Positive Relationships Between Soil Organic Carbon and Tree Physical Structure Highlights Significant Carbon Co-Benefits of Beijing’s Urban Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data Collection
2.3. Calculation of Plot-Level Species Complexity and Forest Spatial Structure Indicators
2.4. Data Analysis
3. Results
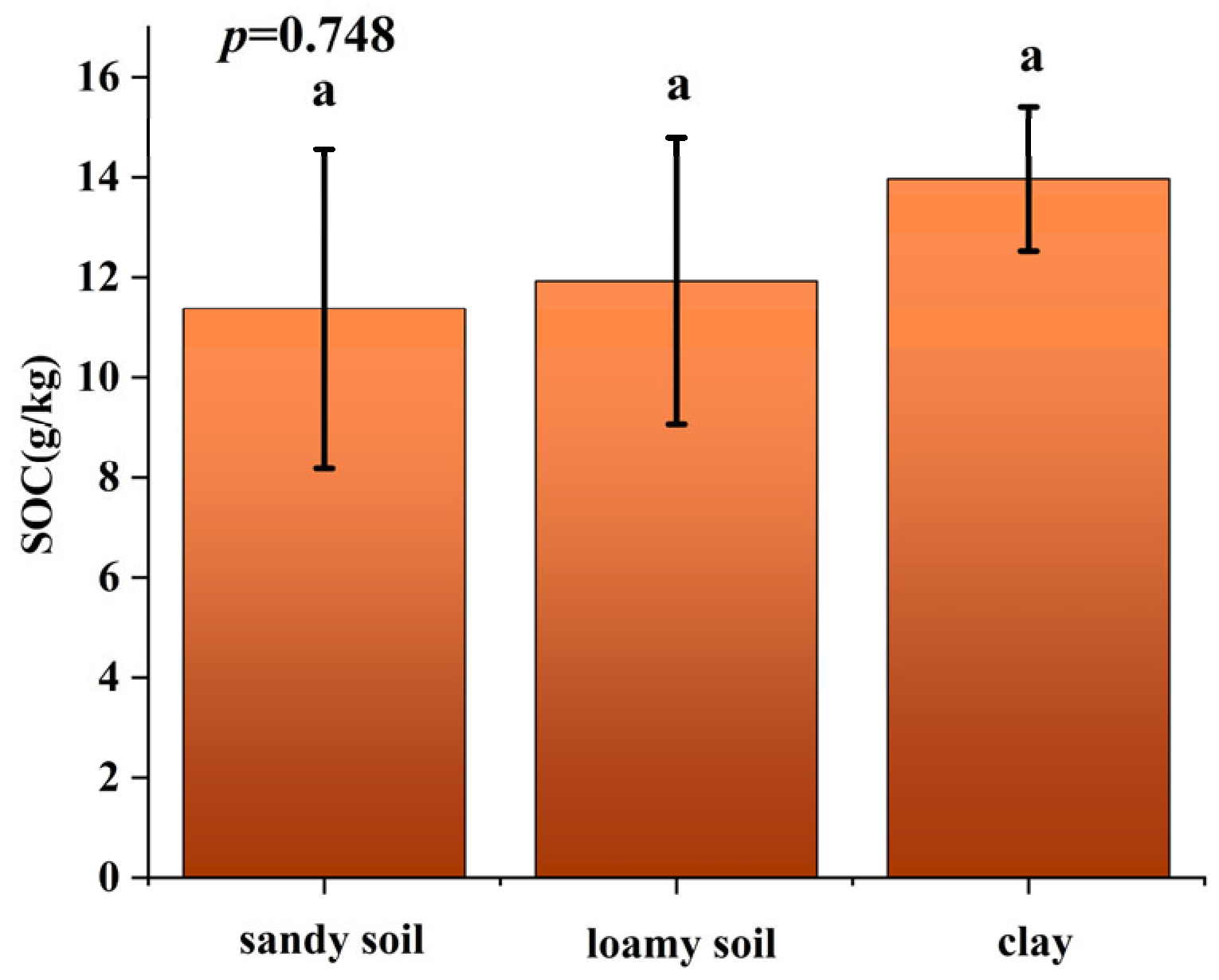
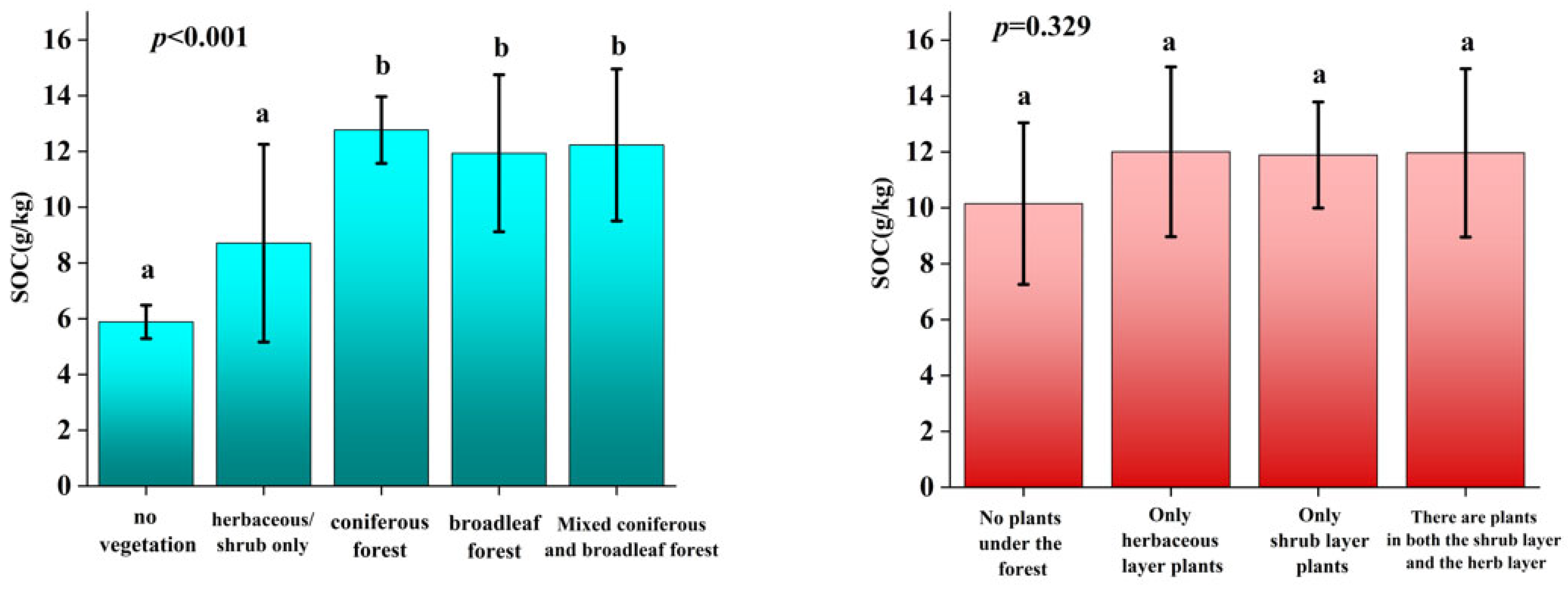
3.1. SOC Distribution by Soil and Vegetation Types
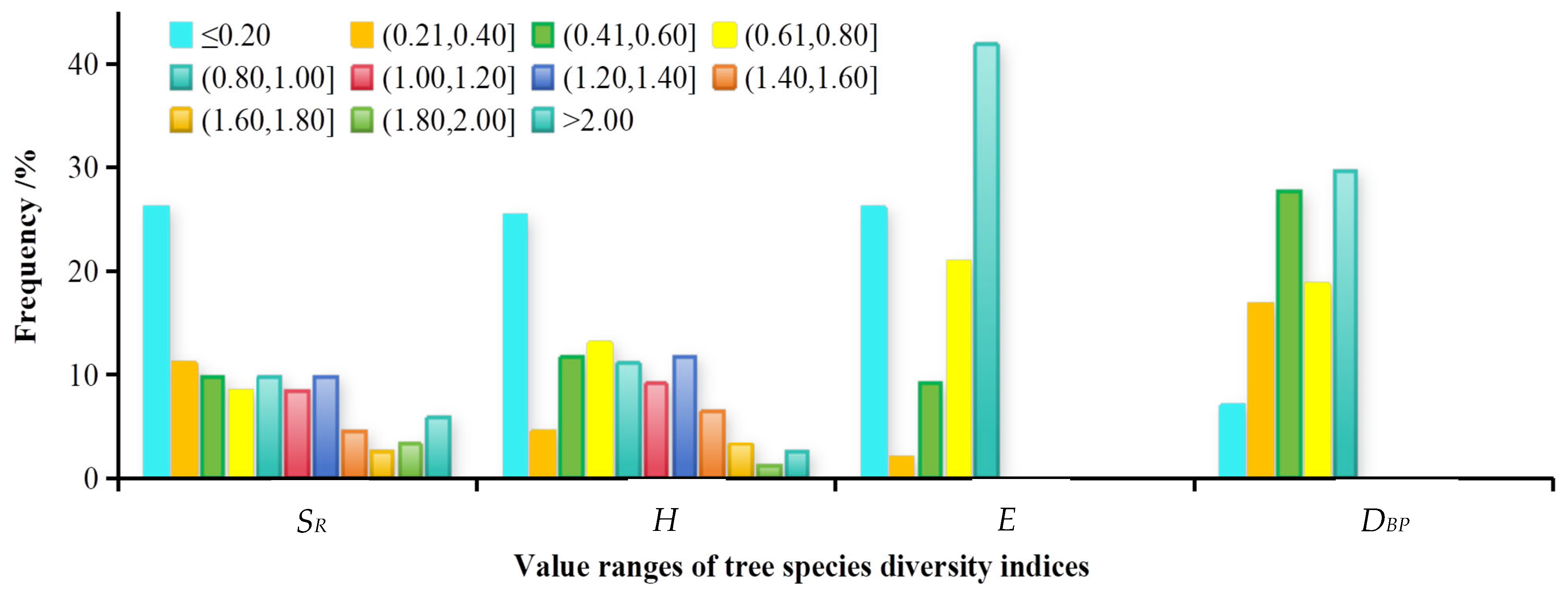
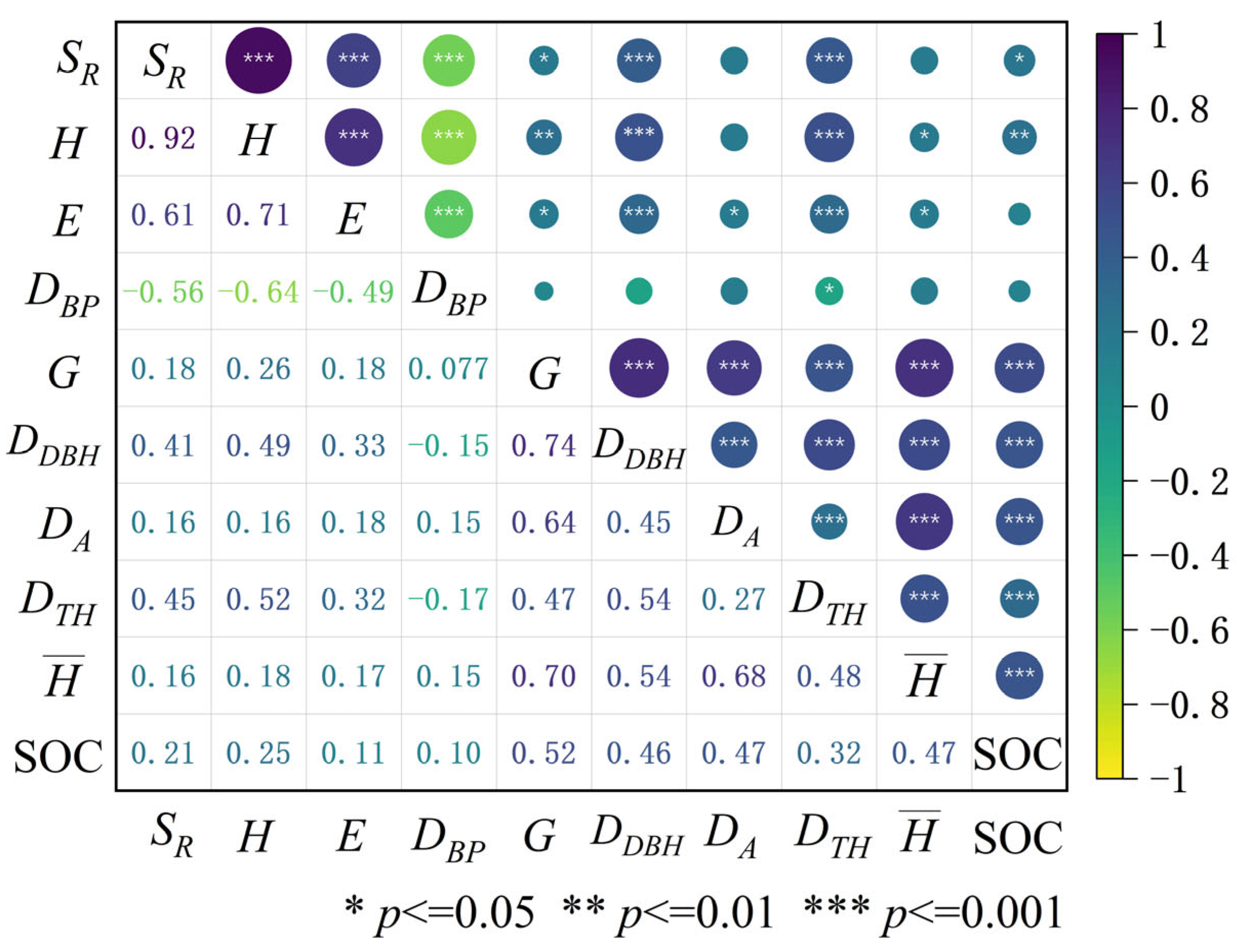
3.2. Relationships Between SOC and Forest Structure and Species Diversity Indicators
4. Discussion
4.1. Effectiveness of Tree Structure Measurements as Indicators of SOC in Urban Areas
4.2. Urban Soil as a Significant Carbon Sink
4.3. Implications for Urban Planning and Greenspace Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Tree Species Name (Scientific Name) | Family | Proportion (%) |
|---|---|---|
| Styphnolobium japonicum | Fabaceae | 12.52 |
| Populus tomentosa | Salicaceae | 11.00 |
| Pinus tabuliformis | Pinaceae | 8.70 |
| Ginkgo biloba | Ginkgoaceae | 7.53 |
| Salix matsudana | Salicaceae | 5.15 |
| Ailanthus altissima | Simaroubaceae | 4.72 |
| Fraxinus pennsylvanica | Oleaceae | 3.98 |
| Juniperus chinensis | Cupressaceae | 3.74 |
| Fraxinus velutina | Oleaceae | 3.39 |
| Eucommia ulmoides | Eucommiaceae | 2.77 |
| Prunus cerasifera ‘Atropurpurea’ | Rosaceae | 2.65 |
| Robinia pseudoacacia | Fabaceae | 2.50 |
