Abstract
UDP-glycosyltransferases (UGTs) play essential roles in various biological processes, such as phytohormone homeostasis, abiotic stress adaptation, and secondary metabolite biosynthesis. Populus euphratica is a model species for investigating stress adaptation; however, the PeUGT gene family has yet to be systematically characterized. Here, we identified 134 UGT genes in P. euphratica. Phylogenetic analysis classified these genes into 16 major groups (A–P), and UGT genes within the same groups showed similar structural characteristics. Tandem duplication events were identified as the predominant mechanism driving the expansion of the PeUGT family. Cis-acting element analysis revealed an enrichment of motifs associated with developmental regulation, light response, phytohormone signaling, and abiotic stress in the promoters of PeUGT genes. Expression profiling demonstrated spatiotemporal regulation of the PeUGT genes under drought stress. Among them, PeUGT110 was significantly induced by PEG treatment in the leaf, root, and stem tissues of P. euphratica. Overexpression of PeUGT110 enhanced drought tolerance in transgenic Arabidopsis. Furthermore, the PeUGT110-OE lines exhibited reduced malonaldehyde accumulation, elevated proline content, higher superoxide dismutase activity, and upregulated expression of stress-related genes under drought stress. The results demonstrated that PeUGT110 plays a critical role in plant drought resistance. These findings establish a foundation for elucidating the function of PeUGT genes.
1. Introduction
With the intensification of climate change, the frequency, intensity, and geographical range of droughts are continuously expanding in most regions of the world, making drought a critical abiotic stress that restricts plant growth, species distribution, and crop productivity [1,2]. Drought stress disrupts plant physiological homeostasis by directly limiting water absorption and transport or indirectly inducing secondary stresses such as high temperature and oxidative damage. Cellular dehydration caused by drought leads to membrane system disruption, enzyme activity inhibition, and stomatal closure, which collectively reduce photosynthetic efficiency [3]. To survive, plants have evolved multi-layered adaptive strategies. At the physiological level, they accumulate osmolytes to maintain cellular osmotic balance and enhance antioxidant systems to scavenge excess reactive oxygen species (ROS), thereby alleviating oxidative injury [4]. At the molecular level, drought signals are transduced via abscisic acid (ABA)-dependent or ABA-independent pathways, activating transcription factors that regulate stress-responsive genes, ultimately improving cellular dehydration tolerance [5].
The UDP-glycosyltransferase (UGT) family is the largest subgroup within the glycosyltransferase superfamily in plants [6]. UGTs are central to the modulation of plant hormones and the synthesis of diverse natural products, such as steroids, terpenoids, phenylpropanoids, and flavonoids [6,7]. A hallmark of UGTs is the conserved C-terminal plant secondary product glycosyltransferase (PSPG) motif, a 44-amino acid sequence critical for glycosyltransferase activity [8]. Structural and functional studies demonstrate that conserved residues within the PSPG motif are essential for binding sugar donors and catalyzing glycosyl group transfer [9,10]. In contrast, the N-terminal region exhibits pronounced sequence divergence, which likely governs substrate specificity by interacting with diverse acceptor molecules, while the catalytic domain enables enzymatic versatility across UGT lineages [11,12].
The UGT gene family has been systematically characterized across diverse plant lineages, and it displays extensive variation in gene number and phylogenetic architecture. In Arabidopsis thaliana, 124 UGT genes are classified into 14 groups (A–N) [13,14], while Linum usitatissimum and Zea mays possess 137 and 147 UGTs grouped into 14 (A–N) and 15 (A–Q) clades, respectively [15,16]. Comparative analyses in Gossypium species (G. raimondii, G. arboreum, G. hirsutum) revealed lineage-specific expansions, with 142, 146, and 196 UGTs partitioned into 16 phylogenetically distinct clades (A–P) [17]. Notably, Acer rubrum contains 249 UGTs subdivided into 18 subgroups (A–R) [18]. Recent studies further highlight this diversification: Populus trichocarpa [19], Melilotus albus [20], and Solanum tuberosum [21] collectively underscore the dynamic evolution of the UGT gene family structure across angiosperms.
Glycosyltransferases catalyze glycosylation by transferring activated sugars from donor molecules (primarily UDP–glucose) to diverse acceptor substrates, while also participating in plant growth and adaptive responses to biotic and abiotic stresses. Ma et al. [22] demonstrated that ugt86a1 knockout mutants exhibit heightened sensitivity to drought and salt stress, whereas UGT86A1-overexpressing lines display robust tolerance, implicating UGT86A1 as a central regulator of abiotic stress responses. Overexpression of AtUGT76E11 in Arabidopsis enhanced salinity and drought resilience while increasing flavonoid accumulation [23]. Arabidopsis UGT79B2 and UGT79B3 improve tolerance to cold, salt, and drought stresses via modulating anthocyanin accumulation [24]. OsUGT83A1 overexpression in rice (Oryza sativa) conferred resistance to drought, salinity, and high-temperature stress [25]. Three UGT genes from Platycodon grandifloras were strongly induced under drought stress [26]. In S. lycopersicum, knockout of SlUDPGT52 via CRISPR/Cas9 enhanced drought tolerance through ROS detoxification [27]. Mechanistically, UGTs regulate stress adaptation via phytohormone modulation. AtUGT74E2 regulates the activity of the auxin indole-3-butyric acid (IBA), resulting in an increase in the intracellular contents of IBA and its glucose conjugate (IBA–glucose), thereby elevating intracellular IBA and IBA–glucose levels to enhance drought resilience [28]. AtUGT76C2 modifies cytokinin through glycosylation, indirectly altering ABA activity to fine-tune drought responses in Arabidopsis [29]. Analysis and functional studies of the UGT gene family are primarily concentrated in herbaceous plants, while research in woody plants remains relatively limited.
P. euphratica Oliv. (Salicaceae, Populus), a dominant tree species in arid desert ecosystems, is renowned for its exceptional tolerance to extreme environmental stresses [30]. As a model species for investigating stress adaptation in woody perennials, P. euphratica has facilitated critical insights into adaptation mechanisms [31,32,33]. Based on its chromosome-scale genome assembly [34], we systematically identified 134 PeUGT genes in P. euphratica and conducted comprehensive analyses of their phylogenetic relationships, chromosomal locations, gene structures, cis-acting regulatory elements, and collinearity. Tissue-specific and stress-responsive expression profiles were further characterized to prioritize candidate genes. Functional validation via heterologous expression of PeUGT110 in Arabidopsis confirmed its role in abiotic stress resilience. This study provides fundamental insights into the UGT gene family in P. euphratica and establishes a framework for investigating their functional roles in drought stress adaptation in woody plants.
