Effects of Different Phosphorus Addition Levels on Physiological and Growth Traits of Pinus massoniana (Masson Pine) Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Harvest and Measurements
2.3. Statistical Analysis
3. Results
3.1. Effect of Phosphorus Addition on Biomass Allocation
3.2. Effect of Phosphorus Addition on the Root Morphology and Architecture
3.3. Effect of Phosphorus Addition on Nutrient Elements and Non-Structural Carbohydrates
3.4. Effect of Phosphorus Addition on the Nutrient Accumulation Ratio
3.5. Correlation Analysis Among the Indicators
4. Discussion
4.1. Responses of Biomass Allocation
4.2. Responses of Root Morphology and Root Architecture
4.3. Responses of Nutrient Elements’ and Non-Structural Carbohydrates’ Allocation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jian, Z.J.; Ni, Y.Y.; Xu, J.; Lei, L.; Zeng, L.X.; Xiao, W.F. Soil fertility in the Pinus massoniana forests of China. Acta Ecol. Sin. 2021, 41, 5279–5288. [Google Scholar] [CrossRef]
- Peng, J.F.; Cui, J.Y.; Li, J.B.; Peng, M.; Ma, Y.T.; Wei, X.X.; Li, J.K.; Li, X.; Liu, Y.M.; Li, J.X. Microenvironmental effects on growth response of Pinus massoniana to climate at its northern boundary in the Tongbai Mountains, Central China. J. For. Res. 2024, 1, 44–57. [Google Scholar] [CrossRef]
- Tie, L.H.; Peñuelas, J.; Huang, C.; Sardans, J.; Bose, A.K.; Ouyang, S.G.; Kong, Y.X.; Guo, Y.; Wu, Y.J.; Cheng, W.; et al. Phosphorus limitation of Pinus massoniana reforestation increases with stand development: Evidence from plant, leaf litter, and soil. Plant Soil 2024, 504, 817–832. [Google Scholar] [CrossRef]
- Lu, L.H.; Cai, D.X.; He, R. Synthetic Analysis on the fertilization effects for young Pinus massoniana plantation. Sci. Silvae Sin. 2004, 40, 99–105. [Google Scholar]
- Liu, Q.H.; Zhou, Z.C.; Zhang, K.M.; Lan, Y.Z.; Wu, J.F.; Nie, G.Q. Influence of phosphorus on growth and wood basic density of Pinus massoniana provenances. J. Zhejiang AF Univ. 2012, 29, 185–191. [Google Scholar]
- Kulmann, M.S.S.; Aguilar, M.V.M.A.; Tassinari, A.; Schwalbert, R.; Tabaldi, L.A.; Araujo, M.M.; Antoniolli, Z.I.; Nicoloso, F.T.; Brunetto, G.; Schumacher, V.M. Effects of increasing soil phosphorus and association with ectomycorrhizal fungi (Pisolithus microcarpus) on morphological, nutritional, biochemical, and physiological parameters of Pinus taeda L. For. Ecol. Manag. 2023, 544, 121207. [Google Scholar] [CrossRef]
