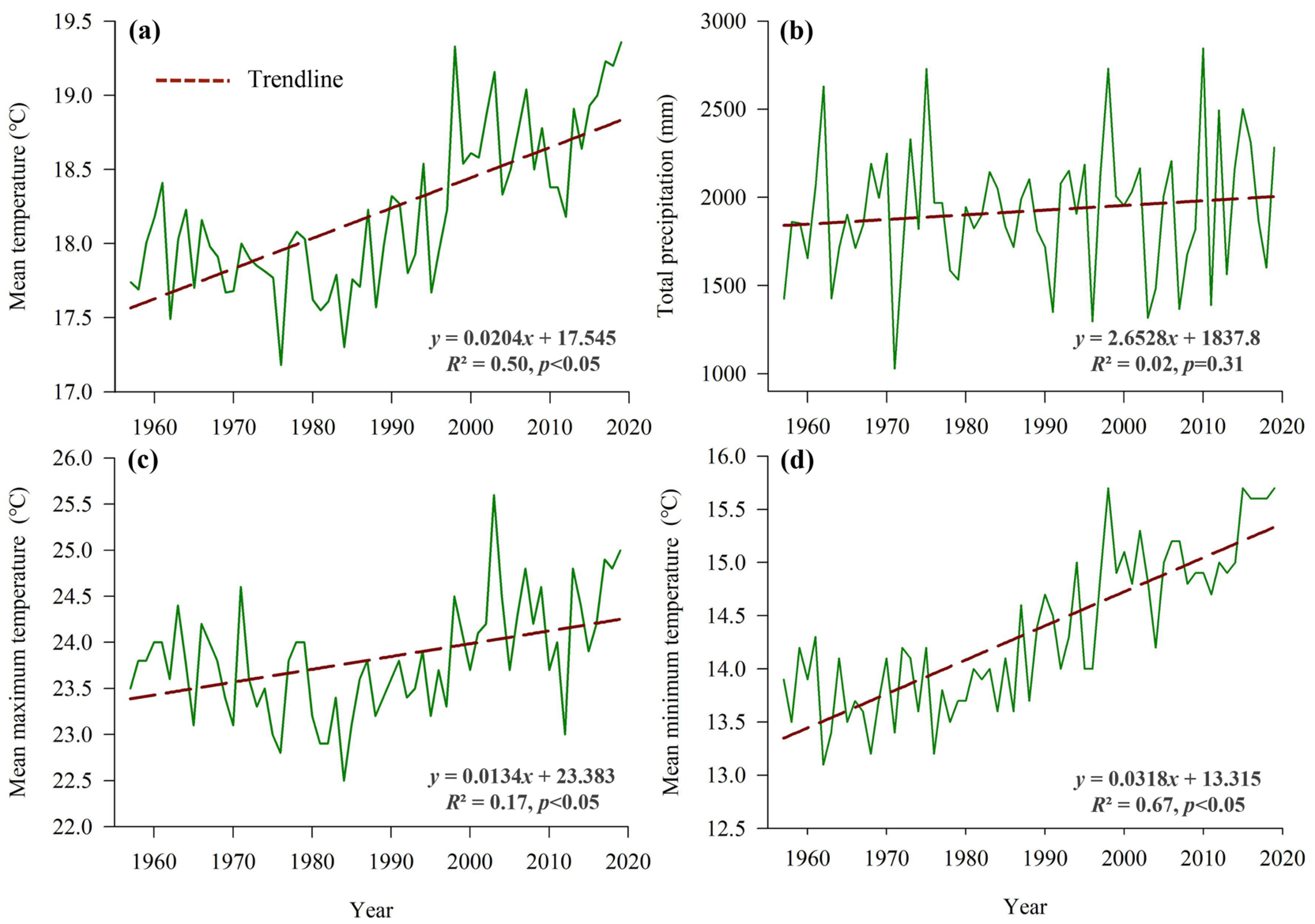
3.1. Climate Change Trends
3.2. Statistics of Chronology Characteristics
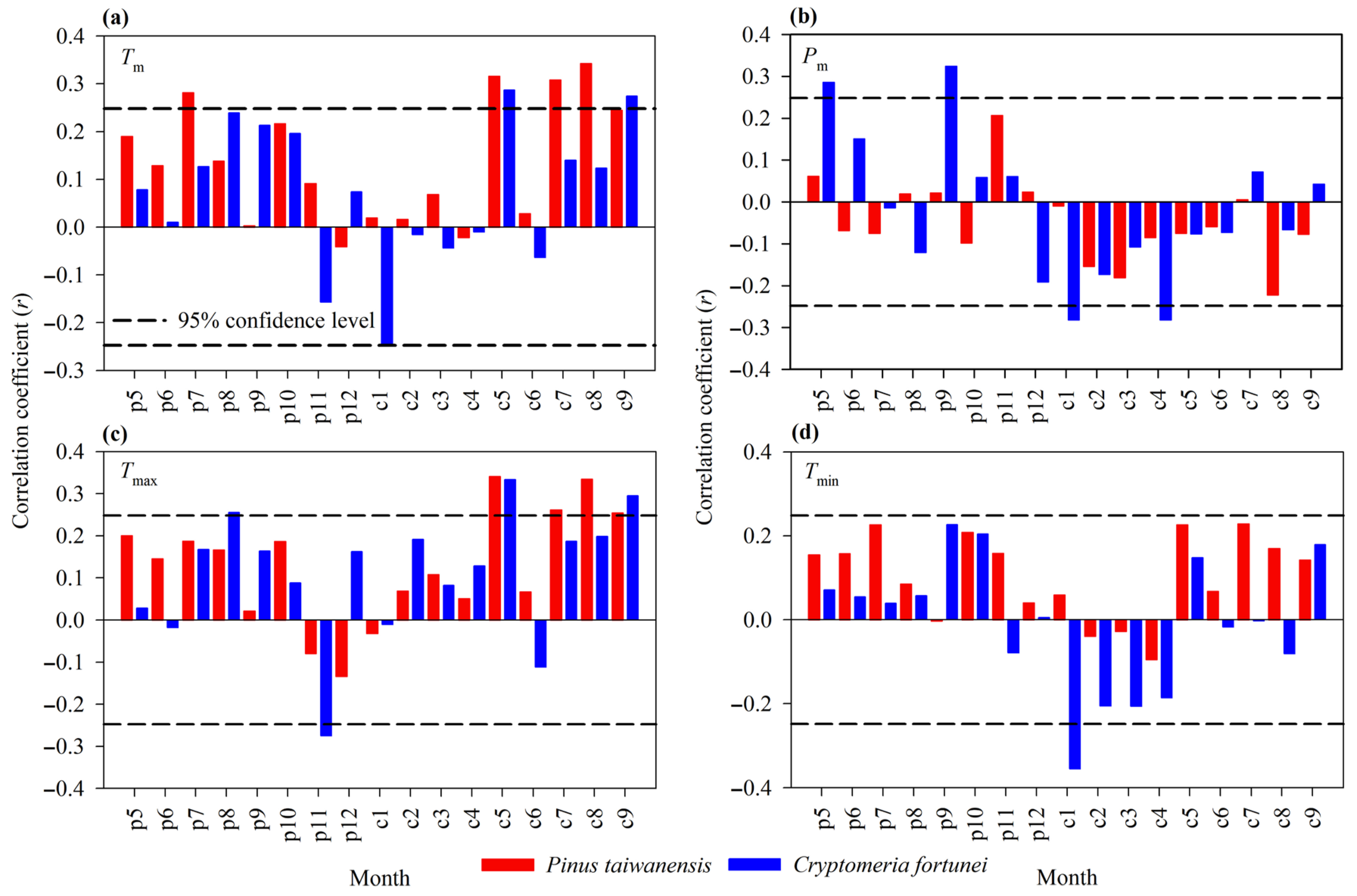
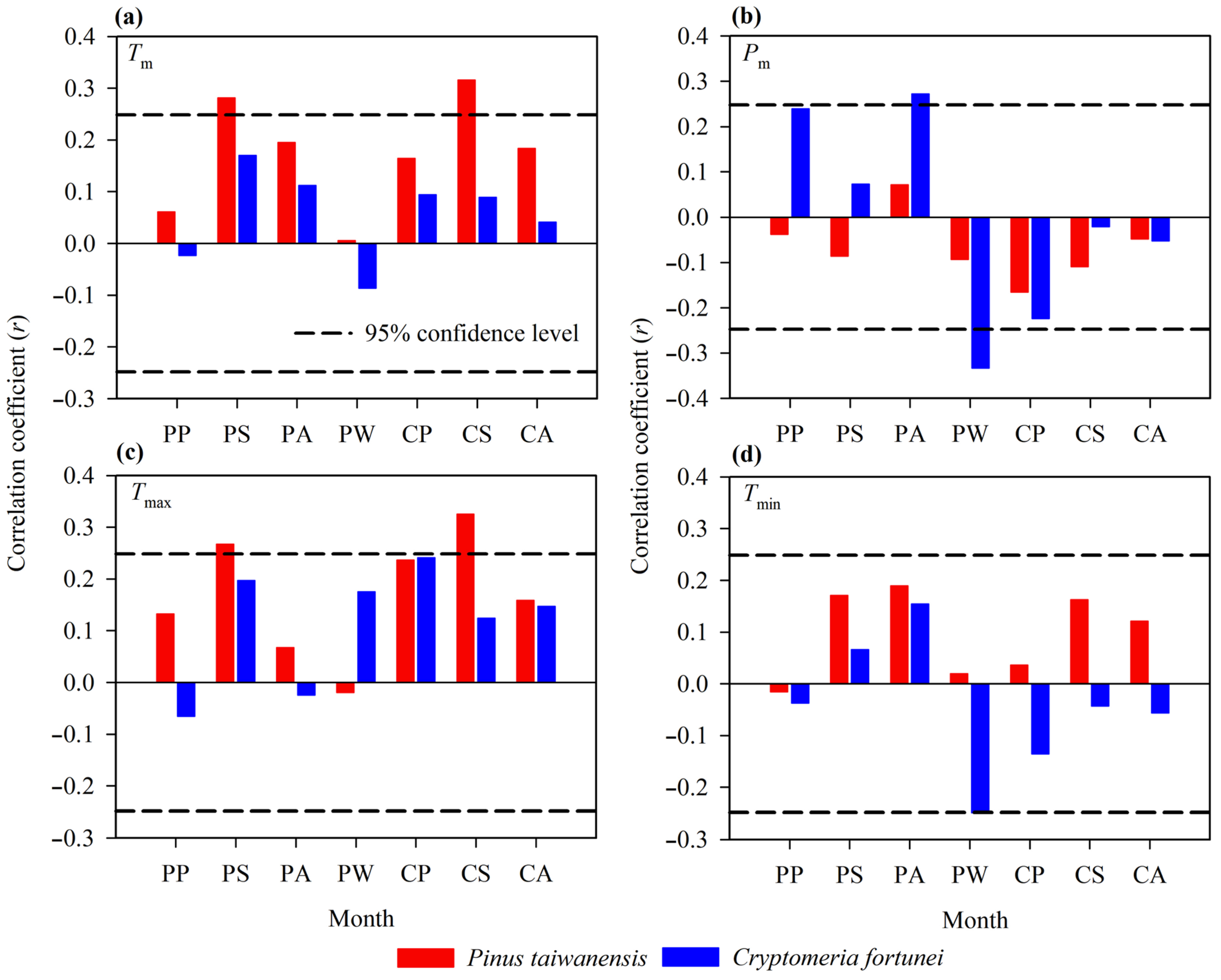
3.3. Relationship Between Tree Growth and Monthly Climatic Factors
3.4. Relationship Between Tree Growth and Seasonal Climatic Factors
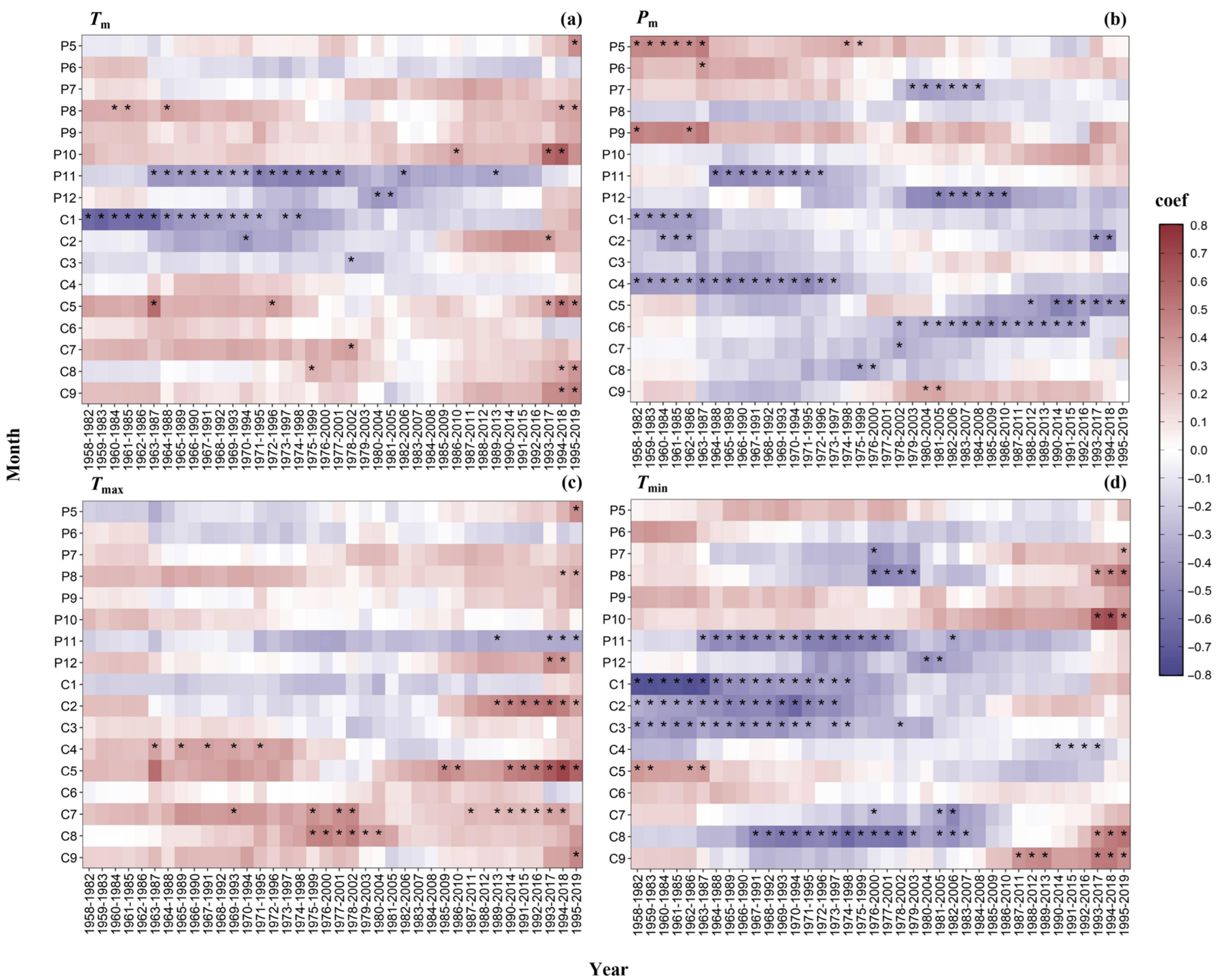
3.5. Temporal Stability Analysis of Tree Growth-Climate Relationships
4. Discussion
4.1. Climate–Growth Relationships Across Tree Species
4.2. Stability of Radial Climate–Growth Relationships Across Tree Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022-Impacts, Adaptation and Vulnerability: WorkingGroup II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814. [Google Scholar] [CrossRef]
- Borgaonkar, H.P.; Sikder, A.B.; Ram, S. High altitude forest sensitivity to the recent warming: A tree-ring analysis of conifers from Western Himalaya, India. Quat. Int. 2011, 236, 158–166. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Konings, A.G.; Shaw, J. Divergent forest sensitivity to repeated extreme droughts. Nat. Clim. Chang. 2020, 10, 1091–1095. [Google Scholar] [CrossRef]
- Gao, L.; Gou, X.; Deng, Y.; Yang, M.; Zhang, F. Assessing the influences of tree species, elevation and climate on tree-ring growth in the Qilian Mountains of northwest China. Trees 2017, 31, 393–404. [Google Scholar] [CrossRef]