2. Materials and Methods
2.1. Identification of PeUGT Proteins
The P. euphratica genome dataset was downloaded from the National Genomics Data Center (NGDC; https://ngdc.cncb.ac.cn, accessed on 4 March 2024), accession number: GWHAAYU00000000. The Hidden Markov Model (HMM) profile of the UGT domain (PF00201) was retrieved from the Pfam database (http://pfam.xfam.org/, accessed on 6 March 2024). Candidate UGT genes were identified using HMMER v3.0 (http://hmmer.org/, accessed on 7 March 2024) with an E-value cutoff of <1 × 10−5 against the P. euphratica proteome. Conserved UGT domains were verified using the NCBI Conserved Domain Search (CD-Search) and Pfam. Proteins lacking the conserved PSPG structural domain were excluded, and the remaining validated members were designated as PeUGT genes used in this study. The basic physicochemical properties of PeUGT proteins including molecular weight (MW), isoelectric point (pI), and amino acid count were predicted using ExPASy ProtParam (https://web.expasy.org/protparam/, accessed on 10 March 2024). The WoLF PSORT (https://wolfpsort.hgc.jp, accessed on 10 March 2024) was utilized to predict the subcellular localization of PeUGT proteins.
2.2. Analyses of Phylogenetic Relationship, Chromosome Location, and Synteny
The UGT protein sequences from P. trichocarpa [19] were aligned to the predicted PeUGTs using the MUSCLE implemented in MEGA 11 [35], and the resulting alignment was used for subsequent phylogenetic analysis. A phylogenetic tree was constructed via the neighbor-joining method (NJ) with 1000 bootstrap replicates in MEGA 11, followed by annotation and visualization on the Interactive Tree of Life (iTOL) (https://itol.embl.de/, accessed on 15 March 2024). Gene structure diagrams for PeUGTs were generated using TBtools-II [36] based on the P. euphratica genome annotation. Comparative analyses incorporated UGT sequences from A. thaliana (downloaded from NCBI: https://www.ncbi.nlm.nih.gov/, accessed on 15 March 2024) and P. trichocarpa (retrieved from Phytozome v13: https://phytozome-next.jgi.doe.gov/, accessed on 15 March 2024). Gene duplication events and collinear relationships among PeUGTs, Arabidopsis, and P. trichocarpa UGT genes were analyzed and visualized using TBtools-II [36].
2.3. Analyses of Gene Structures, Conserved Motifs and Promoter Cis-Acting Element of PeUGT Genes
The exon–intron structures of PeUGT genes were analyzed by Gene Structure Display Server (GSDS 2.0; http://gsds.cbi.pku.edu.cn/, accessed on 16 March 2024). The conserved motifs were predicted using the Multiple Em for Motif Elicitation (MEME) online tools (https://meme-suite.org/meme/tools/meme, accessed on 16 March 2024) with default parameters: maximum motif width of 50 amino acids and 10 motifs identified. Promoter regions (2000 bp upstream of transcription start site) were extracted from the P. euphratica genome data and analyzed for cis-acting elements using PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 17 March 2024). Results were visualized using TBtools-II.
2.4. Expression Patterns of PeUGT Genes in Different Tissues
To investigate the potential PeUGT genes involved in drought stress, we analyzed transcript abundance of all PeUGT genes in two-year-old P. euphratica plants at 0, 5, 10, 15, and 20 days after initiating to 20% PEG-6000-simulated drought stress using RNA-Seq (dataset unpublished). Transcript levels were quantified as log2-transformed FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values, and hierarchical clustering analysis was performed using TBtools-II to generate a heatmap. The PeUGT genes significantly induced by PEG-6000 were considered as potential drought-responsive genes and selected for further functional studies.
2.5. Quantitative Real-Time PCR
Total RNA was isolated from P. euphratica and Arabidopsis tissues using the MiniBEST Plant RNA Extraction Kit (Takara, Beijing, China). Residual genomic DNA was eliminated, and first-strand cDNA synthesis was performed with the PrimeScript™ RT Reagent Kit (with gDNA Eraser; Takara, Beijing, China). Quantitative real-time PCR (qRT-PCR) was conducted in triplicate using SYBR® Premix Ex Taq™ II (Takara, Beijing, China) on a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The thermal cycling protocol comprised an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s (denaturation) and 60 °C for 30 s (annealing/extension). Gene expression levels were calculated by the 2−ΔΔCT method [37], normalized to the internal reference gene AtACTIN2 (for Arabidopsis) and Pe18s rRNA (for P. euphratica). Primer sequences are provided in Table S1.
2.6. Arabidopsis Transformation and Drought Treatment
The coding sequence of PeUGT110 (accession number: POPULUS_EUPHRATICA_25607) was amplified with gene-specific primers (Table S1), verified by Sanger sequencing, and cloned into the pBI121 binary vector under the CaMV 35S promoter. The recombinant plasmid (35S::PeUGT110-GFP) was introduced into Agrobacterium tumefaciens strain GV3101 via electroporation and subsequently transformed into A. thaliana ecotype Col-0 using Agrobacterium-mediated floral dip transformation [38]. To identify PeUGT110 overexpression lines, T0 transgenic seeds were subjected to selection on Murashige and Skoog (1/2 MS) medium supplemented with 50 mg/L kanamycin. Three independent T3 homozygous transgenic lines with higher PeUGT110 transcript levels, identified via qRT-PCR, were used for further analysis.
2.7. Plant Materials and Drought Treatments
Arabidopsis seeds were surface-sterilized with 70% ethanol and 1% sodium hypochlorite, rinsed thoroughly with sterile deionized water, and sown onto MS medium. Seeds were stratified at 4 °C in darkness for 72 h to synchronize germination. Plants were grown in a controlled-environment chamber under the following conditions: 23 °C, 60% relative humidity, 16 h light/8 h dark photoperiod, and light intensity of 450 μmol·m−2·s−1. Seven-day-old seedlings were transplanted into a soil mixture and maintained under identical growth conditions. For drought treatment, four-week-old seedlings were subjected to soil drenching with 20% (w/v) PEG-6000 solution every 2 days. Leaf tissues were harvested at 0, 3, 5, and 7 days post-treatment, immediately frozen in liquid nitrogen, and stored at −80 °C. Proline (Pro) content, malonaldehyde (MDA) content, and superoxide dismutase (SOD) activity were determined using commercial assay kits (Jiancheng, Nanjing, China). Three independent biological replicates were performed for each experiment.
