Patterns in Root Phenology of Woody Plants Across Climate Regions: Drivers, Constraints, and Ecosystem Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection Criteria
2.2. Data Extraction and Standardization
Climate Classification
2.3. Analysis Approach
Statistical Analysis
3. Results
3.1. Patterns in Root Phenology Across Climate Regions
3.1.1. Temperate Regions
3.1.2. Tropical and Subtropical Regions
3.1.3. Continental Regions
3.1.4. Mediterranean Regions
3.1.5. Arctic and Subarctic Regions
3.2. Environmental Controls on Root Phenology
3.2.1. Temperature Effects
3.2.2. Water Availability Effects
3.2.3. Other Environmental Factors
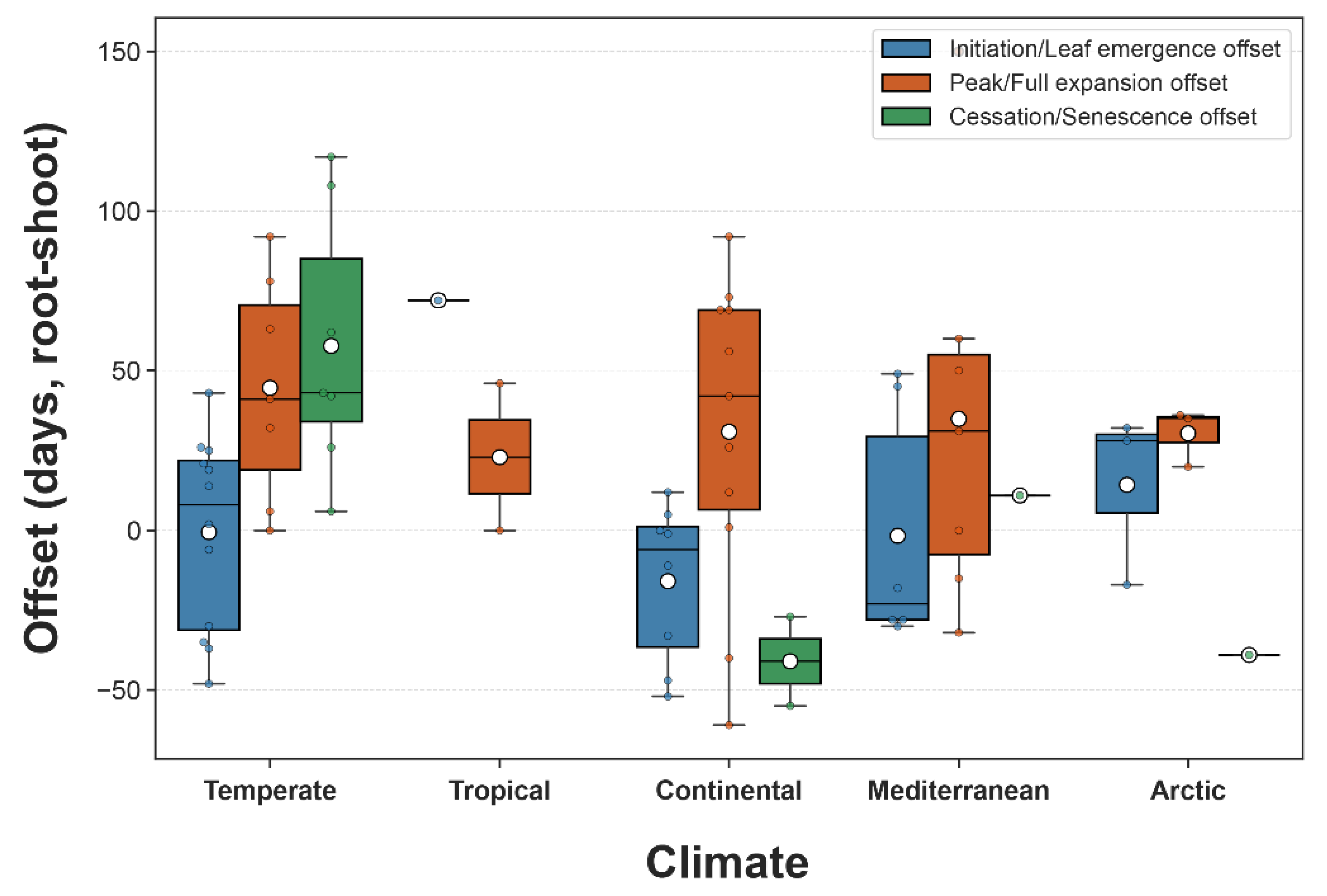
3.3. Patterns of Root-Shoot Temporal Offsets
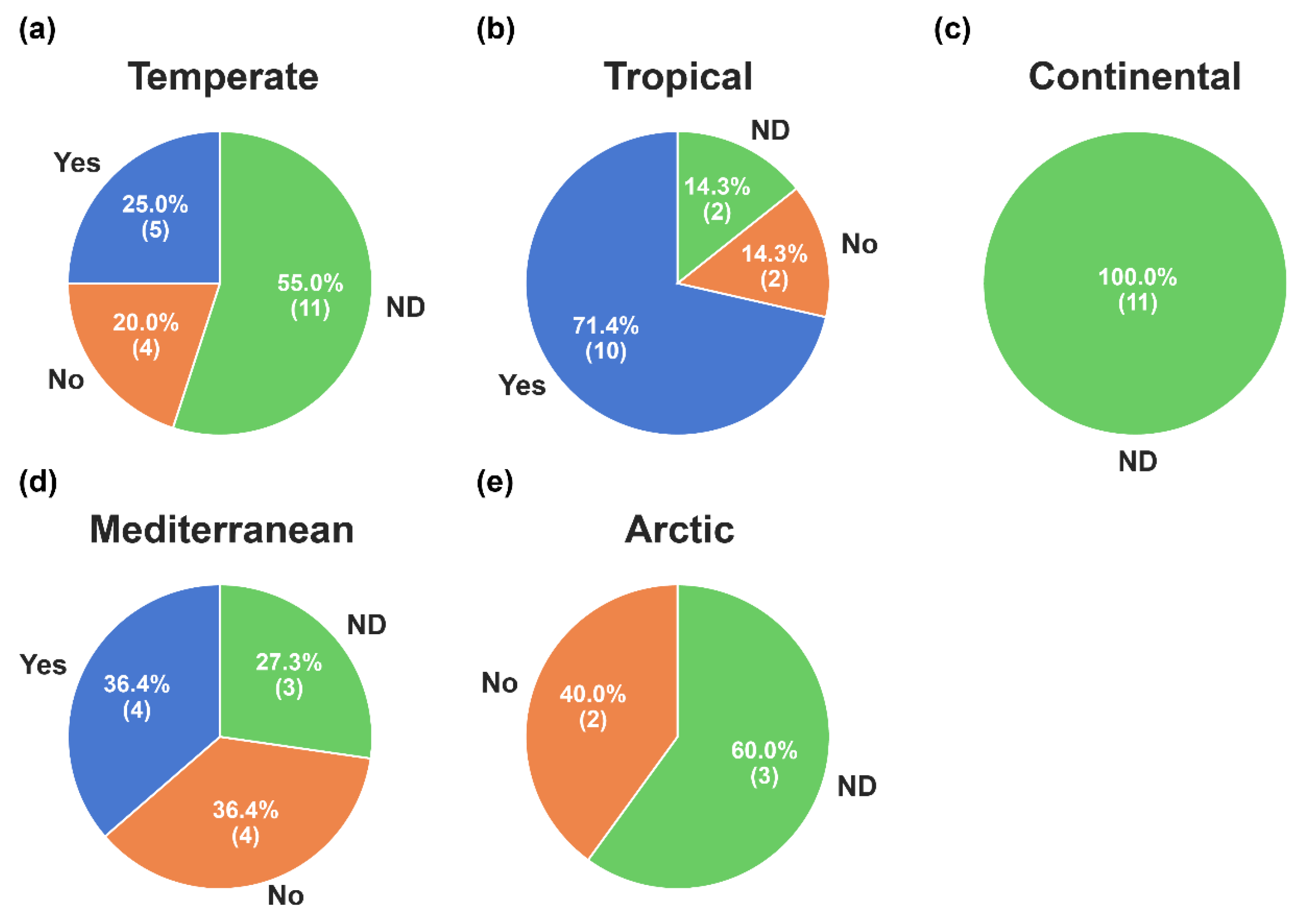
3.4. Winter Root Growth
4. Discussion
4.1. Comparative Analysis of Root Phenological Patterns Across Climate Regions
4.1.1. Phenological Variations in Temperate Regions
4.1.2. Resource-Driven Phenology in Tropical and Subtropical Systems
4.1.3. Bimodal Patterns in Mediterranean Ecosystems
4.1.4. Compressed Phenology in Continental and Arctic Regions
4.2. Hierarchical Control of Environmental Factors
4.3. Ecological and Evolutionary Significance of Root-Shoot Coordination
4.4. Ecosystem Implications of Winter Root Growth
4.5. Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate Change, Phenology, and Phenological Control of Vegetation Feedbacks to the Climate System. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, Soil and Plant Functional Types as Drivers of Global Fine-root Trait Variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Radville, L.; McCormack, M.L.; Post, E.; Eissenstat, D.M. Root Phenology in a Changing Climate. J. Exp. Bot. 2016, 67, 3617–3628. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Richardson, A.D.; Andy Black, T.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S.; et al. Influence of Spring and Autumn Phenological Transitions on Forest Ecosystem Productivity. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef]
- Rewald, B.; Leuschner, C. Does Root Competition Asymmetry Increase with Water Availability? Plant Ecol. Divers. 2009, 2, 255–264. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining Fine Roots Improves Understanding of Below-ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Grace, J.; Rayment, M. Respiration in the Balance. Nature 2000, 404, 819–820. