Higher Winter Precipitation and Temperature Are Associated with Smaller Earlywood Vessel Size but Wider Latewood Width in Quercus faginea Lam.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Sampling, and Measurement
2.2. Chronology Building and Assessment
2.3. Computation of Climate–Growth Relationships
3. Results
3.1. Vessel Distributions and Contribution of the First Row
3.2. Comparative Analysis of Variable Chronologies
3.3. Assessment of Chronology Quality
3.4. Climate–Growth Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RW | Total ring width |
| EW | Earlywood width |
| LW | Latewood width |
| mvd | Mean vessel diameter |
| Mvd | Median vessel diameter |
| q3 | Third quartile of vessel area distribution |
| m95 | 95th percentile of vessel area distribution |
| max | Maximum vessel area |
| nv | Number of vessels |
| tva | Total vessel area |
| nvR | Ratio of vessels in the first row |
| Ks | Specific conductivity |
| Kt | Theoretical conductivity (sum of the fourth power of vessel radii) |
| DH | Hydraulically-weighted diameter |
| EPS | Expressed populations signal |
| SPEI | Standardized precipitation–evapotranspiration index |
References
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- King, G.M.; Gugerli, F.; Fonti, P.; Frank, D.C. Tree growth response along an elevational gradient: Climate or genetics? Oecologia 2013, 173, 1587–1600. [Google Scholar] [CrossRef]
- Fonti, P.; von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- Alla, A.Q.; Camarero, J.J. Contrasting responses of radial growth and wood anatomy to climate in a Mediterranean ring-porous oak: Implications for its future persistence or why the variance matters more than the mean. Eur. J. For. Res. 2012, 131, 1537–1550. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef]