3. Results
3.1. Identification and Characteristics of PeUGTs
The UGT gene family in P. euphratica was systematically identified using a Hidden Markov Model (HMM) profile (PF00201) corresponding to the conserved PSPG motif. A total of 134 PeUGT genes encoding proteins possessing a functional PSPG domain were identified and named PeUGT1 to PeUGT134 based on their chromosomal positions. Physicochemical analyses revealed substantial variation among PeUGT proteins: amino acid lengths ranged from 297 (PeUGT90) to 809 (PeUGT126) residues, molecular weights spanned 32.9 to 90.0 kDa, and theoretical isoelectric points (pI) varied from 4.69 to 9.30. Subcellular localization predictions showed that the majority of PeUGT proteins localized to the chloroplast (87 members), cytosol (22 members), and nucleus (8 members). Comprehensive physicochemical properties are detailed in Table S2.
3.2. Phylogenetic Analysis of UGT Genes
To resolve the evolutionary relationships of UGT genes in P. euphratica, we constructed an NJ phylogenetic tree using MEGA11 with UGT protein sequences from P. euphratica and P. trichocarpa. Phylogenetic analysis classified the 134 PeUGT genes into 16 groups (A–P), displaying considerable variation in group size (Figure 1). Group E, containing 33 members, emerged as the largest lineage, followed by group G (23 members), group L (18 members), group A (14 members), and group D (8 members). The remaining groups (B, C, F, H, I, J, K, M, N, O, P) comprised 2, 2, 5, 4, 5, 1, 5, 5, 1, 5, and 3 members, respectively, with groups J and N representing the smallest lineages (1 member each).
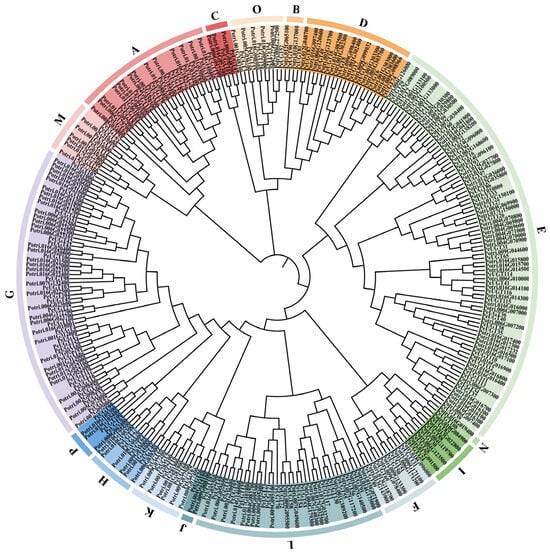
Figure 1.
Phylogenetic analysis of the UGT proteins among P. euphratica and P. trichocarpa. A neighbor-joining phylogenetic tree was reconstructed using MEGA 11, including 134 full-length UGT proteins from P. euphratica and 188 from P. trichocarpa. The UGTs proteins were classified into 16 distinct groups (A–P), with each group represented by arcs in different colors.
3.3. Chromosome Localization and Gene Duplication Analysis of UGT Gene in P. euphratica
Chromosomal localization analysis of PeUGT genes, based on the P. euphratica genome annotation, revealed that 133 genes were unevenly distributed across chromosomes 1–19, while PeUGT134 was localized to an unassembled scaffold (Figure 2). Chromosomes 6 and 16 displayed the highest PeUGT gene densities (each 19 genes), followed by chromosomes 1, 9, and 17. In contrast, chromosomes 15 and 19 each contained only one PeUGT gene. Gene duplication events, including 65 tandem and 46 segmental duplication pairs within the PeUGT family (Figure 3, Table S3), were identified, providing insights into the expansion and diversification of this gene family. To elucidate cross-species evolutionary relationships, synteny analyses were conducted between P. euphratica and two phylogenetically distinct species: Arabidopsis (eudicot model) and P. trichocarpa (closely related woody species). These analyses revealed 34 and 140 homologous UGT gene pairs between P. euphratica and A. thaliana or P. trichocarpa, respectively (Figure 4). The results imply that P. euphratica has a closer evolutionary relationship with P. trichocarpa than with A. thaliana.
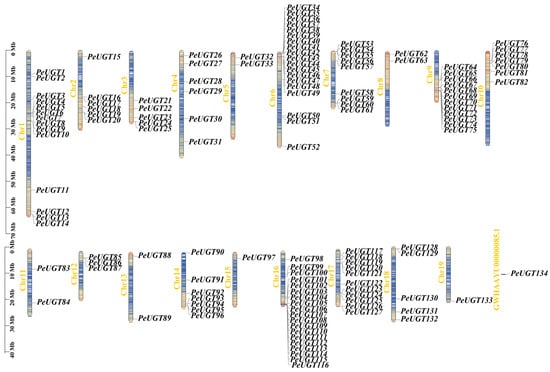
Figure 2.
Chromosomal localization of UGT gene family members in P. euphratica.
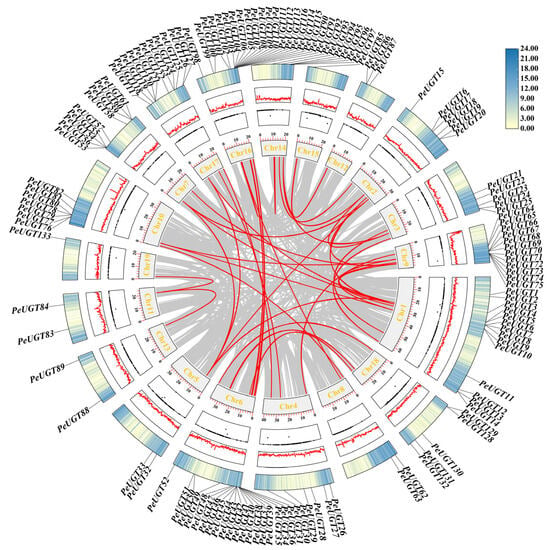
Figure 3.
Genome-wide synteny analysis of PeUGT genes in P. euphratica. Red interchromosomal lines represent segmental duplication events between paralogs. The circular diagram layers (inner to outer) show: the chromosomes, regions of unknown DNA bases (N-clusters), GC content patterns, and the genome-wide gene density.
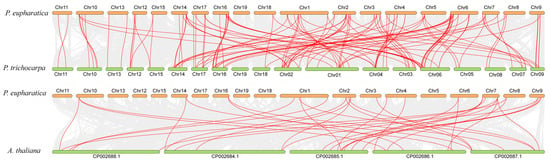
Figure 4.
Collinearity analysis of UGT genes across P. euphratica, P. trichocarpa, and Arabidopsis thaliana. Red lines connect collinear orthologous gene pairs between P. euphratica and P. trichocarpa, and P. euphratica and A. thaliana.
