Change Patterns of Understory Vegetation Diversity and Rhizosphere Soil Microbial Community Structure in a Chronosequence of Phellodendron chinense Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Soil Sampling and Analysis
2.4. Species Diversity Analysis
2.5. High-Throughput Microbial Sequencing
2.6. Data Analysis
- (1)
- [22]
- (2)
- S: total number of species; F1: the number of species consisting of only one individual; F2: the number of species consisting of only two individuals.
3. Results and Analysis
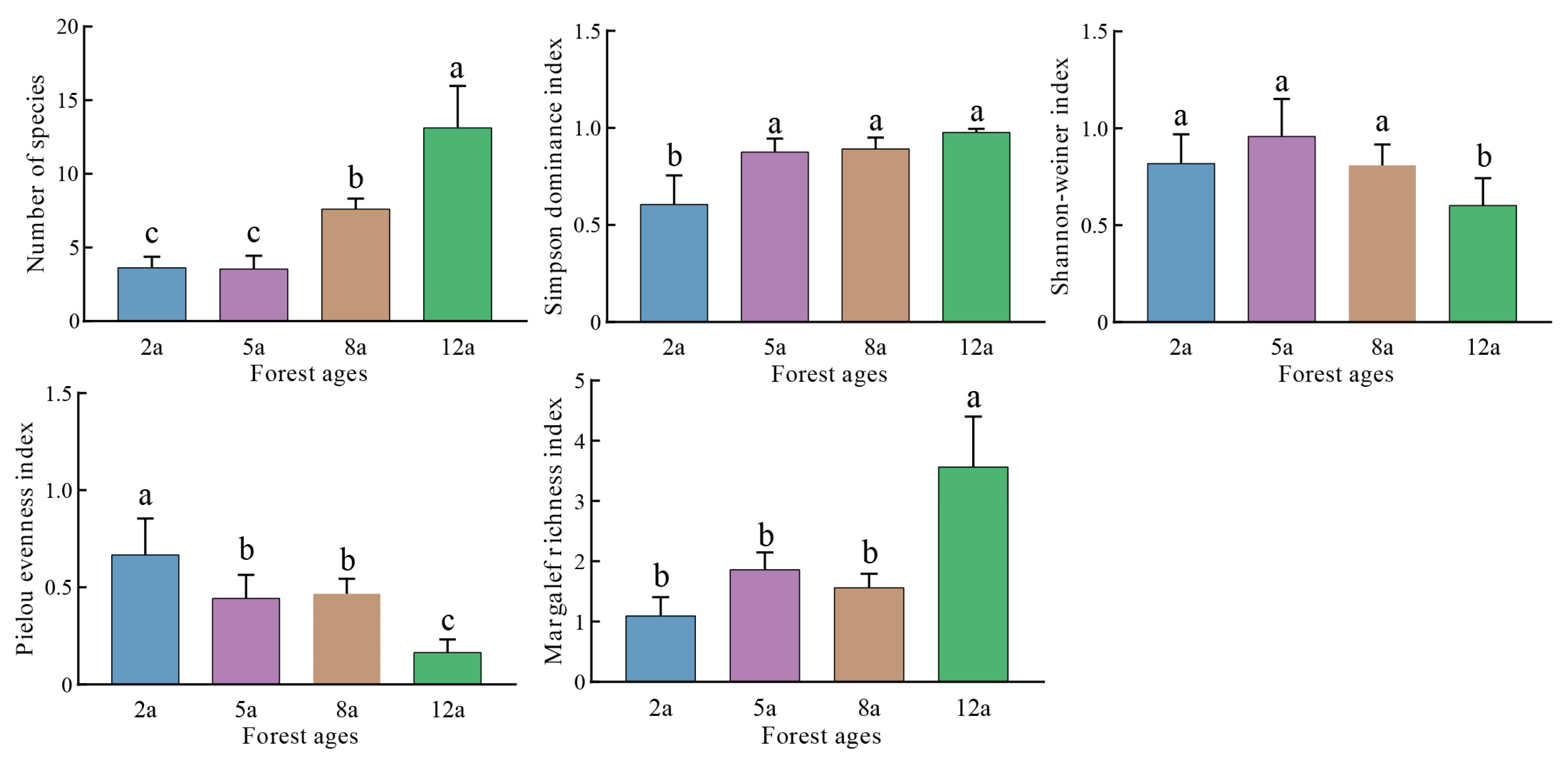
3.1. Understory Species Composition and Plant Diversity Across Stand Ages
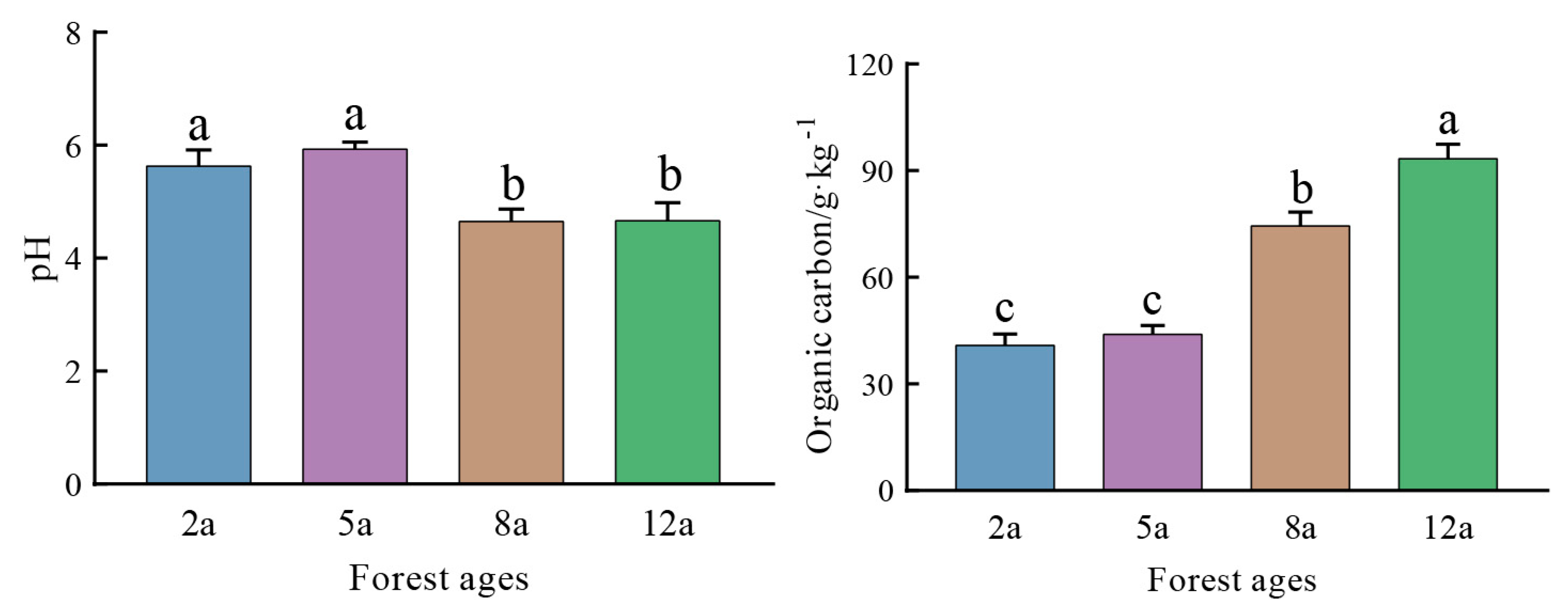
3.2. Variations in Soil Physicochemical Properties Across Stand Ages
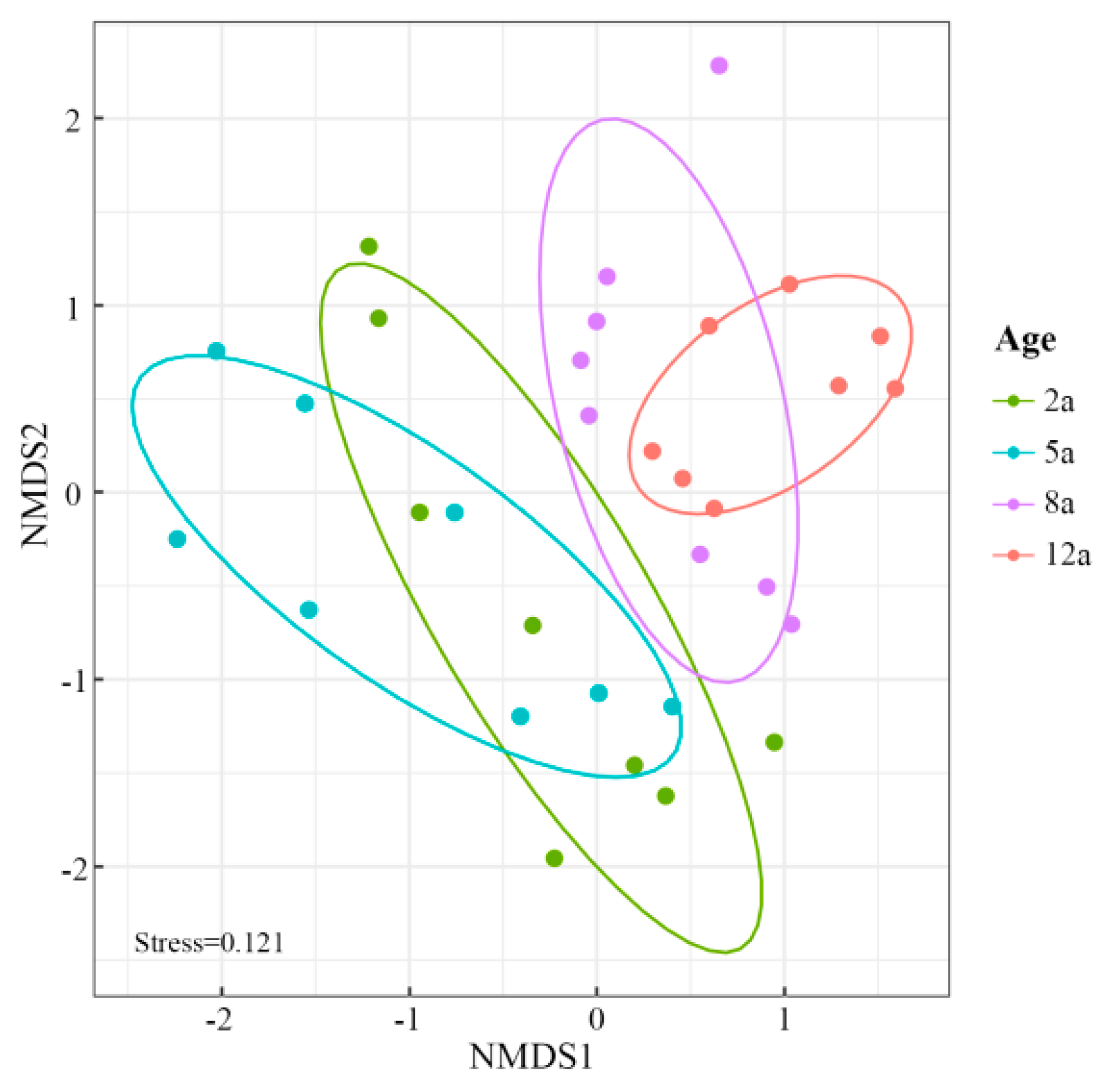
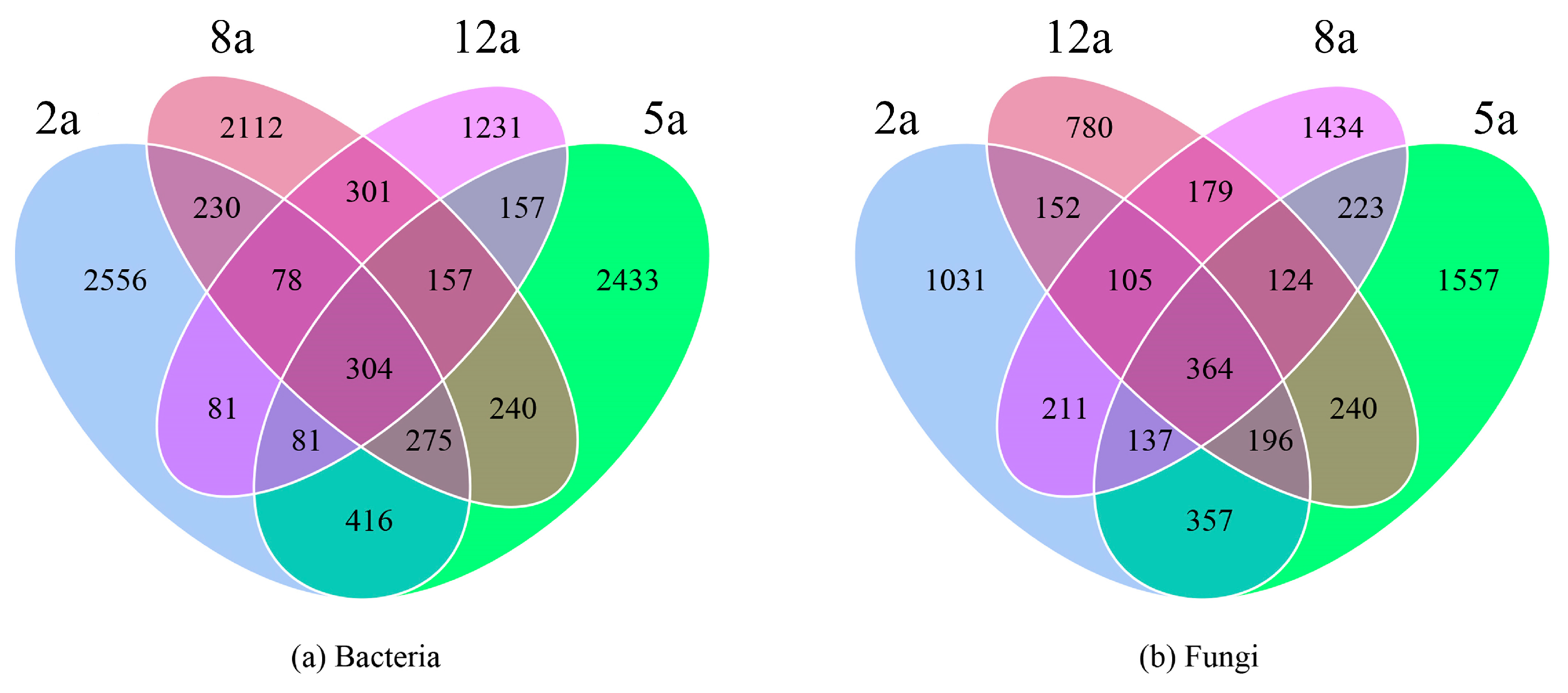
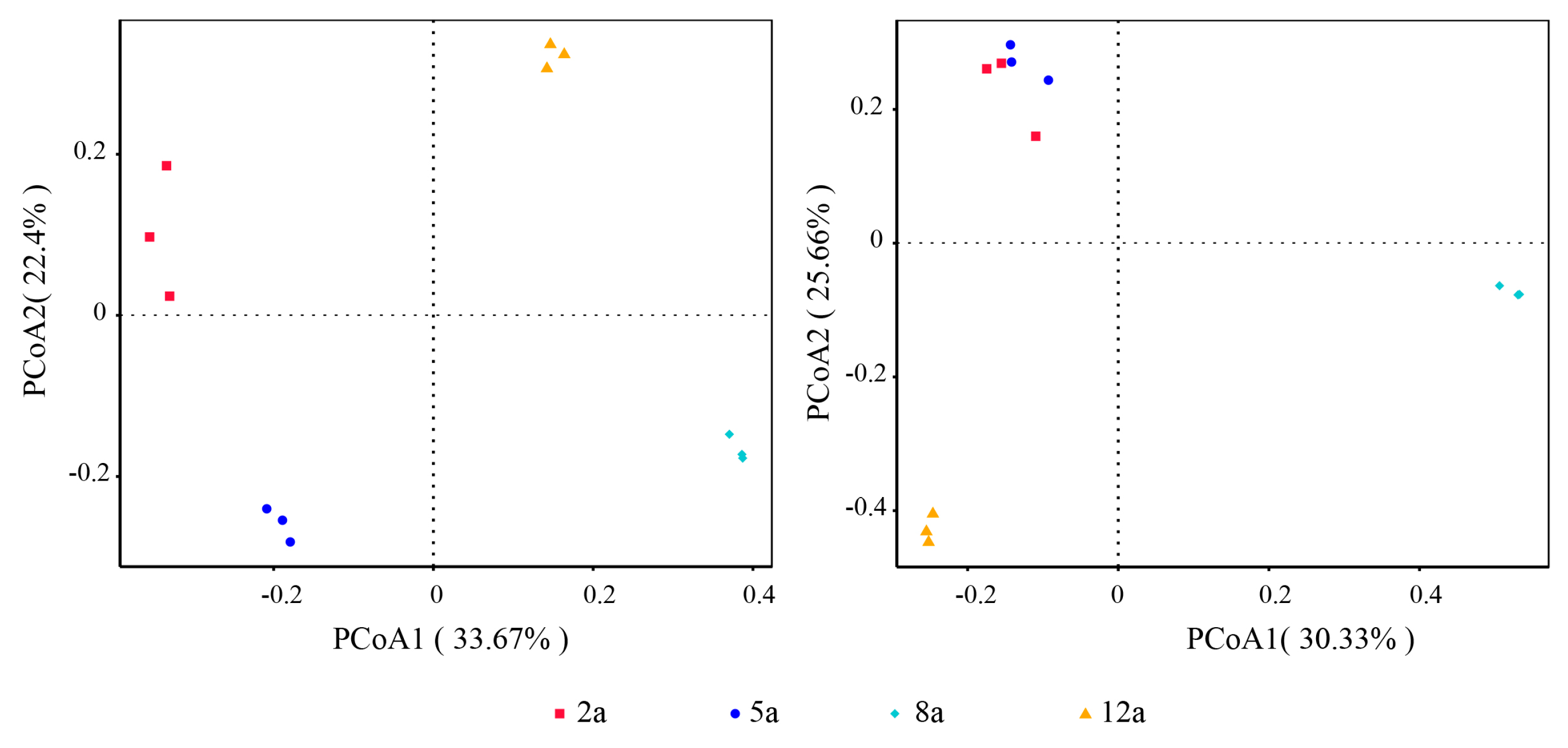
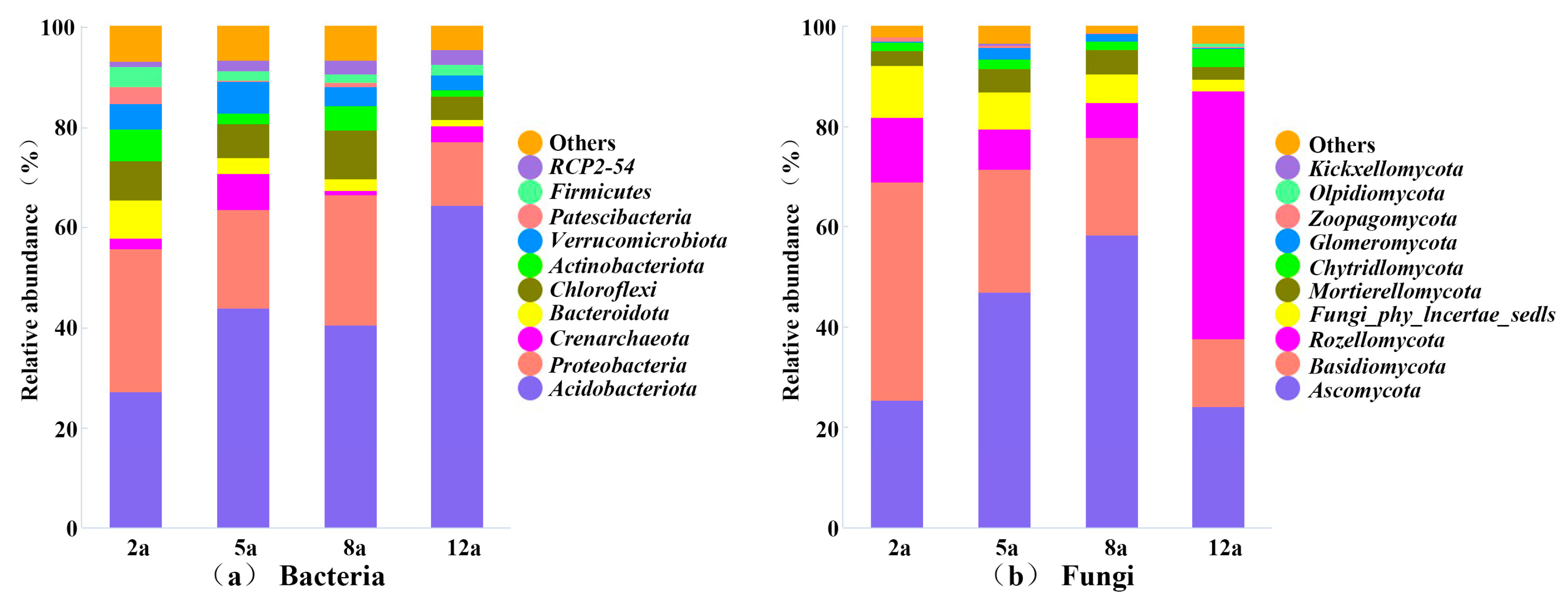
3.3. Rhizosphere Soil Microbial Community Structure Analysis
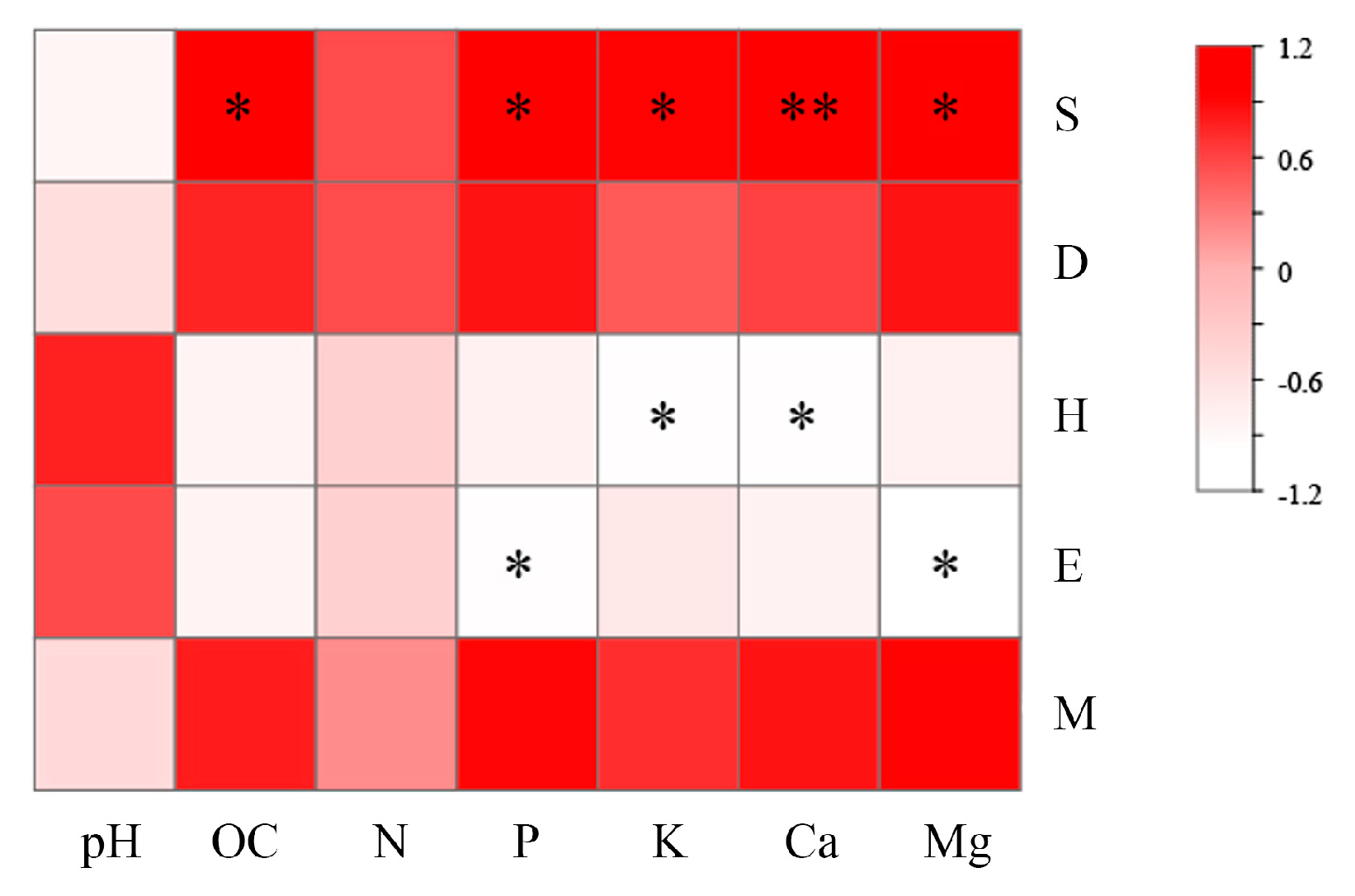
3.4. Correlation Analysis Between Soil Properties and Microbial Community Diversity
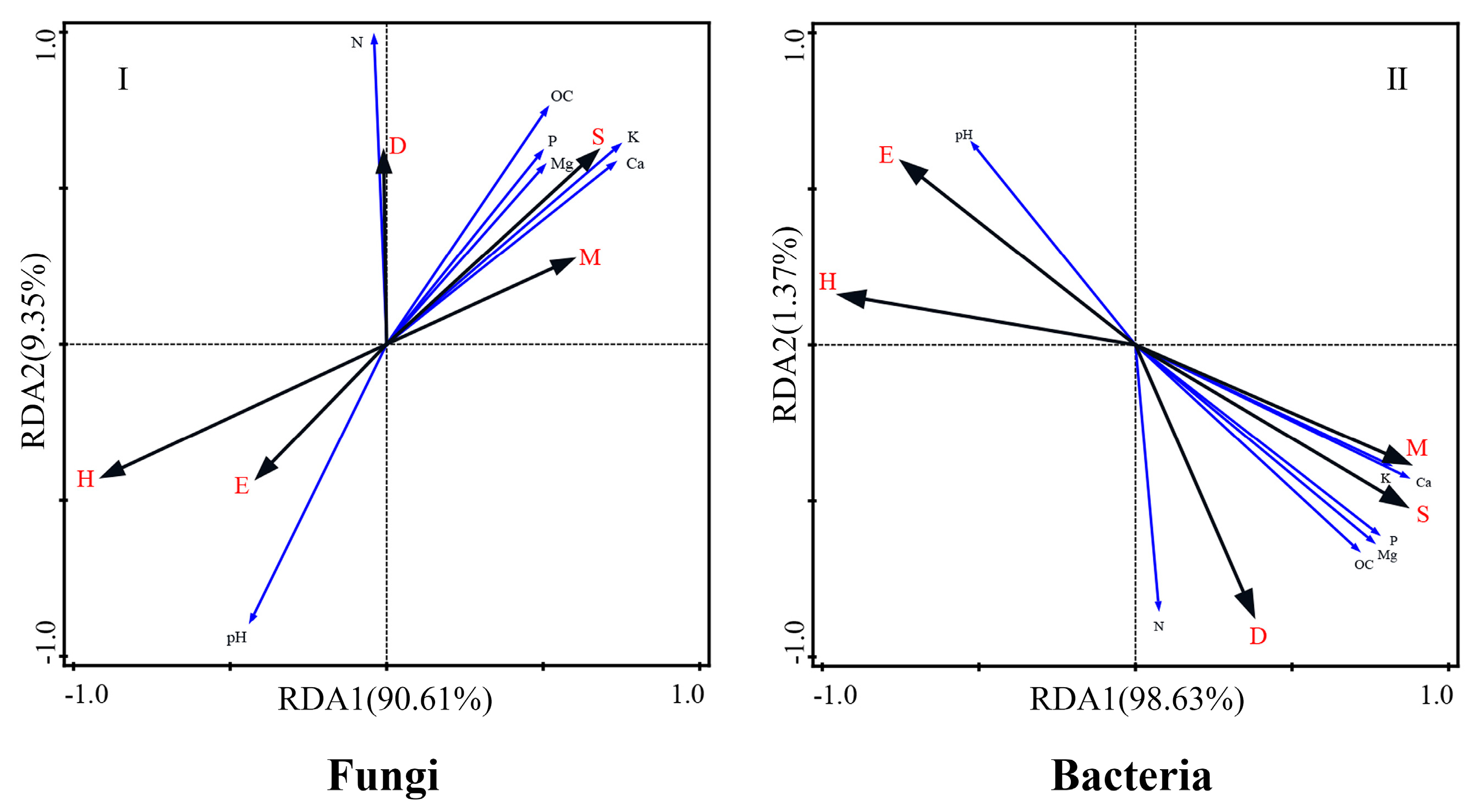
3.5. Key Drivers of Rhizosphere Microbial Community Structure in P. chinense Plantations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, C.; Wang, Y.; Du, H.; Hao, X.J. A New Triterpenoid from the Fruits of Phellodendron chinense var. glabriusculum Schneid. Chin. J. Chem. 2008, 26, 1343–1345. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Shen, Y.; Chen, X.; He, G.; He, X.; Wang, G.; He, H.; Lv, Z. The response of nutrient cycle, microbial community abundance and metabolic function to nitrogen fertilizer in rhizosphere soil of Phellodendron chinense Schneid seedlings. Front. Microbiol. 2023, 14, 1302775. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, H.S.; Kim, Y.; Lee, J.; Kim, B.Y.; Jeong, S.J. Phytochemical quantification and the in vitro acetylcholinesterase inhibitory activity of Phellodendron chinense and its components. Molecules 2017, 22, 925. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, S.; Jiao, J.; Yan, W.; Zeng, B.; He, H.; He, G. Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings. Int. J. Mol. Sci. 2023, 24, 16045. [Google Scholar] [CrossRef]