- Capelo, J.; Catry, F. A distribuição do carvalho-português em Portugal. In Árvores e Florestas de Portugal, Volume 2: Os Carvalhais—Um Património a Conservar; Sande Silva, J., Ed.; Fundação Luso-Americana para o Desenvolvimento: Lisboa, Portugal, 2007; pp. 85–93. [Google Scholar]
- Castro-Díez, P.; Navarro, J. Water relations of seedlings of three Quercus species: Variations across and within species grown in contrasting light and water regimes. Tree Physiol. 2007, 27, 1011–1018. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Lomba, Â.; Almeida, R.S.; Vázquez, F.M.; Garcia, C. Late Quaternary range shifts of marcescent oaks unveil the dynamics of a major biogeographic transition in southern Europe. Sci. Rep. 2020, 10, 21598. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on growth and wood anatomical properties of Quercus faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Tumajer, J.; Serra-Maluquer, X.; Gazol, A.; de Andrés, E.G.; Colangelo, M.; Sangüesa-Barreda, G.; Olano, J.M.; Rozas, V.; García-Plazaola, J.I.; Fernández-Marín, B.; et al. Bimodal and unimodal radial growth of Mediterranean oaks along a coast-inland gradient. Agric. For. Meteorol. 2022, 327, 109234. [Google Scholar] [CrossRef]
- Vieira, J.; Campelo, F.; Nabais, C. Environment controls seasonal and daily cycles of stem diameter variations in Portuguese oak (Quercus faginea L.). Forests 2022, 13, 170. [Google Scholar] [CrossRef]
- García-González, I.; Fonti, P. Ensuring a representative sample of earlywood vessels for dendroecological studies: An example from two ring-porous species. Trees 2008, 22, 237–244. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, L.; Liu, S.; Lei, P.; Zhang, R.; Cherubini, P. Xylem adjustment and growth response of early- and late-successional tree species to rapid warming. Eur. J. For. Res. 2024, 143, 785–801. [Google Scholar] [CrossRef]