- Lloyd, A.H.; Bunn, A.G.; Berner, L. A latitudinal gradient in tree growth response to climate warming in the Siberian taiga. Glob. Chang. Biol. 2011, 17, 1935–1945. [Google Scholar] [CrossRef]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef]
- Cai, Q.; Ma, S.; Sun, L.; Chen, G.; Xiao, J.; Fang, W.; Ji, C.; Tang, Z.; Fang, J. Elevational Patterns of Tree Species Richness and Forest Biomass on Two Subtropical Mountains in China. Forests 2023, 14, 1337. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Q.J.; Meng, S.W.; Zhou, G.; Shah, S.; Xu, Z.Z. Summer temperature variability inferred from tree-ring records in the central Hengduan Mountains, southeastern Tibetan Plateau. Dendrochronologia 2018, 51, 92–100. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, G.; Liu, Q.J. Tree-ring based summer temperature regime reconstruction in XiaoXing Anling Mountains, northeastern China since 1772 CE. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 495, 13–23. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Zhang, T.W. Reconstructed temperature for Yong’an, Fujian, Southeast China: Linkages to the Pacific Ocean climate variability. Glob. Planet. Chang. 2012, 86–87, 11–19. [Google Scholar] [CrossRef]
- Su, J.; Gou, X.; Hille Ris Lambers, J.; Zhang, D.D.; Zheng, W.; Xie, M.; Manzanedo, R.D. Increasing ENSO variability synchronizes tree growth in subtropical forests. Agric. For. Meteorol. 2024, 345, 109830. [Google Scholar] [CrossRef]
- Wang, X.P.; Wang, Z.H.; Fang, J.Y. Mountain ranges and peaks in China. Biodivers. Sci. 2004, 12, 206–212. [Google Scholar] [CrossRef]
- Chen, S.F.; Xu, H.; Lin, W.J.; Zheng, X.; Xu, X.J.; Liu, W.F.; Ding, H.; Chen, S.P. Variation analysis on species diversity of plant communities along the elevation gradient in Wuyishan National Park. J. Plant Resour. Environ. 2023, 32, 1–9. [Google Scholar]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Zhang, T.W. Tree ring-based winter temperature reconstruction for Changting, Fujian, subtropical region of Southeast China, since 1850: Linkages to the Pacific Ocean. Theor. Appl. Climatol. 2011, 109, 141–151. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1968. [Google Scholar]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology; Springer: New York, NY, USA, 1990. [Google Scholar]