- Shinde, S.; Naik, D.; Cumming, J.R. Carbon allocation and partitioning in Populus tremuloides are modulated by ectomycorrhizal fungi under phosphorus limitation. Tree Physiol. 2017, 38, 52–65. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Bai, C.; Liu, Y.F.; Ma, M.Z.; Zhang, S.W.; Liu, H.; Bai, R.; Han, X.R.; Yong, J.W.H. Resilient and sustainable production of peanut (Arachis hypogaea) in phosphorus-limited environment by using exogenous gamma-aminobutyric acid to sustain photosynthesis. Ecotoxicol. Environ. Saf. 2023, 263, 115388. [Google Scholar] [CrossRef]
- Crombez, H.; Motte, H.; Tom, B. Tackling plant phosphate starvation by the roots. Dev. Cell 2019, 48, 599–615. [Google Scholar] [CrossRef]
- Xu, W.F.; Zhang, Q.; Yuan, W.; Xu, F.Y.; Aslam, M.M.A.; Miao, R.; Li, Y.; Wang, Q.W.; Li, X.; Zhang, X.; et al. The genome evolution and low-phosphorus adaptation in white lupin. Nat. Commun. 2020, 11, 1069. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.; Mo, S.; Liu, J.; Xing, Y.; Wang, Y.; Ge, C.; Wang, Y. Mechanism of low phosphorus inducing the main root lengthening of rice. J. Plant Growth Regul. 2021, 18, 1032–1043. [Google Scholar] [CrossRef]
- Gong, H.Q.; Xiang, Y.; Wako, B.K.; Jiao, X.Q. Complementary effects of phosphorus supply and planting density on maize growth and phosphorus use efficiency. Front. Plant Sci. 2022, 13, 983788. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Yang, X.Y.; Liu, H.J.; Wang, W.; Wang, C.; Ding, G.D.; Xu, F.S.; Wang, S.L.; Cai, H.M.; Hammond, J.P.; et al. Local and systemic responses conferring acclimation of Brassica napus roots to low phosphorus conditions. J. Exp. Bot. 2022, 11, 4753–4777. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 977–1178. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Stickland, T.R.; Harvey, M.L.; Wilson, G.W. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Sorgonà, A.; Abenavoli, M.R.; Cacco, G. A comparative study between two citrus rootstocks: Effect of nitrate on the root morpho-topology and net nitrate uptake. Plant Soil 2005, 270, 257–267. [Google Scholar] [CrossRef]
- Xu, J.; Lei, L.; Zeng, L.X.; Jian, Z.J.; Xiao, W.F.; Ni, Y.Y. The combined effect of root morphological and resistance traits alleviated the growth limitations of Pinus massoniana seedlings under low phosphorus conditions. Plant Soil 2024, 507, 807–822. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.F.; Chen, Y.; Sun, M.M.; Wang, Y.; Chen, Y.F. The transcription factor NIGT1.2 modulates both phosphate uptake and nitrate influx during phosphate starvation in Arabidopsis and maize. Plant Cell 2020, 32, 3519–3534. [Google Scholar] [CrossRef]