| Koelreuteria paniculata | Sapindaceae | 2.38 |
| Platycladus orientalis | Cupressaceae | 2.34 |
| Platanus acerifolia | Platanaceae | 2.30 |
| Malus spectabilis | Rosaceae | 2.11 |
| Cedrus deodara | Pinaceae | 1.95 |
| Acer truncatum | Sapindaceae | 1.83 |
| Ulmus pumila | Ulmaceae | 1.76 |
| Echeveria ‘Peach Pride’ | Rosaceae | 1.21 |
| Salix babylonica | Salicaceae | 1.17 |
| Pinus bungeana | Pinaceae | 1.05 |
| Platanus occidentalis | Platanaceae | 0.94 |
| Juglans regia | Juglandaceae | 0.74 |
| Platanus orientalis | Platanaceae | 0.70 |
| Acer pictum subsp. mono | Sapindaceae | 0.70 |
| Toona sinensis | Meliaceae | 0.62 |
| Prunus sibirica | Rosaceae | 0.59 |
| Yulania denudata | Magnoliaceae | 0.55 |
| Lonicera maackii | Caprifoliaceae | 0.55 |
| Aesculus chinensis | Sapindaceae | 0.55 |
| Prunus triloba | Rosaceae | 0.47 |
| Broussonetia papyrifera | Moraceae | 0.43 |
| Populus nigra | Salicaceae | 0.43 |
| Quercus mongolica | Fagaceae | 0.39 |
| Sabina chinensis | Cupressaceae | 0.35 |
| Prunus davidiana | Rosaceae | 0.31 |
| Morus alba | Moraceae | 0.27 |
| Pyrus communis | Rosaceae | 0.27 |
| Yulania denudata | Magnoliaceae | 0.23 |
| Prunus serrulata | Rosaceae | 0.23 |
| Crataegus pinnatifida | Rosaceae | 0.20 |
| Amorpha fruticosa | Fabaceae | 0.20 |
| Populus nigra | Salicaceae | 0.20 |
| Lagerstroemia indica | Lythraceae | 0.20 |
| Pinus armandii | Pinaceae | 0.20 |
| Acer tataricum subsp. ginnala | Sapindaceae | 0.20 |
| Juniperus formosana | Cupressaceae | 0.20 |