3.4. Structure and Conserved Motif Analysis of PeUGT Genes
To characterize the PeUGT gene family, exon–intron architectures and conserved motifs were analyzed. Exon–intron structure analysis revealed substantial variation, with exon numbers ranging from 1 to 8 across the family. Among the 134 PeUGT genes, 35 contained a single exon, 81 had two exons, and 12 possessed three exons. Notably, PeUGT82 and PeUGT88 each harbored four exons, PeUGT91 had six, PeUGT16 had seven, and PeUGT52 and PeUGT126 had eight exons. Phylogenetic analysis indicated that exon–intron structures correlate with group classification, as members within the same clade exhibited similar architectures (Figure 5A,C).
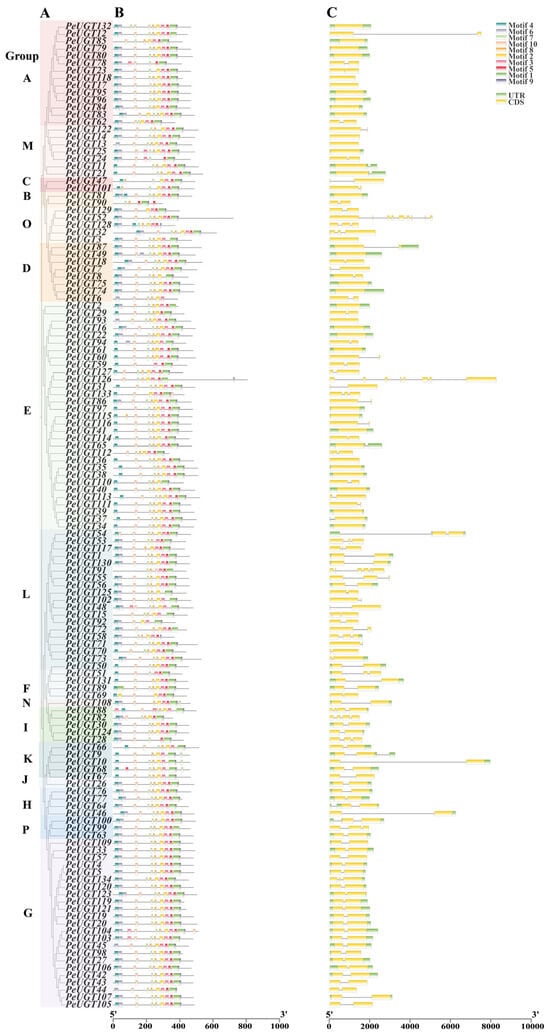
Figure 5.
Phylogenetic tree, conserved motif, and gene structure analysis of the PeUGTs. (A) Phylogenetic analysis of 134 PeUGT genes was performed using the neighbor-joining method in MEGA11. The different colored blocks represent distinct phylogenetic groups (A–P). (B) The conservative motif position of PeUGT proteins. (C) The exon–intron structure analysis of PeUGT genes.
Conserved motifs were predicted using the MEME website. All PeUGT proteins contained Motif 1, which aligns with the PSPG-box sequence (WAPQ-VL-H-AVG-FLTHCGWN-STLES-GVP-WPM-DQ) critical for glycosyltransferase activity. Within the same clade, shared conserved motif compositions and arrangements, most PeUGTs with the predominant order being Motifs 4-6-10-7-8-2-3-5-1-9. However, minor variations in motif order were observed in select PeUGT proteins (Figure 5B). The structural conservation across clades suggests functional similarity among PeUGT family members.
3.5. Cis-Acting Elements Analysis of PeUGT Genes
To explore the potential functions of PeUGT genes, we analyzed cis-acting elements within the 2000 bp promoter regions upstream of each gene. After filtering out generic cis-acting elements, the remaining elements were classified into four functional categories: cell development, phytohormone response, environmental stress response, and light signaling (Figure 6, Table S4). Seven cis-acting elements related to cell development were identified, including the CAT-box, MSA-like, GCN4_motif, CCAAT-box, MBSI, HD-Zip 1, and RY elements. All PeUGT promoters contained elements responsive to jasmonate (JA/MeJA), ABA, gibberellin (GA), auxin (IAA), and salicylic acid (SA). The elements related to environmental stress include the low-temperature response element (LTR), anaerobic induction elements (ARE, GC-motif), drought induction element (MBS), defense and stress response element (TC-rich repeats), circadian rhythm control element, and AT-rich elements. In addition, ten light response elements were detected: GT1 motif, G-Box, Box 4, MRE, ATC motif, Sp1, ATCT motif, ACE, 3-AF binding site, and AAAC motif. These findings suggest that PeUGT expression is likely modulated by diverse environmental cues and phytohormone signaling pathways, aligning with their putative roles in stress adaptation and developmental regulation.
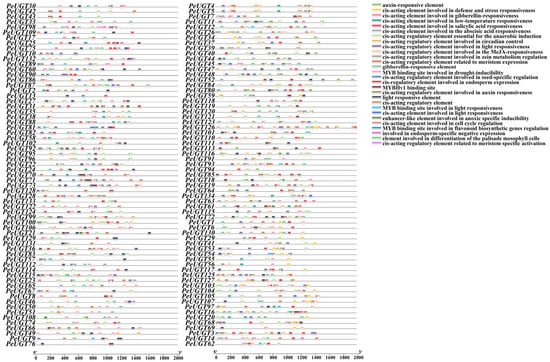
Figure 6.
Cis-acting element distribution in PeUGT promoter regions. Colored boxes represent distinct cis-acting elements.
3.6. Expression of PeUGT Genes in Different Tissues Under Drought Stress
To investigate the organ-specific roles of PeUGT genes under drought stress, we analyzed RNA-Seq data from P. euphratica leaf, root, and stem tissues subjected to 20% PEG-6000 treatment. The expression patterns of 134 PeUGT genes exhibited distinct tissue-specific and time-dependent patterns (Figure 7). In leaf tissues, 37 PeUGT genes were upregulated and 19 downregulated. The leaf tissue exhibited the most significant changes in UGT gene expression, particularly at days 15 and 20. Notably, PeUGT107, PeUGT110, PeUGT118, PeUGT112, PeUGT124, and PeUGT129 were significantly upregulated during these time points, suggesting their potential involvement in leaf adaptive responses to prolonged drought stress.
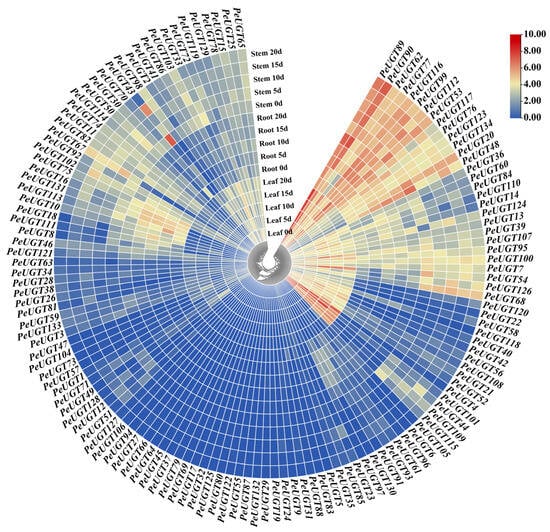
Figure 7.