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef]
- Chen, J.X.; Lian, W.T.; Li, Z.; Guo, X.; Li, Y.N.; Zhao, H.Y.; Yi, K.K.; Li, X.X.; Liao, H. Natural variation in the GmVPE1 promoter contributes to phosphorus re-translocation to seeds and improves soybean yield. Plant Biotechnol. J. 2025, 23, 1359–1372. [Google Scholar] [CrossRef]
- Hayes, P.E.; Nge, F.J.; Cramer, M.D.; Finnegan, P.M.; Fu, P.; Hopper, S.D.; Oliveira, R.S.; Turner, B.L.; Zemunik, G.; Zhong, H.T.; et al. Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant Soil 2021, 462, 67–88. [Google Scholar] [CrossRef]
- Xiao, X.L.; Zhang, J.Q.; Satheesh, V.; Meng, F.X.; Gao, W.L.; Dong, J.S.; Zheng, Z.; An, G.Y.; Nussaume, L.; Liu, D.; et al. SHORT-ROOT stabilizes PHOSPHATE1 to regulate phosphate allocation in Arabidopsis. Nat. Plants 2022, 8, 1074–1081. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Amit, K.; Shahbaz, M.; Koirala, M.; Blagodatskaya, E.; Seidel, S.J.; Kuzyakov, Y.; Pausch, J. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. J. Plant Nutr. Soil Sci. 2019, 182, 945–952. [Google Scholar] [CrossRef]
- Feng, J.G.; Song, Y.J.; Zhu, B. Ecosystem-dependent responses of soil carbon storage to phosphorus enrichment. New Phytol. 2023, 238, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Wu, T.G.; Chu, X.L.; Wang, B.; Wang, X.H.; Zhang, D.B.; Zhou, Z.C. Effects of phosphorus addition and inoculation of Mycorrhizal Fungi on the growth and phosphorus utilization of masson pine container seedlings from dfferent families. For. Res. 2021, 34, 142–151. [Google Scholar]
- Zhang, T.; Wen, X.P.; Ding, G.J. Ectomycorrhizal symbiosis enhances tolerance to low phosphorous through expression of phosphate transporter genes in masson pine (Pinus massoniana). Acta Physiol. Plant. 2017, 39, 101. [Google Scholar] [CrossRef]
- Fan, F.H.; Wang, Q.Z.; Li, H.P.; Ding, G.J.; Wen, X.P. Transcriptome-Wide Identification and Expression Profiles of Masson Pine WRKY Transcription Factors in Response to Low Phosphorus Stress. Plant Mol. Bio. Rep. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Jiao, J.Y.; Chen, T.D.; Chen, Y.L.; Lin, H.; Xu, Q.; Cheng, Y.Z.; Zhao, W.T. Soil nutrient evaluation of alluvial fan in the middle and lower reaches of Lhasa River Basin. J. Plant Nutr. Fertil. 2022, 28, 2082–2096. [Google Scholar]