| Picea asperata | Pinaceae | 0.20 |
| Prunus cerasifera | Rosaceae | 0.16 |
| Euonymus maackii | Celastraceae | 0.16 |
| Malus pumila | Rosaceae | 0.16 |
| Prunus serrulata | Rosaceae | 0.16 |
| Paulownia fortunei | Paulowniaceae | 0.16 |
| Prunus persica ‘Atropurpurea’ | Rosaceae | 0.16 |
| nectarine | Rosaceae | 0.16 |
| Populus canadensis | Salicaceae | 0.12 |
| Prunus pseudocerasus | Rosaceae | 0.12 |
| Pinus parviflora | Pinaceae | 0.12 |
| Morus nigra | Moraceae | 0.12 |
| Prunus persica | Rosaceae | 0.08 |
| Acer palmatum | Sapindaceae | 0.08 |
| Fraxinus rhynchophylla | Oleaceae | 0.08 |
| Ziziphus jujuba | Rhamnaceae | 0.08 |
| Styphnolobium japonicum ‘Pendula’ | Fabaceae | 0.08 |
| Cotinus coggygria | Anacardiaceae | 0.08 |
| Syringa oblata | Oleaceae | 0.08 |
| Taxus wallichiana | Taxaceae | 0.08 |
| Pyrus pyrifolia | Rosaceae | 0.08 |
| Diospyros kaki | Ebenaceae | 0.08 |
| Picea wilsonii | Pinaceae | 0.04 |
| Shrub Layer Species Name (Scientific Name) | Quantity of Sample Plots | Shrub Layer Species Name (Scientific Name) | Quantity of Sample Plots |
|---|---|---|---|
| Buxus megistophylla | 23 | Jasminum nudiflorum | 2 |
| Broussonetia papyrifera | 16 | Lagerstroemia indica | 2 |
| Rosa chinensis | 11 | Malus micromalus | 2 |
| Lonicera japonica | 10 | Juniperus sabina | 2 |
| Buxus sinica | 9 | Rosa xanthina | 2 |
| Ulmus pumila | 8 | Viburnum acerifolium | 1 |
| Morus alba | 6 | Jasminum mesnyi | 1 |
