Abstract
Using metrics from network theory allows us to understand the structural organization of epiphyte communities and identify the host trees (phorophytes) that are fundamental to their establishment. In this study, we applied ecological network metrics to examine the structure of interactions between vascular epiphytes and phorophytes in a woody inselberg community in southeastern Brazil. The recorded network comprised 30 epiphyte species and 13 phorophyte species, exhibiting a nested structure, low specialization (H2′), low connectance, and low modularity, like other epiphyte–phorophyte networks. The main roles in the network were played by the generalist epiphyte Tillandsia loliacea and the lithophytic phorophytes Tabebuia reticulata and Pseudobombax petropolitanum, which interacted with 100% of the recorded epiphytic species. Epiphyte species were organized vertically into modules that correlate with the ecological zones of the phorophytes, suggesting that their distribution responds to microclimatic variation along the vertical gradient. These results reinforce the importance of particular phorophyte species as key structuring agents of epiphytic communities and highlight their central role in extreme environments such as inselbergs.
1. Introduction
The study of interactions between species is fundamental to understanding the mechanisms of ecological community assembly [1]. These interactions, which include mutualism, antagonism, and commensalism, form complex networks that influence ecological dynamics and affect ecosystems’ structure, functioning, and preservation [2]. A classic example of commensal interaction is established between epiphytes and phorophytes [3]. However, little is known about how epiphyte–phorophyte interactions are structured in extreme environments such as inselbergs, particularly about the vertical organization of these interactions within phorophytes and the structural roles that phorophytes play in these networks.
In recent years, there has been a growing interest in understanding the structure of interaction networks between epiphytes and phorophytes [4]. This advance is particularly relevant in terrestrial island ecosystems such as inselbergs [5,6], which function as important refuges for biodiversity despite their isolated nature and extreme environmental conditions. These rocky outcrops are home to both epiphyte species typical of the adjacent forest and endemic and endangered species [7]. In this context, studying epiphyte–phorophyte interactions in inselbergs is fundamental to understanding the ecological patterns that support epiphyte diversity in these ecosystems.
Inselbergs are isolated rocky outcrops of granite and/or gneiss found in various world regions [8]. Due to their physical–environmental heterogeneity, they harbor a variety of plant habitats, including crevices, mats, woody vegetation, forest islands, and vertical rock walls, which support a specialized flora adapted to varying degrees of rock inclination [9,10,11]. In southeastern Brazil, these ecosystems are recognized as one of the world’s main centers of lithophyte plant diversity [12]. Although herbaceous communities, such as monocotyledonous mats, are the primary components of inselberg flora [12,13], woody communities, i.e., woody vegetation established on shallow depressions and forest islands, have been largely neglected [11]. Lithophyte trees, shrubs, and palms play a fundamental role on inselbergs by providing structural support for distinct groups of plants, such as vascular epiphytes [5,6,14,15].
Epiphytes are plants germinating and developing on other plants (phorophytes), usually trees and shrubs, using them as structural support without causing harm [16]. Their distribution on phorophytes occurs horizontally (among different phorophyte species) and vertically (from the base of the trunk to the branches of the canopy). The structure of epiphyte–phorophyte interactions has been analyzed using metrics derived from ecological network theory, such as nestedness, modularity, connectance, and complementary specialization. These networks generally exhibit a nested network structure, which is neither highly specialized nor modular [3,5,6,17,18,19,20], with consistent patterns across different environments. In nested networks, the group of species with fewer interactions (specialists) represents a subset of the interactions established by species with a greater number of interactions (generalists) [21].
Low specialization and modularity may be related to epiphytes’ ability to colonize a wide range of substrates and microenvironments [22]. In ecological networks, modularity refers to the presence of subgroups (or modules) within the network in which species interact more frequently with each other than with species outside the module. In the context of epiphyte–phorophyte interactions, it is important to distinguish between the two types of modularity. Horizontal modularity occurs among different phorophyte species, where specific epiphyte–phorophyte interactions tend to form distinct modules. In contrast, vertical modularity refers to the distribution of epiphytes along the environmental gradient within a single phorophyte, from its base to the upper crown [6,19]. This vertical pattern often reflects variation in microclimatic conditions and substrate characteristics along the host tree [19]. However, little is known about how these modularity patterns, both horizontal and vertical, manifest in commensal networks and influence the structure of these communities.
In addition to network metrics, ecological network analyses allow us to describe how species interact and assess the importance of each species based on its position within the network. For example, species that interact with many others in different parts of the network are considered network hubs, while species whose interactions are concentrated in a single group are referred to as module hubs [23]. Analyzing these positions allows species to be classified into functional categories, such as peripherals, connectors, module hubs, and network hubs [24], providing insights into how different components contribute to community cohesion and resilience.
In this study, we investigated the interaction network structure between epiphytes and phorophytes in woody vegetation on an inselberg in the Brazilian Atlantic Forest (southeastern Brazil), focusing on the structural roles played by species within the network. We hypothesize that (1) the epiphyte–phorophyte network exhibits a nested structure with low specialization and low modularity, features commonly observed in commensal networks; (2) the vertical structure of the network is modular, with modules corresponding to the ecological zones of the phorophytes; (3) epiphyte species richness and abundance vary significantly among the ecological zones of the phorophytes, due to morphologic and microclimatic variation from the base to the upper crown; and (4) generalist and endemic phorophytes from inselbergs occupy central positions in the network because they host a greater number of epiphyte species and establish multiple interactions.
2. Materials and Methods
2.1. Study Site
The study was carried out at Pedra de Santa Angélica (20°43′54″ S, 41°25′58″ W), an Atlantic Forest inselberg located in the southern region of Espírito Santo state, Alegre county, southeastern Brazil (Figure 1). The inselberg is located in a matrix of tropical Semideciduous Seasonal Forest [25], currently fragmented by human activities, mainly pastures for cattle raising, coffee plantations (Coffea canephora Pierre ex A. Froehner), and forestry, mainly Eucalyptus spp. (Myrtaceae). The region has a hot and humid tropical climate, with a dry winter (April to September) and a rainy summer (October to March), with a mean annual temperature of 24 °C and an average annual rainfall of 1293 mm [14].