- Yung, W.S.; Chan, T.F.; Kong, F.; Lam, H.M. The Plant Genome special section: Epigenome and epitranscriptome in plant–environment interactions. Plant Genome 2023, 16, e20404. [Google Scholar] [CrossRef]
- Leonard, S.J.; Dirzo, R.; Eisenhauer, N.; Rebollo, R.; Schädler, M.; Ferlian, O. Explaining variation in plant-herbivore associational effects in a tree biodiversity experiment. J. Ecol. 2023, 111, 2694–2709. [Google Scholar] [CrossRef]
- Cai, X.; Chen, C.; Singh, A.K.; Zhu, X.; Liu, W. Anthropogenic restoration exhibits more complex and stable microbial co-occurrence patterns than natural restoration in rubber plantations. Sci. Total Environ. 2024, 948, 174935. [Google Scholar] [CrossRef]
- Zhou, G.; Lucas-Borja, M.E.; Eisenhauer, N.; Eldridge, D.J.; Liu, S.; Delgado-Baquerizo, M. Understorey biodiversity supports multiple ecosystem services in mature Mediterranean forests. Soil Biol. Biochem. 2022, 172, 108774. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Liu, Q.; Wang, D. Whole-tree harvesting improves the ecosystem N, P and K cycling functions in secondary forests in the Qinling Mountains, China. Front. Plant Sci. 2024, 15, 1394112. [Google Scholar] [CrossRef]
- Fan, C.; Cui, Y.; Zhang, Q.; Yin, N.; Cai, X.; Yuan, X.; Senadheera, S.; Cho, Y.; Ok, Y.S. A critical review of the interactions between rhizosphere and biochar during the remediation of metal(loid) contaminated soils. Biochar 2023, 5, 87. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, Y.; Zhang, F.; Yu, S.; Cui, X.; Wu, Y. A comparative analysis of microbial communities in the rhizosphere soil and plant roots of healthy and diseased yuanyang nanqi (Panax vietnamensis) with Root Rot. Agriculture 2024, 14, 719. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Rubert-Nason, K.; Li, X.; Xie, H.; Bao, X. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, H.; Li, X.; Xie, Y.; Lei, X.; Zheng, Y.; Yang, D.; Wang, F. Spatial heterogeneity and affecting factors of litter organic carbon and total nitrogen over natural spruce-fir mixed forests in northeastern China. Catena 2019, 174, 293–300. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, J.; Robson, T.M.; Kurokawa, H.; Peng, H.; Zhou, L.; Yu, L.; Deng, J.; Wang, Q.W. Autumn sunlight promotes aboveground carbon loss in a temperate mixed forest. Ecol. Process. 2024, 13, 48. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, P.; Zhang, X.; Ou, Z.; Ma, H.; Suo, C.; Ma, J.; Wan, P. Characteristics of different aged plantations of Ormosia hosiei with regards to soil microbial biomass and enzymatic activities. J. For. Res. 2024, 35, 119. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Koranda, M.; Rinnan, R.; Michelsen, A. Close coupling of plant functional types with soil microbial community composition drives soil carbon and nutrient cycling in tundra heath. Plant Soil 2023, 488, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Khan, S.M.; Ahmad, H.; Ahmad, Z.; Page, S. Vegetation mapping and multivariate approach to indicator species of a forest ecosystem: A case study from the Thandiani sub Forests Division (TsFD) in the Western Himalayas. Ecol. Indic. 2016, 71, 336–351. [Google Scholar] [CrossRef]
- Francos, M.; Úbeda, X.; Pereira, P. Impact of bonfires on soil properties in an urban park in Vilnius (Lithuania). Environ. Res. 2020, 181, 108895. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Jiang, J.; Zhou, X.; Niu, X.; Wei, Y.; Ma, J. Evaluating the potential slope plants using new method for soil reinforcement program. Catena 2019, 180, 346–354. [Google Scholar] [CrossRef]
- Chao, A.; Yang, M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Piotto, D.; Magnago, L.F.S.; Montagnini, F.; Ashton, M.S.; Oliver, C.; Thomas, W.W. Nearby mature forest distance and regenerating forest age influence tree species composition in the Atlantic forest of Southern Bahia, Brazil. Biodivers. Conserv. 2021, 30, 2165–2180. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef]
- Brant, R.A.; Edwards, C.E.; Reid, J.L.; Bassüner, B.; Delfeld, B.; Dell, N.; Mangan, S.A.; de la Paz Bernasconi Torres, V.; Albrecht, M.A. Restoration age affects microbial-herbaceous plant interactions in an oak woodland. Ecol. Evol. 2024, 14, e11360. [Google Scholar] [CrossRef]