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil Respiration and the Global Carbon Cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Giardina, C.P.; Coleman, M.D.; Hancock, J.E.; King, J.S.; Lilleskov, E.A.; Loya, W.M.; Pregitzer, K.S.; Ryan, M.G.; Trettin, C.C. The Response of Belowground Carbon Allocation in Forests to Global Change. In Tree Species Effects on Soils: Implications for Global Change; Binkley, D., Menyailo, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; NATO Science Series IV: Earth and Environmental Sciences; Volume 55, pp. 119–154. ISBN 978-1-4020-3445-9. [Google Scholar]
- Schwartz, M.D.; Reed, B.C.; White, M.A. Assessing Satellite-derived Start-of-season Measures in the Conterminous USA. Int. J. Climatol. 2002, 22, 1793–1805. [Google Scholar] [CrossRef]
- Mayer, A. Phenology and Citizen Science. BioScience 2010, 60, 172–175. [Google Scholar] [CrossRef]
- Abdelmajeed, A.Y.A.; Juszczak, R. Challenges and Limitations of Remote Sensing Applications in Northern Peatlands: Present and Future Prospects. Remote Sens. 2024, 16, 591. [Google Scholar] [CrossRef]
- Hendrick, R.L.; Pregitzer, K.S. Applications of Minirhizotrons to Understand Root Function in Forests and Other Natural Ecosystems. Plant Soil 1996, 185, 293–304. [Google Scholar] [CrossRef]
- Graefe, S.; Hertel, D.; Leuschner, C. Estimating Fine Root Turnover in Tropical Forests along an Elevational Transect Using Minirhizotrons. Biotropica 2008, 40, 536–542. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Pude, R.; Köpke, U. Root Growth in Biopores—Evaluation with in Situ Endoscopy. Plant Soil 2013, 371, 179–190. [Google Scholar] [CrossRef]
- Xiankui, Q.; Chuankuan, W. Acclimation and Adaptation of Leaf Photosynthesis, Respiration and Phenology to Climate Change: A 30-Year Larix Gmelinii Common-Garden Experiment. For. Ecol. Manag. 2018, 411, 166–175. [Google Scholar] [CrossRef]
- Burke, M.K.; Raynal, D.J. Fine Root Growth Phenology, Production, and Turnover in a Northern Hardwood Forest Ecosystem. Plant Soil 1994, 162, 135–146. [Google Scholar] [CrossRef]
- Contador, M.L.; Comas, L.H.; Metcalf, S.G.; Stewart, W.L.; Porris Gomez, I.; Negron, C.; Lampinen, B.D. Root Growth Dynamics Linked to Above-Ground Growth in Walnut (Juglans regia). Ann. Bot. 2015, 116, 49–60. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Wang, H.; Dai, X.; Yang, F.; Zhao, M. Response of the Fine Root Production, Phenology, and Turnover Rate of Six Shrub Species from a Subtropical Forest to a Soil Moisture Gradient and Shading. Plant Soil 2016, 399, 135–146. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the Roles of Nonstructural Carbohydrates in Forest Trees—from What We Can Measure to What We Want to Know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Körner, C. Climatic Stress. In Alpine Plant Life; Springer International Publishing: Cham, Switzerland, 2021; pp. 175–201. ISBN 978-3-030-59537-1. [Google Scholar]