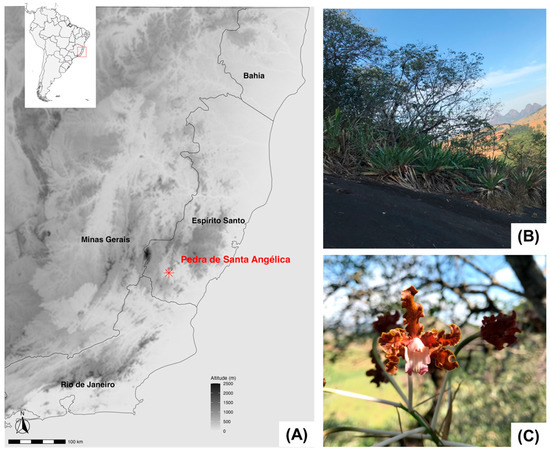
Figure 1.
(A) Location of the study area in the state of Espírito Santo, southeastern Brazil. (B) General view of the woody vegetation on the inselberg, surrounded by exposed rock. (C) Laelia gloriosa (Rchb.f.) L.O.Williams (Orchidaceae), an epiphytic species recorded in this study.
The inselberg habitat type studied is the woody vegetation established in shallow depressions filled with humic Litholic Neosol, in which epiphytic communities develop. This vegetation occurs on a rocky slope between 200 and 420 m above sea level, characterized by deciduous trees and shrubs, with more than 80% of the species shedding their leaves during the dry season. The average height of the trees is approximately 5 m, rarely exceeding 10 m (with a maximum of 16 m), with the dominant tree species being Tabebuia reticulata A.H.Gentry and Pseudobombax petropolitanum A. Robyns [11].
2.2. Data Collection
We register four categories of epiphytes: holoepiphytes (which complete the entire life cycle on phorophytes), hemiepiphytes (which have a life stage as terrestrial plants), facultative epiphytes (capable of developing on other substrates), and accidental epiphytes (terrestrial species that can occasionally occur on other plants) [16]. For every individual epiphyte, we recorded information about its respective phorophytes. Although the degree of dependence on phorophytes may vary among categories, we chose to include all observed interactions to reflect the structural and functional complexity of the studied system, as has been conducted in previous studies [5,6,14,15].
For sampling, we established ten linear transects (50 m × 2 m), systematically distributed with a minimum spacing of 10 m between them [26]. All shrub/tree phorophytes with a diameter at breast height (DBH, measured at 1.30 m) ≥5 cm within these transects were included. Data on the floristic composition and woody vegetation structure are available in Couto et al. [11]. Each epiphytic individual was recorded in one of the five ecological zones of the phorophyte: superficial root, trunk, primary branches, secondary branches, and upper crown (Figure 2), as described in Couto et al. [15]. Data collection involved canopy sampling techniques using specialized tree-climbing methods adapted from mountaineering, which include the use of ropes, harnesses, and other safety equipment to ensure safe access to the canopy and accurate data collection [27]. To count the epiphytes, we used the unit ‘stand’ (sensu Sanford [28]), defined as a group of rosettes (Bromeliaceae), leaves (ferns) or pseudobulbs (Orchidaceae) of a single species, spatially separated from other groups of the same species by areas of the phorophyte either devoid of epiphytes or occupied by other species. Angiosperm family classification followed the APG IV system [29], while monilophytes and lycophytes were classified according to the PPG I system [30].
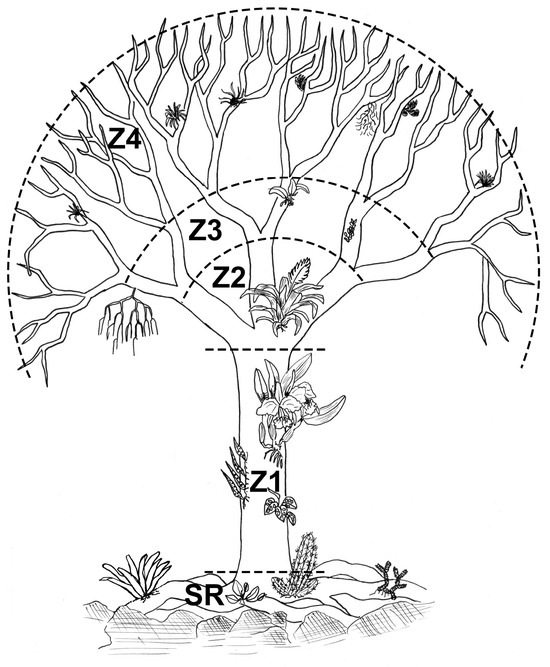
Figure 2.
Vertical ecological zones: (SR) superficial roots; (Z1) trunk; and three crown zones—(Z2) primary branches, (Z3) secondary branches, and (Z4) upper crown, as described by Couto et al. [15].
2.3. Network-Level Metrics
To characterize and analyze the epiphyte–phorophyte interaction network, we constructed three matrices: (1) a qualitative matrix, where 0 indicates absence and 1 indicates presence of interactions; (2) a quantitative matrix with the number of individual epiphytes (i) associated with each phorophyte (j); and (3) a quantitative matrix recording the occurrence of epiphyte species (i) across five ecological zones of the phorophytes (j).
We calculated different structural metrics to characterize the network based on these matrices. Network size was defined as the sum of the number of epiphyte species (E) and phorophyte species (P), reflecting the total species richness involved in the system. Connectance (C) was calculated as the ratio between the number of observed and the maximum possible number of interactions, considering all potential combinations between the two groups [31]. This metric, which ranges from 0 (no interaction) to 1 (all species connected), indicates the density of connections in the network.
The complementary specialization of the network (H2′) is used to evaluate the degree of specialization at the network level. This metric considers the frequency of interactions and ranges from 0 (no specialization, or random interactions) to 1 (maximum specialization, with interactions restricted between specific pairs of species) [32]. Additionally, we calculated the generality and vulnerability, which express, respectively, the weighted average number of phorophyte species with which each epiphyte species interacts and the average number of epiphyte species associated with each phorophyte species [18,33]. Together, these provide insight into the breadth and intensity of group interactions.