- Frainer, A.; McKie, B.G. The legacy of forest disturbance on stream ecosystem functioning. J. Appl. Ecol. 2021, 58, 1511–1522. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, X.; Luan, Y.; Dai, W. Effects of short-term acidification on the adsorption of dissolved organic matter by soil minerals and its mechanism of action. Minerals 2023, 13, 1448. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Wu, D.; He, X.; Jiang, L.; Li, W.; Wang, H.; Lv, G. Root exudates facilitate the regulation of soil microbial community function in the genus Haloxylon. Front. Plant Sci. 2024, 15, 1461893. [Google Scholar] [CrossRef] [PubMed]
- Thaïs, W.; Silva, L.C.R.; Scow, K.M.; Doane, T.A.; Powers, R.F.; Horwath, W.R. Plant-microbe interactions regulate carbon and nitrogen accumulation in forest soils. For. Ecol. Manag. 2017, 384, 415–423. [Google Scholar] [CrossRef]
- Sarah, A.M.; Frindte, K.; Bodelier, P.L.E.; Knief, C. Rice straw serves as additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol. Biochem. 2019, 135, 235–238. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Janssens, I.A.; Deng, Y.; He, X.; Liu, L.; Yi, Y.; Xiao, N.; Wang, X.; Li, C.; et al. Divergent rhizosphere and non-rhizosphere soil microbial structure and function in long-term warmed steppe due to altered root exudation. Glob. Change Biol. 2024, 30, e17111. [Google Scholar] [CrossRef]
- Song, Y.; Zhai, J.; Zhang, J.; Qiao, L.; Wang, G.; Ma, L.; Xue, S. Forest management practices of Pinus tabulaeformis plantations alter soil organic carbon stability by adjusting microbial characteristics on the Loess Plateau of China. Sci. Total Environ. 2021, 766, 144209. [Google Scholar] [CrossRef]
- Street, B.B.; Rice, A.M.; Richardson, J.B. Forest floor and Na azide effect on elements in leachate from contrasting New Hampshire and Virginia forest soils. Soil Sci. Soc. Am. J. 2023, 87, 1444–1457. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, F.; Zhang, X.; Qi, D. Changes in fine root decomposition of primary Pinus koraiensis forest after clear cutting and restoration succession into secondary broad-leaved forest. Appl. Soil Ecol. 2021, 158, 103785. [Google Scholar] [CrossRef]
- Luan, J.; Li, S.; Dong, W.; Liu, Y.; Wang, Y.; Liu, S. Litter decomposition affected by bamboo expansion is modulated by litter-mixing and microbial composition. Funct. Ecol. 2021, 35, 2562–2574. [Google Scholar] [CrossRef]