- Fritts, H.C. Growth-Rings of Trees-Their Correlation Withclimate. Science 1966, 154, 973–979. [Google Scholar] [CrossRef]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y. Two centuries temperature variations over subtropical southeast China inferred from Pinus taiwanensis Hayata tree-ring width. Clim. Dyn. 2016, 48, 1813–1825. [Google Scholar] [CrossRef]
- Wang, F.; Arseneault, D.; Pan, B.; Liao, Q.; Sugiyama, J. Pre-1930 unstable relationship between climate and tree-ring width of Pinus taiwanensis Hayata in southeastern China. Dendrochronologia 2019, 57, 125629. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Wang, H.Q. Tree-ring response of subtropical tree species in southeast China on regional climate and sea-surface temperature variations. Trees 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.; Zhao, B.; Li, Z.; Drew, D.M.; Zhao, X. Species-specific and elevation-differentiated responses of tree growth to rapid warming in a mixed forest lead to a continuous growth enhancement in semi-humid Northeast Asia. For. Ecol. Manag. 2019, 448, 76–84. [Google Scholar] [CrossRef]
- Xiao, S.C.; Ding, A.J.; Tian, Q.Y.; Han, C.; Peng, X.M. Site- and species-specific climatic responses of two co-occurring shrubs in the temperate Alxa Desert Plateau, northwest China. Sci. Total Environ. 2019, 667, 77–85. [Google Scholar] [CrossRef]