Nestedness was assessed using the NODF (Nestedness metric based on Overlap and Decreasing Fill), which considers the overlapping of interactions and the decreasing order of filling the matrix [21]. Values range from 0 (no nestedness) to 100 (maximum nestedness), reflecting the degree of hierarchical organization of the network. Significance was assessed and tested by the Monte Carlo procedure with 1000 randomizations, using the CE null model (null model 2, as Bascompte et al. [34]), with the analyses performed in the software ANINHADO 3.0.2 [35].
Modularity was estimated using QuanBiMo (Quantitative Bipartite Modularity), which is suitable for bipartite and weighted networks. This metric indicates the degree to which the network is divided into modules, subsets of species that interact more frequently with each other than with the rest of the network. Values range from 0 (no modularity) to 1 (maximum modularity) [36].
Except for nestedness, we use the R-package bipartite for all other metrics [37]. The output of the R-package bipartite allows the comparison of observed metrics with a null model (or expected value), resulting in a p-value comparable to the significance test of classical statistical methods [37]. To visualize the vertical network, we used Gephi 0.8.2 beta [38].
2.4. Species-Level Metrics
Species-level metrics help describe patterns of diversity and organization of interactions, allowing the identification of the structural and functional role of each species in the network [39]. The degree (k) (or centrality), for example, corresponds to the total number of interactions that a species establishes, with higher values indicating more connected species and, therefore, potentially more influential in the network structure [40]. Considering the asymmetric nature of the commensal interaction between epiphytes and phorophytes, species strength was calculated only for phorophytes. The species strength (SS) represents the sum of the individual species dependencies (d′) for all species of another group [39]. The dependency value (d′) is calculated as the proportion of each partner’s interactions that are linked to a species, determined using the quantitative interaction matrix. A high value of d′ indicates that the species plays a generalist and functionally important role in the network. Thus, its removal can significantly compromise the structure and functioning of the community, given its central contribution to established interactions [41]. These metrics were performed using the specieslevel() function from the bipartite package in R.
To define the roles of species in the network, we use two metrics: the z-score, which measures species’ connection within the module, and the c-score, which evaluates the distribution of these connections between modules [24]. The cutoff values for z and c were defined by the 95% percentile, enabling the classification of species into four topological roles: peripherals (with few interactions), connectors (linking multiple modules), module hubs (highly connected within their module), and network hubs (highly connected both within and across modules). We also calculated species-level metrics for each species in the networks using R-package bipartite [37].
2.5. Vertical Stratification
Generalized linear models (GLMs) with quasi-Poisson distribution and logarithmic link function were used to analyze differences in epiphyte species richness and abundance among the five ecological zones on the phorophytes (SR, Z1, Z2, Z3, and Z4) [42]. To assess the relationship between the identified modules and the ecological zones of the phorophytes, a Mantel correlation test was performed with 10,000 randomizations [43]. The analysis compared two weighted matrices of epiphyte abundance, one representing module composition (generated by the QuanBiMod algorithm) and the other representing ecological zones. All analyses were conducted in the R environment [44]. We used the R-package RT4Bio [45] for generalized linear models (GLMs), the R-package vegan [46] for the Mantel test, and the R-package bipartite [37] for network modularity analysis using the QuanBiMod algorithm.
3. Results
3.1. Metrics of Network Structure
The interaction network between vascular epiphytes and phorophytes comprised 79 interactions involving 30 species of vascular epiphytes and 13 species of phorophytes (network size S = 43 species; see Figure 3 and Table 1 and Table 2). The network was nested and exhibited low connectance, low complementary specialization, and low overall modularity, with four structural modules identified (Table 3). On average, vascular epiphyte species interacted with 3.52 phorophyte species (generality), while phorophytes hosted, on average, 2.09 epiphyte species (vulnerability).
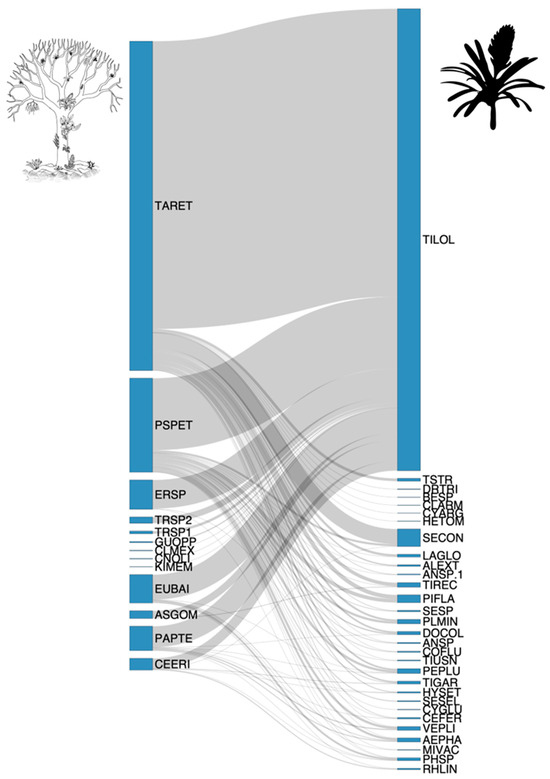
Figure 3.
Bipartite network between epiphytes and phorophytes in woody vegetation on an inselberg in southeastern Brazil. The size of each species’ box represents its abundance in the observed interactions, while the width of the gray lines indicates interaction strength (thicker lines represent stronger interactions). For acronyms, see Table 1 and Table 2.

Table 1.
Vascular epiphytes recorded in the woody vegetation on an inselberg in southeastern Brazil, with respective families, acronyms, ecological categories (Cat.: Epi = holoepiphyte, Aci = accidental epiphyte, Fac = facultative epiphyte, Hem = hemiepiphyte), abundances (Abund), and degree (k).

Table 2.
Phorophyte species sampled in woody vegetation on an inselberg in southeastern Brazil, with respective families, acronyms, abundances (Abund), degree (k), and species strength (SS).

Table 3.
Network-level metrics for the commensal network between epiphytes and phorophytes in the woody vegetation on an inselberg in southeastern Brazil.
