First Assessment of the Biodiversity of True Slime Molds in Swamp Forest Stands of the Knyszyn Forest (Northeast Poland) Using the Moist Chambers Detection Method
Abstract
1. Introduction
2. Materials and Methods
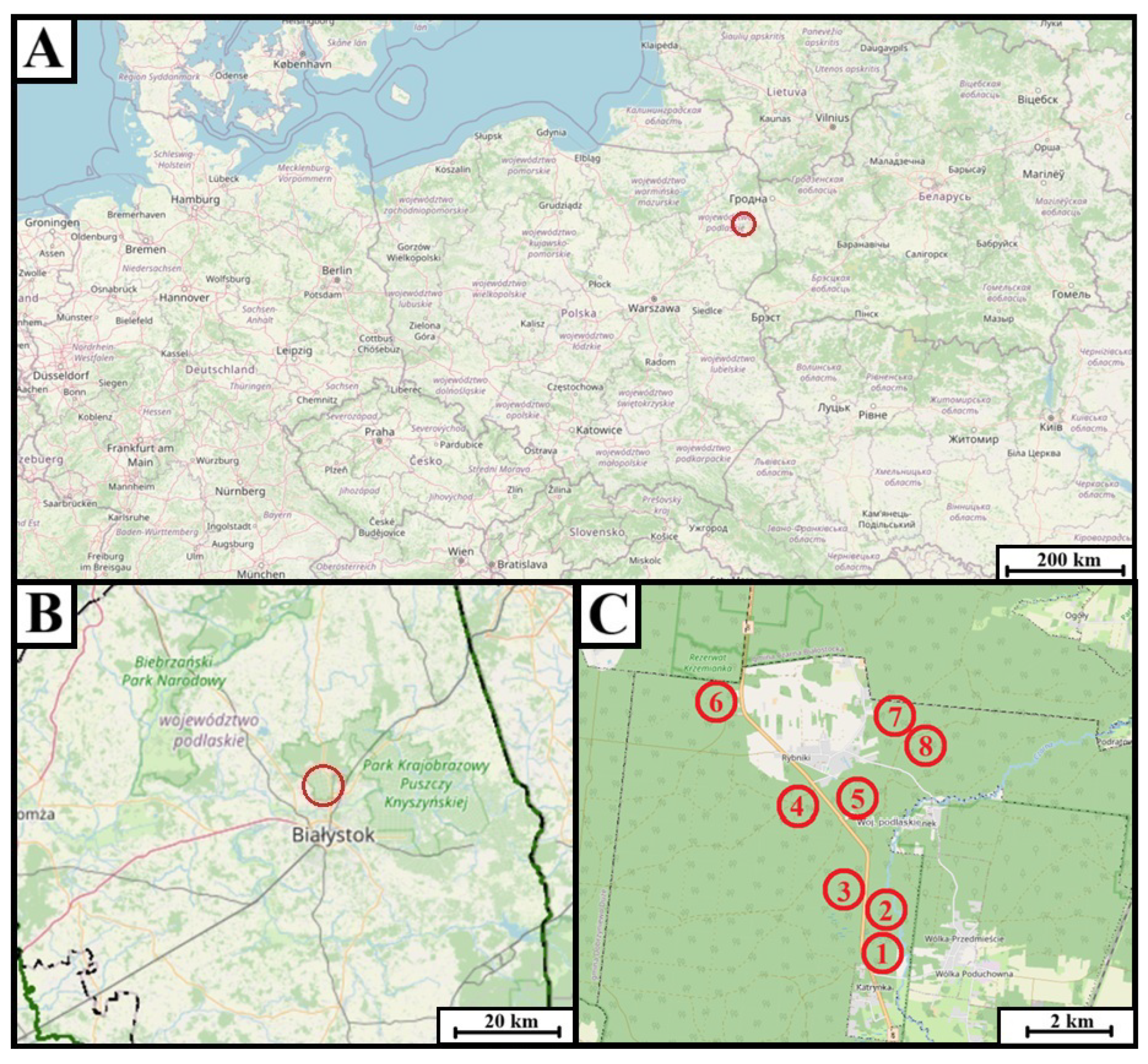
2.1. Study Area
2.2. Field Sampling
2.3. Establishment and Maintenance of Moist Chamber Cultures
2.4. Morphological Character Assessment and Checklist Verification
2.5. Statistical Analysis
3. Results
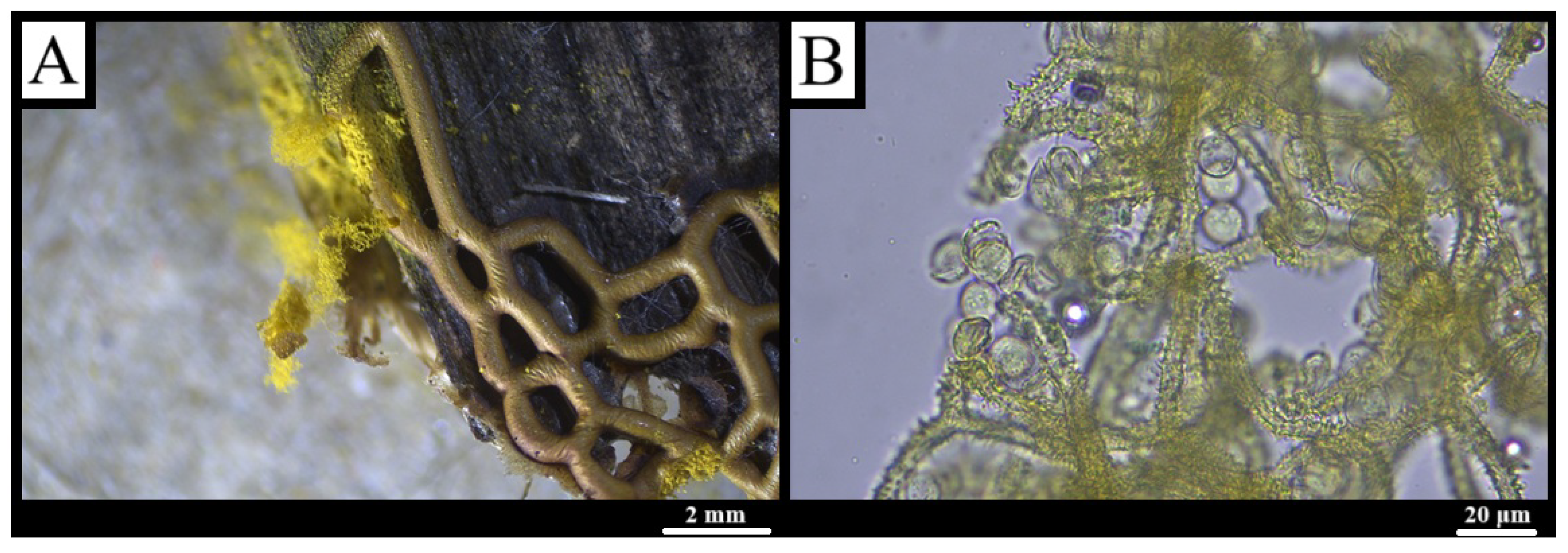
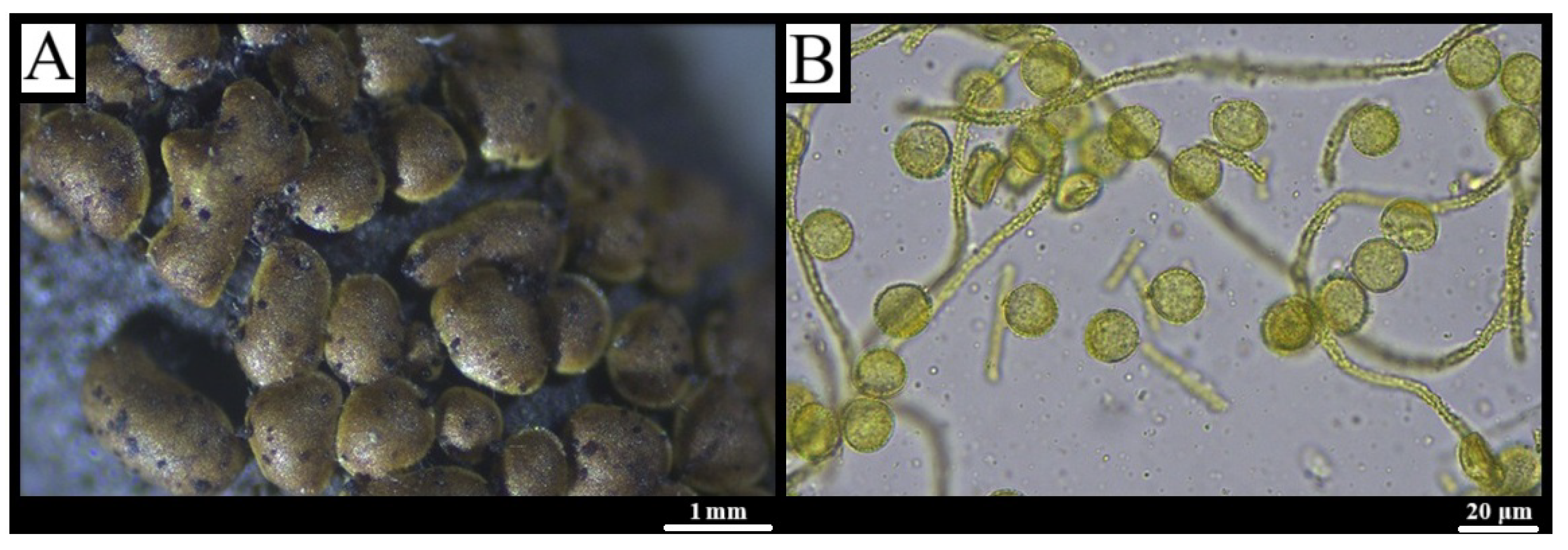
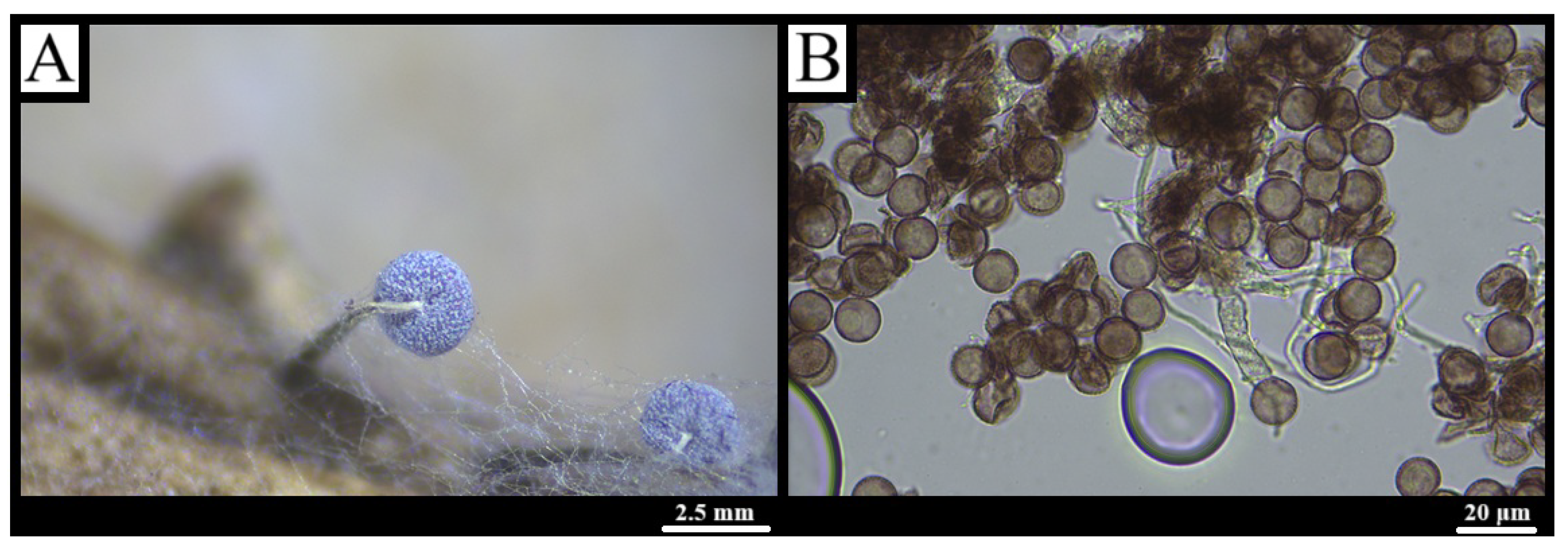
3.1. Taxonomy
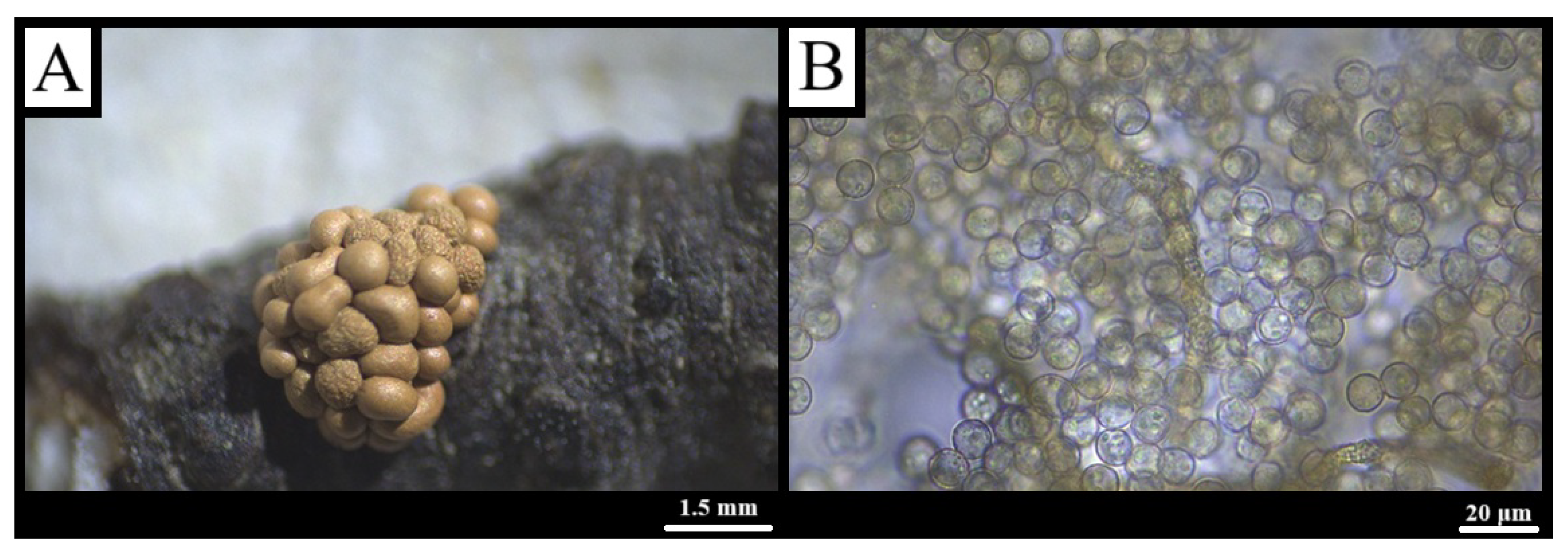
3.1.1. Arcyria cinerea (Bull.) Pers., Synopsis Methodica Fungorum 1: 724 (1801) [40]
3.1.2. Arcyria pomiformis (Leers) Rostaf., Pamiętn. Towarz. Nauk Ścisłych w Paryżu 6(1): 271 (1875) [45]