- Bouma, T.J.; Nielsen, K.L.; Hal, J.V.; Koutstaal, B. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Funct. Ecol. 2001, 15, 360–369. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhou, B.Z.; Ge, X.G.; Cao, Y.H.; Brunner, I.; Shi, J.X.; Li, M.H. Species-Speciffc responses of root morphology of three co-existing tree species to nutrient patches reflect their root foraging strategies. Front. Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef]
- Gao, Z.B.; Wang, H.Y.; Lu, X.T.; Wang, Z.Y. Effects of nitrogen and phosphorus addition on C:N:P stoichiometry in roots and leaves of four dominant plant species in a meadow steppe of Hulunbuir. Chin. J. Ecol. 2017, 36, 80–88. [Google Scholar]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H. Mobile carbohydrates in Himalayan treeline trees I. evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Gu, R.; Lin, L.X.; Russo, S.E. Functional traits and ecological niches as correlates of the interspecific growth–mortality trade-off among seedlings of 14 tropical tree species. Funct. Ecol. 2024, 38, 1888–1901. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, J.Y.; Yang, Y.M.; Yu, D.Y.; Zhang, H.Y.; Zhang, D. Molecular mechanisms underlying plant responses to low phosphate stress and potential applications in crop improvement. New Crops 2025, 2, 100064. [Google Scholar] [CrossRef]
- Zhou, J.F.; Shi, W.H.; Pan, K.T.; Ying, Y.Q.; Sun, C. Effect of low phosphorus stress on growth and nutrient physiology of Phyllostachys edulis seedlings. J. Zhejiang AF Univ. 2022, 39, 1010–1017. [Google Scholar]
- Joshi, S.R.; Morris, J.W.; Tfaily, M.M.; Young, R.P.; McNear, D.H., Jr. Low soil phosphorus availability triggers maize growth stage specific rhizosphere processes leading to mineralization of organic P. Plant Soil 2021, 459, 423–440. [Google Scholar] [CrossRef]
- Yu, Y.C.; Yu, J.; Fang, L.; Jiang, D.F. Organic Acids exudation from the roots of Cunninghamia lanceolata and Pinus massoniana seedlings under low phosphorus stress. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2007, 31, 9–12. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, Y.Q.; Wan, X.R. Effects of different levels of NP nutrition on the dry matter distribution of root and shoot of Chinese Fir, Slash Pine and Timor Mahogany seedlings. Sci. Silvae Sin. 2006, 42, 48–53. [Google Scholar]