- García-González, I.; Souto-Herrero, M. Earlywood anatomy highlights the prevalent role of winter conditions on radial growth of oak at its distribution boundary in NW Iberia. Plants 2023, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Gea-Izquierdo, G.; Fonti, P.; Cherubini, P.; Martín-Benito, D.; Chaar, H.; Cañellas, I. Xylem hydraulic adjustment and growth response of Quercus canariensis Willd. to climatic variability. Tree Physiol. 2012, 32, 401–413. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Castro-Díez, P.; Pérez-Rontomé, C.; Montserrat-Martí, G. Stem xylem features in three Quercus (Fagaceae) species along a climatic gradient in NE Spain. Trees 1997, 12, 90–96. [Google Scholar] [CrossRef]
- Souto-Herrero, M.; Rozas, V.; García-González, I. Earlywood vessels and latewood width explain the role of climate on wood formation of Quercus pyrenaica Willd. across the Atlantic-Mediterranean boundary in NW Iberia. For. Ecol. Manag. 2018, 425, 126–137. [Google Scholar] [CrossRef]
- Akhmetzyanov, L.; Buras, A.; Sass-Klaassen, U.; Den Ouden, J.; Mohren, F.; Groenendijk, P.; García-González, I. Multi-variable approach pinpoints origin of oak wood with higher precision. J. Biogeogr. 2019, 46, 1163–1177. [Google Scholar] [CrossRef]
- Sass, U.; Eckstein, D. The variability of vessel size in beech (Fagus sylvatica L.) and its ecophysiological interpretation. Trees 1995, 9, 247–252. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Fichot, R.; Laurans, F.; Monclus, R.; Moreau, A.; Pilate, G.; Brignolas, F. Xylem anatomy correlates with gas exchange, water-use efficiency and growth performance under contrasting water regimes: Evidence from Populus deltoides x Populus nigra hybrids. Tree Physiol. 2009, 29, 1537–1549. [Google Scholar] [CrossRef]
