Abstract
Despite increasing interest in including indigenous and local people in forest restoration initiatives, their views on which species are most useful, or reasons behind not planting native tree species are often ignored. Focused on south-western Rwanda, this study addressed these knowledge gaps. We carried out 12 focus group discussions with village elders to determine the following: main benefits provided by native forests, the native species they prefer for different uses, and the main barriers to species’ cultivation. Then, considering other key information from the literature, we performed a ranking exercise to determine which native species had the greatest potential for large-scale tree planting initiatives. Our results show that native forests provide 17 benefits to local communities, some of which cannot be replaced by plantations with exotic species. Among the 26 tree species identified as most useful for timber, firewood, medicine and fodder, ten were ranked as with the greatest potential for restoration initiatives. Of these, two had not been included in recent experimental plantations using native species in Rwanda, and none were considered among the priority species for domestication in Africa. Overall, our study highlights the need to better connect the ecological and social dimension of forest reforestation initiatives in multiple contexts.
1. Introduction
Land degradation threatens the lives of over 3 billion people worldwide, reducing their access to basic needs such as water, food, and energy [1]. Thirty African countries have committed to restore degraded or lost forests by participating in the African Forest Landscape Restoration Initiative, which aims to 100 million hectares of land by 2030 [2,3,4]. In the past decade, ecosystem restoration goals have moved from ecological recovery as the main target to holistic landscape restoration as the target, explicitly considering people’s livelihoods [5]. In this more holistic approach, it is essential that restoration planning considers indigenous and local knowledge (ILK, such as that from indigenous and local rural communities) to help achieve not only social validation, but also a greater number of benefits of the restored landscapes [6]. ILK can contribute to all aspects of ecological restoration, from the reconstruction of the reference ecosystem to evaluation of restoration outcomes, including monitoring and adaptive management [7]. In practice, though, ILK is often underrepresented in active restoration planning and monitoring, including in, e.g., species selection [8]. This is the case even if several studies have shown that restoration efforts which include ILK and promote species most useful to local communities are more likely to succeed (e.g., [9]).
Among the most useful species to local communities, there are the so called ‘native’ (or indigenous) multipurpose trees, i.e., trees which may provide food, medicine, firewood, livestock feed, timber and income, often also improving land conditions through nitrogen fixation and increased soil moisture [10]. These tree species also sequester carbon, opening opportunities for payments for ecosystem services [11], and carbon markets. Such tree species are considered key not just for forest restoration purposes, but also for improving Africa’s future food systems, providing environmental, social, and economic benefits, a concept referred to as ‘Land Maxing’ [12].
Rwanda, one of the most densely populated countries in Africa, has committed to restore 2 million hectares by 2030 [2]. So far, it has been promoting exotic fast-growing species, such as Eucalyptus and Grevillea, in its forest restoration efforts [13,14]. In spite of this, there is an increasing government interest in native tree species’ adoption in forest restoration efforts, and in the past five years mixed-species plantations of native species have been established in different sites, to study species’ ecological traits in relation to predicted climate change impacts in mountain regions [15,16]. However, local communities’ preferences for native species have not been investigated, challenging the social acceptability of future forest restoration efforts using native species, including those with ‘optimal’ thermal tolerances in face of raising temperatures (as determined in these ongoing experiments). In Yangambi in DRC, for example, the low adoption of agroforestry practices is related to the choice of species being promoted, as farmers prefer native multipurpose tree species with a known utility for their livelihoods [17].
In this study, focused on local communities’ views in south-western Rwanda, we investigated local communities’ uses and values of both native tree species and native forests. We addressed four research questions:
- Which are the main benefits provided by native forests to local communities?
- Which native species are preferred for timber, firewood, medicine, and fodder?
- What are the main barriers to native tree cultivation for local communities?
- Which of the native species preferred by local communities have the greatest potential for landscape restoration initiatives?
Given that nowadays local communities have no legal access to material benefits from native forests (a national park), we hypothesized that the main benefits identified would be micro-climate regulation and tourism. Farmer communities in neighboring countries place high value on micro-climate regulation, with comments such as ‘the forests attract rains for our crops’ [18,19]. We hypothesized that the preferred species would be those with more uses, and that they would be rather similar to those identified by local communities in Kibira, Burundi [19]—same ethnic groups and a similar forest—but with some differences related to local abundances of certain tree species. Regarding barriers, we expected a lack of space on farms and native species being perceived as slow-growing as the main barriers as other studies with smallholder farmers have documented, e.g., in Nigeria [20]. Although local communities’ uses and values of native tree species are dynamic over time and cannot be transposed to other locations where the socio-economic context is totally different, this research represents a first step for investigating if there is any major misalignment between ongoing national research efforts on native species and local communities’ preferences in one region of Rwanda so that such an issue can be considered and addressed, as trees take time to grow. We compared our findings with previous work in nearby countries, and discuss the implications of our findings for forest restoration in Rwanda and beyond.
2. Materials and Methods
2.1. Study Site
The present study was conducted in twelve villages located around Nyungwe National Park in south-western Rwanda (Figure 1). In this mountainous region, elevation ranges from 1300 m to 2900 m a.s.l. Rainfall and temperature vary with elevation, with the annual rainfall being about 1800 mm [21]. The rainfall regime is bimodal, with long rains in February–June and short rains in September–December. The park mostly contains montane forests with high biodiversity and endemism, including 1105 species of plants, 280 of birds, and 86 of mammals [22]. Nyungwe was declared a national forest reserve by German colonialists in 1933 [23]. In 2004, it became a national park, and hunting, firewood gathering, and artisanal gold mining were prohibited, and law enforcement increased [24]. However, some illegal activities continued [25]. In 2023, Nyungwe was listed as a World Heritage Site [26].
Figure 1.
Location of the 12 villages where focus group discussions were carried out around Nyungwe National Park in south-western Rwanda.
Local communities living in this mountainous region are farmers of Bantu origin and Twa hunter–gatherers of Pygmy origin. Our study focuses on farmers. These are smallholders mostly relying on rain-fed agriculture for their livelihoods, but some have pigs or cows. The average farm size is less than 1 hectare [27]. Population density around the park is high: 336 people per km2 [28]. Nowadays, there is no native forest left outside the park, and Eucalyptus spp. woodlots are the only ‘forested’ patches outside the park.