- Huusko, K.; Manninen, O.H.; Myrsky, E.; Stark, S. Soil fungal and bacterial communities reflect differently tundra vegetation state transitions and soil physico-chemical properties. New Phytol. 2024, 243, 407–422. [Google Scholar] [CrossRef]
- Koutika, L.S.; Cafiero, L.; Bevivino, A.; Merino, A. Organic matter quality of forest floor as a driver of C and P dynamics in acacia and eucalypt plantations established on a Ferralic Arenosols, Congo. For. Ecosyst. 2020, 7, 40. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, W.; Liu, C.; Lei, L.; Jian, Z.; Shen, Y.; Li, M.H. Effects of thinning and understorey removal on soil extracellular enzyme activity vary over time during forest recovery after treatment. Plant Soil 2023, 492, 457–469. [Google Scholar] [CrossRef]
| Forest Age/a | Altitude/m | Slope/° | Mean Height/m | Mean DBH/cm |
|---|---|---|---|---|
| 2 | 925 | 19 | 4.63 | 3.42 |
| 5 | 975 | 22 | 11.27 | 10.30 |
| 8 | 979 | 24 | 14.73 | 14.27 |
| 12 | 1021 | 18 | 15.76 | 17.83 |
| Family | Genus | Species | Important Value | Family | Genus | Species | Important Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 5a | 8a | 12a | 2a | 5a | 8a | 12a | ||||||
| Shrub | Fabaceae | Vicia | Vicia sativa | — | 11.93 | — | — | ||||||
| Viburnaceae | Sambucus | Sambucus williamsii | — | 2.559 | — | 2.057 | Astragalus | Astragalus sinicus | — | — | 7.216 | 5.178 | |
| Anacardiaceae | Rhus | Rhus chinensis | — | — | 2.412 | 2.161 | Geraniaceae | Geranium | Geranium wilfordii | — | 11.701 | — | — |
| Apocynaceae | Periploca | Periploca forrestii | — | 2.814 | — | 2.709 | Lamiaceae | Mentha | Mentha canadensis | — | — | 5.323 | — |
| Melastomataceae | Oxyspora | Oxyspora paniculata | — | — | 5.088 | — | Lindsaeaceae | Odontosoria | Odontosoria chinensis | — | — | — | 3.069 |
| Rosaceae | Rubus | Rubus pluribracteatus | — | — | — | 2.969 | Menispermaceae | Tinospora | Tinospora sagittata | — | — | — | 2.415 |
| Rubus | Rubus swinhoei | — | — | 3.72 | 2.138 | Plantaginaceae | Plantago | Plantago asiatica | 14.784 | — | — | — | |