- Argüelles-Marrón, B.; Meave, J.A.; Luna-Vega, I.; Crispin-DelaCruz, D.B.; Szejner, P.; Ames-Martínez, F.N.; Rodríguez-Ramírez, E.C. Adaptation potential of Neotropical montane oaks to drought events: Wood anatomy sensitivity in Quercus delgadoana and Quercus meavei. Funct. Ecol. 2023, 37, 2040–2055. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall/CRC: New York, NY, USA, 1993. [Google Scholar]
- Canty, A.; Ripley, B. boot: Bootstrap R (S-Plus) Functions, R package version 1.3-31; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://CRAN.R-project.org/package=boot (accessed on 1 May 2025).
- Wei, T.; Simko, V. R Package ‘corrplot’: Visualization of a Correlation Matrix; Version 0.95; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://github.com/taiyun/corrplot (accessed on 1 May 2025).
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Begueria, S.; Trigo, R.; Lopez-Moreno, J.I.; Azorin-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardized Precipitation-Evapotranspiration Index, R package version 1.8.1; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=SPEI (accessed on 1 May 2025).
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time-series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cabon, A.; Fernández-de-Uña, L.; Gea-Izquierdo, G.; Meinzer, F.C.; Woodruff, D.R.; Martínez-Vilalta, J.; De Cáceres, M. Water potential control of turgor-driven tracheid enlargement in Scots pine at its xeric distribution edge. New Phytol. 2020, 225, 209–221. [Google Scholar] [CrossRef]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Granier, C.; Muller, B. Water deficit and growth. Co-ordinating processes without an orchestrator? Curr. Opin. Plant Biol. 2011, 14, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Lis, G.; Olano, J.M.; Rozas, V.; Rossi, S.; Vázquez-Ruiz, R.A.; García-González, I. Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Funct. Ecol. 2017, 31, 592–603. [Google Scholar] [CrossRef]