3.1.3. Arcyria denudata (L.) Wettst., Verh. Zool.-Bot. Ges. Wien 35: 353 (1886) [46]
3.1.4. Arcyodes incarnata (Alb. & Schwein.) O.F. Cook, Science 15: 651 (1902) [47]
3.1.5. Ceratiomyxa fruticulosa var. flexuosa (Lister) G. Lister, Monograph of the Mycetozoa, ed. 2: 26 (1911) [50]
3.1.6. Comatricha nigra (Pers.) J. Schröt., Krypt.-Fl. Schlesien 3(1): 118 (1885) [53]
3.1.7. Cribraria microcarpa (Schrad.) Pers., Synopsis Methodica Fungorum 1: 190 (1801) [40]
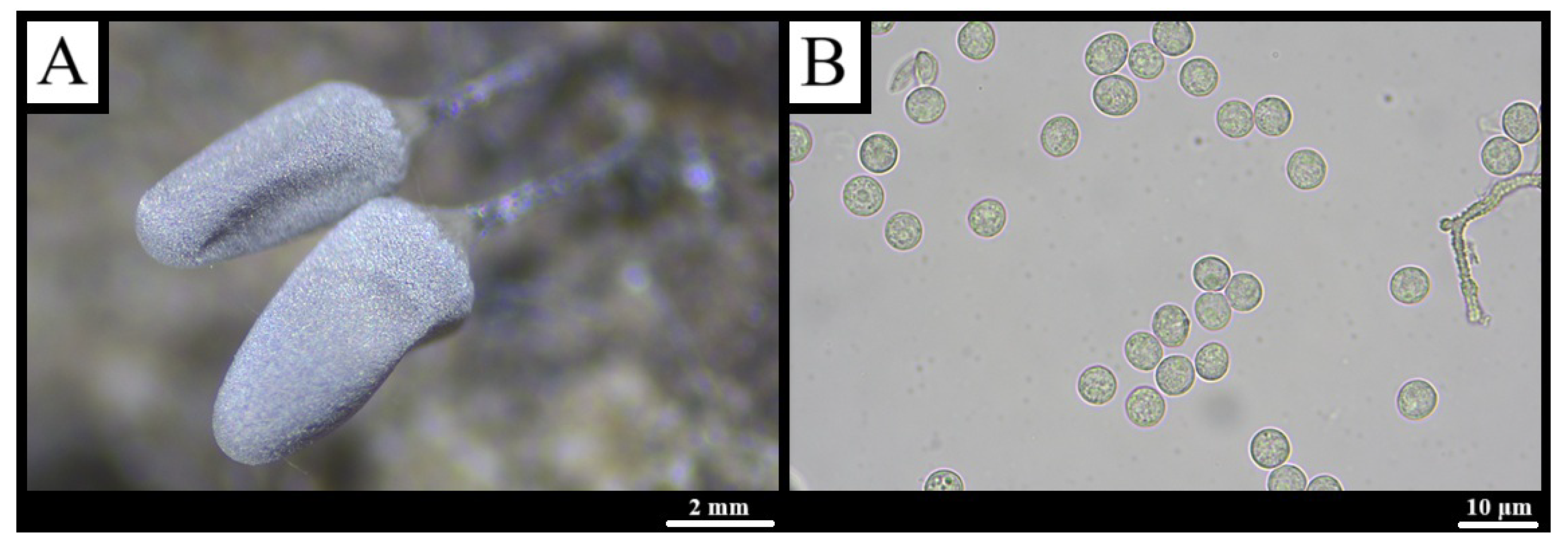
3.1.8. Hemitrichia serpula (Scop.) Rostaf., Versuch eines Systems der Mycetozoen: 14 (1873) [57]
3.1.9. Lamproderma scintillans (Berk. & Broome) Morgan, Journal of the Cincinnati Society of Natural History 16: 131 (1894) [60]
3.1.10. Metatrichia vesparium (Batsch) Nann.-Bremek., Proc. Kon. Ned. Akad. Wetensch. C 69: 348 (1966) [63]