- Makoto, K.; Wilson, S.D.; Sato, T.; Blume-Werry, G.; Cornelissen, J.H.C. Synchronous and Asynchronous Root and Shoot Phenology in Temperate Woody Seedlings. Oikos 2020, 129, 643–650. [Google Scholar] [CrossRef]
- Janos, D.P.; Scott, J.; Bowman, D.M.J.S. Temporal and Spatial Variation of Fine Roots in a Northern Australian Eucalyptus Tetrodonta Savanna. J. Trop. Ecol. 2008, 24, 177–188. [Google Scholar] [CrossRef]
- Bonomelli, C.; Bonilla, C.; Acuña, E.; Artacho, P. Seasonal Pattern of Root Growth in Relation to Shoot Phenology and Soil Temperature in Sweet Cherry Trees (Prunus avium): A Preliminary Study in Central Chile. Cienc. E Investig. Agrar. 2012, 39, 127–136. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Holbrook, N.M.; Gutierrez-Soto, M.V. Dry-Season Leaf Flushing of Enterolobium Cyclocarpum (Ear-Pod tree): Above- and Belowground Phenology and Water Relations. Tree Physiol. 2007, 27, 1561–1568. [Google Scholar] [CrossRef]
- Bazié, P.; Ky-Dembele, C.; Jourdan, C.; Roupsard, O.; Zombré, G.; Bayala, J. Synchrony in the Phenologies of Fine Roots and Leaves of Vitellaria Paradoxa in Different Land Uses of Burkina Faso. Agrofor. Syst. 2019, 93, 449–460. [Google Scholar] [CrossRef]
- Maeght, J.-L.; Gonkhamdee, S.; Clément, C.; Isarangkool Na Ayutthaya, S.; Stokes, A.; Pierret, A. Seasonal Patterns of Fine Root Production and Turnover in a Mature Rubber Tree (Hevea brasiliensis Müll. Arg.) Stand- Differentiation with Soil Depth and Implications for Soil Carbon Stocks. Front. Plant Sci. 2015, 6, 1022. [Google Scholar] [CrossRef]
- Endo, I.; Kume, T.; Kho, L.K.; Katayama, A.; Makita, N.; Ikeno, H.; Ide, J.; Ohashi, M. Spatial and Temporal Patterns of Root Dynamics in a Bornean Tropical Rainforest Monitored Using the Root Scanner Method. Plant Soil 2019, 443, 323–335. [Google Scholar] [CrossRef]
- Noguchi, K.; Han, Q.; Araki, M.G.; Kawasaki, T.; Kaneko, S.; Takahashi, M.; Chiba, Y. Fine-Root Dynamics in a Young Hinoki Cypress (Chamaecyparis obtusa) Stand for 3 Years Following Thinning. J. For. Res. 2011, 16, 284–291. [Google Scholar] [CrossRef]