| Styphnolobium japonicum | 6 | Berberis amurensis | 1 |
| Forsythia suspensa | 6 | Berberis thunbergii ‘Atropurpurea’ | 1 |
| Juniperus procumbens | 6 | Metaplexis hemsleyana | 1 |
| Ailanthus altissima | 5 | Euonymus fortunei | 1 |
| Toona sinensis | 5 | Averrhoa carambola | 1 |
| Cercis chinensis | 4 | Rhamnus leptophylla | 1 |
| Ligustrum vicaryi | 4 | Prunus serrulata | 1 |
| Syringa oblata | 4 | Koelreuteria paniculata | 1 |
| Prunus triloba | 4 | Ziziphus jujuba | 1 |
| Ligustrum ovalifolium | 3 | Weigela florida | 1 |
| Echeveria ‘Peach Pride’ | 3 | Paulownia tomentosa | 1 |
| Ligustrum quihoui | 3 | Amorpha fruticosa | 1 |
| Prunus persica ‘Atropurpurea’ | 3 | Juglans regia | 1 |
| Ginkgo biloba | 3 | Platycladus orientalis | 1 |
| Populus tomentosa | 3 | Robinia pseudoacacia | 1 |
| Prunus cerasifera ‘Atropurpurea’ | 3 | Pyrus communis | 1 |
| Lespedeza chinensis | 2 | Prunus padus | 1 |
| Ilex chinensis | 2 | Sabina chinensis | 1 |
| Kerria japonica | 2 | Tilia tuan | 1 |
| Juniperus chinensis | 2 | Chimonanthus praecox | 1 |
| Hibiscus syriacus | 2 | Fraxinus pennsylvanica | 1 |
| Vitex negundo | 2 |
| Herbaceous Layer Species Name (Scientific Name) | Quantity of Sample Plots | Herbaceous Layer Species Name (Scientific Name) | Quantity of Sample Plots |
|---|---|---|---|
| Crepidiastrum sonchifolium | 45 | Abutilon theophrasti | 2 |
| Chenopodium album | 38 | Oxalis corniculata | 2 |
| Ophiopogon japonicus | 26 | Acalypha australis | 2 |
| Setaria viridis | 23 | Dodonaea viscosa | 1 |