- Dawes, M.A.; Haettenschwiler, S.; Bebi, P.; Hagedorn, F.; Handa, I.T.; Koerner, C.; Rixen, C. Species-specific tree growth responses to 9 years of CO2 enrichment at the alpine treeline. J. Ecol. 2011, 99, 383–394. [Google Scholar] [CrossRef]
- Kašpar, J.; Tumajer, J.; Šamonil, P.; Vašíčková, I. Species-specific climate–growth interactions determine tree species dynamics in mixed Central European mountain forests. Environ. Res. Lett. 2021, 16, 034039. [Google Scholar] [CrossRef]
- Yuan, D.; Zhu, L.; Cherubini, P.; Li, Z.; Zhang, Y.; Wang, X. Species-specific indication of 13 tree species growth on climate warming in temperate forest community of northeast China. Ecol. Indic. 2021, 133, 108389. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Duan, B.; Sun, C. Regional difference of the start time of the recent warming in Eastern China: Prompted by a 165-year temperature record deduced from tree rings in the Dabie Mountains. Clim. Dyn. 2018, 50, 2157–2168. [Google Scholar] [CrossRef]
- Chen, F.; Yu, S.; Yuan, Y.; Wang, H.; Gagen, M. A tree-ring width based drought reconstruction for southeastern China: Links to Pacific Ocean climate variability. Boreas 2016, 45, 335–346. [Google Scholar] [CrossRef]