- Harris, J.R.; Bassuk, N.L.; Zobel, R.W.; Whitlow, T.H. Root and Shoot Growth Periodicity of Green Ash, Scarlet Oak, Turkish Hazelnut, and Tree Lilac. J. Am. Soc. Hortic. Sci. 1995, 120, 211–216. [Google Scholar] [CrossRef]
- Iversen, C.M.; Childs, J.; Norby, R.J.; Ontl, T.A.; Kolka, R.K.; Brice, D.J.; McFarlane, K.J.; Hanson, P.J. Fine-Root Growth in a Forested Bog Is Seasonally Dynamic, but Shallowly Distributed in Nutrient-Poor Peat. Plant Soil 2018, 424, 123–143. [Google Scholar] [CrossRef]
- Steinaker, D.F.; Wilson, S.D.; Peltzer, D.A. Asynchronicity in Root and Shoot Phenology in Grasses and Woody Plants. Glob. Change Biol. 2010, 16, 2241–2251. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Variability in Root Production, Phenology, and Turnover Rate among 12 Temperate Tree Species. Ecology 2014, 95, 2224–2235. [Google Scholar] [CrossRef]
- Côté, B.; Hendershot, W.H.; Fyles, J.W.; Roy, A.G.; Bradley, R.; Biron, P.M.; Courchesne, F. The Phenology of Fine Root Growth in a Maple-Dominated Ecosystem: Relationships with Some Soil Properties. Plant Soil 1998, 201, 59–69. [Google Scholar] [CrossRef]
- Mohamed, A.; Monnier, Y.; Mao, Z.; Jourdan, C.; Sabatier, S.; Dupraz, C.; Dufour, L.; Millan, M.; Stokes, A. Asynchrony in Shoot and Root Phenological Relationships in Hybrid Walnut. New For. 2020, 51, 41–60. [Google Scholar] [CrossRef]
- Palacio, S.; Montserrat-Martí, G. Above and Belowground Phenology of Four Mediterranean Sub-Shrubs. Preliminary Results on Root–Shoot Competition. J. Arid Environ. 2007, 68, 522–533. [Google Scholar] [CrossRef]
- Cuesta, B.; Vega, J.; Villar-Salvador, P.; Rey-Benayas, J.M. Root Growth Dynamics of Aleppo Pine (Pinus halepensis Mill.) Seedlings in Relation to Shoot Elongation, Plant Size and Tissue Nitrogen Concentration. Trees 2010, 24, 899–908. [Google Scholar] [CrossRef]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Onelli, E.; Scippa, G.S.; Chiatante, D. Seasonality of Fine Root Dynamics and Activity of Root and Shoot Vascular Cambium in a Quercus Ilex L. Forest (Italy). For. Ecol. Manag. 2019, 431, 26–34. [Google Scholar] [CrossRef]
- Blume-Werry, G.; Wilson, S.D.; Kreyling, J.; Milbau, A. The Hidden Season: Growing Season Is 50% Longer below than above Ground along an Arctic Elevation Gradient. New Phytol. 2016, 209, 978–986. [Google Scholar] [CrossRef]
- Ma, T.; Parker, T.; Fetcher, N.; Unger, S.L.; Gewirtzman, J.; Moody, M.L.; Tang, J. Leaf and Root Phenology and Biomass of Eriophorum Vaginatum in Response to Warming in the Arctic. J. Plant Ecol. 2022, 15, 1091–1105. [Google Scholar] [CrossRef]
