Coordinated Roles of Osmotic Adjustment, Antioxidant Defense, and Ion Homeostasis in the Salt Tolerance of Mulberry (Morus alba L. ‘Tailai Sang’) Seedlings
Abstract
1. Introduction
1.1. Global Challenge of Soil Salinization and Its Impact on Forestry
1.2. Mulberry (Morus alba L.): Economic and Ecological Importance
1.3. Known Salt-Tolerance Mechanisms in Plants
1.4. Knowledge Gap and Rationale
1.5. Objectives and Hypotheses
- Quantifying the dose-dependent effects of NaCl on the growth and photosynthetic performance (including both gas exchange and chlorophyll fluorescence) of two-year-old Morus alba ‘Tailai Sang’ seedlings.
- Investigating the patterns of osmotic adjustment through the accumulation of proline, soluble sugars, and soluble proteins.
- Characterizing the ion homeostasis strategy by measuring Na+ and K+ distribution in roots and leaves.
- Evaluating the dynamics of the antioxidant system by measuring ROS indicators (MDA, H2O2) and the activities of key antioxidant enzymes (SOD, CAT, APX, GR).
- Establishing a salt tolerance threshold (LC50) based on biomass reduction.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Salt Stress Treatments
2.3. Measurement of Growth and Biomass
2.4. Photosynthetic Gas Exchange and Chlorophyll Fluorescence
2.5. Photosynthetic Pigments and Anthocyanins
2.6. Osmotic Regulators and Ion Content
2.7. Oxidative Stress Markers and Antioxidant Enzymes
2.8. Statistical Analysis
3. Results
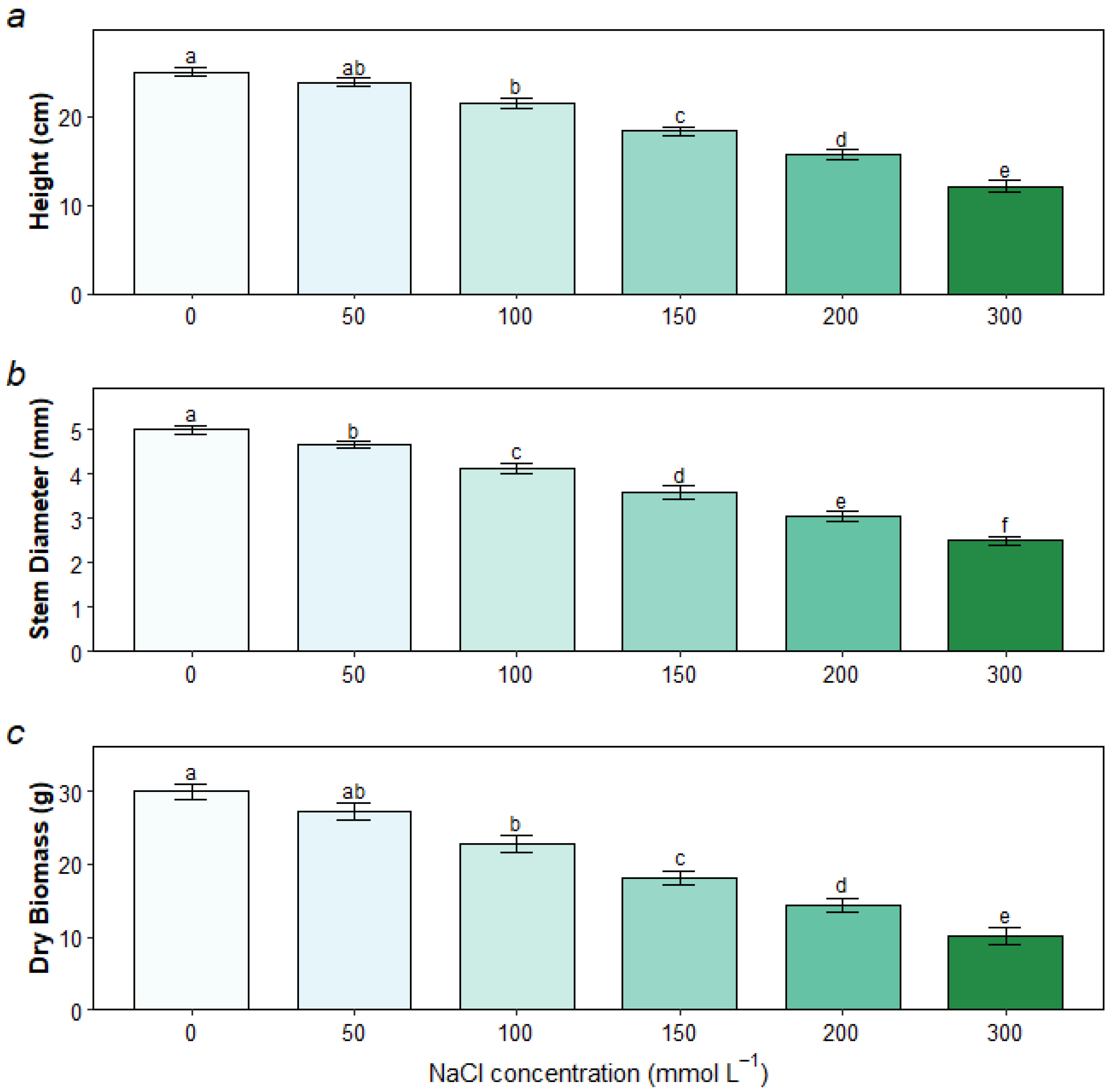
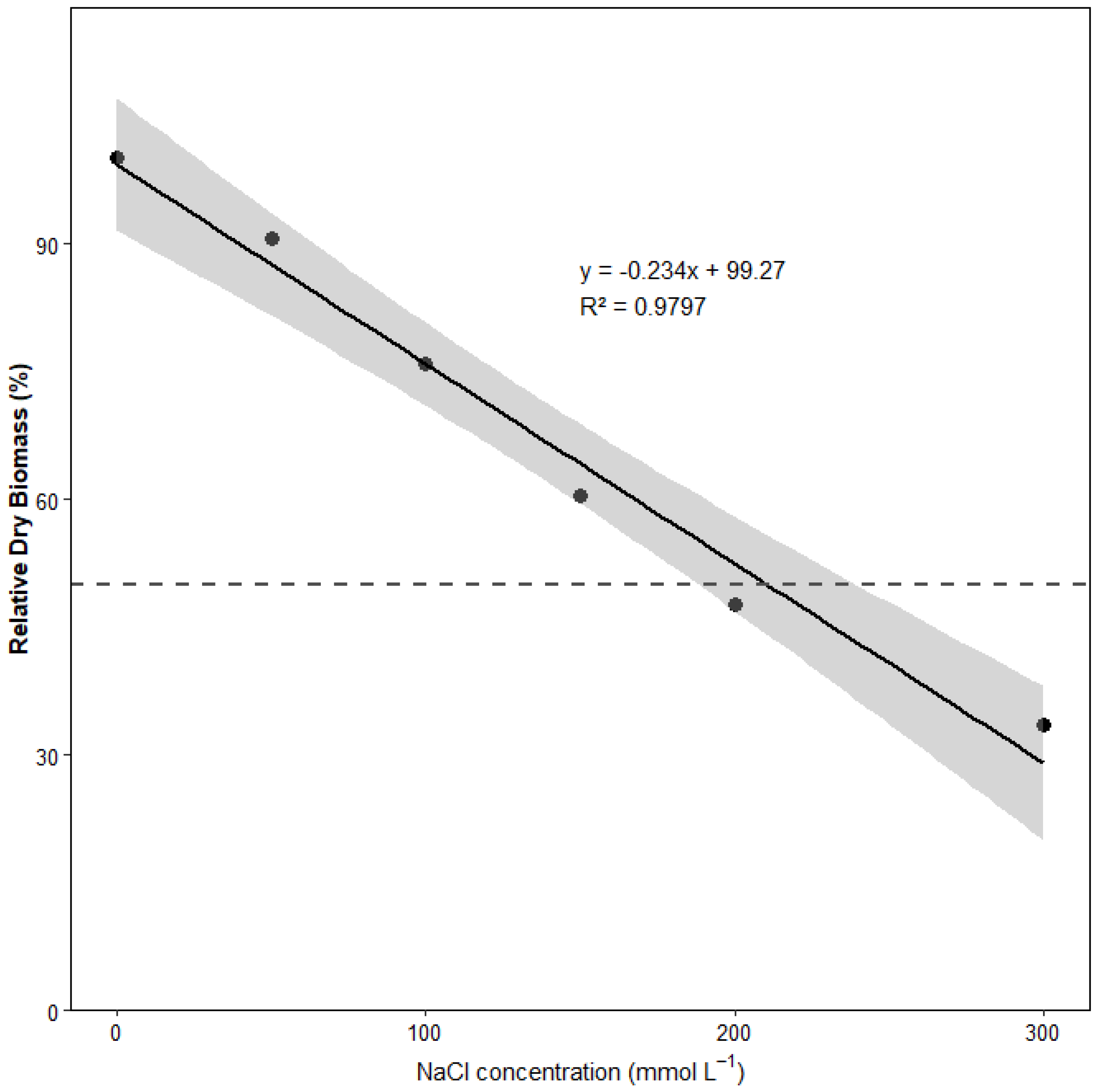
3.1. Growth and Biomass Responses to Salt Stress
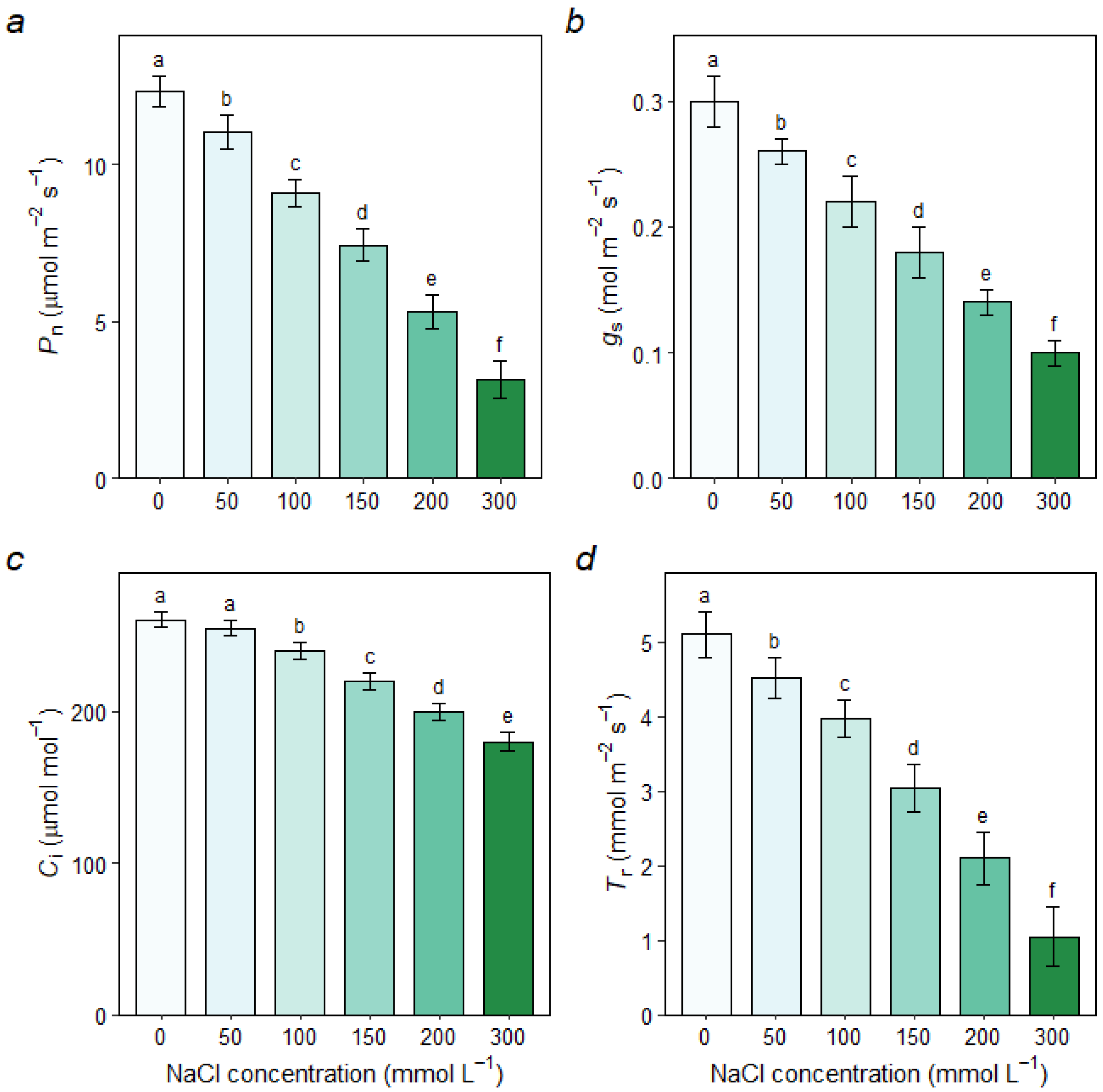
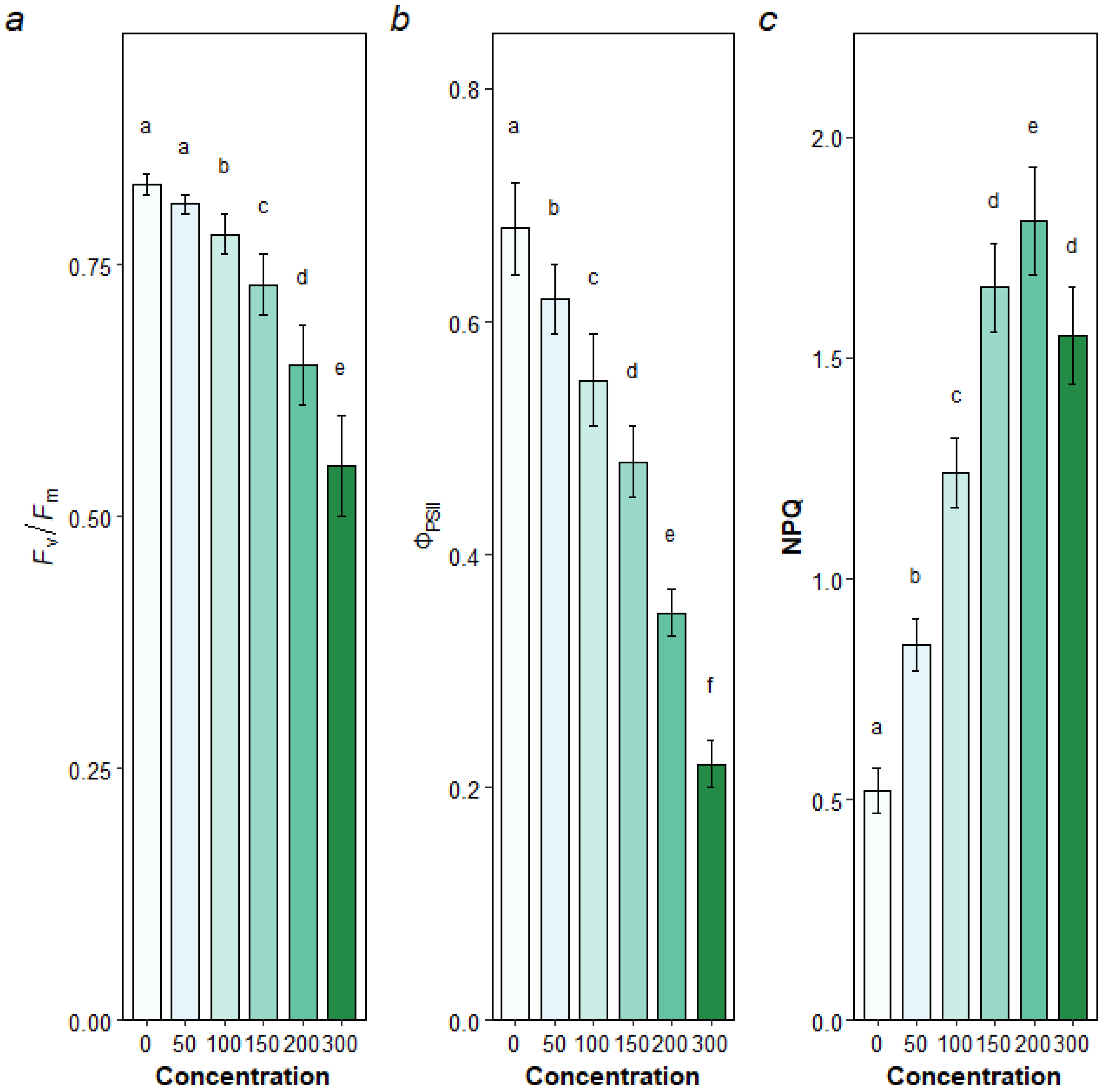
3.2. Photosynthetic Performance Under Salt Stress
3.2.1. Gas Exchange Parameters