Tissue-specific expression profiles of PeUGT genes in response to drought treatment. Transcript levels of the PeUGT genes in leaf, root, and stem were analyzed at 0 day, 5 days, 10 days, 15 days, and 20 days after drought treatment. The blue-to-red color gradient indicates increasing transcript levels (scale: 0–10).
Root tissues displayed differential regulations of 42 upregulated and 24 downregulated PeUGT genes. The expression of PeUGT genes in the root showed a clear time-dependent increase, especially at the later stages of drought stress (15 and 20 days). For instance, PeUGT110 and PeUGT102 showed a notable increase in expression at day 15, while PeUGT89, PeUGT90, and PeUGT124 exhibited a significant upregulation by day 20, indicating their role in maintaining water and ion homeostasis. In stem tissues, 37 PeUGT genes were upregulated and 36 downregulated. Expression remained stable during early drought but increased gradually under prolonged treatment. Genes such as PeUGT102, PeUGT110, PeUGT112, and PeUGT124 showed slight upregulation at days 10 and 15, whereas PeUGT89, PeUGT98, and PeUGT113 displayed enhanced expression at later stages (20 days), indicating their potential regulatory roles in maintaining stem physiological functions during extended water deficit.
To validate the accuracy of the transcriptome data, five candidate PeUGT genes were analyzed in leaf, root, and stem tissues using the RT-qPCR method. As shown in Figure 8, most candidate genes showed strong concordance with the RNA-Seq expression profiles, confirming the reliability of the transcriptomic dataset. Notably, PeUGT110 displayed significant drought-responsive upregulation across roots, stems, and leaves under stress conditions. To further confirm its functional role in drought adaptation, the PeUGT110 gene was prioritized for heterologous overexpression and phenotypic validation in Arabidopsis.
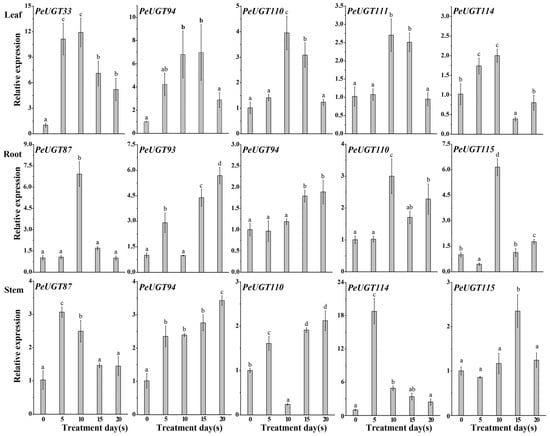
Figure 8.
qRT-PCR analysis of PeUGT gene expression in leaf, root, and stem tissues of P. euphratica under drought stress. Expression levels of eight PeUGT genes in two-year-old P. euphratica plants at 0, 5, 10, 15, and 20 days after initiating to 20% PEG-6000-simulated drought stress via qRT-PCR. Expression levels were normalized against untreated controls (0-day) serving as the baseline (1.0-fold expression). The bars represent the mean ± standard deviation (SD) (n = 3 biological replicates), with letters indicating statistically significant differences determined by one-way ANOVA (p < 0.05).
3.7. Overexpression of the PeUGT110 Gene Improves the Drought Tolerance in Arabidopsis
To further investigate the drought responsiveness of candidate genes, the PeUGT110 gene, which was significantly induced by drought stress, was selected for functional validation. The PeUGT110 gene was cloned and heterologously expressed in Arabidopsis, and five T3 generation homozygous lines were obtained. The expression of PeUGT110 in these lines was examined via qRT-PCR. Three independent overexpression lines (PeUGT110-OE-L1, L2, and L3) with higher expression levels were selected for subsequent experiments (Figure S1). Under normal conditions, the PeUGT110-OE lines exhibited no phenotypic divergence from wild-type (WT) plants. After three days of treatment, neither PeUGT110-OE nor WT plants displayed visible wilting or chlorosis. By day five, leaves of WT plants exhibited obvious wilting and yellowing, while PeUGT110-OE lines showed only mild leaf yellowing without significant wilting. After seven days of drought stress, severe wilting and desiccation were observed in WT plants, whereas PeUGT110-OE lines retained partial leaf turgor, with delayed symptom progression (Figure 9A). These results demonstrate that PeUGT110 overexpression enhances drought tolerance by mitigating leaf dehydration and chlorosis under prolonged water deficit.
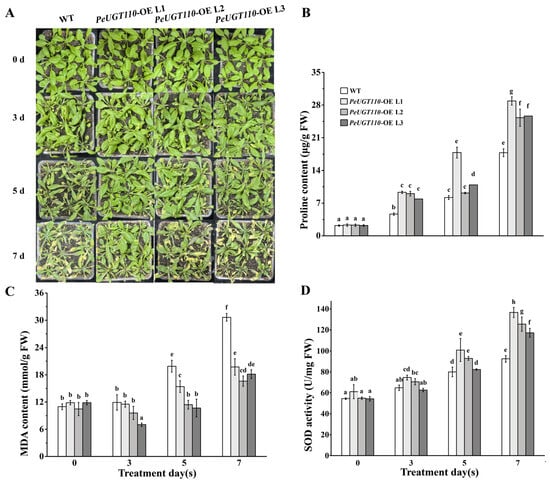
Figure 9.
Overexpression of PeUGT110 in Arabidopsis improved the drought stress. Four-week-old seedlings of PeUGT110-OE lines and wild-type (WT) plants were irrigated with 20% PEG-6000 solution every 2 days instead of water. The plant performance was photographed, and leaves were harvested at 0, 3, 5, and 7 days following drought stress treatment. (A) Phenotype of PeUGT110-OE lines and WT plants under drought stress. (B) Proline (Pro) content. (C) Malonaldehyde (MDA) content. (D) Superoxide dismutase (SOD) activity. The bars represent the mean ± SD (n = 3 biological replicates), with letters indicating statistically significant differences determined by one-way ANOVA (p < 0.05).