- Billings, W.D.; Mooney, H.A. THE ECOLOGY OF ARCTIC AND ALPINE PLANTS. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Miller, K.S.; Geisseler, D. Temperature Sensitivity of Nitrogen Mineralization in Agricultural Soils. Biol. Fertil. Soils 2018, 54, 853–860. [Google Scholar] [CrossRef]
- Gui, D.; Zeng, F.; Liu, Z.; Zhang, B. Root Characteristics of Alhagi Sparsifolia Seedlings in Response to Water Supplement in an Arid Region, Northwestern China. J. Arid Land 2013, 5, 542–551. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, L.; Li, Y. Prolonged Dry Periods between Rainfall Events Shorten the Growth Period of the Resurrection Plant Reaumuria soongorica. Ecol. Evol. 2018, 8, 920–927. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, C.; Zhao, H.; Chen, T.; Yao, X.; Wang, X.; Yang, Y.; Chen, G. Above- and Belowground Phenology Responses of Subtropical Chinese Fir (Cunninghamia lanceolata) to Soil Warming, Precipitation Exclusion and Their Interaction. Sci. Total Environ. 2024, 933, 173147. [Google Scholar] [CrossRef]
- Van Den Driessche, R. Importance of Current Photosynthate to New Root Growth in Planted Conifer Seedlings. Can. J. For. Res. 1987, 17, 776–782. [Google Scholar] [CrossRef]
- Iivonen, S.; Rikala, R.; Vapaavuori, E. Seasonal Root Growth of Scots Pine Seedlings in Relation to Shoot Phenology, Carbohydrate Status, and Nutrient Supply. Can. J. For. Res. 2001, 31, 1569–1578. [Google Scholar] [CrossRef]
- Kou, L.; Li, S.; Wang, H.; Fu, X.; Dai, X. Unaltered Phenology but Increased Production of Ectomycorrhizal Roots of Pinus Elliottii under 4 Years of Nitrogen Addition. New Phytol. 2019, 221, 2228–2238. [Google Scholar] [CrossRef]
- Ding, Y.; Schiestl-Aalto, P.; Helmisaari, H.-S.; Makita, N.; Ryhti, K.; Kulmala, L. Temperature and Moisture Dependence of Daily Growth of Scots Pine (Pinus sylvestris L.) Roots in Southern Finland. Tree Physiol. 2020, 40, 272–283. [Google Scholar] [CrossRef]
- Zadworny, M.; Eissenstat, D.M. Contrasting the Morphology, Anatomy and Fungal Colonization of New Pioneer and Fibrous Roots. New Phytol. 2011, 190, 213–221. [Google Scholar] [CrossRef]
- Du, E.; Fang, J. Linking Belowground and Aboveground Phenology in Two Boreal Forests in Northeast China. Oecologia 2014, 176, 883–892. [Google Scholar] [CrossRef]
- Schenker, G.; Lenz, A.; Korner, C.; Hoch, G. Physiological Minimum Temperatures for Root Growth in Seven Common European Broad-Leaved Tree Species. Tree Physiol. 2014, 34, 302–313. [Google Scholar] [CrossRef]