2.2. Survey of Local Communities’ Views
We organized focus group discussions (FGDs) in 12 villages. Each FGD involved 4–6 male elders who participated on a voluntary basis after being informed of the aim of the study. FGDs first focused on the importance of the native forest (Nyungwe) for their community, including listing all material and nonmaterial benefits (open question, no limit of benefits to be cited). As entry to Nyungwe is prohibited since 2004 (except for tourism or research), we highlighted that we referred all benefits they had access to in the past, before entry was restricted. To compare FGD answers to other studies, we grouped benefits mentioned into the different ecosystem service categories of the Millennium Ecosystem Assessment’s classification [29]. For instance, the answer ‘the forest brings us good rains’ became the ecosystem service micro-climate regulation.
After discussing native forest benefits, FGDs focused on identifying the following: (i) the native species they considered most important for firewood, construction, medicine, and livestock feed (three species for each category); and (ii) the barriers to cultivating these species (see Appendix A for the guiding questionnaire used). At the end of each FGDs, we asked 1–2 FGD participants to stay for a bit longer to clarify if the species’ names mentioned in Kinyarwanda matched the scientific names available from [30] by asking about fruit shape and size or other taxonomic characteristics. We initially considered collecting botanical specimens for plants which we could not identify using this approach, but as all plants mentioned are relatively ‘common’ plants in the region and have well-known uses, such plant collection was not needed at the end. Plant nomenclature follows the standard of Plants of the World Online [31].
Ethical Statement: The protocols for data collection in this study were reviewed and approved by the University of Rwanda Research Directorate. Prior informed consent for participation in FGDs was orally secured as numerous participants were illiterate.
2.3. Survey of Preferred Native Species’ Potential
For all species mentioned in the FGDs, we reviewed existing literature to determine the following: (i) if the species is useful to wildlife (as food or for habitat for, e.g., perching), (ii) if it was easy to cultivate from seed, (iii) if it was a pioneer-light demander (so it could easily grow in an open-canopy land), (iv) if it was known to be nitrogen-fixing, and (v) if it was among those identified as ‘best candidates’ under future climate according to [15]. While (i) can help identify important species for reforestation when habitat for wildlife is important (near parks, corridors, e.g., [32]), characteristics (ii–iv) can help toward native species’ inclusion in agroforestry (e.g., [33]) and (v) would improve the link between ecological (optimal’ thermal tolerances in face of raising temperatures) with social preferences in proposed large-scale reforestation plantations.
2.4. Data Analyses
For the survey of local communities’ views, we use FGD as the main unit of analysis, reporting answers as a percentage all FDGs carried out (n = 12). The importance of each native species mentioned during FGDs was compared using two quantitative indices: the relative frequency of citation (RFC) and the relative importance index (RI), which are robust quantitative methods used in ethno-botanical studies [34,35,36].
For the analysis of preferred native species’ potential in future reforestation programs, a categorical scaling exercise, like the point scoring procedure described in [37] and [33], was used. Characteristics considered important were assessed in a categorical scale, as follows: (a) useful to wildlife (0: none or not reported, 1: yes); (b) easy to cultivate from seed (0: none or not reported, 1: yes); (c) pioneer-light demander (0: no or not reported, 1: yes); (d) nitrogen-fixing (0: no or not reported, 1: yes); and (e) ‘best candidates’ under future climate for montane region (−1: negative decline in tree growth with increased temperature, 0: not investigated, 0.5: increase in tree growth with small increase in temperature but not for larger increase in temperature, 1: increase in tree growth with increased temperature). While (c) and (e) are reported in [15] and (d) in [38], (a) and (b) were gathered from several sources (see Table A1 in Appendix B). For the latter, we mostly consulted online repositories (e.g., PROTA4U, World Agroforestry Centre’s Agroforestry Database), and if information there was unavailable, we searched for scientific publications using species name as the keyword in, e.g., ScienceDirect.com. Our ranking exercise differs from previous ones (e.g., [33]) as it did not include information on species’ abundance in the wild or current management techniques, as all species considered are now only found inside the park, and farmers reported that they do not actively manage any of them in their fields now. Another difference is that it considers species’ climate change adaptation potential, which is very important in montane regions given that the effects of increased temperatures on tree species’ survival have already been reported [39].
The total score for a given species was computed as the RI index multiplied by 10 (to give more weight to local communities’ preferences) plus the sum of scores for each five above mentioned characteristics. RI, reflecting both frequency of citation and the number of use categories, ranges from 0 to 1. Without a multiplier in our ranking exercise, the fact that a species had been cited in 12 different villages or had four different uses (RI closer to 1) would be as important as a species, e.g., providing food to wildlife or not, which seemed unfair given that we wanted to reflect communities’ voices. We initially explored different multipliers (e.g., 2, 5, 10) but decided to use 10, as this multiplier gave twice as much value to local communities’ views as on other characteristics ‘we’ (but maybe not local communities) considered important (because we selected 5 others, so the maximum score for that part was 5).
This might seem a very ‘rough’ rating system, as (1) other characteristics could also have been included in the assessment, (2) the scale used only had 2–3 categories, and (3) the fact that some characteristics might be more important than others were not taken into account. However, the total score of each species is just considered an indication of its potential compared with the other species assessed. Regarding (1), data availability for other characteristics (e.g., ease of propagation from cuttings, pest resistance or market value) was unavailable for most species. Considering (2), we are aware that by using a 2–3 category scale, we are oversimplifying complex biological realities (e.g., easy to cultivate from seed depends on seed pretreatment, soil type, etc.). This simplified scale was also related to lack of data availability, and difficulty in creating a scale comparable across species when the information gathered came from multiple sources. Criterion (e) was gathered from one source [15] in which different species were planted under different temperatures (different elevations), and seedling growth was assessed over time. For this criterion, we were able to create an intermediate category 0.5, which refers to increased growth at middle elevations, but decreased growth at lower elevations (see [15]).
3. Results
3.1. Native Forest Benefits
Study participants identified 17 benefits from native forests, with timber, firewood, wild meat, medicinal resources, and micro-climate regulation being those most cited across the villages studied (Table 1). Interestingly, no cultural benefit (such as ceremonies, identity linkages) was cited in any village studied. Wild meat and minerals were the two forest benefits considered most important in 100% and 75% of the FGDs, respectively. Notably, these have no substitutes in Eucalyptus spp. woodlots.