The analysis of the vertical stratification of the network revealed low modularity (Q = 0.1749, p < 0.01). The network was divided into three modules—SR; Z1; and Z2-Z3-Z4, which comprised between 3 and 17 epiphyte species each (Figure 4). The SR module, corresponding to the superficial root zone, had the highest epiphyte richness (17 species), with a predominance of accidental epiphytes. In the other modules, holoepiphytes predominated, except for a few accidental species, such as Alcantarea extensa, which was recorded in module Z1. Tillandsia loliacea exhibited the highest interaction intensity in the upper crown (Z4), whereas Selaginella convoluta was associated with the superficial root zone (SR).
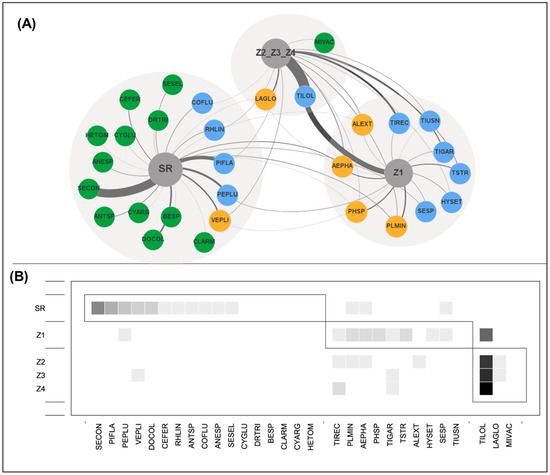
Figure 4.
Modularity of epiphyte and phorophyte species interactions in the woody vegetation on an inselberg in southeastern Brazil. Modules were identified using the QuaBiMo algorithm [36]. (A) Ecological network illustrating the three modules corresponding to ecological zones (SR, Z1-Z2-Z3, and Z4) used by epiphytic species. Network vertices (epiphytes) are shown as colored circles with species acronyms. Colors indicate the number of modules that each species (vertex) belongs to: green for species occurring in only one module (peripheral), blue for species in two modules, and orange for species present in three modules (connector species). Line thickness represents the frequency of interactions within each module. (B) Interaction matrix. Gray boxes outline the three modules, with filled cells inside representing links within modules. Darker gray square cells indicate more frequent interactions. For acronyms, see Table 1.
3.2. Species-Level Descriptors
The epiphytic species with the highest number of phorophyte associations were Tillandsia loliacea, which interacted with 13 phorophyte species, followed by Philodendron sp., associated with five species. Aechmea phanerophlebia and Pecluma plumula each interacted with four phorophyte species. Cereus fernambucensis, Doryopteris collina, Hylocereus setaceus, Selaginella convoluta, Tillandsia gardneri, Tillandsia recurvata, and Vellozia plicata each interacted with three phorophyte species (Figure 3; Table 1). The phorophyte species that interacted with the greatest number of vascular epiphyte species were Tabebuia reticulata (25 species), Pseudobombax petropolitanum (21 species), Parapiptadenia pterosperma (8 species), and both Ceiba erianthos and Eugenia cf. bahiensis with 6 species each (Figure 3; Table 2). The Malvaceae family was the most represented among the phorophytes, with two species sampled.
The number of interactions between epiphytes and individual phorophytes ranged from 1 to 25 (Figure 3; Table 2). Tabebuia reticulata and Pseudobombax petropolitanum stood out as the most generalist phorophytes, with degrees of 25 and 21, respectively. Species strength values further underscored their importance to the network, with values of 16.20 and 8.72, respectively, indicating a strong dependence of epiphytes on these phorophytes and highlighting their central role in maintaining the structure and functioning of the epiphytic community (Table 2).
Both groups, epiphytes and phorophytes, were dominated by peripheral species, with no connector species or network hubs identified. However, one epiphyte species (Tillandsia loliacea) and one phorophyte species (Tabebuia reticulata) played important modular roles, exhibiting high z-score values that characterize them as hubs within their respective modules (Figure 5). Among epiphytes, connectivity values between modules (c) ranged from 0 to 0.67, with no species exceeding the threshold of 0.73. Within-module connectivity values (z) ranged from −0.81 to 3.28, with Tillandsia loliacea as the only species having z > 1.81, indicating its role as the module’s central species. Among phorophytes, connectivity between modules (c) ranged from 0 to 0.61, with no species reaching the threshold of 0.74. Within-module connectivity (z) values ranged from −0.71 to 2.65, with Tabebuia reticulata as the only species exceeding the 1.73 threshold (Figure 5).
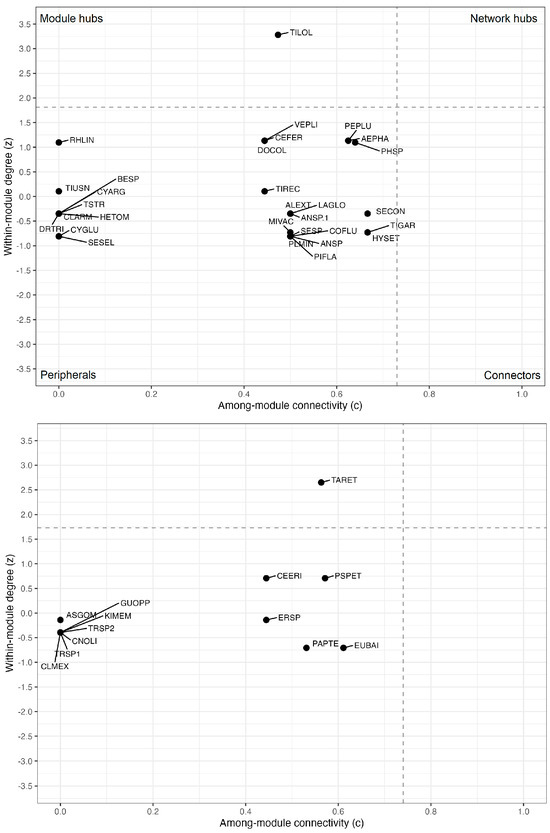
Figure 5.