During the initial three days of drought stress, no significant differences in Pro content, MDA levels, or SOD activity were observed between PeUGT110-OE lines and WT Arabidopsis (Figure 9B–D). After five days, transgenic lines exhibited a gradual increase in Pro accumulation (Figure 9B) and SOD activity (Figure 9D), surpassing WT levels, while MDA content in PeUGT110-OE lines decreased compared to WT. At day seven, Pro and SOD levels rose significantly across all genotypes, but PeUGT110-OE lines maintained higher Pro content and SOD activity relative to WT. Concurrently, MDA accumulation increased in both WT and transgenic plants under drought stress, but the degree of increase in the WT plants was significantly higher than that in the transgenic lines (Figure 9C). These findings suggest that PeUGT110 overexpression mitigates drought-induced oxidative damage by enhancing osmoprotectant synthesis and antioxidant capacity, thereby reducing membrane lipid peroxidation.
3.8. Expression Profiling of Drought Stress-Related Genes in Arabidopsis Overexpressing PeUGT110
To explore the mechanism underlying PeUGT110-mediated drought tolerance, we analyzed the expression profiles of stress-responsive genes in PeUGT110-OE Arabidopsis seedlings under drought conditions. qRT-PCR revealed that PeUGT110-OE lines exhibited significant upregulation of stress-related genes (AtRD22, AtP5CS1, AtAIL1, AtFBS1, AtMYB96, and AtCOR47) compared to WT plants (Figure 10). Expression of AtRD22 and AtP5CS1 in PeUGT110-OE lines exceeded WT levels throughout the stress period, with a sharp increase by day 5 and day 7. Basal expression of AtAIL1 was elevated in PeUGT110-OE lines even under non-stress conditions and further amplified under drought. AtFBS1, encoding an F-box protein responsive to multiple abiotic stresses, was markedly upregulated in transgenic lines under drought. Notably, AtMYB96, a key transcriptional activator of ABA signaling during drought stress, showed pronounced induction in PeUGT110-OE lines, particularly at days 5 and 7 of treatment. The stress-responsive gene AtCOR47 displayed marked induction in transgenic lines across all time points, peaking at day 3. These findings suggest that PeUGT110 regulates a transcriptional network that upregulates osmotic stress-responsive genes, thereby enhancing drought tolerance.
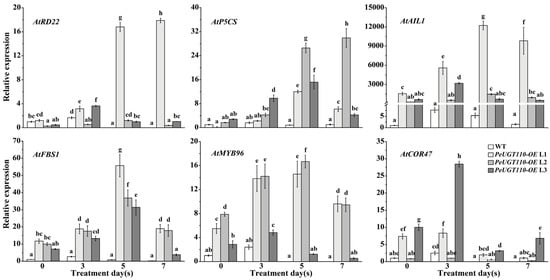
Figure 10.
PeUGT110 improves the upregulation of stress-related genes under drought treatments. qPCR analysis was performed to measure the expression levels of AtRD22, AtP5CS1, AtAIL1, AtFBS1, AtMYB96, and AtCOR47 genes after 0, 3, 5, and 7 days of 20% PEG-6000 treatment in four-week-old PeUGT110-OE lines and WT Arabidopsis. The bars represent the mean ± SD (n = 3 biological replicates), with letters indicating statistically significant differences determined by one-way ANOVA (p < 0.05).
4. Discussion
Plant UGTs, which represent the largest subgroup within the glycosyltransferase family, are ubiquitously distributed across fungi, bacteria, plants, and animals. These enzymes catalyze glycosylation reactions, playing a key role in various metabolic processes [7]. UGT genes play a crucial role in plant growth and in mediating responses to both biotic and abiotic stresses by regulating the biosynthesis of secondary metabolites [6,39]. P. euphratica is an important tree species in desert ecosystems and serves as a model for studying stress responses in woody plants. However, a comprehensive identification and analysis of the PeUGT family members has not yet been conducted. We systematically identified 134 PeUGT genes through genome-wide analysis (Figure 1, Table S2). Functional characterization revealed drought stress-responsive activity in specific PeUGT members, indicating their potential roles in abiotic stress adaptation. The results provide novel genetic resources for enhancing drought tolerance in woody plants through molecular breeding approaches.
In this study, 134 PeUGT genes were unevenly distributed across 19 chromosomes, with a predominance on Chr01 (n = 14), Chr06 (n = 19), and Chr16 (n = 19) (Figure 2). Comparative analysis revealed interspecific variation in UGT family sizes: Nelumbo nucifera (108) [40], Arabidopsis (124) [13], L. usitatissimum (137) [15], P. trichocarpa (191) [19], A. rubrum (249) [18], and S. tuberosum (295) [21]. This result suggests limited evolutionary expansion of the UGT family in P. euphratica. Phylogenetic analysis classified the 134 PeUGT genes into 16 evolutionarily distinct groups (A–P). Five clades (A, D, E, G, L) showed significant expansion, containing 14, 8, 33, 23, and 18 members, respectively, representing the larger clusters in P. euphratica (Figure 1). Similar expansion patterns were reported in Rubus chingii [41], Punica granatum [42], Camellia sinensis [43], and P. trichocarpa [19]. These five rapidly evolving groups (A, D, E, G, L) demonstrate accelerated expansion rates compared to other groups, consistent with evolutionary patterns observed in higher plants [44]. Additionally, gene duplication analysis identified 65 tandem and 46 segmental duplication events, with chromosome 16 showing a notable number of tandem duplications (Figure 3, Table S3). This suggests that tandem duplications may be a primary driver of the PeUGT family expansion. Such duplication patterns highlight potential mechanisms for the expansion and diversification of PeUGT genes, likely facilitated by local genomic rearrangements [39].
Conserved motif arrangements were observed within corresponding groups (Figure 5B), indicating structural conservation of PeUGT proteins among evolutionarily related members. This clade-specific conservation pattern aligns with reports on P. trichocarpa [19], S. tuberosum [21], and N. tabacum [45]. Furthermore, gene structure analysis revealed conserved exon–intron architectures among PeUGT genes within the same phylogenetic clades or subclades, consistent with their evolutionary relationships (Figure 5C). Among the 134 PeUGT genes, 35 lacked introns, while 81 contained a single intron. This intron-poor architecture (86.6% with ≤1 intron) aligns with observations that intron-poor gene families tend to undergo more rapid evolutionary diversification [46]. Additionally, UGT genes with similar exon and intron counts exhibit significant sequence similarities, suggesting their closely linked evolutionary relationships [47]. In the present study, several cis-acting elements related to hormones, light, cell development, and environmental stress were identified in the promoters of the PeUGT genes. This distribution pattern is similar to that reported for Hordeum vulgare [48], Manihot esculenta [49], Arachis hypogaea [50], and O. sativa [51], indicating that these UGT genes likely play crucial roles in the response to diverse abiotic stresses.