3.1.11. Oligonema flavidum (Peck) Peck, Ann. Rep. N.Y. State Mus. Nat. Hist. 31: 42 (1878) [65]
3.1.12. Perichaena depressa (Libert) Rostaf., Versuch eines Systems der Mycetozoen: 112 (1873) [57]
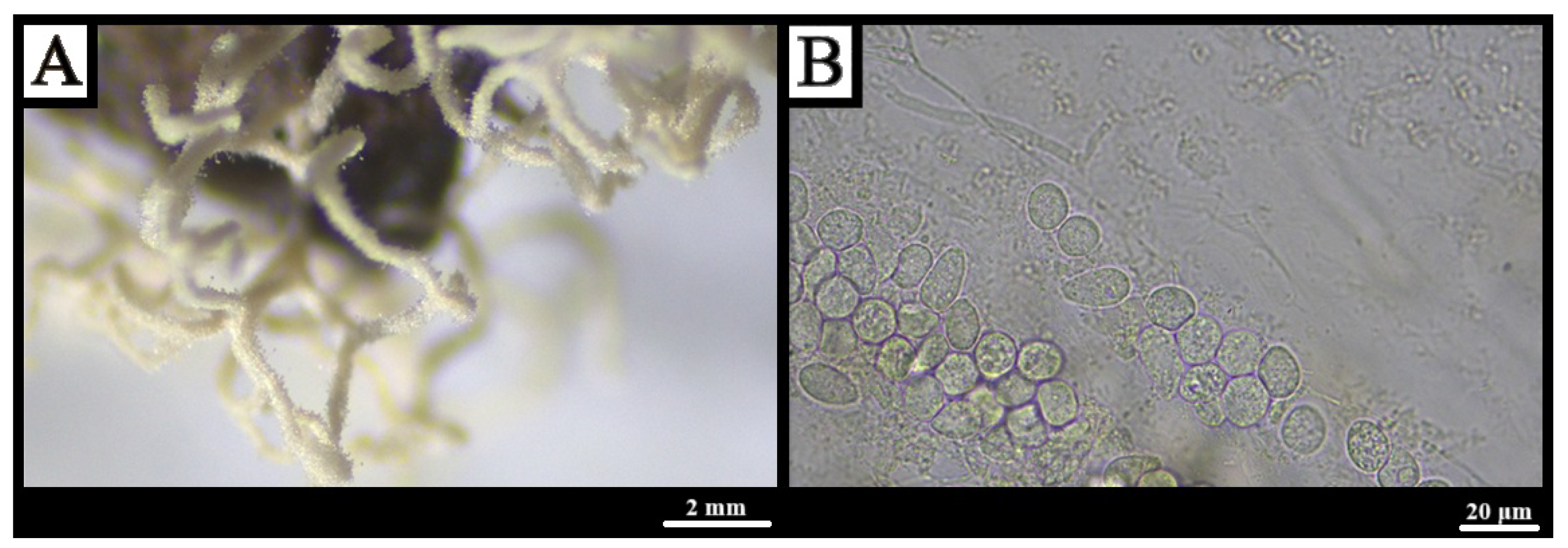
3.1.13. Physarum album (Bull.) Chevall., Flore Générale des Environs de Paris 1: 345 (1826) [71]
3.1.14. Physarum bivalve Pers., Neues Magazin für die Botanik 1: 89 (1794) [76]
3.2. Statistical Analysis
3.2.1. Performance of Moist-Chamber Cultures
3.2.2. Species Incidence Across Host–Substrate Combinations
3.2.3. Host-Specific Richness and Sampling Coverage
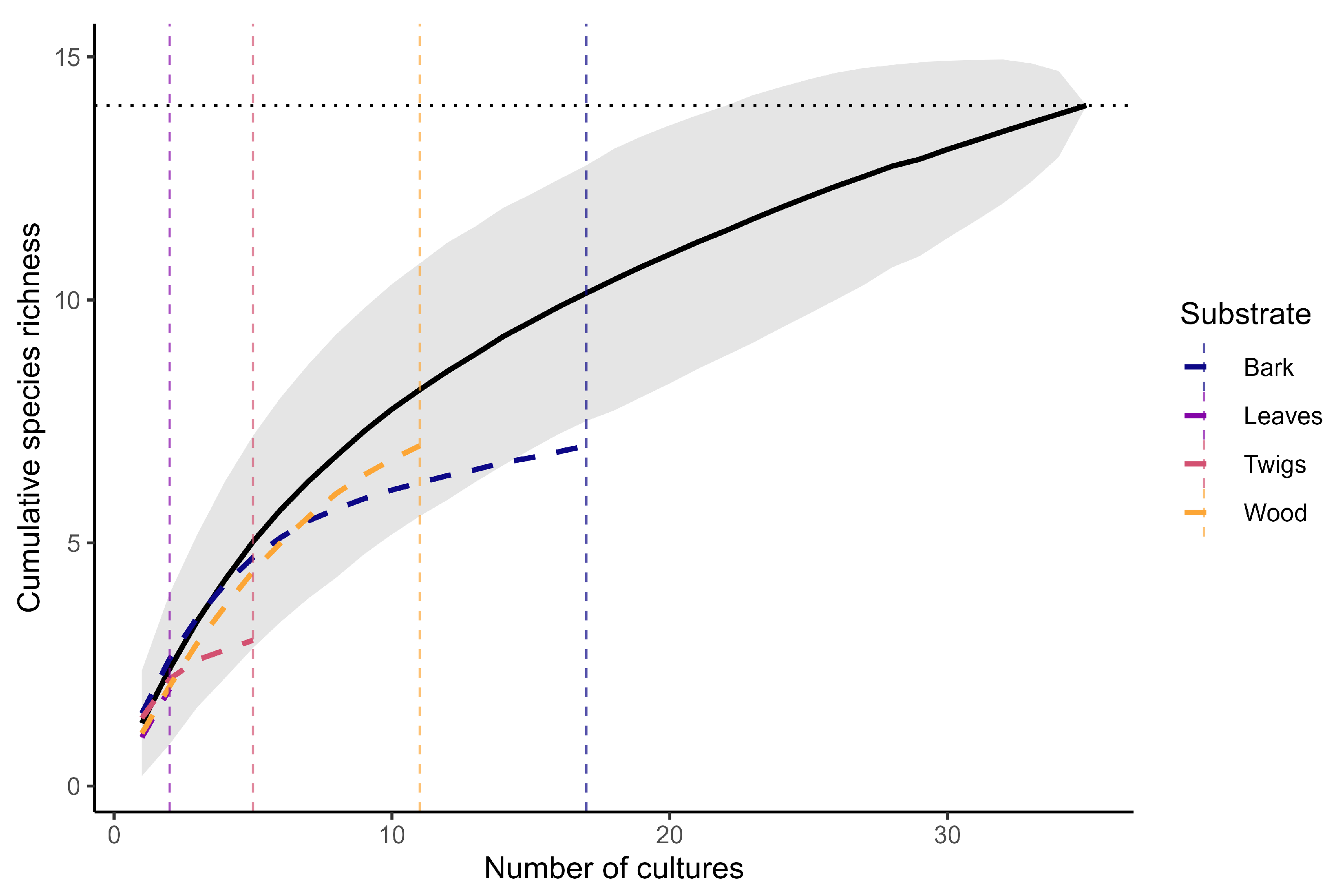
3.2.4. Sampling Completeness and Substrate-Specific Accumulation
3.2.5. Incidence Patterns
4. Discussion
5. Conclusions
- Moist-chamber cultures proved highly effective for uncovering Eumycetozoa in swamp-forest habitats, detecting numerous slime-mold species despite waterlogged substrates and challenging field conditions. The protocol yielded a high proportion of sporulating preparations, sufficient for the species-accumulation curve to approach an asymptote and broadly comparable with success rates reported from other temperate European forests.
- Several taxa—Arcyodes incarnata, Ceratiomyxa fruticulosa var. flexuosa, Oligonema flavidum, and Perichaena depressa—constitute new records for northeastern Poland, highlighting peatland and swamp forests as refugia for regionally rare or under-explored myxomycetes.
- A. incarnata has not been recorded in Poland for several decades, emphasizing the importance of targeted sampling that can reveal taxa assumed to be absent or exceedingly rare in the current myxobiota.