| Taraxacum mongolicum | 22 | Veronica persica | 1 |
| Viola arcuata | 14 | Trigonotis peduncularis | 1 |
| Lolium perenne | 14 | Calamintha debilis | 1 |
| Humulus scandens | 12 | Medicago sativa | 1 |
| Plantago asiatica | 9 | Ligustrum lucidum | 1 |
| Potentilla supina | 8 | Imperata cylindrica | 1 |
| Lepidium apetalum | 8 | Nepeta cataria | 1 |
| Poa annua | 7 | Cervus canadensis | 1 |
| Artemisia argyi | 7 | Cirsium vlassovianum | 1 |
| Cirsium arvense | 7 | Ixeris chinensis | 1 |
| Hosta plantaginea | 5 | Lythrum salicaria | 1 |
| Digitaria sanguinalis | 5 | Chloris virgata | 1 |
| Iris tectorum | 5 | Vincetoxicum chinense | 1 |
| Artemisia annua | 5 | Vallisneria natans | 1 |
| Inula japonica | 4 | Oenothera biennis | 1 |
| Potentilla chinensis | 4 | Hedyotis auricularia | 1 |
| Calystegia hederacea | 4 | Melica scabrosa | 1 |
| Rubia cordifolia | 4 | Artemisia mongolica | 1 |
| Hemerocallis fulva | 4 | Prunella vulgaris | 1 |
| Eleusine indica | 4 | Hemisteptia lyrata | 1 |
| Duchesnea indica | 3 | Rumex crispus | 1 |
| Phyllostachys edulis | 3 | Cynanchum bungei | 1 |
| Hemerocallis citrina | 3 | Ipomoea nil | 1 |
| Iris lactea | 3 | Elymus kamoji | 1 |
| Bassia scoparia | 3 | Aster hispidus | 1 |
| Convolvulus arvensis | 3 | Leptopus chinensis | 1 |
| Carex duriuscula | 3 | Ceratopteris thalictroides | 1 |
| Lespedeza bicolor | 3 | Solanum nigrum | 1 |
| Ophiopogon bodinieri | 3 | Galinsoga quadriradiata | 1 |
| Persicaria lapathifolia | 3 | Eriochloa villosa | 1 |