| Herb | Poaceae | Microstegium | Microstegium vimineum | — | — | 18.499 | 9.164 | ||||||
| Umbelliferae | Centella | Centella asiatica | — | — | 15.565 | 5.345 | Alopecurus | Alopecurus aequalis | — | — | 10.375 | 4.158 | |
| Oenanthe | Oenanthe javanica | — | — | 3.207 | 3.235 | Chasmanthium | Chasmanthium latifolium | — | — | 18.414 | 7.726 | ||
| Araliaceae | Hydrocotyle | Hydrocotyle nepalensis | — | 4.911 | — | — | Setaria | Setaria palmifolia | — | 7.373 | — | — | |
| Asparagaceae | Asparagus | Asparagus cochinchinensis | — | — | 3.236 | 3.002 | Pennisetum | Pennisetum alopecuroides | — | — | — | 2.759 | |
| Asteraceae | Pseudognaphalium | Pseudognaphalium affine | 24.22 | 9.287 | — | — | Arthraxon | Arthraxon hispidus | — | — | — | 12.096 | |
| Erigeron | Erigeron sumatrensis | 7.88 | 14.961 | — | — | Polygonaceae | Rumex | Rumex obtusifolius | — | — | — | 2.617 | |
| Youngia | Youngia japonica | 15.451 | — | 11.948 | 4.951 | Ranunculaceae | Ranunculus | Ranunculus repens | 18.338 | 11.691 | 13.548 | 9.558 | |
| Sonchus | Sonchus asper | — | 7.449 | — | — | Ranunculus | Ranunculus muricatus | — | 5.781 | — | — | ||
| Aster | Aster indicus | 14.467 | 6.211 | — | — | Ranunculus | Ranunculus cantoniensis | — | 8.481 | — | — | ||
| Artemisia | Artemisia indica | — | — | 20.641 | — | Rosaceae | Duchesnea | Duchesnea indica | 11.92 | — | — | — | |
| Athyriaceae | Anisocampium | Anisocampium niponicum | — | — | — | 3.919 | Rubus | Rubus corchorifolius | — | — | — | 6.509 | |
| Brassicaceae | Cardamine | Cardamine flexuosa | — | 14.387 | — | — | Geum | Geum aleppicum | — | — | — | 4.688 | |
| Caryophyllaceae | Cerastium | Cerastium glomeratum | 23.58 | 13.608 | 12.928 | 5.506 | Selaginellaceae | Selaginella | Selaginella delicatula | — | — | — | 3.176 |
| Stellaria | Stellaria alsine | — | — | 19.051 | 10.001 | Selaginella | Selaginella doederleinii | — | — | — | 2.548 | ||
| Stellaria | Stellaria media | — | 15.884 | — | — | Thelypteridaceae | Parathelypteris | Parathelypteris glanduligera | — | — | 6.759 | — | |
| Stellaria | Stellaria vestita | — | — | 16.203 | — | Urticaceae | Gonostegia | Gonostegia hirta | — | — | 11.253 | 6.894 | |