- Ai, Z.Z.; Yuan, J.; Hang, M.L.; Tan, X.F. Effects of phosphorus on root/shoot ratio and root morphology of Camellia oleifera seedlings under aluminum toxicity. Jiangsu Agric. Sci. 2017, 45, 106–108. [Google Scholar]
- Han, C.; Song, M.; Du, H.; Zeng, F.P.; Peng, W.X.; Wang, H.; Chen, L.; Su, L. Biomass and carbon storage in roots of Cunninghamia lanceolata and Pinus massoniana plantations at different stand ages in Guangxi. Acta Ecol. Sin. 2017, 37, 2282–2289. [Google Scholar] [CrossRef][Green Version]
- Bom, F.; Williams, A.; Borrell, A.K.; Raymond, N.; Bell, M. Phosphorus management is key to effective deployment of root ideotypes in complex soil environments. Plant Soil 2023, 489, 323–340. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, P.F.; Zou, X.H.; Wang, P.; Ma, J.; Ma, X.Q. Relationship between Growth and Endogenous Hormones of Chinese Fir seedlings under low phosphorus stress. Sci. Silvae Sin. 2016, 52, 57–66. [Google Scholar]
- Chen, Y.L.; Li, X.L.; Zhou, X.Y. Effects of phosphate deficiency on the growth and acid phosphatase activity of Larix gmelini seedlings. J. Beijing For. Univ. 2006, 28, 46–50. [Google Scholar]
- Chen, X.Y.; Ren, H.; Zhang, J.W.; Zhao, B.; Ren, B.Z.; Wan, Y.S.; Liu, P. Deep phosphorus fertilizer placement increases maize productivity by improving root-shoot coordination and photosynthetic performance. Soil Tillage Res. 2024, 235, 105915. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant. 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Liu, B.; Xu, W.Y.; Niu, Y.X.; Li, Q.Y.; Cao, B.L.; Qi, J.Y.; Zhao, Y.D.; Zhou, Y.L.; Song, L.; Cui, D.K.; et al. TaTCP6 is required for efficient and balanced utilization of nitrate and phosphorus in wheat. Nat. Commun. 2025, 16, 1683. [Google Scholar] [CrossRef]
- Ni, H.J.; Zhao, J.C.; Yang, Z.Y. The effects of different moso bamboo densities on the physiological growth of Indocalamus latifolius cultivated in moso bamboo Forests. Forests 2025, 16, 636. [Google Scholar] [CrossRef]
- Xu, F.L.; Zhao, Y.F.; Zhang, P.; Wang, W.L.; Yu, Q.M.; Wang, W.D.; Wang, G.X. Effects of fertilization on nitrogen and phosphorus content in roots, stems and leaves of Larix principis-rupprechtii plantation. Sci. Silvae Sin. 2014, 50, 139–143. [Google Scholar]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.B.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.P.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Dong, Y.F.; Xu, T.; Wang, Y.P.; Wang, H.T.; Duan, B.L. Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New For. 2017, 48, 587–607. [Google Scholar] [CrossRef]