| Erigeron canadensis | 2 | Impatiens balsamina | 1 |
| Orychophragmus violaceus | 2 | Capsella bursa-pastoris | 1 |
| Phragmites australis | 2 | Arabidopsis thaliana | 1 |
| Gaillardia pulchella | 2 | Alternanthera philoxeroides | 1 |
| Artemisia capillaris | 2 | Tragopogon orientalis | 1 |
| Zea mays | 2 | Rehmannia glutinosa | 1 |
| Saussurea japonica | 2 | Plantago depressa | 1 |
| Lactuca indica | 2 | Paederia foetida | 1 |
| Cynodon dactylon | 2 | Cosmos bipinnatus | 1 |
| Adiantum capillus-veneris | 2 | Klasea centauroides | 1 |
| Sonchus oleraceus | 2 | Ipomoea purpurea | 1 |
| Bothriospermum chinense | 2 | Acorus calamus | 1 |
| Forest types | Proportion of sampling plots (%) |
| No tree | 4.5 |
| Coniferous forest | 3.8 |
| Broadleaved forest | 58.4 |
| Mixed coniferous and broadleaved forest | 33.0 |
| Understory composition | Proportion of sampling plots (%) |
| No vegetation under the forest | 11 |
| Only herbaceous plants in the understory | 28 |
| Only shrubs in the understory | 8.8 |
| The understory had both shrubs and herbaceous vegetation | 53.2 |
| Soil type | Proportion of sampling plots (%) |
| Sandy soil | 35.4 |
| Loamy soil | 62.8 |
| Clay | 1.8 |
References
- United Nations Department of Economic and Social Affairs (UN DESA). The 2018 Revision of the World Urbanization Prospects 2018. Available online: https://www.un.org/zh/desa/68-world-population-projected-live-urban-areas-2050-says-un (accessed on 16 May 2018).