- Hamrick, J.L. Response of forest trees to global environmental changes. For. Ecol. Manag. 2004, 197, 323–335. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Toledo, M.; Poorter, L.; Peña-Claros, M.; Alarcón, A.; Balcázar, J.; Leaño, C.; Licona, J.C.; Llanque, O.; Vroomans, V.; Zuidema, P.; et al. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 2011, 99, 254–264. [Google Scholar] [CrossRef]
- Trahan, M.W.; Schubert, B.A. Temperature-induced water stress in high-latitude forests in response to natural and anthropogenic warming. Glob. Chang. Biol. 2016, 22, 782–791. [Google Scholar] [CrossRef]
- Fonti, P.; Jansen, S. Xylem plasticity in response to climate. New Phytol. 2012, 195, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate Change Impacts in Alpine Environments. Geogr. Compass. 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
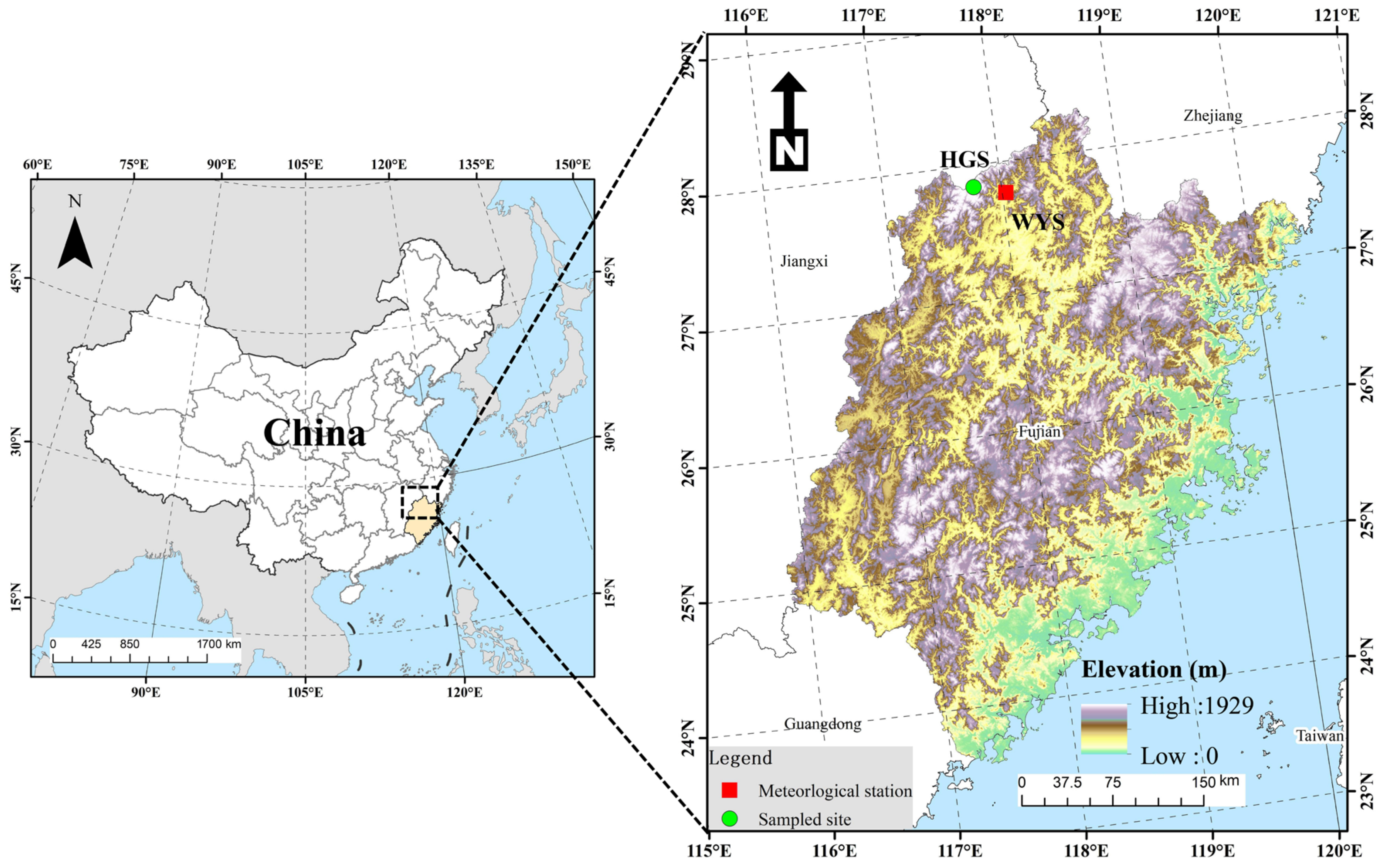


| Tree Species | Latitude | Longitude | Elevation (m) | DBH (cm) | Slope (°) |
|---|---|---|---|---|---|
| Pinus taiwanensis | 27°50′25.42″ N | 117°45′25.57″ E | 1863 | 33.7 | 44 |
| Cryptomeria fortunei | 27°50′39.60″ N | 117°45′39.88″ E | 1782 | 47.3 | 37 |
| Tree-Ring Parameters | P. taiwanensis | C. fortunei |
|---|---|---|
| Sample size (tree/core) | 45/45 | 45/45 |
| Mean | 0.9956 | 0.9839 |
| Time span | 1912–2023 | 1857–2023 |
| Standard deviation (SD) | 0.2537 | 0.3246 |
| Mean sensitivity (MS) | 0.1722 | 0.1810 |