- Yu, Q.S.; Ni, X.F.; Ji, C.J.; Zhu, J.L.; Tang, Z.Y.; Fang, J.Y. Effects of 10-year nitrogen and phosphorus additions on leaf non-structural carbohydrates of dominant plants in tropical rainforests in Jianfengling, Hainan. Chin. J. Plant Ecol. 2024, 48, 690–700. [Google Scholar]
- Hammond, J.P.; White, P.J. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 2011, 156, 1033–1040. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Yamagishi, M.; Osaki, M.; Masuda, K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. J. Exp. Bot. 2008, 59, 2749–2756. [Google Scholar] [CrossRef]
- Vriet, C.; Smith, A.M.; Wang, T.L. Root starch reserves are necessary for vigorous re-growth following cutting back in Lotus japonicus. PLoS ONE 2014, 31, e87333. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamazaki, Y.; Kobayashi, A.; Higashitani, A.; Takahashi, H. Hydrotropism Interacts with gravitropism by degrading amyloplasts in seedling roots of arabidopsis and radish. Plant Physiol. 2003, 132, 805–810. [Google Scholar] [CrossRef]

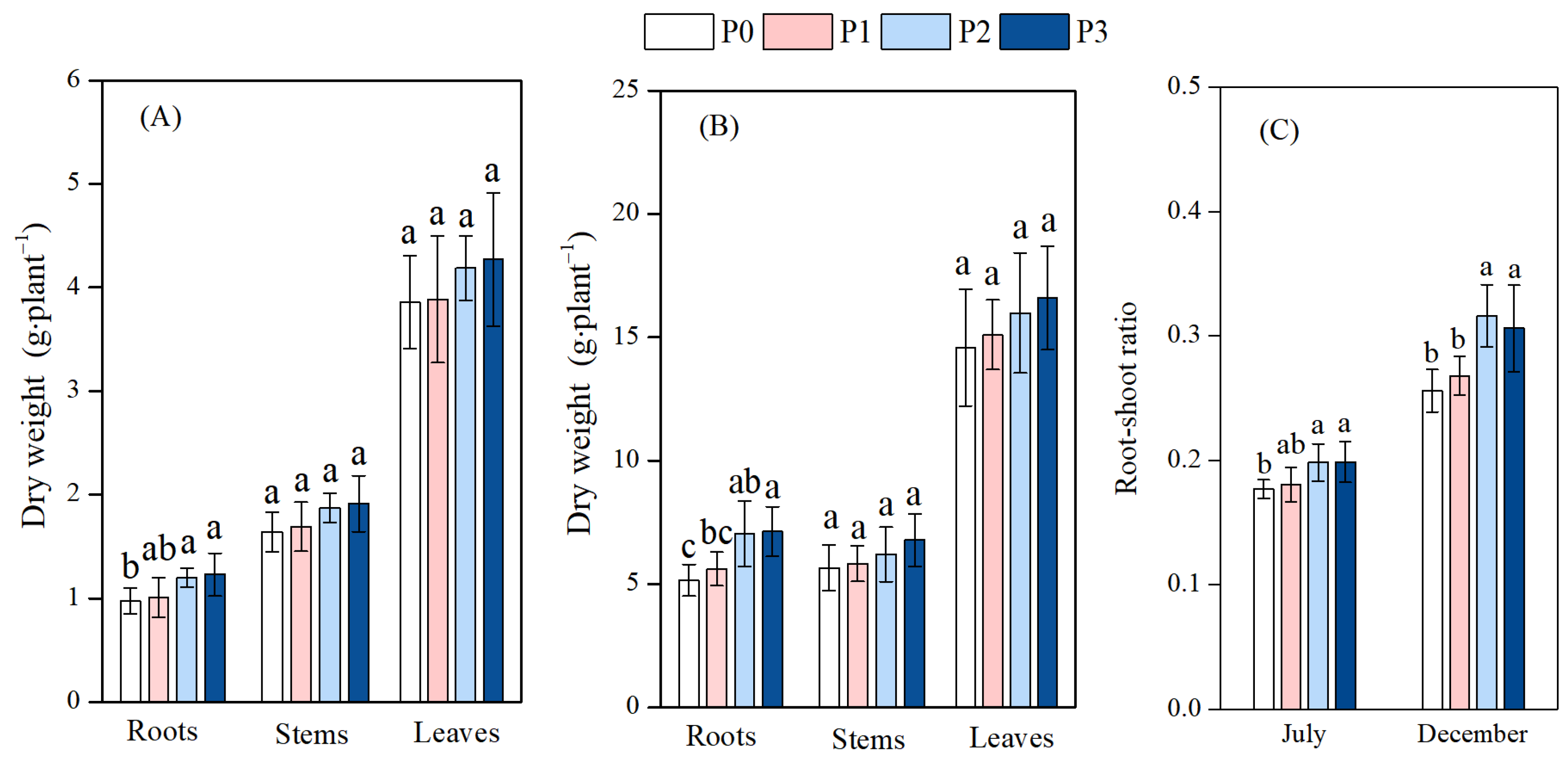
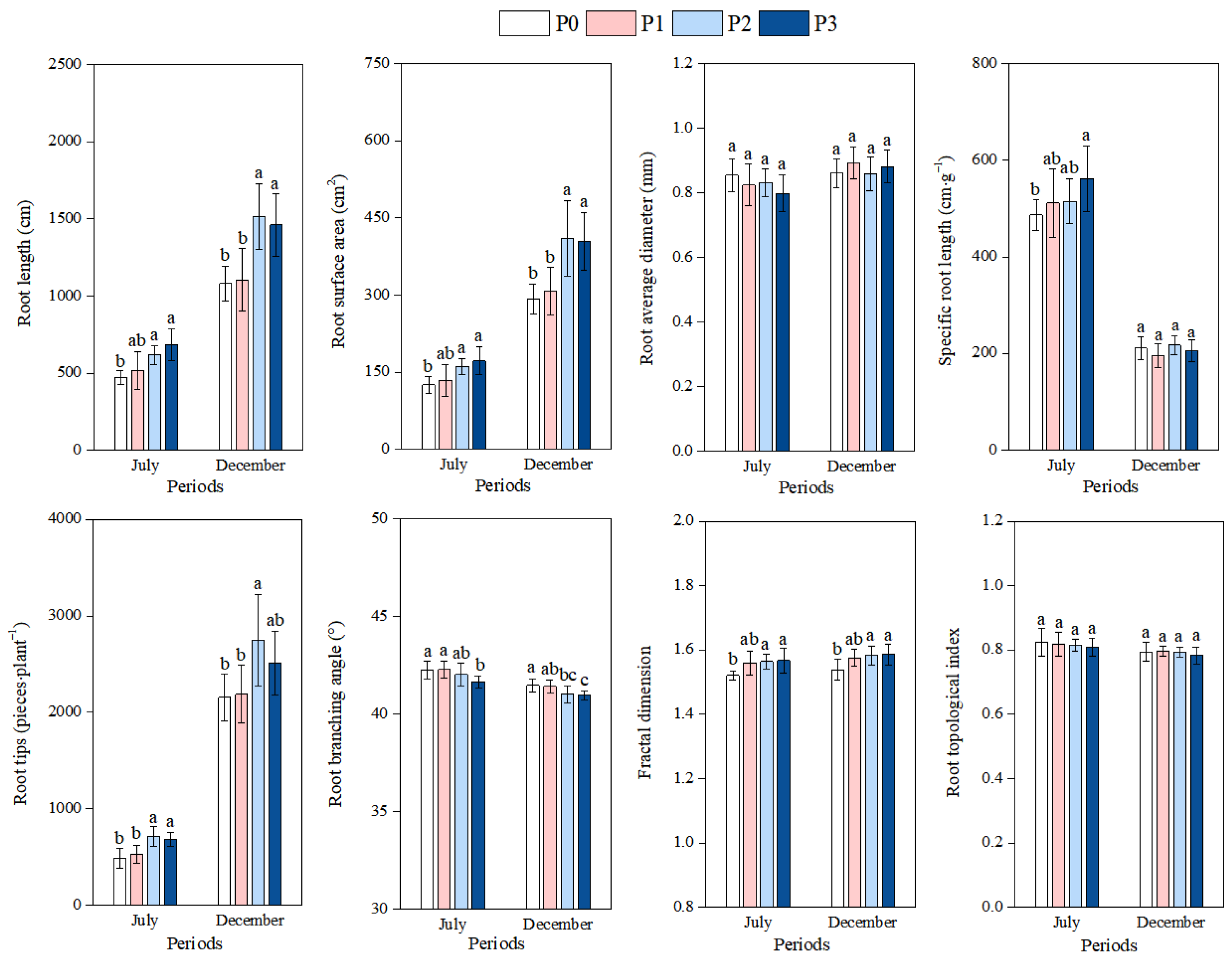
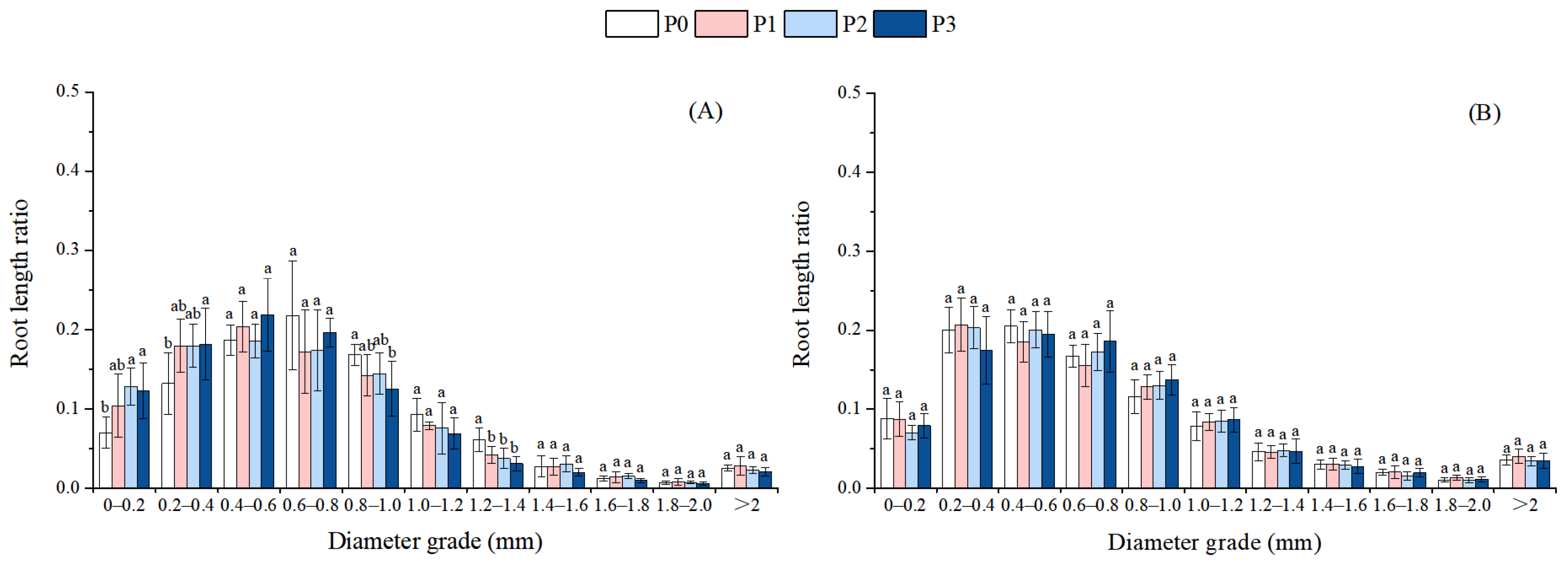

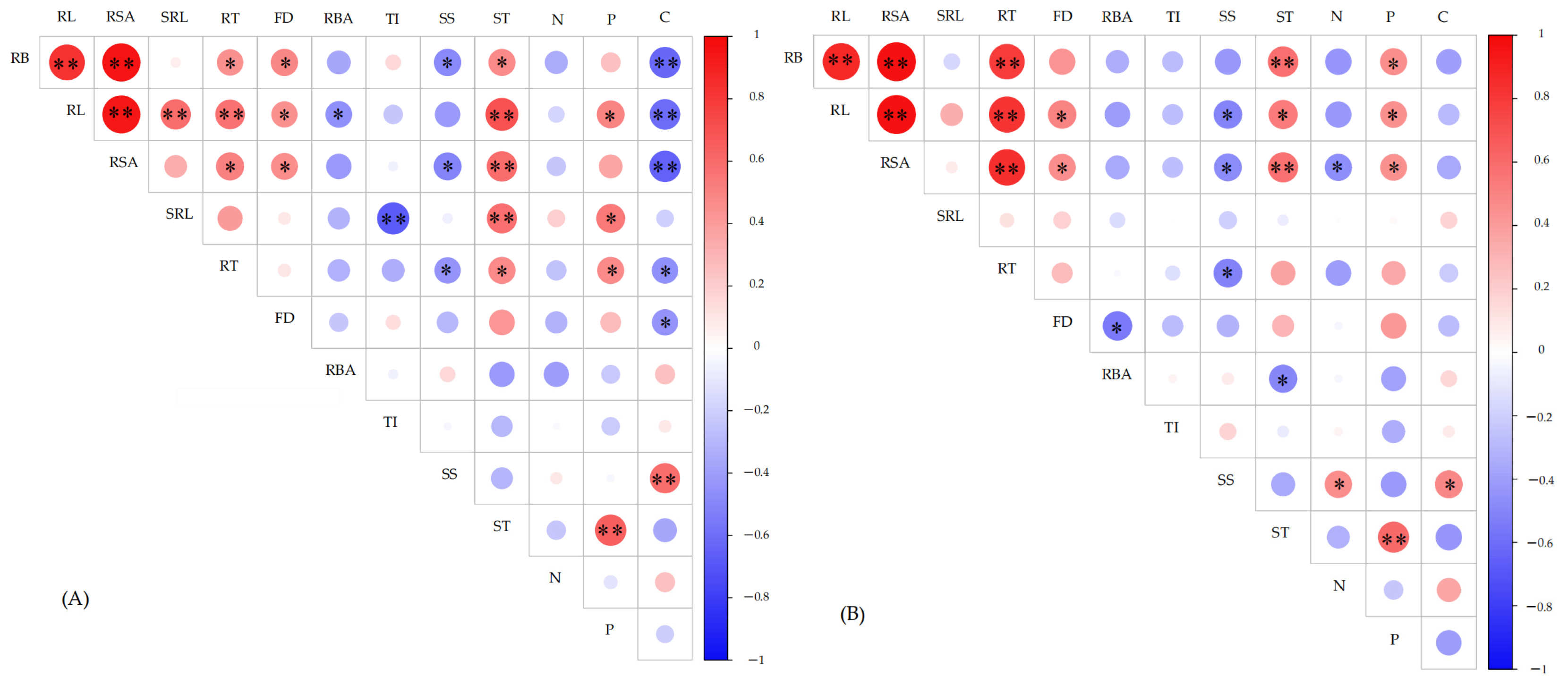
| Periods | Nutrient Content and Ratio | Treatments | Leaves | Stems | Roots |
|---|---|---|---|---|---|
| July | P (mg g−1) | P0 | 0.945 ± 0.044 b | 0.560 ± 0.033 b | 0.633 ± 0.023 b |
| P1 | 1.026 ± 0.044 ab | 0.612 ± 0.026 ab | 0.696 ± 0.032 a | ||
| P2 | 1.136 ± 0.096 a | 0.656 ± 0.071 a | 0.725 ± 0.048 a | ||
| P3 | 1.162 ± 0.086 a | 0.679 ± 0.066 a | 0.739 ± 0.062 a | ||
| N (mg g−1) | P0 | 13.04 ± 0.65 a | 4.61 ± 0.22 a | 8.20 ± 0.66 a | |
| P1 | 12.02 ± 1.08 ab | 4.18 ± 0.36 ab | 7.99 ± 0.53 a | ||
| P2 | 11.78 ± 0.58 b | 3.99 ± 0.31 b | 7.81 ± 0.68 a | ||
| P3 | 11.40 ± 0.96 b | 3.97 ± 0.25 b | 7.75 ± 0.41 a | ||
| C (mg g−1) | P0 | 526.8 ± 7.2 a | 561.4 ± 20.9 a | 545.8 ± 8.3 a | |
| P1 | 513.6 ± 8.7 ab | 549.2 ± 15.9 ab | 537.2 ± 14.1 ab | ||
| P2 | 501.8 ± 14.2 b | 537.8 ± 19.0 ab | 530.2 ± 18.3 ab | ||
| P3 | 506.4 ± 16.0 b | 525.2 ± 23.8 b | 525.6 ± 17.1 b | ||