Roles of epiphytic species (top) and phorophyte species (bottom) in the modular and overall network structure in woody vegetation on an inselberg in southeastern Brazil. z-scores represent within-module degree (i.e., number of interactions within a module), while c-score indicates among-module connectivity. Dashed lines represent threshold values for c and z, defining four topological roles: peripheral species (bottom left, low z, and low c), connectors (bottom right, low z, and high c), module hubs (top left, high z, and low c), and network hubs (top right, high z, and high c). Threshold values were defined by the 95th percentile: for epiphytes, c = 0.73, and z = 1.81; for phorophytes, c = 0.74 and z = 1.73.
3.3. Vertical Stratification
Epiphyte richness and abundance varied significantly across phorophyte ecological zones (GLMs; richness: F = 2.77, p < 0.001; abundance: F = 25.46, p < 0.001). Species richness decreased along the vertical gradient of phorophytes, with the superficial root zone (SR) exhibiting the highest richness (23 species; 77%), followed by the trunk (Z1: 14 species; 47%), primary branches (Z2: 13 species; 43%), secondary branches (Z3: 11 species; 37%), and upper crown (Z4: 7 species; 23%). Of the seven species recorded in the outer crown, five belonged to the genus Tillandsia. Contrast analyses revealed that the superficial root zone differed significantly from the other zones (p < 0.05). The upper crown had the highest abundance (52%) and also differed significantly from other ecological zones (p < 0.05). In this zone, Tillandsia loliacea accounted for 59% of the recorded individuals. Additionally, the superficial root and trunk zones differed significantly from zones Z2, Z3, and Z4 (p < 0.05). Epiphyte abundance followed an inverse gradient relative to species richness, with the highest abundance in the upper crown, followed by the secondary branches, primary branches, stem, and lastly the superficial roots.
4. Discussion
This study analyzed the horizontal and vertical structure of vascular epiphyte–phorophyte interaction networks on an Atlantic Forest inselberg, highlighting species’ structural roles. Although the network exhibited typical patterns of commensal interactions, such as nestedness, low connectance, low specialization, and low modularity [4,5,6,17,18,19,20], our results showed generalist interaction patterns with preferences for particular phorophyte species. The lithophytes tree Tabebuia reticulata and Pseudobombax petropolitanum, endemic to Atlantic Forest inselbergs, stood out by hosting all recorded epiphyte species. This suggests that the loss of these key phorophytes could disproportionately impact the epiphyte community. Additionally, the vertical networks exhibited low modularity, reflecting the spatial occupation patterns across the ecological zones of the phorophytes. This likely relates to characteristics of the inselberg environment, where vegetation is sparse, trees are relatively small, and there is typically no continuous crown, conditions that limit vertical stratification and may constrain the formation of distinct vertical modules. Notably, species richness was highest in the superficial roots, while abundance was greatest in the upper crown. These findings advance our understanding of species interactions in tropical inselbergs and unique and threatened ecosystems that remain underrepresented in ecological studies, especially within the Atlantic Forest Domain [47,48].
The epiphyte–phorophyte network showed low connectance and a low degree of specialization, consistent with other commensal networks described in inselbergs [5,6] and in various ecosystems [17,18,19,49,50]. These patterns are influenced by both the low specificity of epiphytes and the environmental constraints of rocky outcrops, such as water scarcity, high radiation, and shallow soils. Similar patterns have also been observed in other low-intimacy mutualistic interactions, such as those between plants and nectarivorous or seed-dispersing ants [32,51]. Since epiphytes establish themselves wherever diaspores arrive [52,53], this may further contribute to the low level of specialization observed.
Nonetheless, epiphyte distribution is not entirely random [3,52,53]. Variations in ecological traits among epiphytic species can lead to distinct patterns of phorophyte colonization. As noted by Sáyago et al. [49] and Taylor et al. [54], low specialization may reflect selection based on phorophyte structural attributes such as size, bark texture, and crown architecture, which differ among individuals and species. Additionally, other ecological filters contribute to this non-randomness. Successful epiphyte colonization depends not only on the arrival of propagules but also on the success of germination and survival. Microenvironmental conditions on the surface of phorophytes, such as the water retention capacity of the bark, nutrient accumulation, and light availability, can act as post-dispersal filters, favoring the establishment of certain species. In our study, many phorophytes hosted only a single epiphyte species, whereas T. reticulata and P. petropolitanum hosted most of the epiphytic community (25 and 21 species, respectively), likely due to structural differences related to age and size [5,17,18,49,55,56].
The nested pattern observed supports the hypothesis that commensalistic epiphyte–phorophyte networks tend to be nested [3,4,5,6,17,18,19], a pattern consistent with interactions among orchids [20,52], bromeliads [49], and even bryophytes [50]. This pattern is also consistent with those found in plant–animal mutualistic networks [40], suggesting that common structural features may emerge across different types of ecological interactions. However, the mechanisms driving these patterns may be different. For epiphytes, nestedness is often attributed to species’ relative abundance, as more abundant species have a higher likelihood of interacting with a broad range of partners due to increased encounter rates. Such abundance-driven patterns can resemble random associations, not necessarily reflecting strong ecological preferences or filtering [3,18]. Nonetheless, in studies including ours, nestedness exceeds chance expectations, suggesting that factors beyond species abundance contribute to shaping the network structure [3,18,49]. Ecological succession plays a key role, where pioneer species facilitate the establishment of others, along with phorophyte traits such as bark size and texture. Larger phorophytes typically host more diverse epiphytic communities, including species shared with smaller phorophytes [3], which reinforces the nested pattern.
Horizontal modularity in epiphyte–phorophyte networks is generally low [4,17,20,50]. According to Naranjo et al. [4], this low modularity can be attributed to the reduced specialization levels and the lack of coevolutionary processes between the species involved. In our study, the epiphyte–phorophyte network observed in the study exhibited low overall modularity. Horizontal modularity was also low, although slightly higher than expected by chance, suggesting the presence of some level of non-random interaction structure. A key factor that may influence this low modular organization is the dispersal mode of many epiphytes, especially anemochory, which is common in families such as Bromeliaceae, Orchidaceae, and Polypodiaceae [57]. Wind dispersal facilitates random colonization across different phorophytes, reducing partner fidelity and limiting the formation of well-defined modules [20]. In some cases, however, species with more restricted dispersal, such as Tillandsia (five species of which were recorded in our study), may promote local aggregation patterns that contribute to modularity. Even so, these effects are likely modulated by broader ecological and environmental factors that reinforce low modularity overall.