Table 1.
Benefits from native forests mentioned by study participants with regard to the percent the focus group discussions carried out citing each (n = 12).
3.2. Native Forest Species and Barriers to Their Cultivation
Study participants cited 33 native species, including 26 trees, 1 liana, 1 fern, and 5 herbaceous plants (Figure 2). The seven species perceived as most useful were as follows: Ocotea usambarensis and Faurea saligna (for timber); Polyscias fulva and Macaranga kilimadscharica (for firewood); Carapa grandiflora and Maesa lanceolata (for medicine); and the liana Sericostachys scandens for fodder (Figure 2). The seven species perceived as most useful were also those highest ranked using the relative frequency of citation (RFC) and the relative importance index (RI), but using the RI, Syzygium guineense was also ranked among the highest (see Table 2). Several species were identified as the most useful for two or more uses (Figure 2).
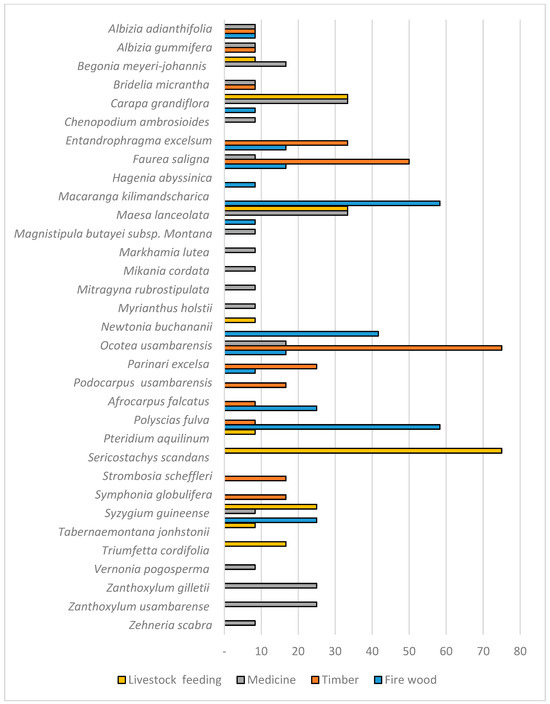
Figure 2.
Preferred native forest species for firewood, timber, medicine, and fodder regarding the percent the focus group discussions carried out citing each (n = 12).

Table 2.
Ranking of preferred native tree species using relative frequency of citation (RFC) and relative importance index (RI). Species are arranged in decreasing order of RI. * = herbaceous plant; + = liana and # = fern.
With regard to barriers to native tree cultivation, five barriers were mentioned, including the following: government rules on which crops to cultivate (91% of FGDs), the perception that native tree species were slow-growing (83%), lack of space on their farms (66%), lack of seed availability (66%), and lack of knowledge on how to cultivate these species (16%).
3.3. Preferred Native Species’ Potential in Restoration
Our ranking exercise indicated that five of the seven most preferred species (O. usambarensis, F. saligna, P. fulva, C. grandiflora, M. lanceolata) had the greatest potential, together with S. guineense, Albizia adianthifolia, A. gummifera, Bridelia micrantha, and Newtonia buchananii. Most of these ten species provided food for wildlife and were relatively easy to cultivate from seed (Table 3). Five were pioneer species which could be easily grown in the sun (P. fulva, M. lanceolata, B. micrantha, A. adianthifolia, A. gummifera), and the latter two were also N-fixers. Regarding their climate change adaptation potential, five species (A. gummifera, B. micrantha, P. fulva, M. lanceolata, N. buchananii) showed some potential to increase growth under warmer temperatures (in previous work, see Table 3), three species had shown negative growth (C. grandiflora, F. saligna, S. guineense), and two species had not been investigated, including the species ranked the highest in our exercise (O. usambarensis).

Table 3.
Native tree species’ potential for landscape restoration, identified using data from focus group discussions and a literature review. RI: relative importance index. For references used to create this table, see Appendix B. The sign: refer to the no mention in the literature cited.
4. Discussion
4.1. Native Forest Benefits
Study participants identified 17 benefits from native forests, including previsioning and regulating ecosystem services, but no cultural services. The latter was rather surprising, as local communities in Kibira NP in Burundi (native forest contiguous to Nyungwe) mentioned tourism and the aesthetic value of native forests, while local communities near Kahuzi NP in eastern DRC (about 30km from Nyungwe) mentioned tourism and ceremonies [18,19]. In a survey carried out at the same study site in 2012, local communities mentioned tourism as a forest benefit [40], but these authors sampled different villages to those we sampled. If inquired about tourism, participants mentioned that ‘Yes, there are tourists visiting the park, but we do not get any benefit from them, so it is not a native forest benefit’ (elder comment in FGD3). Other authors have highlighted the discontentment with current payment for tourism schemes around Nyungwe National Park [24].
The approach we used to gather information on cultural benefits from the native forest (FGDs) may not have provided all the nuances desired on this aspect. Indeed, other authors have highlighted that complementary methods such as walking interviews can help reveal more ‘immediate, intuitive, and grounded’ experiences in nature, deepening relational aspects and cultural values [41]. However, as access to the park is restricted (other than tourism, which has an expensive entry fee), this was complicated to implement in practice. It is also possible that we used a rather ‘restrictive’ approach to define cultural benefits from native forests, and that we should have considered the ‘cultural’ dimension of other forest benefits mentioned in the FGDs. In a recent survey in Volcanoes National Park (northern Rwanda), for example, traditional values and practices considered by researchers included the use of medicinal plants, hunting to maintain indigenous practices, and the use of bamboo for making traditional household items [42]. If we consider these three aspects, then local communities reported cultural values associated with the native forest of Nyungwe. Indeed, during FGD5, an elder mentioned the cultural link to medicinal plant use, commenting that ‘we are no longer in contact with our forest which helped us for various problems in the past, we are afraid for our kids if they will be able to know which plants were used in the treatment of diseases such as the madness caused by ghosts (abazimu)’.
Wild meat and minerals were the two native forest benefits considered most important in the villages studied. While minerals do not specifically relate to native forest species compared to exotic species plantations, wild meat does, as leaves, fruits, and seeds from several native forest species are consumed by animals (see Table 3).