Tissue-specific gene expression patterns often reflect functional specialization in plants. For instance, in G. hirsutum, elevated expression of GhUGT genes in leaves and stems suggests their involvement in cotton growth regulation [52]. Similarly, HvUGT genes in H. vulgare exhibit organ-specific expression profiles (roots, leaves, and buds) and drought-inducible activation [48]. RNA sequencing analysis revealed tissue-specific drought responses among PeUGT genes in P. euphratica, with 56, 66, and 73 PeUGT genes showing stress-induced expression in leaves, stems, and roots, respectively (Figure 7). Notably, PeUGT80, PeUGT94, PeUGT110, and PeUGT114 were significantly upregulated across all three tissues, indicating that these genes play crucial roles in drought tolerance.
Drought stress can induce morphological changes such as leaf wilting and apical bending. During this process, excessive accumulation of ROS leads to oxidative damage, including protein oxidation and lipid peroxidation, with a concomitant increase in MDA content [4]. To cope with drought stress, plants employ osmotic regulation as a mechanism. Pro, a key organic osmotic regulator, helps prevent tissue dehydration by reducing cellular osmotic potential and stabilizing macromolecular structures. Additionally, SOD acts as the first line of defense, maintaining ROS levels within a safe threshold to avoid oxidative damage [1,3]. In this study, the PeUGT110-OE lines exhibited drought-tolerant phenotypes including improved growth status, reduced MDA accumulation, elevated proline content, and enhanced SOD activity compared to WT plants under drought stress (Figure 9). These physiological markers suggest that PeUGT110 plays a positive role in drought tolerance. This finding aligns with functional reports in other species: UGT76C2-overexpressing O. sativa shows enhanced ROS scavenging with Pro and soluble sugar accumulation [25]; AhUGT75A overexpression in Arabidopsis reduces MDA content and improves drought tolerance [50]; GmUGT73F4 transgenic Arabidopsis exhibits elevated antioxidant enzyme activities under heat stress [53]; and Z. mays UGTs mitigate oxidative stress through flavonoid-mediated ROS reduction [54]. These results indicate that PeUGT110 enhances drought resilience through dual mechanisms: osmotic adjustment via proline accumulation and oxidative stress mitigation through antioxidant enzyme activation.
5. Conclusions
The UGT genes play critical roles in plant growth, development, and responses to abiotic stress. In this study, a comprehensive analysis of the UGT gene family in P. euphratica was conducted, revealing its role in response to drought stress. A total of 134 PeUGT genes were identified and found to be unevenly distributed across the 19 chromosomes. These genes were grouped into 16 phylogenetic groups, with group E showing significant expansion. These genes exhibited tissue-specific and time-dependent expression under drought stress. Crucially, PeUGT110 was demonstrated to be a central drought-tolerance regulator, conferring enhanced resilience through glycosylation-mediated osmoprotection and antioxidant potentiation. Our findings not only advance the understanding of UGT-mediated stress adaptation but also establish a foundation for developing drought-resistant plants via targeted genetic engineering or breeding strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f16081214/s1, Figure S1: Expression analysis of PeUGT110 in transgenic Arabidopsis lines; Table S1: The primer sequences used in this study; Table S2: Physical and chemical properties of UGT proteins in P. euphratica; Table S3: The tandem and segmental duplication gene pairs of UGTs. Table S4: Promoter cis-acting elements of PeUGT genes.
Author Contributions
Conceptualization, G.W., J.A., Q.H., J.X. and J.L.; methodology, G.W., J.A. and Q.H.; investigation, J.A., Q.H., J.X. and J.L.; writing—original draft preparation, G.W., J.A. and Q.H.; writing—review and editing, G.W. and J.A.; supervision, G.W.; funding acquisition, G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research Project of Qinghai Province Science Foundation, grant number 2020-ZJ-968Q.
Data Availability Statement
The transcriptome data used in this study is available from the corresponding author upon reasonable request (email: wanggaini07@163.com).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought stress in plants: An overview. Plant responses to drought stress: From morphological to molecular features. Int. J. Agric. Biol. 2012, 11, 100–105. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Bowles, D.; Lim, E.-K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Jones, P. Glycosyltransferases in plant natural product synthesis: Characterization of a supergene family. Trends Plant Sci. 2000, 5, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.; Møller, B.L.; Bak, S. On the origin of family 1 plant glycosyltransferases. Phytochemistry 2003, 62, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Osmani, S.A.; Bak, S.; Møller, B.L. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 2009, 70, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Offen, W.; Martinez-Fleites, C.; Yang, M.; Kiat-Lim, E.; Davis, B.G.; Tarling, C.A.; Ford, C.M.; Bowles, D.J.; Davies, G.J. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006, 25, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Yonekura-Sakakibara, K.; Hanada, K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011, 66, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Tian, L. Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J. 2019, 100, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Li, Y.; Lim, E.-K.; Bowles, D.J. Higher plant glycosyltransferases. Genome Biol. 2001, 2, reviews3004.1. [Google Scholar] [CrossRef] [PubMed]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Gupta, V.S. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genom. 2012, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Wang, Y.; Dong, R.; Yu, H.; Hou, B. Genome-wide identification and phylogenetic analysis of Family-1 UDP glycosyltransferases in maize (Zea mays). Planta 2014, 239, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pang, C.; Fan, S.; Song, M.; Yu, J.; Wei, H.; Ma, Q.; Li, L.; Zhang, C.; Yu, S. Genome-wide analysis of the family 1 glycosyltransferases in cotton. Mol. Genet. Genom. 2015, 290, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Kamal, K.A.; Shah, F.A.; Zhao, Y.; Chen, Z.; Fu, S.; Zhu, Z.; Ren, J.; Liu, H. Genome-wide identification of the UGT genes family in Acer rubrum and role of ArUGT52 in anthocyanin biosynthesis under cold stress. BMC Plant Biol. 2025, 25, 288. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.M.; Khan, U.M.; Nawaz, S.; Saleem, F.; Ahmed, N.; Rana, I.A.; Atif, R.M.; Shaheen, N.; Seo, H. Genome wide analysis of family-1 UDP glycosyltransferases in Populus trichocarpa specifies abiotic stress responsive glycosylation mechanisms. Genes 2022, 13, 1640. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Yan, Q.; Wu, F.; Wang, Y.; Wang, S.; Zong, X.; Zhou, P.; Zhang, J. Genome-wide analysis of the UDP-glycosyltransferase family reveals its roles in coumarin biosynthesis and abiotic stress in Melilotus albus. Int. J. Mol. Sci. 2021, 22, 10826. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, J.; Sheng, S.; Wang, D.; Wang, T.; Wang, N.; Chen, A.; Wang, L.; Peng, Y.; Ma, Y. Genome-wide characterization of Solanum tuberosum UGT gene family and functional analysis of StUGT178 in salt tolerance. BMC Genom. 2024, 25, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, G.; Zhao, S.; Zhang, F.; Ma, X.; Liu, C.; Ding, Y.; Hou, B. Arabidopsis glycosyltransferase UGT86A1 promotes plant adaptation to salt and drought stresses. Physiol. Plant. 2025, 177, e70050. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.M.; Meng, X.F.; Lin, J.S.; Li, Y.J.; Hou, B.K. Ectopic expression of glycosyltransferase UGT76E11 increases flavonoid accumulation and enhances abiotic stress tolerance in Arabidopsis. Plant Biol. 2018, 20, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, X.; Yu, H.; Dong, N.; Li, J.; Chang, X.; Wang, J.; Jiang, C.; Liu, J.; Chi, X. Genome-wide analysis of UDP-glycosyltransferases family and identification of UGT genes involved in drought stress of Platycodon grandiflorus. Front. Plant Sci. 2024, 15, 1363251. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, X.; Hou, H.; Fan, Y.; Wang, L.; Zeng, H.; Chen, X.; Ding, Y.; Hu, X.; Yan, Q. Genome-wide characterization of the tomato UDP-glycosyltransferase gene family and functional identification of SlUDPGT52 in drought tolerance. Hortic. Adv. 2023, 1, 14. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; Van De Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.M.; Kojima, M.; Sakakibara, H.; Hou, B.K. N-glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 2200–2213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Yan, H.; Arndt, S.K. Leaf and whole tree adaptations to mild salinity in field grown Populus euphratica. Tree Physiol. 2009, 29, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Xu, J.; Shao, X.; Luo, W.; Niu, Z.; Gao, C.; Wan, D. A novel gene coding γ-aminobutyric acid transporter may improve the tolerance of Populus euphratica to adverse environments. Front. Plant Sci. 2019, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ma, J.; Niu, Z.; Bai, X.; Lei, W.; Shao, X.; Chen, N.; Zhou, F.; Wan, D. Tissue-specific transcriptome analysis reveals multiple responses to salt stress in Populus euphratica seedlings. Genes 2017, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Li, G.; Hu, H.; Lv, J.; Zheng, Q.; Liu, J.; Wan, D. A gene that underwent adaptive evolution, LAC2 (LACCASE), in Populus euphratica improves drought tolerance by improving water transport capacity. Hortic. Res. 2021, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Zhang, J.; Ma, X.; Li, Y.; Li, M.; Wang, D.; Kang, M.; Wu, H.; Yang, Y. Improved genome assembly provides new insights into genome evolution in a desert poplar (Populus euphratica). Mol. Ecol. Resour. 2020, 20, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Su, Y.; Chen, N.; Shen, S. Genome-wide analysis of the UGT gene family and identification of flavonoids in Broussonetia papyrifera. Molecules 2021, 26, 3449. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Lu, M.; Chen, J.; Shi, T. Gene expression and evolution of Family-1 UDP-glycosyltransferases—Insights from an aquatic flowering plant (sacred lotus). Aquat. Bot. 2020, 166, 103270. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Shen, M.; Li, Q.; Wang, S.; Jiang, J.; Zeng, W. Identification and functional verification of the glycosyltransferase gene family involved in flavonoid synthesis in Rubus Chingii Hu. Plants 2024, 13, 1390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, Y.; Ke, D.; Teng, Y.; Chen, Y.; Langjia, R. Molecular identification and characterization of UDP-glycosyltransferase (UGT) multigene family in pomegranate. Horticulturae 2023, 9, 540. [Google Scholar] [CrossRef]
- Cui, L.; Yao, S.; Dai, X.; Yin, Q.; Liu, Y.; Jiang, X.; Wu, Y.; Qian, Y.; Pang, Y.; Gao, L. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea (Camellia sinensis). J. Exp. Bot. 2016, 67, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Qu, X.; Wu, F.; Li, X.; Ren, M.; Tong, Y.; Wu, X.; Yang, A.; Chen, Y. Genome-wide analysis of UDP-glycosyltransferases family and identification of UGT genes involved in abiotic stress and flavonol biosynthesis in Nicotiana tabacum. BMC Plant Biol. 2023, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, Y.; Zhang, Y.; Guo, J.; Li, K.; Fu, W.; Jia, Z.; Li, W.; Tran, L.-S.P.; Jia, K.-P. Genome-wide identification, characterization and expression profiles of the CCD gene family in Gossypium species. 3 Biotech 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lu, Q.; Liu, R.; Gong, J.; Gong, W.; Liu, A.; Ge, Q.; Li, J.; Shang, H.; Li, P. Genome-wide characterization of the UDP-glycosyltransferase gene family in upland cotton. 3 Biotech 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Admas, T.; Cheng, B.; Meng, Y.; Pan, R.; Zhang, W. UGT gene family identification and functional analysis of HvUGT1 under drought stress in wild barley. Physiol. Mol. Biol. Plants 2024, 30, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dai, J.; Chen, Z.; Tie, W.; Yan, Y.; Yang, H.; Zeng, J.; Hu, W. Comprehensive analysis and expression profiles of cassava UDP-glycosyltransferases (UGT) family reveal their involvement in development and stress responses in cassava. Genomics 2021, 113, 3415–3429. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Liu, Y.; Yao, R.; He, D.; Yan, L.; Chen, Y.; Huai, D.; Wang, Z.; Yu, B.; Kang, Y. Genome-wide analysis of UDP-glycosyltransferase gene family and identification of a flavonoid 7-O-UGT (AhUGT75A) enhancing abiotic stress in peanut (Arachis hypogaea L.). BMC Plant Biol. 2023, 23, 626. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, S.R.; Zhao, G.; Yi, J.; Yoo, Y.; Jin, P.; Lee, S.W.; Jung, K.h.; Zhang, D.; An, G. Rice glycosyltransferase1 encodes a glycosyltransferase essential for pollen wall formation. Plant Physiol. 2013, 161, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, L.; Huang, H.; Zhang, Y.; Wang, J.; Lu, X.; Wang, S.; Wang, D.; Chen, X.; Chen, C. Genome-wide identification, evolution and function analysis of UGTs superfamily in cotton. Front. Mol. Biosci. 2022, 9, 965403. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qian, P.; Ye, M.; Mu, K.; Wang, S.; Chen, M.; Ma, H. GmUGT73F4 plays important roles in enhancing seed vitality and tolerance to abiotic stresses in transgenic Arabidopsis. Plant Cell Tissue Organ Cult. 2022, 150, 313–328. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Xie, J.; Bu, J.; Li, L.; Xin, X.; Zhao, Y.; Zhang, H.; Yang, L.; Wang, J. Transcriptomic profiling of the high-vigour maize (Zea mays L.) hybrid variety response to cold and drought stresses during seed germination. Sci. Rep. 2021, 11, 19345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).