- Tyree, M.T.; Cochard, H. Summer and winter embolism in oak: Impact on water relations. Ann. For. Sci. 1996, 53, 173–180. [Google Scholar] [CrossRef]
- Lupi, C.; Morin, H.; Deslauriers, A.; Rossi, S. Xylem phenology and wood production: Resolving the chicken-or-egg dilemma. Plant Cell Environ. 2010, 33, 1721–1730. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem network transport capacity: A Review of embolism repair in vascular plants. Front. Plant Sci. 2013, 4, 108. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.-L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; Rozas, V.; Vázquez-Ruiz, R.A.; García-González, I. Do ring-porous oaks prioritize earlywood vessel efficiency over safety? Environmental effects on vessel diameter and tyloses formation. Agric. For. Meteorol. 2018, 248, 205–214. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Kudo, K.; Yamagishi, Y.; Nakamura, Y.; Nakaba, S.; Begum, S.; Nugroho, W.D.; Arakawa, I.; Kitin, P.; Funada, R. Winter-spring temperature pattern is closely related to the onset of cambial reactivation in stems of the evergreen conifer Chamaecyparis pisifera. Sci. Rep. 2020, 10, 14341. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, D.; Regev, L.; Boaretto, E.; Carrer, M. Xylem anatomical traits reveal different strategies of two Mediterranean oaks to cope with drought and warming. Environ. Exp. Bot. 2017, 133, 128–138. [Google Scholar] [CrossRef]
- Castagneri, D.; Carrer, M.; Regev, L.; Boaretto, E. Precipitation variability differently affects radial growth, xylem traits and ring porosity of three Mediterranean oak species at xeric and mesic sites. Sci. Total Environ. 2020, 699, 134285. [Google Scholar] [CrossRef]