It is important to consider the scale of restoration initiatives and the type of benefit they can provide when compared to ‘native forests’. While planting a few native trees in farmers’ fields can provide, e.g., some medicinal resources, it is unlikely that these few trees provide enough habitat to sustain wildlife populations to be hunted if there is no native forest nearby. Large-scale reforestation initiatives are better placed to provide wild meat, but such interventions in the already highly populated landscapes of Rwanda are challenging. Remarkably, though, an expansion of the buffer zone of Volcanoes National Park is already in progress (A.C.-S. Pers. Obs. 2024). The focus of this expansion is providing habitat for threatened mountain gorillas, not hunting.
It is also critical to acknowledge that some native forest benefits can be replaced by exotic species and that is why these exotic species have been planted in Rwanda. They can provide firewood and poles for construction or wood for making, e.g., furniture. Some species such as such as Eucalyptus spp. can also provide nectar for honey production, and others can be used as fodder. However, certain exotic species might reduce soil fertility or water availability in an area compared to native species. Therefore, it is important to consider the potential trade-offs between the services provided by different species, as some of these may apply at different spatial scales (e.g., water purification, soil formation).
4.2. Native Forest Species and Barriers to Their Cultivation
Study participants cited 26 native forest species as being among the most useful for timber, firewood, and medicine. Among these, 17 were also among the most useful to local communities in Kibira NP in Burundi, and five in Kahuzi NP in DRC [18,19]. The similarity in species’ preferences in with Burundi is likely to be related to a similar forest composition and cultural groups, while the forest composition and cultural groups in eastern DRC are more different. Preferences in useful plant species are known to be related not only to their abundance in a specific area and the availability of replacement by other species with same usage, but also cultural preferences, e.g., [33]. As we hypothesized, the most preferred species had several uses. That is why there were very small differences between their rankings using the relative frequency of citation (RFC) or the relative importance index (RI). Other authors have reported similar findings, highlighting that often, the more versatile a plant, the more widespread its usefulness, e.g., [34]. This is not always the case, as sometimes a species can be cited by many respondents but might only have one particular use. That is why using different indices to explore species’ importance is recommended [34]. Several species cited in this study are maintained in agroforestry systems elsewhere because of their usefulness to local communities. For example, Albizia adianthifolia, Bridelia micrantha, and Faurea saligna are commonly found in traditional agroforestry systems in South Africa [43], although the preferred use for A. adianthifolia and B. micrantha is fodder and firewood (respectively), uses which were not mentioned in our study. In Uganda, F. saligna, B. micrantha, and A. adianthifolia are used in agroforestry practices and various uses like fire wood, timber, bee forage, and charcoal [44].
In terms of species most useful for fodder, the liana Sericostachys scandens was the species most cited. This species, native to Africa, can dominate forest gaps and hamper tree seedling establishment [45,46]. Although its use as fodder was not reported before, it seems that local communities in our study area are making the most of this plant resource which has become increasingly abundant in the past decades [47].
With regard to barriers to native tree cultivation, study participants highlighted the following: i) native tree species are slow-growing, ii) there is a lack of space on their farms, and iii) the government rules on farm use. The first two barriers have been reported in other studies, e.g., in Sierra Leone [33], in Rwanda and Uganda [48,49]. The latter barrier (government rules on farm use) is likely to be specific for Rwanda, as strict government rules on farming practices currently determine which crops are to be cultivated in each agroecological zone, and prohibit, e.g., intercropping [50]. Although such rules do not prohibit native tree cultivation in farmers’ fields, they do not encourage them either. The perception that ‘native tree species being slow-growing’ is not necessarily true for all native species, as some pioneer ones are known to grow up to 4 or 5 m in just two years [15].
4.3. Preferred Native Species’ Potential in Restoration
Our ranking exercise indicated that ten species had the greatest potential for landscape restoration initiatives in the region studied in Rwanda. These included five pioneer (A. adianthifolia, A. gummifera, B. micrantha, P. fulva, M. lanceolata) and five late-successional (C. grandiflora, F. saligna, N. buchananii, O. usambarensis, S. guineense) species. For the latter group, their light requirements should be considered, as some shading might be needed to ensure their saplings’ survival. Mixed-species plantations (where pioneers are likely to grow faster and help shade the non-pioneer) or the enrichment planting of such species in existing exotic species’ plantations could be an option, but future research is needed to help guide best planting—and plantation management—practices. Regarding native species’ inclusion into farmers’ fields (e.g., agroforestry), the barriers local communities had identified should be addressed. One option for addressing, e.g., ‘native tree species being slow growing’ could be to organize field visits to the existing experimental trial RwandaTree Project [16]. For the other two main barriers (lack of space on their farms and government rules on farm use related to main crops grown), a change in government’ rules—or incentives—would be needed, requiring more coordination between different government initiatives.
Interestingly, none of the ten ‘best candidates’ species we identified are among the species selected as ‘priority’ trees for domestication in Africa [51]. In fact, only Prunus africana (cited by study respondents, but not among top-ranked) is among these priority species [51]. This is likely to be related to the regional divide in domestication research pm the continent (either the humid lowlands of West and Central Africa, or the highlands and drylands of East Africa (see [51])), and lack of attention to the ‘humid’ highlands and montane forests of the Albertine Rift, which comprise Uganda, Rwanda, Burundi, DRC, and Tanzania. Other authors have highlighted the need to consider more biomes in domestication research across the African continent, e.g., [33]. This finding is of key importance as it hinders effective, locally adapted restoration and domestication efforts in the Albertine Rift region, missing key opportunities not just for local communities’ livelihoods, but also biodiversity conservation and climate change mitigation. International and national actors in the Albertine Rift countries should carefully consider such implications, reflecting on how local voices have been excluded so far. Our study provides a tangible roadmap for integrating local knowledge to overcome this disconnect.
The ranking exercise we used, which combines farmers’ preferences and a review of species’ characteristics, including the potential to adapt to warmer temperatures, is relatively easy to carry out and could be used in other parts of the world. Apart from helping guide future landscape restoration efforts (e.g., forest restoration or agroforestry programs), such a ranking exercise can also contribute to identify important research gaps. For example, it helped highlight that O. usambarensis potential for climate change adaptation has not been investigated yet in ongoing field trials in the country, which is unfortunate as this was the species rankest the highest among study participants.