| N/P | P0 | 13.81 ±0.31 a | 8.24 ± 0.24 a | 12.96 ± 0.92 a | |
| P1 | 11.72 ± 1.06 b | 6.83 ± 0.63 b | 11.50 ± 0.90 ab | ||
| P2 | 10.42 ± 0.93 c | 6.10 ± 0.23 c | 10.79 ± 0.74 b | ||
| P3 | 9.82 ± 0.63 c | 5.89 ± 0.68 c | 10.57 ± 1.27 b | ||
| C/N | P0 | 40.47 ± 1.22 b | 121.9 ± 7.2 a | 66.98 ± 6.03 a | |
| P1 | 42.97 ± 3.51 ab | 132.3 ± 13.0 a | 67.46 ± 5.02 a | ||
| P2 | 42.66 ± 1.94 ab | 135.5 ± 11.9 a | 68.22 ± 5.58 a | ||
| P3 | 44.62 ± 3.12 a | 132.7 ± 10.5 a | 67.92 ± 3.02 a | ||
| C/P | P0 | 472.7 ± 35.5 a | 819.3 ± 65.5 a | 700.7 ± 44.7 a | |
| P1 | 410.3 ± 26.6 b | 767.0 ± 41.0 ab | 594.2 ± 27.6 b | ||
| P2 | 378.1 ± 19.0 bc | 695.2 ± 62.5 bc | 571.3 ± 35.6 b | ||
| P3 | 366.5 ± 20.4 c | 616.4 ± 43.4 c | 552.7 ± 36.8 b | ||
| December | P (mg g−1) | P0 | 1.098 ± 0.077 c | 0.661 ± 0.045 c | 0.750 ± 0.039 b |
| P1 | 1.220 ± 0.085 b | 0.706 ± 0.034 bc | 0.866 ± 0.031 a | ||
| P2 | 1.296 ± 0.040 ab | 0.768 ± 0.062 ab | 0.895 ± 0.060 a | ||
| P3 | 1.340 ± 0.075 a | 0.823 ± 0.056 a | 0.910 ± 0.050 a | ||
| N (mg g−1) | P0 | 12.10 ± 0.51 a | 4.17 ± 0.35 a | 7.54 ± 0.63 a | |
| P1 | 10.51 ± 0.66 b | 3.70 ± 0.25 b | 7.39 ± 0.54 a | ||
| P2 | 10.16 ± 0.89 b | 3.43 ± 0.29 b | 6.99 ± 0.58 a | ||
| P3 | 9.77 ± 0.50 b | 3.46 ± 0.22 b | 7.09 ± 0.23 a | ||
| C (mg g−1) | P0 | 517.2 ± 13.1 a | 539.4 ± 15.8 a | 524.0 ± 15.4 a | |
| P1 | 498.0 ± 9.1 b | 524.8 ± 9.7 ab | 513.8 ± 13.3 ab | ||
| P2 | 490.4 ± 17.3 b | 516.6 ± 9.4 bc | 509.8 ± 9.0 ab | ||
| P3 | 489.2 ± 10.9 b | 505.4 ± 15.0 c | 501.6 ± 18.8 b | ||
| N/P | P0 | 11.06 ± 0.79 a | 6.30 ± 0.21 a | 10.08 ± 1.08 a | |
| P1 | 8.65 ± 0.85 b | 5.23 ± 0.13 b | 8.56 ± 0.88 b | ||
| P2 | 7.84 ± 0.72 bc | 4.46 ± 0.21 c | 7.81 ± 0.35 b | ||
| P3 | 7.30 ± 0.47 c | 4.23 ± 0.45 c | 7.80 ± 0.43 b | ||
| C/N | P0 | 42.82 ± 2.42 b | 130.1± 11.3 b | 69.93 ± 6.43 a | |
| P1 | 47.52 ± 2.69 a | 142.6 ± 10.8 ab | 69.77 ± 4.89 a | ||
| P2 | 48.52 ± 4.02 a | 151.8 ± 14.7 a | 73.27 ± 5.49 a | ||
| P3 | 50.19 ± 2.63 a | 146.2 ± 7.2 a | 70.79 ± 1.47 a | ||
| C/P | P0 | 472.9 ± 35.8 a | 819.3 ± 65.5 a | 700.7 ± 44.7 a | |
| P1 | 409.6 ± 27.0 b | 745.2 ± 38.9 b | 594.2 ± 27.6 b | ||
| P2 | 378.8 ± 20.0 bc | 676.6 ± 59.4 bc | 571.3 ± 35.6 b | ||
| P3 | 365.9 ± 20.2 c | 616.4 ± 43.4 c | 552.7 ± 36.8 b |
| Periods | Non-Structural Carbohydrate Content and Ratio | Treatments | Leaves | Stems | Roots |
|---|---|---|---|---|---|
| July | Soluble sugar (%) | P0 | 11.33 ± 0.76 a | 7.54 ± 0.47 a | 7.94 ± 0.66 a |