| Crassulaceae | Sedum | Sedum emarginatum | 37.972 | 19.268 | — | — | Pilea | Pilea japonica | — | 8.907 | — | — | |
| Sedum | Sedum bulbiferum | — | — | — | 13.188 | Urtica | Urtica fissa | — | — | 2.651 | — | ||
| Sedum | Sedum makinoi | 22.213 | 19.971 | — | — | Violaceae | Viola | Viola diffusa | — | 9.504 | 8.674 | — | |
| Dennstaedtiaceae | Pteridium | Pteridium aquilinum | 13.683 | 7.867 | — | — | Viola | Viola philippica | — | — | — | 4.133 | |
| Dryopteridaceae | Dryopteris | Dryopteris erythrosora | — | — | — | 3.701 | Viola | Viola grypoceras | — | 8.769 | — | — | |
| Mineral Nutrients/mg·kg−1 | Age/a | |||
|---|---|---|---|---|
| 2 | 5 | 8 | 12 | |
| N | 245.27 ± 13.55 c | 276.48 ± 6.39 c | 778.61 ± 14.33 a | 515.46 ± 9.29 b |
| P | 60.68 ± 17.20 d | 86.36 ± 5.29 c | 115.90 ± 3.93 b | 169.64 ± 4.09 a |
| K | 159.30 ± 30.25 c | 133.5 ± 11.35 c | 183.61 ± 6.39 b | 216.56 ± 11.34 a |
| Ca | 73.89 ± 4.93 c | 46.63 ± 6.91 d | 134.92 ± 5.29 b | 242.81 ± 7.04 a |
| Mg | 9.11 ± 0.72 d | 37.72 ± 2.29 c | 61.01 ± 10.33 b | 120.53 ± 4.72 a |
| Microbial | Age | Observed Species | Shannon | Simpson | Chao1 | ACE | Goods Coverage |
|---|---|---|---|---|---|---|---|
| Bacteria | 2a | 1789 ± 14.189 | 8.358 ± 0.579 | 0.995 ± 0.002 | 1805.343 ± 3.495 | 1788.356 ± 13.241 | 0.998 ± 0.001 |
| 5a | 1983 ± 56.367 | 9.295 ± 0.432 | 0.991 ± 0.007 | 2008.521 ± 37.658 | 1985.412 ± 48.753 | 0.998 ± 0.001 | |
| 8a | 1901 ± 70.684 | 9.557 ± 0.097 | 0.995 ± 0.002 | 1944.853 ± 63.303 | 1922.374 ± 67.841 | 0.997 ± 0.001 | |
| 12a | 1265 ± 76.350 | 7.612 ± 0.065 | 0.992 ± 0.001 | 1287.828 ± 90.214 | 1253.691 ± 71.368 | 0.998 ± 0.001 | |
| Fungus | 2a | 1343 ± 15.674 | 5.973 ± 0.215 | 0.942 ± 0.001 | 1410.258 ± 5.364 | 1427.158 ± 12.159 | 0.998 ± 0.001 |
| 5a | 1670 ± 42.387 | 6.242 ± 0.104 | 0.931 ± 0.001 | 1850.911 ± 21.325 | 1857.843 ± 38.279 | 0.997 ± 0.001 | |
| 8a | 1597 ± 48.651 | 7.498 ± 0.085 | 0.961 ± 0.005 | 1697.818 ± 46.351 | 1709.224 ± 42.781 | 0.998 ± 0.001 | |
| 12a | 1127 ± 62.963 | 6.632 ± 0.112 | 0.926 ± 0.003 | 1219.064 ± 74.218 | 1230.163 ± 55.355 | 0.998 ± 0.001 |
| Index | Shannon | Simpson | Chao1 | ACE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fungus | Bacteria | Fungus | Bacteria | Fungus | Bacteria | Fungus | Bacteria | ||