5. Study Limitations
Our study has some limitations which could be addressed through future work. Due to financial and time limitations to organize separate FGDs, we only investigated the view of Bantu male elders, but not those of females or younger generations, or BaTwa, and therefore, the views reported here are not representative of the local communities studied. For example, females often possess unique knowledge regarding medicinal plants used to treat common ailments among children, which could alter species prioritization. Another limitation is that we specifically asked about the three most preferred species for each selected use (timber, firewood, medicine, and fodder) to reduce the list of options, but future work should consider free listing of all species useful for such purposes. We focused on native forests and species, but it would have been interesting to explicitly ask participants about the benefits provided by exotic species’ woodlots. We focused on villages around Nyungwe, and although the same cultural group studied is found across Rwanda, future work is needed for prioritizing native tree species for reforestation in other agroclimatic zones, as, e.g., ‘optimal’ thermal tolerances in face of raising temperatures differ at different elevations.
6. Conclusions
Our study aimed to contribute to holistic landscape restoration targets in Rwanda, explicitly considering local communities’ views and preferences. Our results show that native forests and native forest species are very important to local communities, and that some of the benefits they provide cannot be replaced in exotic plantations. Therefore, future plantations using native species have strong potential for acceptance in the region, provided that the practical and policy-level barriers identified by local communities, such as restrictive government regulations on land use and the perception of slow growth rates, are actively mitigated through targeted interventions and supportive frameworks. Local communities identified 33 native forest species as most useful to them. Of these, five pioneer and five non-pioneer tree species were the highest ranked for future landscape restoration initiative. Interestingly, two of such species have not been included in recent experimental plantings using native species in Rwanda, and none were considered among the priority species for domestication in Africa. Overall, our study supports previous evidence of the importance of integrating local communities into restoration efforts, and provides an easy-to-use tool for native tree species ranking to help guide interventions across the African continent and beyond.
Author Contributions
Conceptualization, A.C.-S.; Data gathering, F.B. and P.M.; Data analysis A.C.-S. and F.B., with support from all co-authors; writing—original draft preparation, A.C.-S., G.I., M.M., B.A.K., and F.B.; funding acquisition, A.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
Fieldwork was partially funded by L’Oréal Women in Science UK Award for Sustainable Development given to A.C.-S.
Data Availability Statement
Data for this study can be made available upon reasonable request.
Acknowledgments
We thank all study participants, especially the village chiefs who helped organize the focus group discussions. We thank the authorities of Nyamasheke and Rusizi districts for authorization and facilities. We also thank three anonymous reviewers for their constructive feedback.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Guiding Questionnaire for the Focus Group Discussions
- Part 1. The Native Forest.
- Was the native forest important for your community when you were young and you were allowed to go in? Yes/No
- Why was it important? (open question, just tick those mentioned or add in ‘other’)
Service Answer Micro-climate regulation (e.g., the forest attracts the rain) Air purification (e.g., the forest has fresh air) Erosion control Water purification Soil formation (e.g., the soil under forest is good for agriculture) Provisioning water Poles Bamboo Timber for furniture Firewood Medicine resources Baskets, ropes, tools Candles Meat from wild animals Caterpillars Mushrooms Wild fruits Honey Termites Edible leaves Crabs Small fish Fodder Minerals Shelter during conflict Tourism Identity Shelter (home) Ceremonies - Which of all these benefits that have been mentioned were the two most important for your community and why?
- ......................................... Reason/.........................................
- ......................................... Reason/.........................................
- Did you feel ‘attached’ to this forest? For example, is your identity linked with this forest? (Give other three examples).
- Example 1.....................................................................................................................................
- Example 2.....................................................................................................................................
- Example 3.....................................................................................................................................
- Part 2. Preferred Plant Species
- 5.
- Which three plant species from the forest were the most important for your community for firewood?...........................................................................................................
- 6.
- Which tree plant species (three) from the forest were the most important for your community for poles?.......................................................................................................
- 7.
- Which tree plant species (three) from the forest are the most important for medicine?........................................................................................................................................
- 8.
- Which tree species from the forest are the most important for livestock feeding?
- a)
- .....................................................................................................................................
- b)
- .....................................................................................................................................
- c)
- .....................................................................................................................................
- 9.
- Which are top tree species (three) could be important for your community to be promoted in agroforestry of those elders used before?
- a)
- .....................................................................................................................................
- b)
- .....................................................................................................................................
- c)
- .....................................................................................................................................
- 10.
- Why are people in your community not cultivating these native useful trees?
- 1.
- Lack of seeds;
- 2.
- Lack of knowledge on how to cultivate;
- 3.
- Lack of space;
- 4.
- They grow too slowly;
- 5.
- Government rules
- 6.
- Other: state
- 11.
- Is there anything else you would like to add with regard to the importance of the native forest and the plant species found inside?
Appendix B

Table A1.
References used to fill in Table 3. The sign: refer to the no mention in the literature cited.
Table A1.
References used to fill in Table 3. The sign: refer to the no mention in the literature cited.