- The overall rarity of newly documented species, both nationally and continentally, underlines the urgent need for expanded inventories and systematic culturing studies in wet forest habitats. Such investigations will likely continue to uncover additional, previously unrecorded slime molds, further enriching our knowledge of Eumycetozoa diversity in Poland and throughout Europe.
- A chi-square test confirmed a significant association between the substrate type and culture success, with bark markedly outperforming other microhabitats, whereas leaves and needles were least suitable.
- By contrast, once sporulation was triggered, the number of species per culture remained similar across substrates, indicating that competitive assembly processes quickly narrow the community composition regardless of the microhabitat.
- Rarefaction modeling showed that the current sampling effort captured almost the entire resident species pool overall, but alder substrates in particular still harbor an appreciable fraction of undiscovered diversity, warranting further attention.
- The complementary distribution of taxa across bark, wood, twigs, and leaves underscores the necessity of multi-substrate sampling in any future monitoring program.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Inventory of Moist-Chamber Cultures Established from Knyszyn Forest Samples
| Culture Code | Sampling Locality | Forest Sub-Compartment | Plant Association | Coordinates | Field Sampling Date | Culture Start Date | Culture Maintenance End Date | Host Tree | Substrate |
|---|---|---|---|---|---|---|---|---|---|
| MXM1-AG-D | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM1-AG-K | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM1-AG-L | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM1-AG-G | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM1-BP-D | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM1-BP-K | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM1-BP-L | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM1-BP-G | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM1-PA-D | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM1-PA-K | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM1-PA-L | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM1-PA-G | Knyszyn Forest | 01-08-2-05-138a | Sphagno girgensohnii–Piceetum | 53.237284, 23.151192 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM2-AG-D | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM2-AG-K | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM2-AG-L | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM2-AG-G | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM2-BP-D | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM2-BP-K | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM2-BP-L | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM2-BP-G | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM2-PA-D | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM2-PA-K | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM2-PA-L | Knyszyn Forest | 01-08-2-02-76g | Sphagno girgensohnii–Piceetum | 53.254823, 23.143253 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM2-PA-G | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM3-AG-D | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM3-AG-K | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM3-AG-L | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM3-AG-G | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM3-BP-D | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM3-BP-K | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM3-BP-L | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM3-BP-G | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM3-PA-D | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM3-PA-K | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM3-PA-L | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM3-PA-G | Knyszyn Forest | 01-08-2-02-8i | Ribeso nigri–Alnetum | 53.272998, 23.116182 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM4-AG-D | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM4-AG-K | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM4-AG-L | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM4-AG-G | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM4-BP-D | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM4-BP-K | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM4-BP-L | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM4-BP-G | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM4-PA-D | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM4-PA-K | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM4-PA-L | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM4-PA-G | Knyszyn Forest | 01-28-1-05-31f | Fraxino–Alnetum | 53.278592, 23.135752 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM5-AG-D | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM5-AG-K | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM5-AG-L | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM5-AG-G | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM5-BP-D | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM5-BP-K | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM5-BP-L | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM5-BP-G | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM5-PA-D | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM5-PA-K | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM5-PA-L | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM5-PA-G | Knyszyn Forest | 01-28-1-06-7a | Fraxino–Alnetum | 53.274692, 23.155493 | 10 Dec 2024 | 10 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM6-AG-D | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM6-AG-K | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM6-AG-L | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM6-AG-G | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM6-BP-D | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM6-BP-K | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM6-BP-L | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM6-BP-G | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM6-PA-D | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM6-PA-K | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM6-PA-L | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM6-PA-G | Knyszyn Forest | 01-08-2-02-75D | Ribeso nigri–Alnetum | 53.878910, 21.248893 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM7-AG-D | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM7-AG-K | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM7-AG-L | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM7-AG-G | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM7-BP-D | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM7-BP-K | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM7-BP-L | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM7-BP-G | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM7-PA-D | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM7-PA-K | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM7-PA-L | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM7-PA-G | Knyszyn Forest | 01-08-2-05-125D | Sphagno girgensohnii–Piceetum | 53.878205, 21.202524 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