- Bleicher, N.; von Arx, G. A case study on the climate-growth signal stability and plasticity in European oak from ecologically contrasting sites—Implications for dendroclimatological studies. Trees 2025, 39, 49. [Google Scholar] [CrossRef]
- Skiadaresis, G.; Schwarz, J.; Stahl, K.; Bauhus, J. Groundwater extraction reduces tree vitality, growth and xylem hydraulic capacity in Quercus robur during and after drought events. Sci. Rep. 2021, 11, 5149. [Google Scholar] [CrossRef]
- Peters, R.L.; Steppe, K.; Cuny, H.E.; De Pauw, D.J.; Frank, D.C.; Schaub, M.; Rathgeber, C.B.K.; Cabon, A.; Fonti, P. Turgor—A limiting factor for radial growth in mature conifers along an elevational gradient. New Phytol. 2021, 229, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Yasue, K.; Hosoo, Y.; Funada, R. Relationship between formation of earlywood vessels and leaf phenology in two ring-porous hardwoods, Quercus serrata and Robinia pseudoacacia, in early spring. J. Wood Sci. 2015, 61, 455–464. [Google Scholar] [CrossRef]
- Kitin, P.; Funada, R. Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA J. 2016, 37, 315–331. [Google Scholar] [CrossRef]
- Michelot, A.; Bréda, N.; Damesin, C.; Dufrene, E. Differing growth responses to climatic variations and soil water deficits of Fagus sylvatica, Quercus petraea and Pinus sylvestris in a temperate forest. For. Ecol. Manag. 2012, 265, 161–171. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Chamberlain, C.J.; Wieringa, M.M.; Aubrecht, D.M.; Carbone, M.S.; Walker, J.C.; Xu, X.; Czimczik, C.I.; Richardson, A.D. Seasonal fluctuation of nonstructural carbohydrates reveals the metabolic availability of stemwood reserves in temperate trees with contrasting wood anatomy. Tree Physiol. 2020, 40, 1355–1365. [Google Scholar] [CrossRef]