3.2.2. Chlorophyll Fluorescence Parameters
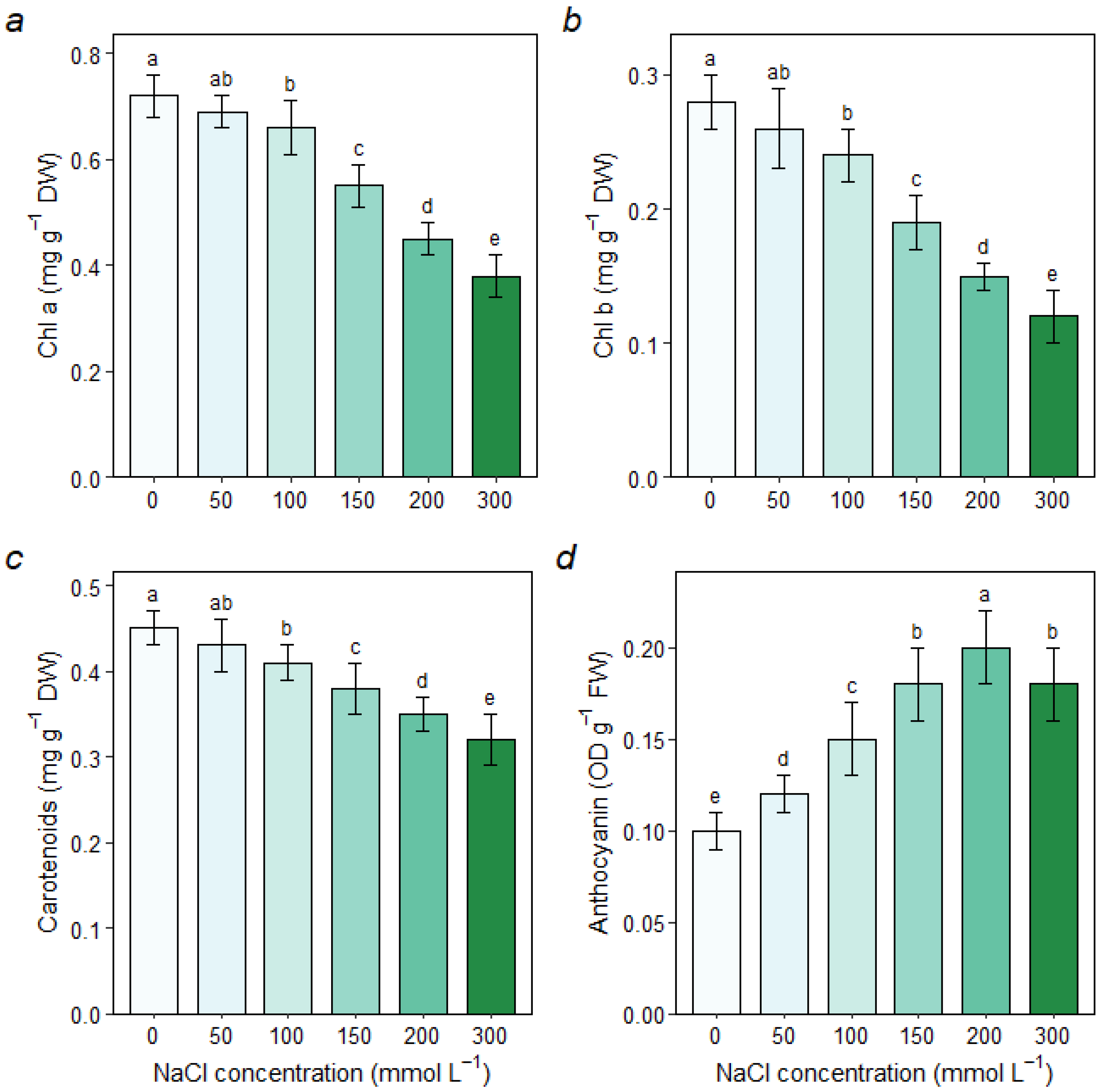
3.3. Changes in Photosynthetic Pigments
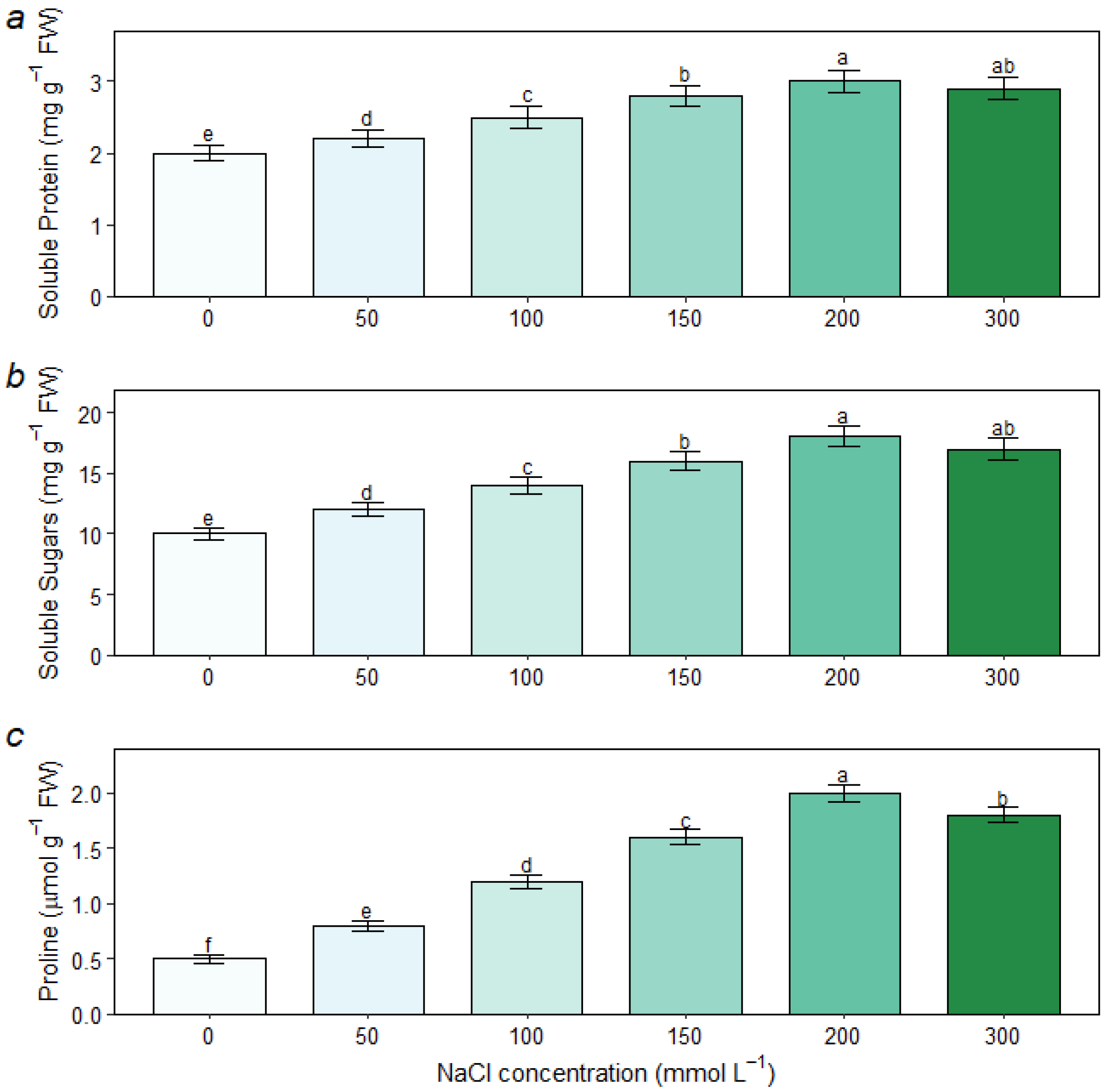
3.4. Accumulation of Osmotic Regulators
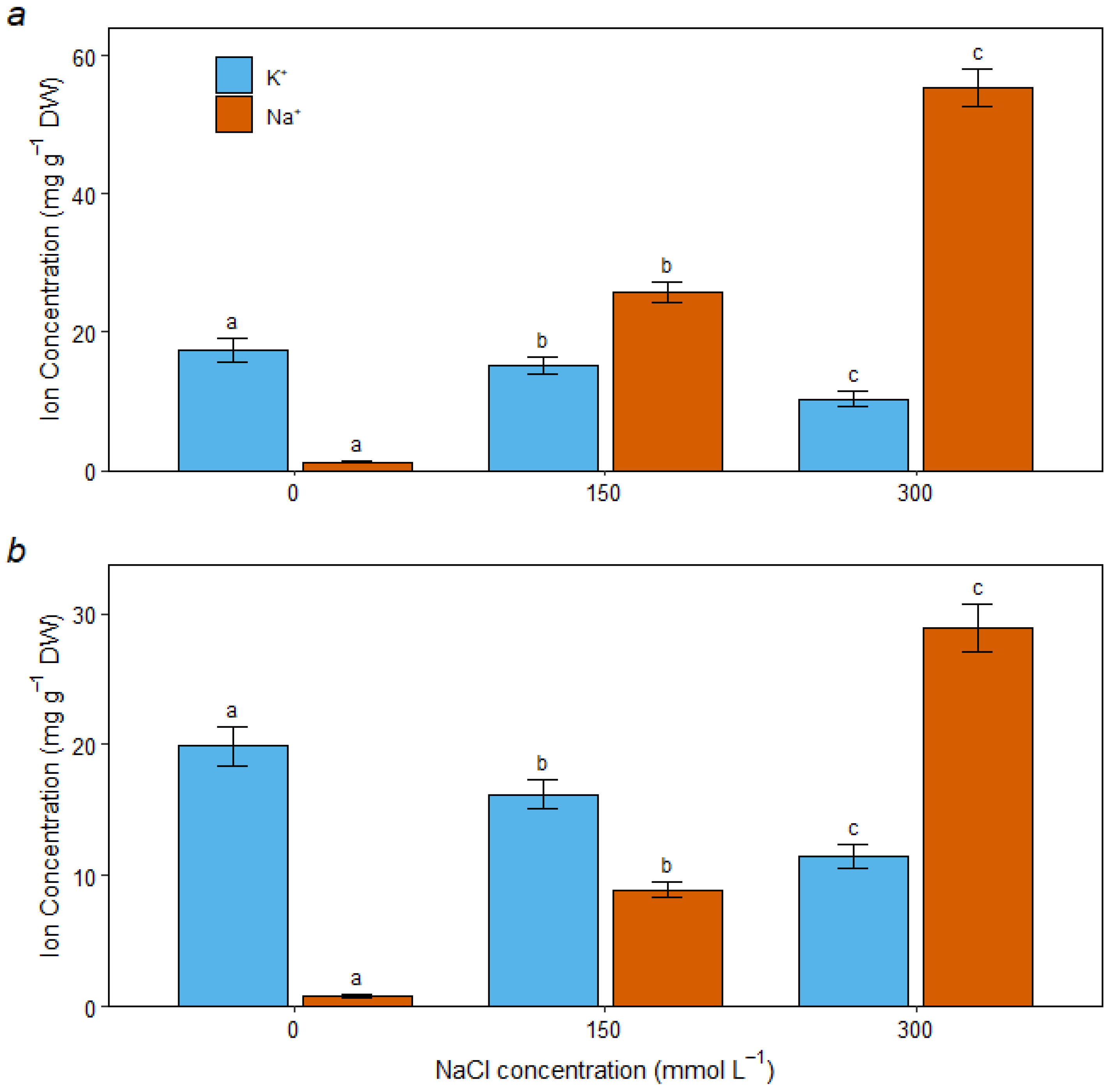
3.5. Ion Homeostasis Under Salt Stress
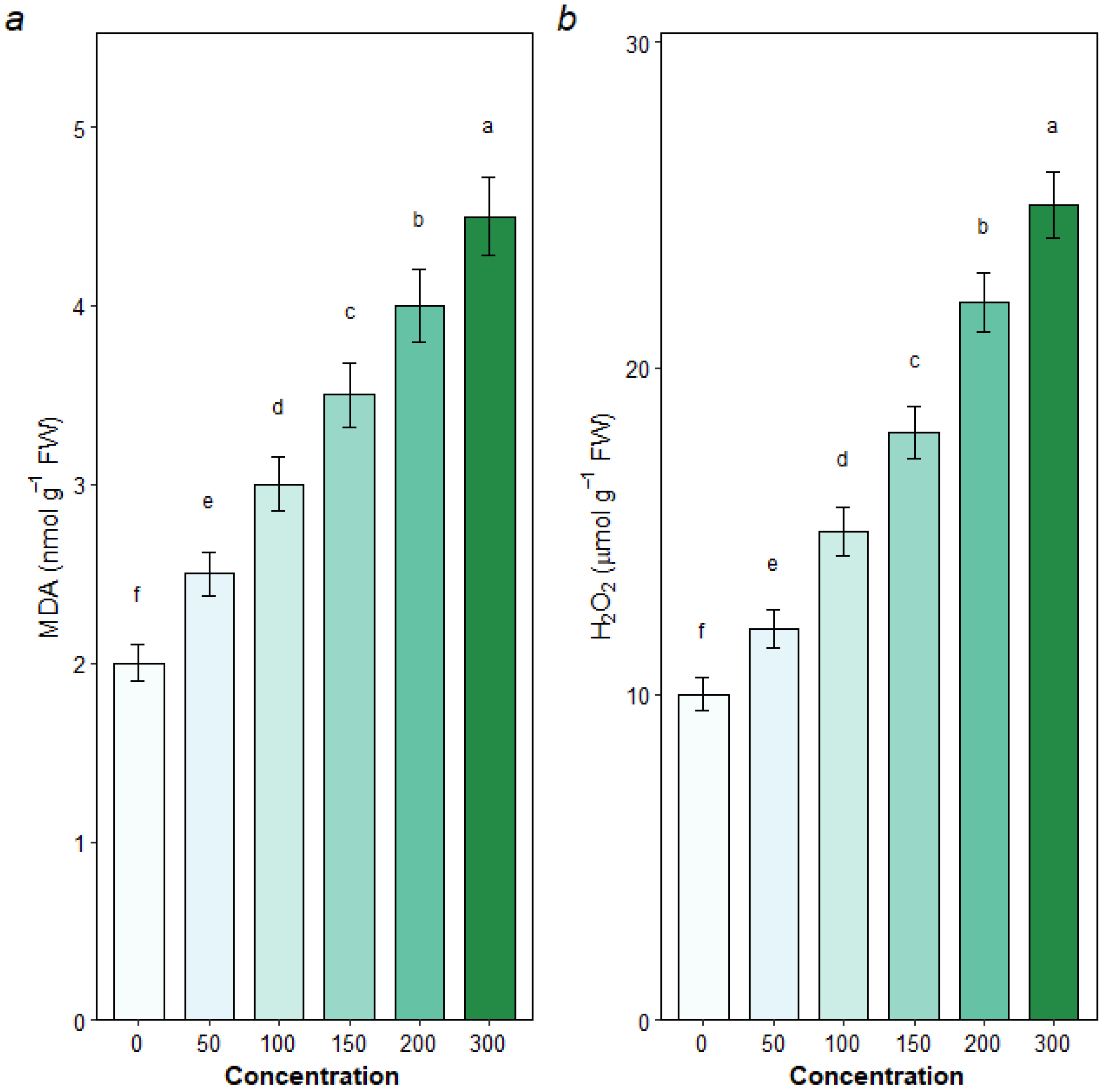
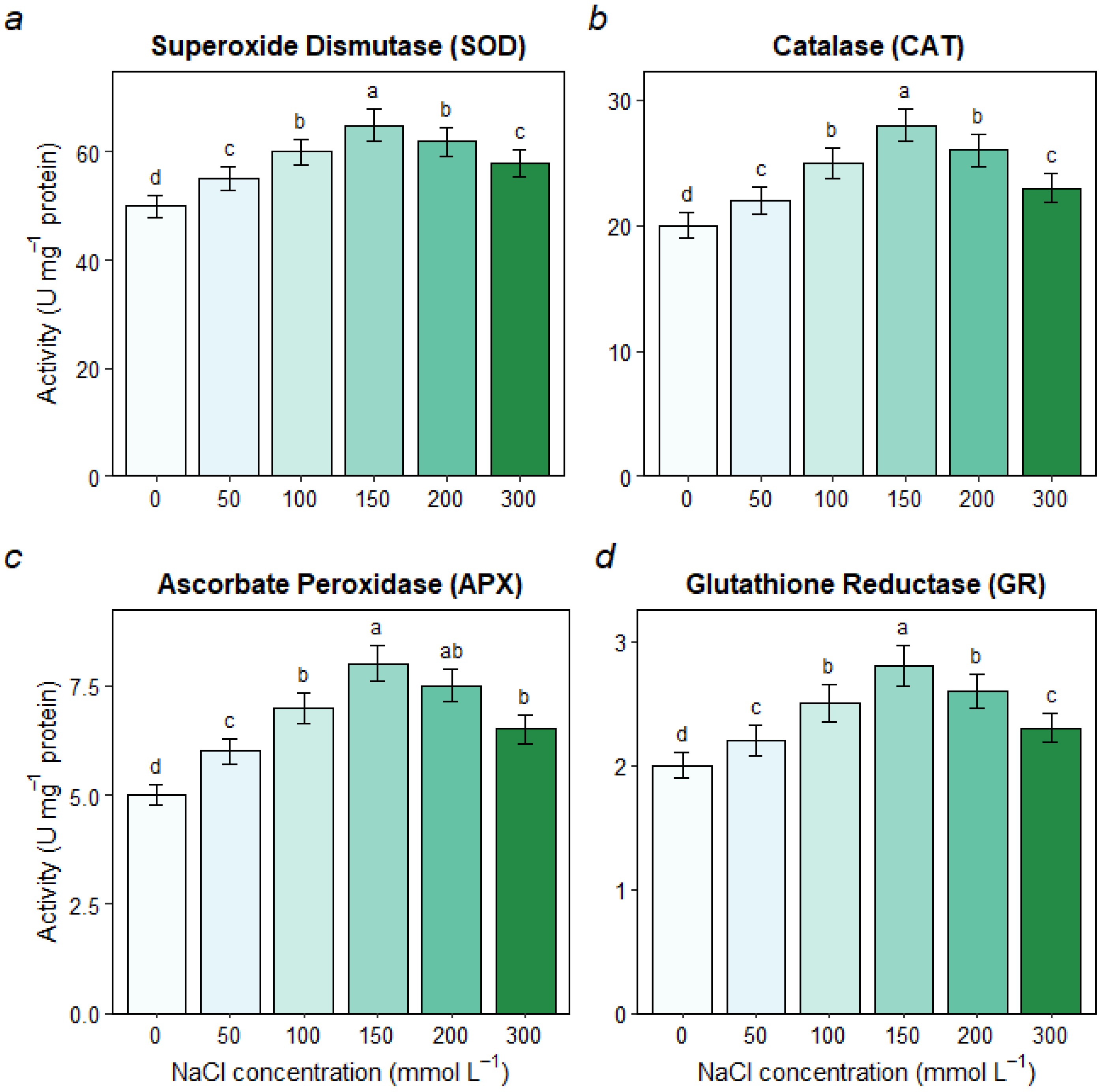
3.6. Oxidative Stress and Antioxidant Enzyme Activities
3.7. Estimated Salt Tolerance Threshold
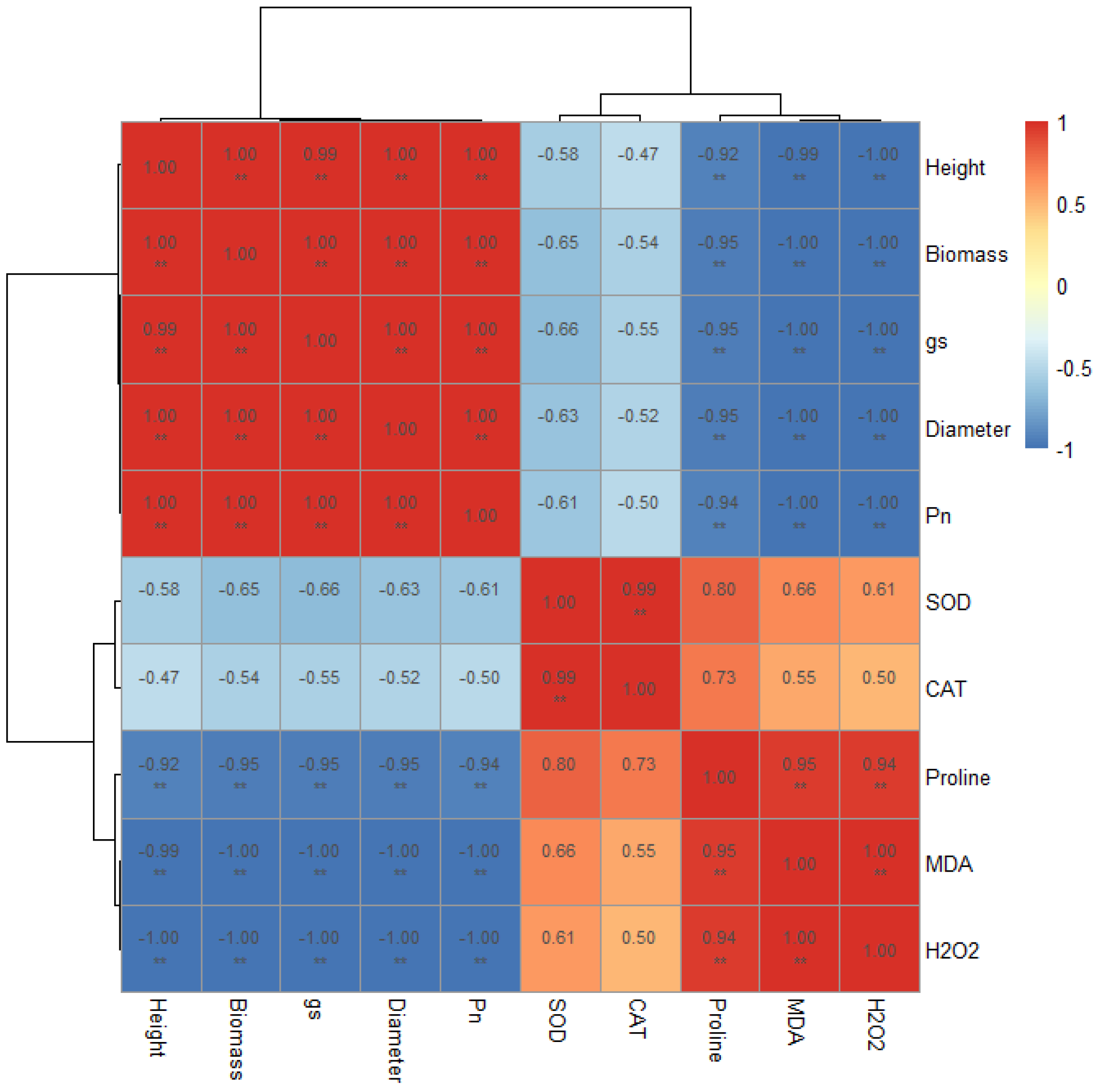
3.8. Correlation Analysis Among Measured Parameters
4. Discussion
4.1. Growth Inhibition Is a Consequence of Impaired Photosynthesis
4.2. Osmotic Adjustment and Ion Homeostasis Are Core Tolerance Strategies
4.3. The Antioxidant System Provides Inducible but Saturable Defense
4.4. Integrated Stress Response Network Revealed by Correlation Analysis