- Zhao, D.; Cai, J.; Xu, Y.; Liu, Y.; Yao, M. Carbon Sinks in Urban Public Green Spaces under Carbon Neutrality: A Bibliometric Analysis and Systematic Literature Review. Urban For. Urban Green. 2023, 86, 128037. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Liu, S.; Wang, J.; McDowell, W.H.; Huang, K.; Raymond, P.A.; Yang, Z.; Xia, X. Globally Elevated Greenhouse Gas Emissions from Polluted Urban Rivers. Nat. Sustain. 2024, 7, 938–948. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wu, P.; Feng, K.; Hubacek, K.; Li, X.; Sun, L. Impacts of Urban Expansion on Terrestrial Carbon Storage in China. Environ. Sci. Technol. 2019, 53, 6834–6844. [Google Scholar] [CrossRef] [PubMed]
- Esperon-Rodriguez, M.; Tjoelker, M.G.; Lenoir, J.; Baumgartner, J.B.; Beaumont, L.J.; Nipperess, D.A.; Power, S.A.; Richard, B.; Rymer, P.D.; Gallagher, R.V. Climate Change Increases Global Risk to Urban Forests. Nat. Clim. Change 2022, 12, 950–955. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Y.; Liu, Y.; Li, X. Exploring the Correlation between Waterbodies, Green Space Morphology, and Carbon Dioxide Concentration Distributions in an Urban Waterfront Green Space: A Simulation Study Based on the Carbon Cycle. Sustain. Cities Soc. 2023, 98, 104831. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Giannico, V.; Jim, C.Y.; Sanesi, G.; Lafortezza, R. Urban Forests, Ecosystem Services, Green Infrastructure and Nature-Based Solutions: Nexus or Evolving Metaphors? Urban For. Urban Green. 2019, 37, 3–12. [Google Scholar] [CrossRef]
- Zhao, J.; Davies, C.; Veal, C.; Xu, C.; Zhang, X.; Yu, F. Review on the Application of Nature-Based Solutions in Urban Forest Planning and Sustainable Management. Forests 2024, 15, 727. [Google Scholar] [CrossRef]
- Pan, C.; Zhou, G.; Shrestha, A.K.; Chen, J.; Kozak, R.; Li, N.; Li, J.; He, Y.; Sheng, C.; Wang, G. Bamboo as a Nature-Based Solution (NbS) for Climate Change Mitigation: Biomass, Products, and Carbon Credits. Climate 2023, 11, 175. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, S.; Sahu, N.; Manika, N.; Singh, S.N.; Anto, S.; Kumar, R.; Husain, R.; Verma, A.K.; Pandey, N. Assessment of Carbon Sequestration Potential of Tropical Tree Species for Urban Forestry in India. Ecol. Eng. 2022, 181, 106692. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, S.; Zhao, S. Valuing Urban Green Spaces in Mitigating Climate Change: A City-wide Estimate of Aboveground Carbon Stored in Urban Green Spaces of China’s Capital. Glob. Change Biol. 2019, 25, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, A.; Talvitie, I.; Ottelin, J.; Heinonen, J.; Junnila, S. Carbon Sequestration and Storage Potential of Urban Residential Environment—A Review. Sustain. Cities Soc. 2022, 84, 104027. [Google Scholar] [CrossRef]
- Strohbach, M.W.; Arnold, E.; Haase, D. The Carbon Footprint of Urban Green Space—A Life Cycle Approach. Landsc. Urban Plan. 2012, 104, 220–229. [Google Scholar] [CrossRef]
- Churkina, G.; Brown, D.G.; Keoleian, G. Carbon Stored in Human Settlements: The Conterminous United States. Glob. Change Biol. 2010, 16, 135–143. [Google Scholar] [CrossRef]
- Guo, H.; Du, E.; Terrer, C.; Jackson, R.B. Global Distribution of Surface Soil Organic Carbon in Urban Greenspaces. Nat. Commun. 2024, 15, 806. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Wang, Y.; Hao, J.; Liu, Y.; Bohnett, E.; Zhang, K. Variation of Carbon Density Components with Overstory Structure of Larch Plantations in Northwest China and Its Implication for Optimal Forest Management. For. Ecol. Manag. 2021, 496, 119399. [Google Scholar] [CrossRef]
- Rödig, E.; Cuntz, M.; Rammig, A.; Fischer, R.; Taubert, F.; Huth, A. The Importance of Forest Structure for Carbon Fluxes of the Amazon Rainforest. Environ. Res. Lett. 2018, 13, 054013. [Google Scholar] [CrossRef]
- Wang, Y.; Brandt, M.; Zhao, M.; Xing, K.; Wang, L.; Tong, X.; Xue, F.; Kang, M.; Jiang, Y.; Fensholt, R. Do Afforestation Projects Increase Core Forests? Evidence from the Chinese Loess Plateau. Ecol. Indic. 2020, 117, 106558. [Google Scholar] [CrossRef]