- Malyshev, A.V.; Blume-Werry, G.; Spiller, O.; Smiljanić, M.; Weigel, R.; Kolb, A.; Nze, B.Y.; Märker, F.; Sommer, F.C.J.; Kinley, K.; et al. Warming Nondormant Tree Roots Advances Aboveground Spring Phenology in Temperate Trees. New Phytol. 2023, 240, 2276–2287. [Google Scholar] [CrossRef]
- Germon, A.; Cardinael, R.; Prieto, I.; Mao, Z.; Kim, J.; Stokes, A.; Dupraz, C.; Laclau, J.-P.; Jourdan, C. Unexpected Phenology and Lifespan of Shallow and Deep Fine Roots of Walnut Trees Grown in a Silvoarable Mediterranean Agroforestry System. Plant Soil 2016, 401, 409–426. [Google Scholar] [CrossRef]
- Smith, M.S.; Fridley, J.D.; Goebel, M.; Bauerle, T.L. Links between Belowground and Aboveground Resource-Related Traits Reveal Species Growth Strategies That Promote Invasive Advantages. PLoS ONE 2014, 9, e104189. [Google Scholar] [CrossRef] [PubMed]
- Mickelbart, M.V.; Robinson, P.W.; Witney, G.; Arpaia, M.L. ‘Hass’ Avocado Tree Growth on Four Rootstocks in California. II. Shoot and Root Growth. Sci. Hortic. 2012, 143, 205–210. [Google Scholar] [CrossRef]
- Menzel, A. Trends in Phenological Phases in Europe between 1951 and 1996. Int. J. Biometeorol. 2000, 44, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D. Flowering Phenology, Fruiting Success and Progressive Deterioration of Pollination in an Early-Flowering Geophyte. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3187–3199. [Google Scholar] [CrossRef]
- Austen, E.J.; Rowe, L.; Stinchcombe, J.R.; Forrest, J.R.K. Explaining the Apparent Paradox of Persistent Selection for Early Flowering. New Phytol. 2017, 215, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, E.A.; Tingey, D.T.; Rygiewicz, P.T.; Johnson, M.G.; Olszyk, D.M. Contributions of Current Year Photosynthate to Fine Roots Estimated Using a 13C-Depleted CO2 Source. Plant Soil 2002, 247, 233–242. [Google Scholar] [CrossRef]
- Lynch, D.J.; Matamala, R.; Iversen, C.M.; Norby, R.J.; Gonzalez-Meler, M.A. Stored Carbon Partly Fuels Fine-root Respiration but Is Not Used for Production of New Fine Roots. New Phytol. 2013, 199, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, A.; Högberg, P. Natural Abundance of 13C in CO2 Respired from Forest Soils Reveals Speed of Link between Tree Photosynthesis and Root Respiration. Oecologia 2001, 127, 305–308. [Google Scholar] [CrossRef]
- Huang, R.; Huang, J.; Zhang, C.; Ma, H.; Zhuo, W.; Chen, Y.; Zhu, D.; Wu, Q.; Mansaray, L.R. Soil Temperature Estimation at Different Depths, Using Remotely-Sensed Data. J. Integr. Agric. 2020, 19, 277–290. [Google Scholar] [CrossRef]