| MXM8-AG-D | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Wood |
| MXM8-AG-K | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Bark |
| MXM8-AG-L | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Leaves |
| MXM8-AG-G | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Alnus glutinosa | Twigs |
| MXM8-BP-D | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Wood |
| MXM8-BP-K | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Bark |
| MXM8-BP-L | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Leaves |
| MXM8-BP-G | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Betula pubescens | Twigs |
| MXM8-PA-D | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Wood |
| MXM8-PA-K | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Bark |
| MXM8-PA-L | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Leaves |
| MXM8-PA-G | Knyszyn Forest | 01-08-2-05-124D | Sphagno girgensohnii–Piceetum | 53.851152, 21.207244 | 19 Dec 2024 | 19 Dec 2024 | 25 Mar 2025 | Picea abies | Twigs |
References
- Tekle, Y.I.; Wang, F.; Wood, F.C.; Anderson, O.R.; Smirnov, A. New insights on the evolutionary relationships between the major lineages of Amoebozoa. Sci. Rep. 2022, 12, 11173. [Google Scholar] [CrossRef]
- Baldauf, S.L. A Search for the Origins of Animals and Fungi: Comparing and Combining Molecular Data. Am. Nat. 1999, 154, S178–S188. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Rojas, C.E. Myxomycetes: Biology, Systematics, Biogeography and Ecology, 1st ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Lado, C.; Eliasson, U. Taxonomy and Systematics: Current Knowledge and Approaches on the Taxonomic Treatment of Myxomycetes: Updated Version. In Myxomycetes; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 8; pp. 269–324. [Google Scholar] [CrossRef]
- Keller, H.W.; Everhart, S.E.; Kilgore, C.M. The Myxomycetes: Introduction, Basic Biology, Life Cycles, Genetics, and Reproduction. In Myxomycetes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–45. [Google Scholar] [CrossRef]
- Stephenson, S.L. Past and Ongoing Field-Based Studies of Myxomycetes. Microorganisms 2023, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Janik, P.; Ronikier, A. Checklist of Myxomycetes (Amoebozoa) of the Polish Tatra Mts. Acta Mycol. 2023, 58, 1–13. [Google Scholar] [CrossRef]
- Clark, J.D.; Haskins, E.F. Reproductive systems in the myxomycetes: A review. Mycosphere 2010, 1, 337–353. [Google Scholar]
- Baba, H.; Sevindik, M.; Dogan, M.; Akgül, H. Antioxidant, antimicrobial activities and heavy metal contents of some Myxomycetes. Fresenius Environ. Bull. 2020, 29, 7840–7846. [Google Scholar]
- Stephenson, S.L. From morphological to molecular: Studies of myxomycetes since the publication of the Martin and Alexopoulos (1969) monograph. Fungal Divers. 2011, 50, 21–34. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Feest, A. Ecology of Soil Eumycetozoans. Acta Protozool. 2012, 51, 201–208. [Google Scholar] [CrossRef]
- Pawłowicz, T.; Wilamowski, K.; Puchlik, M.; Żebrowski, I.; Micewicz, G.M.; Gabrysiak, K.A.; Borowik, P.; Malewski, T.; Zapora, E.; Wołkowycki, M.; et al. Biologically Active Compounds in True Slime Molds and Their Prospects for Sustainable Pest and Pathogen Control. Int. J. Mol. Sci. 2025, 26, 1951. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, C.; Li, Y.; Stephenson, S.L.; Liu, P. Slime molds (Myxomycetes) causing a “disease” in crop plants and cultivated mushrooms. Front. Plant Sci. 2024, 15, 1411231. [Google Scholar] [CrossRef]
- Keller, H.W.; Brooks, T.E. Corticolous Myxomycetes I: Two New Species of Didymium. Mycologia 1973, 65, 286–294. [Google Scholar] [CrossRef]
- de Basanta, D.W.; Estrada-Torres, A. Techniques for Recording and Isolating Myxomycetes. In Myxomycetes; Academic Press: Cambridge, MA, USA, 2017; pp. 333–363. [Google Scholar] [CrossRef]
- Rollins, A.W.; Stephenson, S.L. Myxomycete Assemblages Recovered from Experimental Grass and Forb Microhabitats Placed Out and Then Recollected in the Tallgrass Prairie Preserve, OK. Southeast. Nat. 2016, 15, 681–688. [Google Scholar] [CrossRef]
- Bordelon, A.P.; Keller, H.W.; Scarborough, A.R. An Inexpensive Moist Chamber Culture Technique for Finding Microbiota on Live Tree Bark. Appl. Plant Sci. 2024, 12, e11578. [Google Scholar] [CrossRef]
- Czerwiński, A. (Ed.) Puszcza Knyszyńska. Monografia Przyrodnicza; Zespół Parków Krajobrazowych w Supraślu: Supraśl, Poland, 1995. [Google Scholar]
- Wołkowycki, D.; Łaska, G. Puszcza Knyszyńska. In Śladami Mistrzów. Miejsca Fascynacji Prekursorów Polskiej Geobotaniki; Polskie Towarzystwo Botaniczne i Wydawnictwo SGGW: Warszawa, Poland, 2024; pp. 351–360. [Google Scholar] [CrossRef]
- Kołos, A. Park Krajobrazowy Puszczy Knyszyńskiej. Przyr. Pol. 1989, 1, 10–12. [Google Scholar]
- Stephenson, S.L.; Schnittler, M.; Novozhilov, Y.K. Myxomycete Diversity and Distribution from the Fossil Record to the Present. Biodivers. Conserv. 2008, 17, 285–301. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Stempen, H. Myxomycetes: A Handbook of Slime; Timber Press, Inc.: Portland, OR, USA, 2000. [Google Scholar]
- Baba, H.; Sevindik, M. Myxomycetes (Myxogastria) of Türkiye: A checklist 2023. Mycopath 2023, 21, 53–67. [Google Scholar]
- Keller, H.W.; Kilgore, C.M.; Everhart, S.E.; Carmack, G.J.; Crabtree, C.D.; Scarborough, A.R. Myxomycete Plasmodia and Fruiting Bodies: Unusual Occurrences and User-Friendly Study Techniques. Fungi 2008, 1, 24–37. [Google Scholar]
- Ślusarczyk, D.M. First Record of Slime Molds in Biebrza National Park (NE Poland). Acta Mycol. 2021, 56. [Google Scholar] [CrossRef]
- Krzysztofiak, L. Śluzowce Myxomycetes, Grzyby Fungi i Mszaki Bryophyta Wigierskiego Parku Narodowego; Stowarzyszenie, Człowiek i Przyroda”: Suwałki, Poland, 2010. [Google Scholar]