| Food for Wildlife | Easy to Cultivate from Seed | |
|---|---|---|
| Ocotea usambarensis Engl. | [52] | [52] |
| Maesa lanceolata Forssk. | - | [44] |
| Albizia adianthifolia (Schumach)W.Wight | [53] | [53] |
| Faurea saligna Harv. | - | [54] |
| Carapa grandiflora Sprague | [55] | [55] |
| Albizia gummifera (J.F.Gmel.) C.A.Sm. | [56] | [56] |
| Polyscias fulva (Hiern) Harms | - | [57] |
| Syzygium guineense (Wild.) DC. | [58] | - |
| Newtonia buchananii (Baker) G.C.C. Gilbert & Boutique | [59] | [59] |
| Bridelia micrantha (Hochst.) Baill. | [60] | [60] |
| Afrocarpus falcatus (Thunb.) C.N. Page | [61] | [61] |
| Parinari excelsa Sabine | [62] | - |
| Entandrophragma excelsum (Dawe & Sprague) Sprague | [63] | [63] |
| +Sericostachys scandens Gilg & Lopr. | [47] | - |
| Markhamia lutea (Benth.) K.Schum. | - | [64] |
| Macaranga kilimandscharica Pax | [65] | [65] |
| Zanthoxylum gilletii (De wild.) P.G.Waterman | [66] | [66] |
| Zanthoxylum usambarense (Engl.) Kokwaro | - | [67] |
| *Begonia meyeri-johannis Engl. | [68] | - |
| Hagenia abyssinica (Bruce) J.F.Gmel. | [69] | [69] |
| Podocarpus usambarensis Pilg. | [44] | [44] |
| *Chenopodium ambrosioides L. | - | [70] |
| Mitragyna rubrostipulata (K. Schum.) Havil. | [44] | [44] |
| Myrianthus holstii Engl. | [71] | [71] |
| #Pteridium aquilinum (L.) Kuhn | [72] | - |
| Strombosia scheffleri Engl. | - | [73] |
| Symphonia globulifera L.f. | [74] | [74] |
| *Triumfetta cordifolia A. Rich | [75] | [75] |
| Tabernaemontana jonhstonii (Stapf) Pichon | - | [76] |
References
- Melo, A.; Albuquerque, J.; Siqueira, R.; Silva, E.; Medeiros, R.; Souza, K.; Souza, L.; Gonçalves, A.; Soares, M. Occurrence of noxious weeds under different soil management systems. Appl. Ecol. Environ. Res. 2020, 19, 2061–2072. [Google Scholar] [CrossRef]
- Afr100: Project/Restoring-Relationships-People-and-Planet-Democratic-Republic-Congowww. Afr100. Available online: https://afr100.org/ (accessed on 31 March 2025).
- Vinceti, B.; Valette, B.; Bougma; Turillazzi, A. How is forest landscape restoration being implemented in Burkina Faso? Overview of ongoing initiatives. Sustainability 2020, 12, 10430. [Google Scholar] [CrossRef]
- Parr, C.L.; Beest, T.; Steevens, N. Conflation of reforestation with restoration is widespread. Science 2024, 383, 698–701. [Google Scholar] [CrossRef]
- Chazdon, R.; Brancalion, P. Restoring forests as a means to many ends: An urgent need to replenish tree canopy cover calls for holistic approaches. Science 2019, 365, 24–25. [Google Scholar] [CrossRef]
- McElwee, P.; Fernández-Llamazares, A.; Aumeeruddy-Thomas, Y.; Babai, D.; Bates, P.; Galvin, K.; Liu, G.M.J.; Molnár, Z.; Ngo, T.; Reyes-García, V.; et al. Working with Indigenous and local knowledge (ILK) in large-scale ecological assessments: Reviewing the experience of the IPBES Global Assessment. J. Appl. Ecol. 2020, 57, 1666–1676. [Google Scholar] [CrossRef]
- Uprety, Y.; Asselin, H.; Bergeron, Y.; Doyon, F.; Boucher, J.F. Contribution of traditional knowledge to ecological restoration: Practices and applications. Ecoscience 2012, 19, 225–237. [Google Scholar] [CrossRef]
- Reyes-García, V.; Fernández-Llamazares, A.; McElwee, P.; Öllerer, M.Z.K.; Wilson, J.; Brondizio, E. The contributions of indigenous peoples and local communities to ecological restoration. Restor. Ecol. 2019, 27, 3–8. [Google Scholar] [CrossRef]
- Sena, P.; Gonçalves-Souza, P.; Gonçalves, P.; Ferreira, P.; Melo, F. Biocultural restoration improves delivery of ecosystem services in social-ecological landscapes. Restor. Ecol. 2021, 30, e13599. [Google Scholar] [CrossRef]
- Leakey, R.R.; Akinnifesi, F.K. Towards a domestication strategy for indigenous fruit trees in the tropics. In Indigenous Fruit Trees in the Tropics: Domestication, Utilization and Commercialization; ICFRAF, Ed.; CABI: Nairobi, Kenya, 2008; p. 720. [Google Scholar]
- Atiojio, A.; Tchamba, M.; Njimbe, H. Carbon sequestration potential of community forests and its implications for REDD+ initiatives in Cameroon. Afr. J. Environ. Sci. Technol. 2014, 8, 1–8. [Google Scholar]
- Leakey, R.R.B.; Tienteu Avana, M.-L.; Awazi, N.P.; Assogbadjo, A.E.; Mabhaudhi, T.; Hendre, P.S.; Degrande, A.; Hlahla, S.; Manda, L. The Future of Food: Domestication and commercialization of indigenous food crops in Africa over the third decade (2012–2021). Sustainability 2022, 14, 2355. [Google Scholar] [CrossRef]
- Ruticumugambi, J.A.; Kaplin, B.A.; Blondeel, H.; Mukuralinda, A.; Ndoli, A.; Verdoodt, A.; Rutebuka, J.; Imanirareba, E.; Uwizeyimana, V.; Gatesi, J.; et al. Diversity and composition of agroforestry species in two agro-ecological zones of Rwanda. Agrofor. Syst. 2024, 98, 1421–1443. [Google Scholar] [CrossRef]
- Mukeshimana, F.; Rizinjirabake, F. Perception and knowledge of local community on the use of indigenous tree species for ecosystem restoration in Gasabo district, Rwanda. Rwanda J. Eng. Sci. Technol. Environ. 2024, 6, 1–19. [Google Scholar] [CrossRef]
- Ntirugulirwa, B.; Zibera, E.; Nkuba, E.; Manishimwe, A.; Nsabimana, D.; Uddling, J.; Wallin, G. Contrasting growth and mortality responses of different species lead to shifts in tropical montane tree community composition in a warmer climate. Biogeosciences 2023, 20, 5125–5149. [Google Scholar] [CrossRef]
- RwandaTree. Available online: http://www.rwandatree.com (accessed on 15 July 2024).