- Onwuka, B. Effects of Soil Temperature on Some Soil Properties and Plant Growth. Adv. Plants Agric. Res. 2016, 6, 89–93. [Google Scholar] [CrossRef]
- Reich, P.B. Phenology of Tropical Forests: Patterns, Causes, and Consequences. Can. J. Bot. 1995, 73, 164–174. [Google Scholar] [CrossRef]
- Linares-Palomino, R.; Oliveira-Filho, A.T.; Pennington, R.T. Neotropical Seasonally Dry Forests: Diversity, Endemism, and Biogeography of Woody Plants. In Seasonally Dry Tropical Forests; Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press/Center for Resource Economics: Washington, DC, USA, 2011; pp. 3–21. ISBN 978-1-61091-021-7. [Google Scholar]
- Valeriano, C.; Gutiérrez, E.; Colangelo, M.; Gazol, A.; Sánchez-Salguero, R.; Tumajer, J.; Shishov, V.; Bonet, J.A.; Martínez De Aragón, J.; Ibáñez, R.; et al. Seasonal Precipitation and Continentality Drive Bimodal Growth in Mediterranean Forests. Dendrochronologia 2023, 78, 126057. [Google Scholar] [CrossRef]
- Canadell, J.; Zedler, P.H. Underground Structures of Woody Plants in Mediterranean Ecosystems of Australia, California, and Chile. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Arroyo, M.T.K., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; Ecological Studies; Volume 108, pp. 177–210. ISBN 978-1-4612-7560-2. [Google Scholar]
- Chapin, F.S.; Jefferies, R.L.; Reynolds, J.F.; Shaver, G.R.; Svoboda, J.; Chu, E.W. Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective; Elsevier Science: Oxford, UK, 1991; Physiological Ecology; ISBN 978-0-323-13842-0. [Google Scholar]
- Post, E.; Forchhammer, M.C.; Bret-Harte, M.S.; Callaghan, T.V.; Christensen, T.R.; Elberling, B.; Fox, A.D.; Gilg, O.; Hik, D.S.; Høye, T.T.; et al. Ecological Dynamics Across the Arctic Associated with Recent Climate Change. Science 2009, 325, 1355–1358. [Google Scholar] [CrossRef]
- Nord, E.A.; Lynch, J.P. Plant Phenology: A Critical Controller of Soil Resource Acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef]
- McCormack, M.L.; Iversen, C.M. Physical and Functional Constraints on Viable Belowground Acquisition Strategies. Front. Plant Sci. 2019, 10, 1215. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer New York: New York, NY, 2011; ISBN 978-1-4419-9503-2. [Google Scholar]