- Gough, C.M.; Flower, C.E.; Vogel, C.S.; Dragoni, D.; Curtis, P.S. Whole-ecosystem labile carbon production in a north temperate deciduous forest. Agric. For. Meteorol. 2009, 149, 1531–1540. [Google Scholar] [CrossRef]
- Barbaroux, C.; Bréda, N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol. 2002, 22, 1201–1210. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, M.H.; Whitehead, D.; Tissue, D.T.; Schuster, W.S.F.; Brown, K.J.; Griffin, K.L. Responses of leaf respiration to temperature and leaf characteristics in three deciduous tree species vary with site water availability. Tree Physiol. 2001, 21, 571–578. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Jackson, M.B. Plant adaptations to anaerobic stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Colangelo, M.; Gazol, A.; Camarero, J.J.; Borghetti, M.; Sanchez-Salguero, R.; Matias, L.; Castellaneta, M.; Nola, P.; Ripullone, F. Earlywood vessel characteristics are early indicators of drought-induced decline in ring-porous oak species within the Mediterranean Basin. Sci. Total Environ. 2025, 980, 179565. [Google Scholar] [CrossRef] [PubMed]

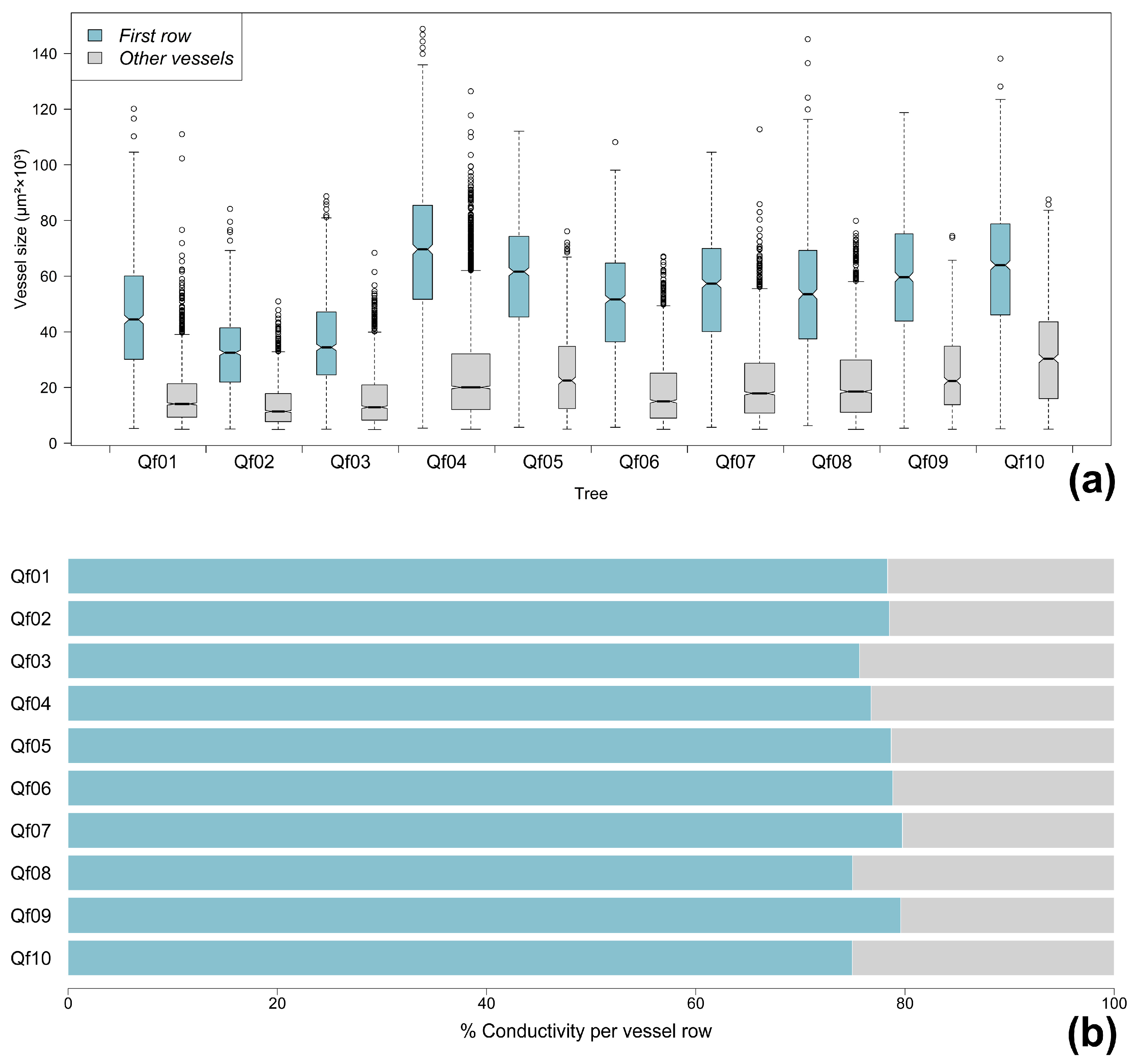
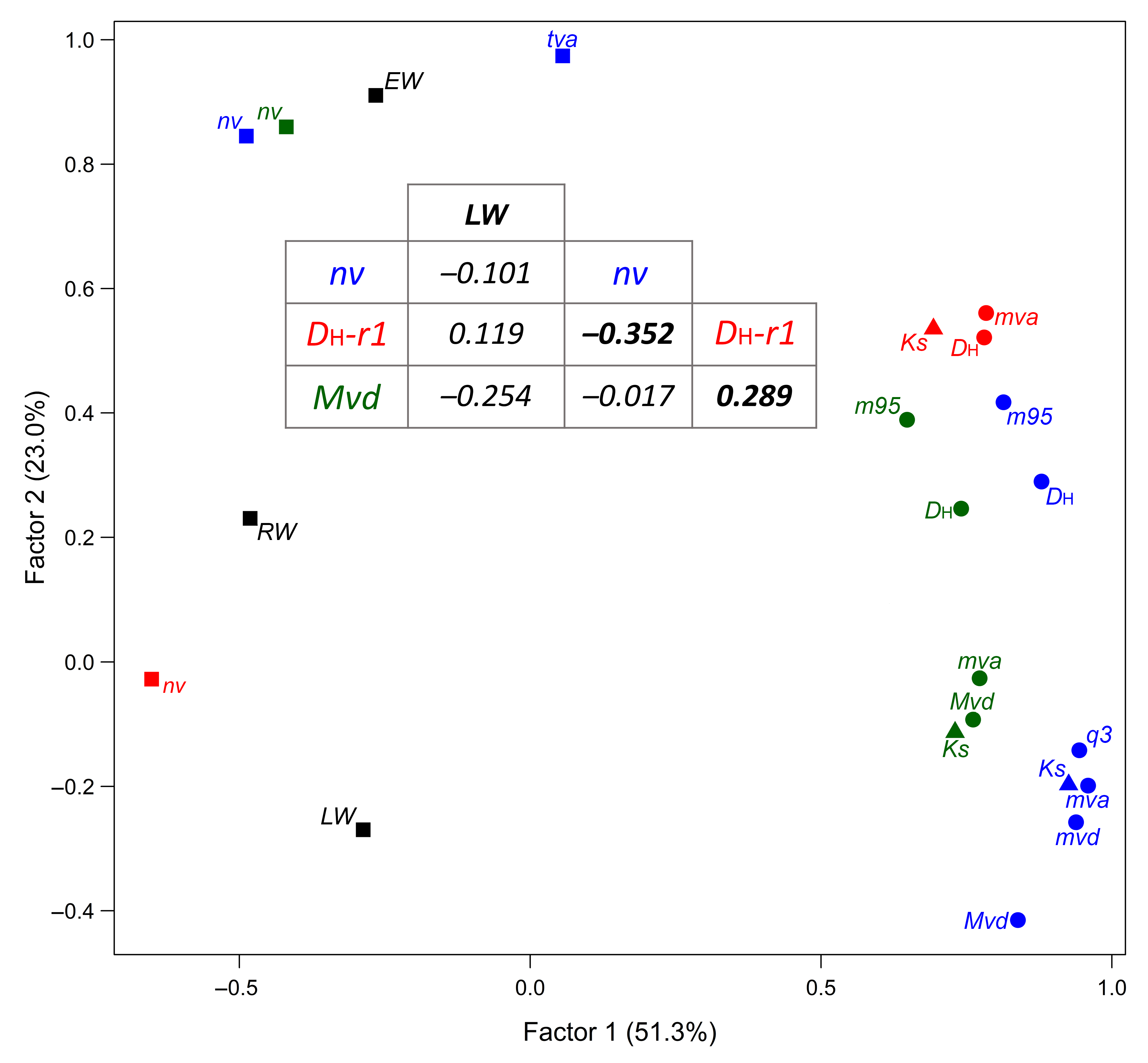
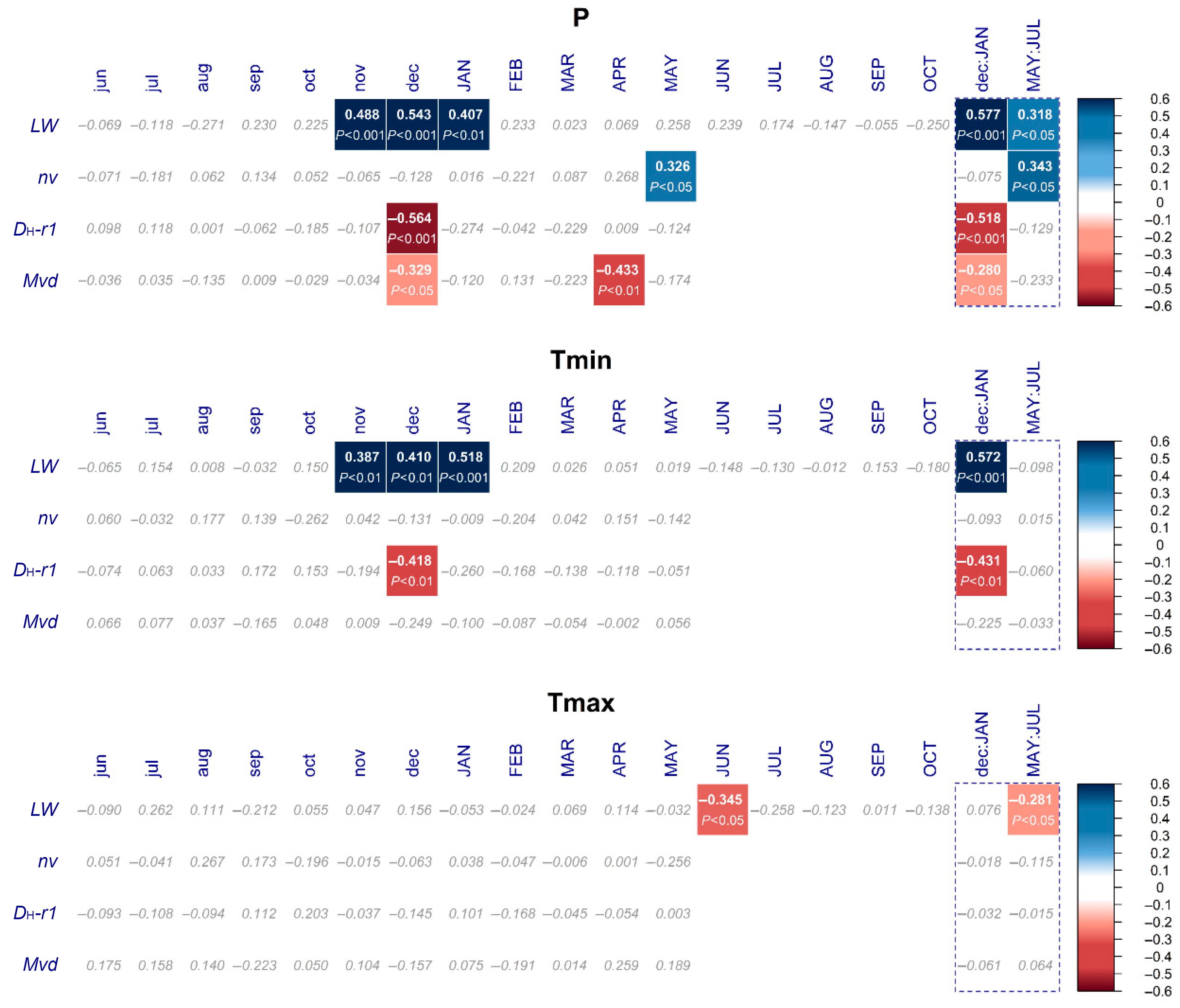
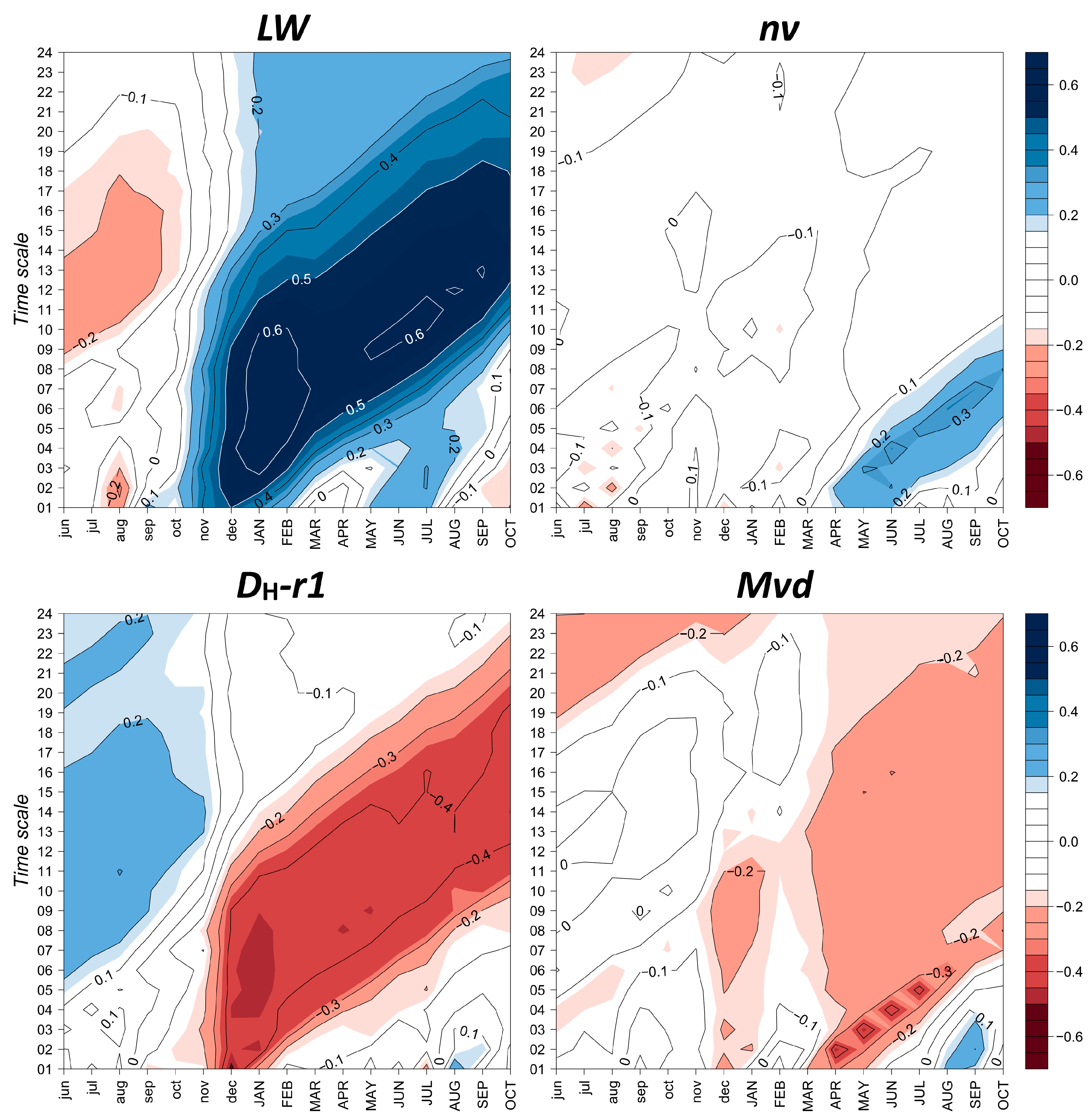
| (a) | Variable | Effect | Estimate | Std Error | t-Value | p-Value |
| Row | −243,313.44 | 73,199.660 | −3.324 | 9.19 × 10−4 | ||
| mva | Year | 121.30 | 26.070 | 4.653 | 3.69 × 10−6 | |
| Row × Year | 137.85 | 36.870 | 3.739 | 1.95 × 10−4 | ||
| Row | −303.89 | 222.779 | −1.364 | 0.17 n.s. | ||
| DH | Year | 0.57 | 0.079 | 7.205 | 1.12 × 10−12 | |
| Row × Year | 0.19 | 0.112 | 1.714 | 0.09 n.s. | ||
| Row | −1934.00 | 241.400 | −8.015 | 3.00 × 10−15 | ||
| nv | Year | −1.06 | 0.086 | −12.296 | <2.00 × 10−16 | |
| Row × Year | 0.96 | 0.122 | 7.919 | 6.23 × 10−15 | ||
| Row | −7875.14 | 1525.738 | −5.162 | 2.94 × 10−7 | ||