Vertical modularity in the epiphyte–phorophyte network was also low, despite the identification of modules aligned with the phorophyte ecological zones. More than half of the species (57%) occurred in two or more modules, reinforcing the pattern of low compartmentalization, consistent with findings from other studies [6,19,50]. This probably reflects the microclimatic gradient provided by phorophytes, ranging from the wetter and shaded base to the more exposed upper crown [53,58]. Epiphyte abundance was higher at the crown zones (Z2, Z3, and Z4), while species richness was concentrated in the basal regions (SR and Z1), where environmental conditions are less severe and organic substrate tends to accumulate [6,15]. Phorophytes with exposed surface roots, such as Pseudobombax petropolitanum and Tabebuia reticulata, were particularly important for vascular epiphytes, especially facultative and accidental species that colonize the surface roots zone (SR) [5,6]. These exposed roots facilitate the accumulation of organic matter and moss mats, enhancing water and nutrient retention [15] and enabling the establishment of lithophytic species like Selaginella convoluta, Doryopteris collina, Vellozia plicata, and Alcantarea extensa, common in granitoid rock outcrops in southeastern Brazil [59] and exhibiting adaptations such as CAM metabolism and desiccation tolerance [8].
In contrast, the upper crown, although responsible for 52% of the total epiphyte abundance, presented lower species richness, possibly due to more adverse environmental conditions, including greater exposure to wind, intense radiation, and limited accumulation of organic substrate [60]. Under these conditions, small atmospheric bromeliads predominated, defined as species that do not have a water reservoir and absorb moisture and nutrients directly from the atmosphere through specialized leaf trichomes. These species, such as T. loliacea, T. recurvata, T. usneoides, T. gardneri, and T. stricta are well adapted to these environments through xeromorphic characteristics, CAM metabolism, and desiccation tolerance [16,22]. Their small size also facilitates the colonization of thin branches unsuitable for larger epiphytes [61]. In general, these bromeliads occur across various xeric habitats, including seasonal forests, tropical savannas, and woody vegetation on inselbergs [5,6,62,63]. Tillandsia loliacea, in particular, was present in all phorophytes analyzed, with high abundance in the upper crown, probably reflecting the xeric conditions of the inselberg environment [6]. Its wide geographic distribution and persistence in disturbed areas around inselbergs further reinforce its adaptability [64].
The lithophytes tree Tabebuia reticulata and Pseudobombax petropolitanum stood out in the network by exhibiting the highest degree and structural importance (i.e., high SS, c-score, and z-score), interacting with all recorded epiphytic species. T. reticulata, in addition to being the most abundant tree in the study area [11], and P. petropolitanum, with its extensive superficial roots exposed over rocky outcrops [14,15], offer broad spatial availability and diverse microsites—due to their large size, crown architecture, and bark roughness—which especially favor accidental and facultative epiphytes, commonly found in inselberg environments [5,15,65].
The predominance of peripheral species and the absence of hubs in the network confirm patterns observed in other plant–plant interactions, such as between epiphytic bryophytes and their phorophytes [50]. This structure may reflect ecological specialization or environmental and spatial constraints that limit the distribution of epiphytes. Within the modules, Tillandsia loliacea assumed a central role, reflecting its broad ecological tolerance and ability to colonize diverse microhabitats, a typical characteristic of atmospheric bromeliads. In general, few phorophytes play central structural roles while the majority remain peripheral, a pattern also recurrent in pollination and seed dispersal networks [24,66], highlighting a common structural feature among different types of ecological interactions.
5. Conclusions
The observed low specialization and nested structure indicate that many epiphytes can establish on multiple phorophyte species, potentially reducing their sensitivity to the loss of individual phorophyte and, consequently, lowering conservation concern at the species level. However, phorophytes with favorable structural traits provide better conditions for epiphyte establishment and survival. Notably, Tabebuia reticulata and Pseudobombax petropolitanum, both endemic to Atlantic Forest inselberg vegetation, hosted the entire recorded epiphytic community, playing central roles in maintaining it. Given that P. petropolitanum is listed as endangered in Brazil, it should be a conservation priority in inselberg flora management. The absence of network centers and the predominance of peripheral species suggest that the network may be vulnerable to local disturbances, with ecological interactions at risk even without immediate species extinction. The network’s modular structure reflects vertical microenvironmental gradients of phorophytes, confirming that light, moisture, and substrate availability can influence the distribution and interactions between species, especially in extreme habitats such as inselbergs. These findings emphasize the importance of conserving species and the ecological interactions that sustain community diversity and function. Future research should explore the role of structural factors in network organization to inform more integrated and effective conservation strategies.
Author Contributions
Conceptualization, T.M.F.; methodology and investigation, T.M.F. and D.R.C.; data curation and analyses, T.M.F.; writing—original draft, T.M.F.; writing—review and editing, T.M.F., D.R.C. and M.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author/the first author upon reasonable request.