- Katayi, A.L.; Kafuti, C.; Kipute, D.D.; Mapenzi, N.; Nsimba, S.M.H.; Mampeta, W.S. Factors inciting agroforestry adoption based on trees outside forest in Biosphere of Yangambi landscape (Democratic Republic of Congo). Agroforest. Syst. 2023, 97, 1157–1168. [Google Scholar] [CrossRef]
- Cuni-Sanchez, A.; Imani, G.; Bulonvu, F.; Batumike, R.; Baruka, G.; Burgess, N.K.J.; Marchant, R. Social perceptions of forest ecosystem services in the Democratic Republic of Congo. Hum. Ecol. 2019, 47, 839–853. [Google Scholar] [CrossRef]
- Ndayizeye, G.; Imani, G.; Nkengerutse, J.; Iramapagarikiye, R.; Ndihokubwayo, N.; Niyongabo, F.; Cuni-Sanchez, A. Ecosystem services from mountain forests: Local communities’ views in Kibira National Park, Burundi. Ecosyst. Serv. 2020, 45, 10117. [Google Scholar] [CrossRef]
- Oakhena, I.F.; Awe, F.; Adeteju, O.L.; Omolora, O.T.; Olubunmi, O.D. Assessing the impact of adoption of agroforestry technology on food production and poverty reduction among farming households in Oyo State, Nigeria. Acta Fytotechn. Zootechn. 2021, 24, 25–34. [Google Scholar]
- Sun, C.; Kaplin, B.A.; Kristensen, K.; Munyaligoga, V.; Mvukiyumwami, J.; KaKajondo, K.; Moermond, T. Tree phenology in a tropical montane forest in Rwanda. Biotropica 1997, 28, 668–681. [Google Scholar] [CrossRef]
- Plumptre, A.; Davenport, T.; Behangana, M.; Kityo, R.; Eilu, G.; Ssegawa, P.; Ewango; Pilgrim, J.D.; Wilson, M.; Languy, M. The biodiversity of the Albertine Rift. Biol. Conserv. 2007, 137, 178–194. [Google Scholar] [CrossRef]
- Masozera, M.K. The Conservation of Mountain Gorillas in Bwindi Impenetrable National Park, Uganda: An Analysis of Institutional Challenges and Opportunities for Sustainable Livelihoods. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2002. [Google Scholar]
- Gross-Camp, N.; Martin, A.; Mcguire, S.; Kebede, M.; Munyarukaza, J. Payments for ecosystem services in an African protected area: Exploring issues of legitimacy, fairness, equity and effectiveness. Oryx 2012, 46, 24–33. [Google Scholar] [CrossRef]
- Roca, R.; Zuňiga, C. Floristic inventory of tropical forest in Rwanda 20 years after artisanal gold-mining. Trop. Resour. 2016, 35, 8–17. [Google Scholar]
- Nyungwe National Park. Available online: https://www.whc.unesco.org/en/list/1697/documents/ (accessed on 13 June 2024).
- Nkurunziza, A.; Mutaganzwa, D.; Ndayitwayeko, W.; Nkengurutse, J.; Kaplin, B.A.; Zafra-Calvo, N.; Cuni-Sanchez, A. Local observations of climate change and adaptation responses: A case study in the mountain region of Burundi-Rwanda. Land 2023, 12, 329. [Google Scholar] [CrossRef]
- RoR. Republic of Rwanda: Rwanda Vision 2020; Ministry of Finance and Economic Planning: Kigali, Rwanda, 2014. [Google Scholar]
- MEA. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Troupin, G.; Bridson, D.M. Flore des Plantes Ligneuses du Rwanda; Musée Royal de l’Afrique centrale: Tervuren, Belgium, 1982; Volume 8. [Google Scholar]
- Available online: http://www.plantsoftheworldonline.org/ (accessed on 4 May 2025).
- Mariscal, A.; Tigabu, M.; Savadogo, P.; Odén, P. Regeneration status and role of traditional ecological knowledge for cloud forest ecosystem restoration in Ecuador. Forests 2022, 13, 92. [Google Scholar] [CrossRef]
- Jusu, A.; Cuni-Sanchez, A. Priority indigenous fruit trees in the African rainforest zone: Insights from Sierra Leone. Genet. Resour. Crop Evol. 2017, 64, 745–760. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo De Santayana, M. Cultural importance indices: A comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Pardo De Santayana, M. Las Plantas en la Cultura Tradicional de la Antigua Merindad de Campoo. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2003. [Google Scholar]
- Reyes-García, V.; Martin, N.; McDade, T.; Tanner, S.; Vadez, V. Concepts and methods in studies measuring individual ethnobotanical knowledge. J. Ethnobiol. 2007, 27, 182–203. [Google Scholar] [CrossRef]
- Brehm, J.; Maxted, N.; Martins-Louçaõ, M.; Ford-Lloyd, V. New approaches for establishing conservation priorities for socio-economically important plant species. Biodivers. Conserv. 2010, 19, 2715–2740. [Google Scholar] [CrossRef]
- Sprent, J.I. Legume Nitrogen Fixation: A Global Perspective; John Wiley& Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cuni-Sanchez, A.; Martin, E.H.; Uzabaho, E.; Ngute, A.S.K.; Bitariho, R.; Kayijamahe, C.; Marshall, A.R.; Mohamed, N.A.; Mseja, G.A.; Nkwasibwe, A.; et al. Evidence of thermophilization in Afromontane forests. Nat. Commun. 2024, 15, 5554. [Google Scholar] [CrossRef]
- Dawson, N.; Martin, A. Assessing the contribution of ecosystem services to human wellbeing: A disaggregated study in western Rwanda. Ecol. Econ. 2015, 117, 62–72. [Google Scholar] [CrossRef]
- Teff-Seker, Y.; Orenstein, D.E. The ‘desert experience’: Evaluating the cultural ecosystem services of drylands through walking and focusing. People Nat. 2019, 1, 234–248. [Google Scholar] [CrossRef]
- Sabuhoro, E.; Lyakurwa, G. Perceived life satisfaction and illegal forest use in the Virunga landscape of Rwanda and Uganda. Forests 2024, 15, 53. [Google Scholar] [CrossRef]
- Makhubele, L.; Chirwa, P.; Sheppard, J.; Tshidzumba, R.; Araia, M.; Kahle, H.-P. Conservation of tree species richness in a traditional agroforestry landscape in the Vhembe biosphere reserve, South Africa. Forests 2022, 13, 1766. [Google Scholar] [CrossRef]
- Katende, A.B.; Birnie, A.; Tengnas, B. Useful Trees and Shrubs for Uganda. Identification and Management for Agricultural and Pastoral Communities; Regional Land Management Unit, RELMA: Nairobi, Kenya, 1995. [Google Scholar]
- Habonayo, R.; Azihou, A.; Dassou, G.; Habyarimana, F.; Amadou, A.; Habimana, B. Influence of the invasive liana Sericostachys scandens Gilg & Lopr. (Amaranthaceae) on the floristic diversity of the woody community in the Kibira National Park in Burundi. Int. J. Innov. Sci. Res. 2019, 44, 159–170. [Google Scholar]