- Pregitzer, K.S.; King, J.S.; Burton, A.J.; Brown, S.E. Responses of Tree Fine Roots to Temperature. New Phytol. 2000, 147, 105–115. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate Change Fingerprints in Recent European Plant Phenology. Glob. Change Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, S.; Blume-Werry, G.; Peters, B.; Smiljanić, M.; Kreyling, J. Patterns and Drivers in Spring and Autumn Phenology Differ Above- and Belowground in Four Ecosystems under the Same Macroclimatic Conditions. Plant Soil 2019, 445, 217–229. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation Between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting Depth and Soil Moisture Control Mediterranean Woody Seedling Survival during Drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Smithwick, E.A.H.; Lucash, M.S.; McCormack, M.L.; Sivandran, G. Improving the Representation of Roots in Terrestrial Models. Ecol. Model. 2014, 291, 193–204. [Google Scholar] [CrossRef]
- Warren, J.M.; Hanson, P.J.; Iversen, C.M.; Kumar, J.; Walker, A.P.; Wullschleger, S.D. Root Structural and Functional Dynamics in Terrestrial Biosphere Models—Evaluation and Recommendations. New Phytol. 2015, 205, 59–78. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T.; Yavitt, J.B. Environmental Control of Fine Root Dynamics in a Northern Hardwood Forest. Glob. Change Biol. 2003, 9, 670–679. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D. Impacts of Environmental Factors on Fine Root Lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; Sloan, V.L.; Sullivan, P.F.; Euskirchen, E.S.; McGuire, A.D.; Norby, R.J.; Walker, A.P.; Warren, J.M.; Wullschleger, S.D. The Unseen Iceberg: Plant Roots in Arctic Tundra. New Phytol. 2015, 205, 34–58. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Rewald, B.; Sandén, H.; Godbold, D.L. Patterns in Root Phenology of Woody Plants Across Climate Regions: Drivers, Constraints, and Ecosystem Implications. Forests 2025, 16, 1257. https://doi.org/10.3390/f16081257
Guo Q, Rewald B, Sandén H, Godbold DL. Patterns in Root Phenology of Woody Plants Across Climate Regions: Drivers, Constraints, and Ecosystem Implications. Forests. 2025; 16(8):1257. https://doi.org/10.3390/f16081257
Chicago/Turabian StyleGuo, Qiwen, Boris Rewald, Hans Sandén, and Douglas L. Godbold. 2025. "Patterns in Root Phenology of Woody Plants Across Climate Regions: Drivers, Constraints, and Ecosystem Implications" Forests 16, no. 8: 1257. https://doi.org/10.3390/f16081257
APA StyleGuo, Q., Rewald, B., Sandén, H., & Godbold, D. L. (2025). Patterns in Root Phenology of Woody Plants Across Climate Regions: Drivers, Constraints, and Ecosystem Implications. Forests, 16(8), 1257. https://doi.org/10.3390/f16081257