- Bochynek, A. First Polish Records of Myxomycetes Rare in Europe. Acta Soc. Bot. Pol. 2015, 84, 443–448. [Google Scholar] [CrossRef]
- Drozdowicz, A.; Ronikier, A.; Stojanowska, W.; Panek, E. Myxomycetes of Poland—A checklist: Krytyczna Lista Śluzowców Polski; Biodiversity of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2003; Volume 10, p. 103. [Google Scholar]
- Slusarczyk, D. Some Observations of Slime Moulds on Wood and Litter in Beech Forests. Acta Mycol. 2010, 45, 239–246. [Google Scholar] [CrossRef]
- Salamaga, A. Oligonema Flavidum (Myxomycetes): A Species New Poland. Pol. Bot. J. 2013, 58, 747–749. [Google Scholar] [CrossRef]
- Drozdowicz, A.; Szolc, P.; Bochynek, A.; Salamaga, A. Myxomycetes of the Lipówka Reserve in the Niepołomice Old Growth Forest (S Poland). Acta Mycol. 2012, 47, 97–107. [Google Scholar] [CrossRef]
- Lawrynowicz, M.; Slusarczyk, D.; Salamaga, A. Revised Data on the Occurrence of Myxomycetes in Central Poland. Acta Mycol. 2011, 46, 223–232. [Google Scholar] [CrossRef]
- Bochynek, A.; Drozdowicz, A. Biota Śluzowców (Myxomycetes) Lasu Użytkowanego Gospodarczo w Okolicy Przysiółka Wyrchczadeczka (Beskid Śląski). Sylwan 2012, 156, 57–63. [Google Scholar]
- Gierczyk, B.; Kujawa, A. Contribution to the Knowledge of Mycobiota of the Wielkopolski National Park (W Poland). Acta Mycol. 2020, 55, 5515. [Google Scholar] [CrossRef]
- Salamaga, A.M. The Myxobiota of the Łagiewnicki Forest in Łódź (Central Poland). Acta Mycol. 2021, 56, 561. [Google Scholar] [CrossRef]
- Narkiewicz, C.; Kita, W.; Pusz, W.; Panek, E. Grzyby i Śluzowce. In Przyroda Karkonoskiego Parku Narodowego; Knapik, R., Migoń, P., Raj, A., Eds.; Karkonoski Park Narodowy: Jelenia Góra, Poland, 2019; pp. 339–358. [Google Scholar]
- Wilga, M.S.; Ciechanowski, M. Ostoja Grzybów Wielkoowocnikowych i Śluzowców w Lasach Oliwskich (Trójmiejski Park Krajobrazowy). Chrońmy Przyr. Ojczystą 2007, 63, 82–101. [Google Scholar]
- Bochynek, A.; Drozdowicz, A. Martwe Drewno jako Mikrosiedlisko Śluzowców w Wybranych Zbiorowiskach Leśnych w Polskich Karpatach. Rocz. Bieszczadzkie 2011, 19, 165–179. [Google Scholar]
- Ronikier, A.; Ronikier, M.; Drozdowicz, A. Diversity of Nivicolous Myxomycetes in the Gorce Mountains—A Low-Elevation Massif of the Western Carpathians. Mycotaxon 2008, 103, 337–352. [Google Scholar] [CrossRef]
- Persoon, C.H. Synopsis Methodica Fungorum; Henricum Dieterich: Göttingen, Germany, 1801; Volume 1, p. 190. [Google Scholar] [CrossRef]
- Neubert, H.; Nowotny, W.; Baumann, K. Die Myxomyceten, Band 1: Ceratiomyxales, Echinosteliales, Liceales, Trichiales; Die Myxomyceten; Karlheinz Baumann Verlag: Gomaringen, Germany, 1993; Volume 1, p. 344. [Google Scholar]
- Kryvomaz, T.I.; Michaud, A.; Minter, D.W. Arcyria denudata . Descr. Fungi Bact. 2012, 192, 1–7. [Google Scholar] [CrossRef]
- Kryvomaz, T.I.; Michaud, A.; Minter, D.W. Arcyria cinerea . Descr. Fungi Bact. 2020, 222, 1–11. [Google Scholar] [CrossRef]
- Farr, M.L. Arcyria cinerea and A. Pomiformis Revised. Mycologia 1962, 54, 516–520. [Google Scholar] [CrossRef]
- Rostafiński, J. Arcyria pomiformis. Pamiętnik Tow. Nauk Ścisłych W Paryżu 1875, 6, 271. [Google Scholar] [CrossRef]
- Wettstein, R.v. Arcyria denudata. Verhandlungen Der Zool.-Bot. Ges. Wien 1886, 35, 353. [Google Scholar]
- Cook, O.F. Arcyodes, a new genus of Myxomycetes. Science 1902, 15, 651. [Google Scholar]
- Kunttu, P.; Varis, E.; Kunttu, S. New Records of Myxomycetes to the Åland Islands. Memo. Soc. Pro Fauna Et Flora Fenn. 2014, 90, 1–4. [Google Scholar]
- Stojanowska, W. Slime Moulds Flora of the Kaczawskie Mts. on the Background of Silesian Myxomycetes. Acta Univ. Wratislav. Pr. Bot. 1972, 171, 9–75. [Google Scholar]
- Lister, A.; Lister, G. A Monograph of the Mycetozoa, 2nd ed.; British Museum: London, UK, 1911. [Google Scholar] [CrossRef]
- Silva, C.F.D.; Cavalcanti, L.D.H. Myxobiota of the Brazilian Atlantic Forest: Species on Oil Palm Tree (Elaeis Guineensis, Arecaceae). Rodriguésia 2010, 61, 575–583. [Google Scholar] [CrossRef]
- Scheetz, R.W.; Nelson, R.K.; Carlson, E.C. Scanning Electron Microscopy of Ceratiomyxa Fruticulosa. Can. J. Bot. 1980, 58, 392–400. [Google Scholar] [CrossRef]
- Schröter, J. Kryptogamen-Flora von Schlesien. Die Pilze Schlesiens; J. U. Kern’s Verlag: Breslau, Poland, 1885; Volume 3, p. 118. [Google Scholar] [CrossRef]
- Neubert, H.; Nowotny, W.; Baumann, K.; Marx, H. Die Myxomyceten, Band 3: Stemonitales; Die Myxomyceten; Karlheinz Baumann Verlag: Gomaringen, Germany, 2000; Volume 3, p. 391. [Google Scholar][Green Version]
- Goodwin, D.C. Morphogenesis of the Sporangium of Comatricha. Am. J. Bot. 1961, 48, 148–154. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, J.H.; Chen, Y.F.; Yang, F.H. Myxomycetes of Taiwan (XVII): Four New Records of Cribraria. Taiwania 2006, 51, 214–218. [Google Scholar] [CrossRef]
- Rostafiński, J. Versuch Eines Systems der Mycetozoen; F. Wolff: Strassburg, France, 1873. [Google Scholar][Green Version]
- Phate, P.; Mishra, R.L. Culture of Hemitrichia Serpula Wide Range Agar Mediu. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 480–488. [Google Scholar]
- Kryvomaz, T.I.; Michaud, A.; Minter, D.W. Hemitrichia serpula. Descr. Fungi Bact. 2020, 222. [Google Scholar] [CrossRef]
- Morgan, A.P. The Myxomycetes of the Miami Valley, Ohio. J. Cincinnati Soc. Nat. Hist. 1894, 16, 131. [Google Scholar]
- Kowalski, D.T. The Species of Lamproderma. Mycologia 1970, 62, 621–672. [Google Scholar] [CrossRef]
- Dagamac, N.H.A.; Rea-Maminta, M.A.D.; Batungbacal, N.S.; Jung, S.H.; Bulang, C.R.T.; Cayago, A.G.R.; dela Cruz, T.E.E. Diversity of Plasmodial Slime Molds (Myxomycetes) in Coastal, Mountain, and Community Forests of Puerto Galera, Oriental Mindoro, the Philippines. J. Asia-Pac. Biodivers. 2015, 8, 322–329. [Google Scholar] [CrossRef]
- Nannenga-Bremekamp, N.E. Notes on Myxomycetes XI. Proc. K. Ned. Akad. Van Wet. Ser. C 1966, 69, 348. [Google Scholar]
- Kryvomaz, T.I.; Michaud, A.; Minter, D.W. Metatrichia vesparium. Descr. Fungi Bact. 2012, 192, 1916. [Google Scholar] [CrossRef]
- Peck, C.H. New species of Myxomycetes. Annu. Rep. N. Y. State Mus. Nat. Hist. 1878, 31, 42. [Google Scholar]
- de Haan, M.; De Pauw, S.; Bogaerts, A. A Study of the Genus Oligonema (Myxomycota) in Belgium. Syst. Geogr. Plants 2004, 74, 251–260. [Google Scholar] [CrossRef]
- de Holanda Cavalcanti, L.; Costa, A.A.A.; Barbosa, D.I.; de Almeida Neves Nepomuceno Agra, L.; Bezerra, A.C.C. Distribution and Occurrence of Oligonema (Trichiales, Myxomycetes) in Brazil. Braz. J. Bot. 2015, 38, 187–191. [Google Scholar] [CrossRef]