Acknowledgments
We thank João Mario C. Covre and Roberto A. C. Jerônimo Júnior for their assistance in the field. We thank Joelcio Freitas for creating the vectors and the tree illustration used in the figures. We are also grateful to the Jaccoud family for granting access to their private property for our research. Fieldwork was authorized by the Authorization and Information System in Biodiversity (SISBIO), under the Chico Mendes Institute for Biodiversity Conservation (ICMBio). T.M.F. and D.R.C. acknowledge the support of research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Institutional Training Program—PCI/INMA, grants: D.R.C. #313848/2025-4 and T.M.F. #313866/2025-2), within the Brazilian Ministry of Science, Technology and Innovation (MCTI). MLG is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (“Bolsa de Produtividade em Pesquisa”). We also extend our thanks to the staff of the MBML herbarium for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Bascompte, J. Disentangling the web of life. Science 2009, 325, 416–419. [Google Scholar] [CrossRef]
- Burns, K.C. Network properties of an epiphytic meta-community? J. Theor. Biol. 2007, 249, 307–313. [Google Scholar] [CrossRef]
- Naranjo, C.; Iriondo, J.M.; Riofrio, M.L.; Lara-Romero, C. Evaluating the structure of commensalistic epiphyte-phorophyte networks: A comparative perspective of biotic interactions. AoB Plants 2019, 11, lz011. [Google Scholar] [CrossRef] [PubMed]
- Francisco, T.M.; Couto, D.R.; Evans, D.M.; Garbin, M.L.; Ruiz-Miranda, C.R. Structure and robustness of an epiphyte–phorophyte commensalistic network in a neotropical inselberg. Austral Ecol. 2018, 43, 903–914. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Trindade, M.S. Commensalistic epiphyte–phorophyte networks in woody vegetation of tropical inselbergs: Patterns of organization and structure. Austral Ecol. 2022, 47, 911–927. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Moreira, M.M.; Fontana, A.P.; Fraga, C.N. Inselbergs from Brazilian Atlantic Forest: High biodiversity refuges of vascular epiphytes from Espírito Santo. Biodivers. Conserv. 2023, 32, 2561–2584. [Google Scholar] [CrossRef]
- Barthlott, W.; Porembski, S. Why study inselbergs. In Inselbergs—Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–6. [Google Scholar]
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccationtolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Couto, D.R.; Porembski, S.; Barthlott, W.; de Paula, L.F.A. Hyperepilithics—An overlooked life form of vascular plants on tropical vertical rock walls. Austral Ecol. 2023, 48, 1074–1082. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; de Paula, L.F.A.; Paula, R.R.; Nascimento, M.T. Woody vegetation on tropical inselbergs: Floristic-structural characterization and aboveground carbon storage. J. Mt. Sci. 2025, 22, 1517–1534. [Google Scholar] [CrossRef]
- Porembski, S. Tropical inselbergs: Habitat types, adaptive strategies and diversity patterns. Braz. J. Bot. 2007, 20, 579–586. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Manhães, V.C.; Dias, H.M.; Pereira, M.C.A. Floristic composition of a neotropical inselberg from the state of Espírito Santo, Brazil: A relevant area for conservation. Check List 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Couto, D.R.; Dias, H.M.; Pereira, M.C.A.; Fraga, C.N.; Pezzopane, J.E.M. Vascular epiphytes on Pseudobombax (Malvaceae) in rocky outcrops (inselbergs) in Brazilian Atlantic Rainforest: Basis for conservation of a threatened ecosystem. Rodriguésia 2016, 67, 583–601. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Garbin, M.L.; Dias, H.M.; Pereira, M.C.A.; Menini Neto, L.; Pezzopane, J.E.M. Surface roots as a new ecological zone for occurrence of vascular epiphytes: A case study on Pseudobombax trees on inselbergs. Plant Ecol. 2019, 220, 1071–1084. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Klein, V.P.; Francisco, T.M.; Quaresma, A.C.; Piedade, M.T.F. Structure of epiphyte–phorophyte networks and their robustness to species loss in white-sand ecosystems in the Amazon. Biotropica 2025, 57, e70005. [Google Scholar] [CrossRef]
- Ceballos, S.J.; Chacoff, N.P.; Malizia, A. Interaction network of vascular epiphytes and trees in a subtropical forest. Acta Oecol. 2016, 77, 152–159. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Garbin, M.L.; Muylaert, R.L.; Ruiz-Miranda, C.R. Low modularity and specialization in a commensalistic epiphyte–phorophyte network in a tropical cloud forest. Biotropica 2019, 51, 509–518. [Google Scholar] [CrossRef]
- Zottarelli, H.G.S.; Molina, J.M.P.; Ribeiro, J.E.L.S.; Sofia, S.H. A Commensal network of epiphytic orchids and host trees in an Atlantic Forest Remnant: A case study revealing the important role of large trees in the network structure. Austral Ecol. 2018, 44, 114–125. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Mello, M.A.; Marquitti, M.D.; Guimarães, P.R.; Viktoria, E.K.; Jordano, P.; Martinez, M.A. Modularity of seed dispersal: Differences in structure and robustness between bat- and bird-fruit networks. Oecologia 2011, 167, 131–140. [Google Scholar] [CrossRef]
- Salazar-Rivera, G.I.; Dáttilo, W.; Castillo-Campos, G.; Flores-Estévez, N.; Ramírez García, B.; Ruelas Inzunza, E. The frugivory network properties of a simplified ecosystem: Birds and plants in a Neotropical periurban park. Ecol. Evol. 2020, 10, 8579–8591. [Google Scholar] [CrossRef]
- Veloso, H.P.; Rangel-Filho, A.L.R.; Lima, J.C.A. Classificação da Vegetação Brasileira Adaptada a um Sistema Universal; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 1991.
- Gentry, A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 1988, 75, 1–34. [Google Scholar] [CrossRef]
- Perry, D.R. A method of access into the crowns of emergent and canopy trees. Biotropica 1978, 10, 155–157. [Google Scholar] [CrossRef]
- Sanford, W.W. Distribution of epiphytic orchids in semi- deciduous tropical forest in southern Nigeria. J. Ecol. 1968, 56, 697–705. [Google Scholar] [CrossRef]
- APG-Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- PPG, I. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Jordano, P. Patterns of mutualistic interactions in pollination and seed dispersal: Connectance, dependence asymmetries, and coevolution. Am. Nat. 1987, 129, 657–677. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. Ecology 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bersier, L.F.; Banasek-Richter, C.; Cattin, M.F. Quantitative descriptors of food-web matrices. Ecology 2002, 83, 2394–2407. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Melian, C.J.; Olesen, J.M. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef]
- Guimarães, P.R., Jr.; Guimarães, P. Improving the analyses of nestedness for large sets of matrices. Environ. Modell. Softw. 2006, 21, 1512–1513. [Google Scholar] [CrossRef]
- Dormann, C.F.; Strauss, R.A. Method for detecting modules in quantitative bipartite networks. Methods Ecol. Evol. 2014, 5, 90–98. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 2. [Google Scholar]
- Cherven, K. Network Graph Analysis and Visualization with Gephi; Packt Publishing Limited: Birmingham, UK, 2013. [Google Scholar]
- Dehling, D.M. The structure of ecological networks. In Ecological Networks in the Tropics; Dáttilo, W., Rico-Gray, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 29–42. [Google Scholar]
- Bascompte, J.; Jordano, P. The structure of plant-animal mutualistic networks. In Ecological Networks: Linking Structure to Dynamics in Food Webs; Pascual, M., Dunne, J., Eds.; Santa Fe Institute Studies in the Sciences of Complexity; Oxford University Press: Oxford, UK, 2006; pp. 143–159. [Google Scholar]
- Vázquez, D.P.; Melián, C.J.; Williams, N.M.; Blüthgen, N.; Krasnov, B.R.; Poulin, R. Species abundance and asymmetric interaction strength in ecological networks. Oikos 2007, 116, 110–1127. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models: Monographs on Statistics and Applied Probability, 2nd ed.; Chapman & Hall/CRC: London, UK, 1989. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology: Developments in Environmental Modelling, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Reis, R., Jr.; Oliveira, M.L.; Borges, G.R.A. RT4Bio: R Tools for Biologists, R package version 1.0; the Laboratory of Behavioral Ecology and Computational Biology: Montes Claros, Brazil, 2015.