4.5. Salt Tolerance Threshold and Implications for Cultivation
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Song, J.; Chen, M.; Feng, G.; Jia, Y.; Wang, B.; Zhang, F. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant Soil 2009, 314, 133–141. [Google Scholar] [CrossRef]
- Wen, Z.G. Physiological Responses of Broussonetia papyrifera Seedlings to Salt Stress. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2018. [Google Scholar]
- Ci, L.; Yang, X. Desertification and Its Control in China; Higher Education Press: Beijing, China, 2010. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and Proteomic Responses of Mulberry Trees (Morus alba L.) to Combined Salt and Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Mogili, T.; Sivaprasad, V. Improvement of abiotic stress adaptive traits in mulberry (Morus spp.): An update on biotechnological interventions. 3 Biotech 2017, 7, 214. [Google Scholar] [CrossRef]
- He, N.; Zhang, C.; Qi, X.; Zhao, S.; Tao, Y.; Yang, G.; Lee, T.H.; Wang, X.; Cai, Q.; Li, D.; et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013, 4, 2445. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z. Study on Characteristics of Drought and Salt Tolerance of Several Species of Poplars and Their Effects on Soil Microbial Communities. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Gomathinayagam, M.; Panneerselvam, R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under Drought Stress. Comptes Rendus Biol. 2008, 331, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1938, 347, 1–39. [Google Scholar]
- Wang, Y.L. Coupling Response Mechanism of Leaf Structure and Leaf Physiology of Two Poplar Varieties to Drought Stress. Ph.D. Thesis, Shanxi Agricultural University, Taigu, China, 2019. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lei, M.; Zhu, C.; Liu, Y.; Karthikeyan, A.S.; Bressan, R.A.; Raghothama, K.G.; Liu, D. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol. 2011, 189, 1084–1095. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Feng, Z.T.; Deng, Y.Q.; Fan, H.; Sun, Q.J.; Sui, N.; Wang, B.S. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica 2014, 52, 313–320. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Zheng, L.; Meng, Y.; Ma, J.; Zhao, X.; Cheng, T.; Ji, J.; Chang, E.; Meng, C.; Deng, N.; Chen, L.; et al. Transcriptomic analysis reveals importance of ROS and phytohormones in response to short-term salinity stress in Populus tomentosa. Front. Plant Sci. 2015, 6, 678. [Google Scholar] [CrossRef]
- Xu, N.; Wei, X.; Wang, Y.; Dong, J.; Yang, X. Mechanism of Arbuscular Mycorrhizal Fungi in Enhancing Lead Stress Resistance in Poplar Trees. Forests 2025, 16, 82. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulfate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms of plant salt tolerance by transcriptomics. Front. Plant Sci. 2018, 9, 77. [Google Scholar]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Daur, I.; Nawaz, M.A.; Ahmad, M.Q.; Rana, I.A.; Atif, R.M.; Yang, S.H.; Chung, G. Redox and ionic homeostasis regulations against oxidative, salinity and drought Stress in wheat (A Systems Biology Approach). Front. Genet. 2017, 8, 141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Wang, T.; Wang, Y.; Dong, J.; Shaopeng, Y. Coordinated Roles of Osmotic Adjustment, Antioxidant Defense, and Ion Homeostasis in the Salt Tolerance of Mulberry (Morus alba L. ‘Tailai Sang’) Seedlings. Forests 2025, 16, 1258. https://doi.org/10.3390/f16081258
Xu N, Wang T, Wang Y, Dong J, Shaopeng Y. Coordinated Roles of Osmotic Adjustment, Antioxidant Defense, and Ion Homeostasis in the Salt Tolerance of Mulberry (Morus alba L. ‘Tailai Sang’) Seedlings. Forests. 2025; 16(8):1258. https://doi.org/10.3390/f16081258
Chicago/Turabian StyleXu, Nan, Tiane Wang, Yuan Wang, Juexian Dong, and Yu Shaopeng. 2025. "Coordinated Roles of Osmotic Adjustment, Antioxidant Defense, and Ion Homeostasis in the Salt Tolerance of Mulberry (Morus alba L. ‘Tailai Sang’) Seedlings" Forests 16, no. 8: 1258. https://doi.org/10.3390/f16081258
APA StyleXu, N., Wang, T., Wang, Y., Dong, J., & Shaopeng, Y. (2025). Coordinated Roles of Osmotic Adjustment, Antioxidant Defense, and Ion Homeostasis in the Salt Tolerance of Mulberry (Morus alba L. ‘Tailai Sang’) Seedlings. Forests, 16(8), 1258. https://doi.org/10.3390/f16081258