- Keller, H.W.; Brooks, T.E. A New Species of Perichaena Decaying Leaves. Mycologia 1971, 63, 657–663. [Google Scholar] [CrossRef]
- Keller, H.W. The Genus Perichaena (Myxomycetes): A Taxonomic and Cultural Study. Ph.D. Thesis, The University of Iowa, Iowa City, IA, USA, 1971. [Google Scholar]
- Stephenson, S.L.; Urban, L.A.; Rojas, C.; McDonald, M.S. Myxomycetes Associated with Woody Twigs. Rev. Mex. De Micol. 2008, 27, 21–28. [Google Scholar]
- Chevallier, F.F. Flore Générale des Environs de Paris; Ferra Jeune: Paris, France, 1826; Volume 1, p. 345. [Google Scholar] [CrossRef]
- Neubert, H.; Nowotny, W.; Baumann, K.; Marx, H. Die Myxomyceten, Band 2: Physarales; Die Myxomyceten; Karlheinz Baumann Verlag: Gomaringen, Germany, 1995; Volume 2, p. 368. [Google Scholar]
- Gao, Y.; Tao, W.; Yan, S.Z.; Chen, S.L. The Life Cycle of Didymium Laxifilum Physarum Album Oat Agar Cult. J. Eukaryot. Microbiol. 2017, 64, 457–463. [Google Scholar] [CrossRef]
- Eroğlu, G. A New Species of Physarum (Physaraceae) Turkey. Phytotaxa 2023, 618, 99–102. [Google Scholar] [CrossRef]
- Sevindik, M.; Baba, H.; Bal, C.; Colak, O.F.; Akgül, H. Antioxidant, Oxidant and Antimicrobial Capacities of Physarum album. J. Bacteriol. Mycol. Open Access 2018, 6, 317–320. [Google Scholar] [CrossRef]
- Persoon, C.H. Dispositio Methodica Fungorum. Neues Mag. Für Die Bot. 1794, 1, 89. [Google Scholar] [CrossRef]
- Moreno, G.; López-Villalba, Á.; Castillo, A.; Deschamps, J.R. Critical Revision of Some Myxomycetes in the Argentinian Herbaria BAFC and LIL – 5. Mycotaxon 2020, 135, 729–751. [Google Scholar] [CrossRef]
- García-Martín, J.M.; Mosquera, J.; Lado, C. Morphological and Molecular Characterization of a New Succulenticolous Physarum (Myxomycetes, Amoebozoa) Unique Polyg. Spores Linked Chain. Eur. J. Protistol. 2018, 63, 13–25. [Google Scholar] [CrossRef]
- Baba, H.; Sevindik, M. The roles of myxomycetes in ecosystems. J. Bacteriol. Mycol. Open Access 2018, 6, 165–166. [Google Scholar] [CrossRef]










| Substrate Type (Tree) | Success (%) | Mean Species SD | Median (IQR) | ||
|---|---|---|---|---|---|
| Bark—Alnus glutinosa | 8 | 4 | 50.0 | 1.5 1.0 | 1.0 (1–1) |
| Leaves—A. glutinosa | 8 | 1 | 12.5 | 1.0 0.0 | 1.0 (1–1) |
| Twigs—A. glutinosa | 8 | 2 | 25.0 | 1.5 0.7 | 1.5 (1–1) |
| Wood—A. glutinosa | 8 | 4 | 50.0 | 1.0 0.0 | 1.0 (1–1) |
| Bark—Betula pubescens | 8 | 6 | 75.0 | 1.7 0.5 | 2.0 (1–2) |
| Leaves—B. pubescens | 8 | 0 | 0.0 | — | — |
| Twigs—B. pubescens | 8 | 1 | 12.5 | 1.0 0.0 | 1.0 (1–1) |
| Wood—B. pubescens | 8 | 3 | 37.5 | 1.0 0.0 | 1.0 (1–1) |
| Bark—Picea abies | 8 | 7 | 87.5 | 1.3 0.8 | 1.0 (1–1) |
| Leaves—P. abies | 8 | 1 | 12.5 | 1.0 0.0 | 1.0 (1–1) |
| Twigs—P. abies | 8 | 2 | 25.0 | 1.5 0.7 | 1.5 (1–1) |
| Wood—P. abies | 8 | 4 | 50.0 | 1.2 0.5 | 1.0 (1–1) |
| All substrates combined | 96 | 35 | 36.5 | 1.3 0.6 | 1.0 (1–1) |
| Species | AG Bark | AG Leaves | AG Twigs | AG Wood | BP Bark | BP Leaves | BP Twigs | BP Wood | PA Bark | PA Leaves | PA Twigs | PA Wood | Row Total | Non Zero Cols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arcyodes incarnata | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Arcyria cinerea | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 8 | 3 |
| Arcyria denudata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 |
| Arcyria pomiformis | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 5 | 3 |
| Ceratiomyxa fruticulosa var. flexuosa | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 |
| Comatricha nigra | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 8 | 4 |
| Cribraria microcarpa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 2 |
| Hemitrichia serpula | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 2 |
| Lamproderma scintillans | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Metatrichia vesparium | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Oligonema flavidum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Perichaena depressa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Physarum album | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 2 | 0 | 0 | 2 | 0 | 9 | 4 |
| Physarum bivalve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Tree species | Chao1 ± SE | Coverage | Species (Frequency) | ||
|---|---|---|---|---|---|
| Alnus glutinosa | 32 | 9 | 33.5 ± 31.1 | 26.9% | Arcyodes incarnata (3.1%), Ceratiomyxa fruticulosa var. flexuosa (3.1%), Comatricha nigra (15.6%), Cribraria microcarpa (3.1%), Hemitrichia serpula (6.3%), Lamproderma scintillans (3.1%), Metatrichia vesparium (3.1%), Oligonema flavidum (3.1%), Perichaena depressa (3.1%) |
| Betula pubescens | 32 | 4 | 4.5 ± 1.3 | 88.9% | Arcyria cinerea (12.5%), Arcyria pomiformis (6.3%), Ceratiomyxa fruticulosa var. flexuosa (3.1%), Physarum album (21.9%) |
| Picea abies | 32 | 8 | 8.7 ± 1.3 | 92.3% | Arcyria cinerea (12.5%), Arcyria denudata (6.3%), Arcyria pomiformis (9.4%), Comatricha nigra (9.4%), Cribraria microcarpa (6.3%), Hemitrichia serpula (3.1%), Physarum album (6.3%), Physarum bivalve (3.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowicz, T.; Żebrowski, I.; Micewicz, G.M.; Puchlik, M.; Wilamowski, K.; Sztabkowski, K.; Oszako, T. First Assessment of the Biodiversity of True Slime Molds in Swamp Forest Stands of the Knyszyn Forest (Northeast Poland) Using the Moist Chambers Detection Method. Forests 2025, 16, 1259. https://doi.org/10.3390/f16081259
Pawłowicz T, Żebrowski I, Micewicz GM, Puchlik M, Wilamowski K, Sztabkowski K, Oszako T. First Assessment of the Biodiversity of True Slime Molds in Swamp Forest Stands of the Knyszyn Forest (Northeast Poland) Using the Moist Chambers Detection Method. Forests. 2025; 16(8):1259. https://doi.org/10.3390/f16081259
Chicago/Turabian StylePawłowicz, Tomasz, Igor Żebrowski, Gabriel Michał Micewicz, Monika Puchlik, Konrad Wilamowski, Krzysztof Sztabkowski, and Tomasz Oszako. 2025. "First Assessment of the Biodiversity of True Slime Molds in Swamp Forest Stands of the Knyszyn Forest (Northeast Poland) Using the Moist Chambers Detection Method" Forests 16, no. 8: 1259. https://doi.org/10.3390/f16081259
APA StylePawłowicz, T., Żebrowski, I., Micewicz, G. M., Puchlik, M., Wilamowski, K., Sztabkowski, K., & Oszako, T. (2025). First Assessment of the Biodiversity of True Slime Molds in Swamp Forest Stands of the Knyszyn Forest (Northeast Poland) Using the Moist Chambers Detection Method. Forests, 16(8), 1259. https://doi.org/10.3390/f16081259