| Kt | Year | 2.37 | 0.543 | 4.366 | 1.39 × 10−5 | |
| Row × Year | 4.20 | 0.768 | 5.470 | 5.67 × 10−8 | ||
| (b) | Standard deviation | mva | DH | nv | Kt | |
| Tree | 7996.46 | 670.82 | 1095.45 | 989.95 | ||
| Residual | 8897.32 | 911.05 | 1225.00 | 1047.60 | ||
| LW | nv | DH-r1 | Mvd-nr1 | |
|---|---|---|---|---|
| Sens (raw) | 0.439 | 0.285 | 0.073 | 0.149 |
| Sens (std) | 0.417 | 0.291 | 0.072 | 0.147 |
| AR1 (raw) | 0.284 | 0.498 | 0.344 | 0.215 |
| AR1 (std) | −0.035 | 0.099 | −0.011 | −0.009 |
| Rbar | 0.306 | 0.198 | 0.357 | 0.120 |
| RvsM | 0.600 | 0.517 | 0.644 | 0.455 |
| EPS | 0.815 | 0.711 | 0.847 | 0.571 |
| SNR | 4.40 | 2.47 | 5.55 | 1.33 |
| %Var | 39.9% | 32.4% | 45.2% | 23.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, I.; Campelo, F.; Vieira, J.; Nabais, C. Higher Winter Precipitation and Temperature Are Associated with Smaller Earlywood Vessel Size but Wider Latewood Width in Quercus faginea Lam. Forests 2025, 16, 1252. https://doi.org/10.3390/f16081252
García-González I, Campelo F, Vieira J, Nabais C. Higher Winter Precipitation and Temperature Are Associated with Smaller Earlywood Vessel Size but Wider Latewood Width in Quercus faginea Lam. Forests. 2025; 16(8):1252. https://doi.org/10.3390/f16081252
Chicago/Turabian StyleGarcía-González, Ignacio, Filipe Campelo, Joana Vieira, and Cristina Nabais. 2025. "Higher Winter Precipitation and Temperature Are Associated with Smaller Earlywood Vessel Size but Wider Latewood Width in Quercus faginea Lam." Forests 16, no. 8: 1252. https://doi.org/10.3390/f16081252
APA StyleGarcía-González, I., Campelo, F., Vieira, J., & Nabais, C. (2025). Higher Winter Precipitation and Temperature Are Associated with Smaller Earlywood Vessel Size but Wider Latewood Width in Quercus faginea Lam. Forests, 16(8), 1252. https://doi.org/10.3390/f16081252