| Autocorrelation order 1 | 0.5921 | 0.7118 |
| All series mean correlation (R) | 0.300 | 0.254 |
| Signal-to-noise ratio (SNR) | 15.415 | 10.871 |
| Expressed population signal (EPS) | 0.939 | 0.916 |
| Variance in first eigenvector | 35.84% | 37.35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Yu, J.; Hu, Y.; Zhou, X.; Ding, H.; Ge, X. Climate–Growth Sensitivity Reveals Species-Specific Adaptation Strategies of Montane Conifers to Warming in the Wuyi Mountains. Forests 2025, 16, 1299. https://doi.org/10.3390/f16081299
Zheng X, Yu J, Hu Y, Zhou X, Ding H, Ge X. Climate–Growth Sensitivity Reveals Species-Specific Adaptation Strategies of Montane Conifers to Warming in the Wuyi Mountains. Forests. 2025; 16(8):1299. https://doi.org/10.3390/f16081299
Chicago/Turabian StyleZheng, Xiao, Jian Yu, Yaping Hu, Xu Zhou, Hui Ding, and Xiaomin Ge. 2025. "Climate–Growth Sensitivity Reveals Species-Specific Adaptation Strategies of Montane Conifers to Warming in the Wuyi Mountains" Forests 16, no. 8: 1299. https://doi.org/10.3390/f16081299
APA StyleZheng, X., Yu, J., Hu, Y., Zhou, X., Ding, H., & Ge, X. (2025). Climate–Growth Sensitivity Reveals Species-Specific Adaptation Strategies of Montane Conifers to Warming in the Wuyi Mountains. Forests, 16(8), 1299. https://doi.org/10.3390/f16081299