- Ndabaga, M.; Basile, H.; Nicolas, B.; Habiyaremye, M.F.; Lejoly, J.; Pierre, M. Life strategy traits of the liana Sericostachys scandens spreading in the montane forests in the Kahuzi-Biega National Park (DR Congo). J. Mt. Sci. 2012, 9, 665–675. [Google Scholar] [CrossRef]
- Miller, A.; Debra, J.; Uwingeneye, G.; Ndayishimiye, D.; Cyril, C.; Grueter, C. Diet and use of fallback foods by Rwenzori black-and-white colobus (Colobus angolensis ruwenzorii) in Rwanda: Implications for super group formation. Int. J. Primatol. 2020. [Google Scholar] [CrossRef]
- Umuhoza, E.; Mugunga, C.; William, A.; Hagumubuzima, F.; Bizimana, E. Farmer’s perception and adoption of agroforestry technologies in eastern Rwanda. J. Res. For. Wildl. Environ. 2023, 15, 144–157. [Google Scholar]
- Tumuhe, L.; Nyamaizi, S. Analysis of tree species, preferences by farmers in Albertine rift, Uganda. Environ. Earth Ecol. 2020, 4, 7–14. [Google Scholar] [CrossRef]
- Clay, N.; King, B. Smallholders’ uneven capacities to adapt to climate change amid Africa’s ‘green revolution’: Of Case study of Rwanda’s crop intensification program. World Dev. 2019, 116, 1–14. [Google Scholar] [CrossRef]
- Leakey, R. From ethnobotany to mainstream agriculture: Socially modified Cinderella species capturing ‘trade-ons’ for ‘land maxing. Planta 2022, 250, 949–970. [Google Scholar] [CrossRef]
- Okeyo, J.M. Ocotea usambarensis Engl. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 31 May 2025).
- Lemmens, R.H.M.J. “Albizia adianthifolia (Schumach.)” W. Wight. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2007; Available online: http://www.prota4u.org/search.asp (accessed on 16 May 2025).
- Lemmens, R.H.M.J. Faurea saligna Harv. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2011; Available online: http://www.prota4u.org/search.asp (accessed on 16 May 2025).
- Nyiramana, A.; Mendoza, I.; Beth, A.K.; Forget, P.-M. Evidence for seed dispersal by rodents in tropical montane forest in Africa. Biotropica 2011, 43, 654–657. [Google Scholar] [CrossRef]
- Maroyi, A. Albizia gummifera. (J. F. Gmel.) C. A. Sm. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2007; Available online: http://www.prota4u.org/search.asp (accessed on 16 May 2025).
- Lemmens, R.H.M.J. “Polyscias fulva (Hiern)” Harms. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2009. [Google Scholar]
- Maroyi, A. “Syzygium guineense(Willd.)” DC. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
- Mairura, F.S. “Newtonia buchananii (Baker f.)” G.C.C. Gilbert & Boutique. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 17 May 2025).
- Bosch, C.H. Bridelia micrantha (Hochst.) Baill. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2012; Available online: http://www.prota4u.org/search.asp (accessed on 16 May 2025).
- Aerts, R. Afrocarpus falcatus (Thunb.) C.N.Page. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 5 June 2025).
- Oyen, L.P.A. Parinari excelsa Sabine. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2012; Available online: http://www.prota4u.org/search.asp (accessed on 17 May 2025).
- Lemmens, R.H.M.J. Entandrophragma excelsum (Dawe & Sprague) Sprague. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 7 June 2025).
- Maroyi, A. Markhamia lutea (Benth.) K.Schum. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2012; Available online: http://www.prota4u.org/search.asp (accessed on 17 May 2025).
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestry Database: A Tree Reference and Selection Guide Version 4.0. World Agroforestry Centre, Kenya. 2009. Available online: https://www.worldagroforestry.org/output/agroforestree-database (accessed on 16 May 2025).
- Okeyo, M.M. “Zanthoxylum gilletii (De Wild.)” P.G.Waterman. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
- Matu, E.N. “Zanthoxylum gilletii (De Wild.)” P.G.Waterman. In Record from PROTA4U; Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2011; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
- Ardhaka, I.M.; Ardi, W.H.; Undaharta, N.K.E.; Tirta, I.G. A new species Begonia from Manusela National Park, Seram. Reinwardtia 2016, 15, 61–64. [Google Scholar] [CrossRef]
- Legesse, N. A Selection of Ethiopia’s Indigenous Trees: Biology, Uses and Propagation Techniques; Addis Ababa University Press: Addis Ababa, Ethiopia, 2010; ISBN 978-99944-52-27-9. [Google Scholar]
- Vàzquez-Yanes, C.; Orozco-Segovia, A. Ecological significance of light controlled seed germination in two contrasting tropical habitats. Oecologia 1990, 83, 171–175. [Google Scholar] [CrossRef]
- Nishimwe, G.A.; Dahlin, S.; Niyitanga, F.; Asudi, G.O.; Augustino, S. Pre-sowing treatments for a better germination of Myrianthus holstii seeds. For. Trees Livelihoods 2024. [Google Scholar] [CrossRef]
- van der Burg, W.J. Pteridium aquilinum (L.) Kuhn. In Record from PROTA4U; Grubben, G.J.H., Denton, O.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2004; Available online: http://www.prota4u.org/search.asp (accessed on 17 May 2025).
- Obeng, E.A. Strombosia scheffleri Engl. In Record from PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2012; Available online: http://www.prota4u.org/search.asp (accessed on 17 May 2025).
- Oyen, L.P.A. Symphonia globulifera L.f. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2005; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
- Jiofack Tafokou, R.B. Triumfetta cordifolia A.Rich. In Record from PROTA4U; Brink, M., Achigan-Dako, E.G., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2010; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
- Lemmens, R.H.M.J. “Tabernaemontana stapfiana” Britten. In Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2006; Available online: http://www.prota4u.org/search.asp (accessed on 18 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).