| Soil nutrients | pH | −0.789 | 0.228 | −0.315 | −0.288 | 0.458 | 0.546 | 0.460 | 0.549 |
| SOC | 0.635 | −0.400 | −0.049 | −0.088 | −0.513 | −0.726 | −0.519 | −0.732 | |
| N | 0.986 * | 0.267 | 0.619 | 0.343 | 0.016 | −0.093 | 0.014 | −0.098 | |
| P | 0.498 | −0.455 | −0.274 | −0.336 | −0.480 | −0.766 | −0.489 | −0.773 | |
| K | 0.477 | −0.603 | −0.036 | 0.133 | −0.758 | −0.836 | −0.761 | −0.838 | |
| Ca | 0.419 | −0.639 | −0.207 | −0.080 | −0.729 | −0.885 | −0.734 | −0.888 | |
| Mg | 0.451 | −0.480 | −0.330 | −0.385 | −0.485 | −0.780 | −0.494 | −0.788 | |
| Species diversity | S | 0.454 | −0.582 | −0.231 | −0.169 | −0.652 | −0.855 | −0.658 | −0.860 |
| D | 0.576 | −0.006 | −0.216 | −0.574 | 0.043 | −0.359 | 0.033 | −0.371 | |
| H | −0.226 | 0.792 | 0.231 | −0.067 | 0.896 | 0.940 | 0.899 | 0.939 | |
| E | −0.311 | 0.447 | 0.505 | 0.612 | 0.366 | 0.721 | 0.377 | 0.730 | |
| M | 0.127 | −0.643 | −0.632 | −0.600 | −0.544 | −0.848 | −0.553 | −0.855 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Song, P.; Zhang, Z.; Gong, Q.; Wu, J.; Sun, Z. Change Patterns of Understory Vegetation Diversity and Rhizosphere Soil Microbial Community Structure in a Chronosequence of Phellodendron chinense Plantations. Forests 2025, 16, 1298. https://doi.org/10.3390/f16081298
Xie C, Song P, Zhang Z, Gong Q, Wu J, Sun Z. Change Patterns of Understory Vegetation Diversity and Rhizosphere Soil Microbial Community Structure in a Chronosequence of Phellodendron chinense Plantations. Forests. 2025; 16(8):1298. https://doi.org/10.3390/f16081298
Chicago/Turabian StyleXie, Chuan, Peng Song, Zhiyu Zhang, Qiuping Gong, Jiaojiao Wu, and Zhipeng Sun. 2025. "Change Patterns of Understory Vegetation Diversity and Rhizosphere Soil Microbial Community Structure in a Chronosequence of Phellodendron chinense Plantations" Forests 16, no. 8: 1298. https://doi.org/10.3390/f16081298
APA StyleXie, C., Song, P., Zhang, Z., Gong, Q., Wu, J., & Sun, Z. (2025). Change Patterns of Understory Vegetation Diversity and Rhizosphere Soil Microbial Community Structure in a Chronosequence of Phellodendron chinense Plantations. Forests, 16(8), 1298. https://doi.org/10.3390/f16081298