| P1 | 10.51 ± 0.73 a | 6.91 ± 0.47 ab | 7.80 ± 0.45 a | ||
| P2 | 9.41 ± 0.44 b | 6.54 ± 0.53 b | 7.41 ± 0.74 a | ||
| P3 | 9.32 ± 0.58 b | 6.25 ± 0.46 b | 7.29 ± 0.52 a | ||
| Starch (%) | P0 | 5.34 ± 0.44 c | 6.80 ± 0.41 c | 7.71 ± 0.47 c | |
| P1 | 6.28 ± 0.42 b | 7.07 ± 0.42 c | 7.88 ± 0.26 c | ||
| P2 | 6.47 ± 0.23 ab | 8.03 ± 0.59 b | 9.13 ± 0.72 b | ||
| P3 | 6.87 ± 0.51 a | 10.32 ± 0.75 a | 12.26 ± 0.94 a | ||
| Sugar/starch | P0 | 2.126 ± 0.099 a | 1.114 ± 0.119 a | 1.105 ± 0.115 a | |
| P1 | 1.680 ± 0.173 b | 0.978 ± 0.059 b | 0.991 ± 0.073 b | ||
| P2 | 1.454 ± 0.066 c | 0.819 ± 0.095 c | 0.814 ± 0.080 c | ||
| P3 | 1.363 ± 0.134 c | 0.607 ± 0.045 d | 0.596 ± 0.039 d | ||
| December | Soluble sugar (%) | P0 | 12.31 ± 0.86 a | 8.79 ± 0.59 a | 9.42 ± 0.72 a |
| P1 | 10.93 ± 0.45 b | 7.98 ± 0.50 b | 9.12 ± 0.49 a | ||
| P2 | 9.89 ± 0.62 c | 6.93 ± 0.50 c | 8.75 ± 0.66 a | ||
| P3 | 9.80 ± 0.45 c | 6.64 ± 0.35 c | 8.62 ± 0.58 a | ||
| Starch (%) | P0 | 5.43 ± 0.25 b | 6.32 ± 0.47 c | 6.38 ± 0.51 d | |
| P1 | 6.55 ± 0.47 a | 6.77 ± 0.30 c | 7.13 ± 0.38 c | ||
| P2 | 7.13 ± 0.60 a | 7.44 ± 0.48 b | 8.26 ± 0.37 b | ||
| P3 | 7.04 ± 0.50 a | 8.77 ± 0.62 a | 10.11 ± 0.75 a | ||
| Sugar/starch | P0 | 2.269 ± 0.110 a | 1.392 ± 0.034 a | 1.478 ± 0.082 a | |
| P1 | 1.673 ± 0.073 b | 1.182 ± 0.117 b | 1.281 ± 0.073 b | ||
| P2 | 1.395 ± 0.146 c | 0.937 ± 0.121 c | 1.064 ± 0.122 c | ||
| P3 | 1.398 ± 0.137 c | 0.759 ± 0.063 d | 0.855 ± 0.068 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, H. Effects of Different Phosphorus Addition Levels on Physiological and Growth Traits of Pinus massoniana (Masson Pine) Seedlings. Forests 2025, 16, 1265. https://doi.org/10.3390/f16081265
Yang Z, Wang H. Effects of Different Phosphorus Addition Levels on Physiological and Growth Traits of Pinus massoniana (Masson Pine) Seedlings. Forests. 2025; 16(8):1265. https://doi.org/10.3390/f16081265
Chicago/Turabian StyleYang, Zhenya, and Hui Wang. 2025. "Effects of Different Phosphorus Addition Levels on Physiological and Growth Traits of Pinus massoniana (Masson Pine) Seedlings" Forests 16, no. 8: 1265. https://doi.org/10.3390/f16081265
APA StyleYang, Z., & Wang, H. (2025). Effects of Different Phosphorus Addition Levels on Physiological and Growth Traits of Pinus massoniana (Masson Pine) Seedlings. Forests, 16(8), 1265. https://doi.org/10.3390/f16081265