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package, R package version 2.6-4; R Foundation: Vienna, Austria, 2022.
- Porembski, S.; Silveira, F.A.O.; Fiedler, P.L.; Watve, A.; Rabarimanarivo, M.; Kouame, F.; Hopper, S.D. Worldwide destruction of inselbergs and related rock outcrops threatens a unique ecosystem. Biodivers. Conserv. 2016, 25, 2827–2830. [Google Scholar] [CrossRef]
- Scarano, F.R. Plant communities at the periphery of the Atlantic rain forest: Rare-species bias and its risks for conservation. Biol. Conserv. 2009, 142, 1201–1208. [Google Scholar] [CrossRef]
- Sáyago, R.; Lopezaraiza-Mikel, M.; Quesada, M.; Alvarez-Anorve, M.Y.; Cascante-Marin, A.; Bastida, J.M. Evaluating factors that predict the structure of a commensalistic epiphyte-phorophyte network. Proc. R. Soc. B. 2013, 280, 20122821. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-X.; Shen, T.; Quan, D.-L.; Nakamura, A.; Song, L. Structuring interaction networks between epiphytic bryophytes and their hosts in Yunnan, SW China. Front. For. Glob. Change 2021, 4, 716278. [Google Scholar] [CrossRef]
- Dáttilo, W.; Vizentin-Bugoni, J.; Debastiani, V.J.; Jordano, P.; Izzo, T.J. The influence of spatial sampling scales on ant–plant interaction network architecture. J. Anim. Ecol. 2019, 88, 903–914. [Google Scholar] [CrossRef]
- Silva, I.A.; Ferreira, A.W.C.; Lima, M.I.S.; Soares, J.J. Networks of epiphytic orchids and host trees in Brazilian gallery forests. J. Trop. Ecol. 2010, 26, 127–137. [Google Scholar] [CrossRef]
- Wagner, K.; Mendieta-Leiva, G.; Zotz, G. Host Specificity in vascular epiphytes: A review of methodology, empirical evidence and potential mechanisms. AoB Plants 2015, 7, plu092. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Saldaña, A.; Zotz, G.; Kirby, C.; Díaz, I.; Burns, K. Composition patterns and network structure of epiphyte–host interactions in Chilean and New Zealand temperate forests. N. Z. J. Bot. 2016, 54, 204–222. [Google Scholar] [CrossRef]
- Laube, S.; Zotz, G. Neither host-specific nor random: Vascular epiphytes on three tree species in a Panamanian lowland forest. Ann. Bot. 2006, 97, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Burns, K. Epiphyte community development throughout tree ontogeny: An island ontogeny framework. J. Veg. Sci. 2015, 26, 902–910. [Google Scholar] [CrossRef]
- Cascante-Marin, A.; von Meijenfeldt, N.; de Leeuw, H.M.H.; Wolf, J.H.D.; Oostermeijer, J.G.B.; den Nijs, J.C.M. Dispersal limitation in epiphytic bromeliad communities in a Costa Rican fragmented montane landscape. J. Trop. Ecol. 2009, 25, 63–73. [Google Scholar] [CrossRef]
- Sanger, J.C.; Kirkpatrick, J.B. Fine partitioning of epiphyte habitat within Johansson zones in tropical Australian rain forest trees. Biotropica 2016, 49, 27–34. [Google Scholar] [CrossRef]
- De Paula, L.F.A.; Azevedo, L.O.; Mauad, L.P.; Cardoso, L.J.T.; Braga, J.M.A.; Kollmann, L.J.C.; Fraga, C.N.; Menini Neto, L.; Labiak, P.H.; Mello-Silva, R.; et al. Sugarloaf Land in south-eastern Brazil: A tropical hotspot of lowland inselberg plant diversity. Biodivers. Data J. 2020, 8, e53135. [Google Scholar] [CrossRef] [PubMed]
- Krömer, T.; Kessler, M.; Gradstein, S.R. Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: The importance of the understory. Plant Ecol. 2007, 189, 261–278. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Olmsted, I.C. Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) in Mexico. Biotropica 1992, 24, 402–407. [Google Scholar] [CrossRef]
- Vergara-Torres, C.A.; Pacheco-Álvarez, M.C.; Flores-Palacios, A. Host preference and host limitation of vascular epiphytes in a tropical dry forest of central Mexico. J. Trop. Ecol. 2010, 26, 563–570. [Google Scholar] [CrossRef]
- Callaway, R.M.; Reinhart, K.O.; Tucker, S.C.; Pennings, S.C. Effects of epiphytic lichens on host preference of the vascular epiphyte Tillandsia usneoides. Oikos 2003, 94, 433–441. [Google Scholar] [CrossRef]
- Smith, L.B.; Downs, R.J. Tillandsioideae (Bromeliaceae). In Flora Neotropica. Monograph; Hafner Press: New York, NY, USA, 1977. [Google Scholar]
- Francisco, T.M.; Couto, D.R.; Garbin, M.L.; Misaki, F.; Ruiz-Miranda, C.R. Role of spatial and environmental factors in structuring vascular epiphyte communities in two neotropical ecosystems. Plant Ecol. Evol. Syst. 2021, 51, 125621. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).