- Forrester, D.I.; Baker, T.G.; Elms, S.R.; Hobi, M.L.; Ouyang, S.; Wiedemann, J.C.; Xiang, W.; Zell, J.; Pulkkinen, M. Self-Thinning Tree Mortality Models That Account for Vertical Stand Structure, Species Mixing and Climate. For. Ecol. Manag. 2021, 487, 118936. [Google Scholar] [CrossRef]
- Berzaghi, F.; Bretagnolle, F.; Durand-Bessart, C.; Blake, S. Megaherbivores Modify Forest Structure and Increase Carbon Stocks through Multiple Pathways. Proc. Natl. Acad. Sci. USA 2023, 120, e2201832120. [Google Scholar] [CrossRef] [PubMed]
- Hanggara, B.B.; Murdiyarso, D.; Ginting, Y.R.S.; Widha, Y.L.; Panjaitan, G.Y.; Lubis, A.A. Effects of Diverse Mangrove Management Practices on Forest Structure, Carbon Dynamics and Sedimentation in North Sumatra, Indonesia. Estuar. Coast. Shelf Sci. 2021, 259, 107467. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, F.; Song, T.; Wang, K.; Du, H. Stand Structure and Abiotic Factors Modulate Karst Forest Biomass in Southwest China. Forests 2020, 11, 443. [Google Scholar] [CrossRef]
- Picard, N. Asymmetric Competition Can Shape the Size Distribution of Trees in a Natural Tropical Forest. For. Sci. 2019, 65, 562–569. [Google Scholar] [CrossRef]
- Farrior, C.E.; Dybzinski, R.; Levin, S.A.; Pacala, S.W. Competition for Water and Light in Closed-Canopy Forests: A Tractable Model of Carbon Allocation with Implications for Carbon Sinks. Am. Nat. 2013, 181, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Prior, L.D.; Bowman, D.M.J.S. Across a Macro-Ecological Gradient Forest Competition Is Strongest at the Most Productive Sites. Front. Plant Sci. 2014, 5, 260. [Google Scholar] [CrossRef]
- Weng, E.; Dybzinski, R.; Farrior, C.E.; Pacala, S.W. Competition Alters Predicted Forest Carbon Cycle Responses to Nitrogen Availability and Elevated CO2: Simulations Using an Explicitly Competitive, Game-Theoretic Vegetation Demographic Model. Biogeosciences 2019, 16, 4577–4599. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, C.; Kundzewicz, Z.W.; Lv, G. Distribution Pattern of Tugai Forests Species Diversity and Their Relationship to Environmental Factors in an Arid Area of China. PLoS ONE 2020, 15, e0232907. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N.; et al. The Positive Effect of Plant Diversity on Soil Carbon Depends on Climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant Diversity Enhances Productivity and Soil Carbon Storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Tree Diversity Increases Decadal Forest Soil Carbon and Nitrogen Accrual. Nature 2023, 618, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased Microbial Growth, Biomass, and Turnover Drive Soil Organic Carbon Accumulation at Higher Plant Diversity. Glob. Change Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jaen, M.C.; Potvin, C. Can We Predict Carbon Stocks in Tropical Ecosystems from Tree Diversity? Comparing Species and Functional Diversity in a Plantation and a Natural Forest. New Phytol. 2011, 189, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, J.; Bao, H.; Wu, Y.; Shen, L.; Xu, X.; Chen, Z.; Smith, P.; Abdalla, M. A Systematic Analysis and Review of Soil Organic Carbon Stocks in Urban Greenspaces. Sci. Total Environ. 2024, 948, 174788. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, M.; Cong, Y.; Lv, H.; Yuan, Z. Soil Organic Carbon Storage in Urban Green Space and Its Influencing Factors: A Case Study of the 0–20 Cm Soil Layer in Guangzhou City. Land 2022, 11, 1484. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Z.; Hao, Z.; Bian, Q.; Wei, K.; Wang, C. Effects of Urban Forest Types and Traits on Soil Organic Carbon Stock in Beijing. Forests 2021, 12, 394. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Zhang, S.; Gao, H.; Huang, J.; Sun, S.; Song, X.; Fry, E.; Tian, H.; Xia, X. Urban Development Enhances Soil Organic Carbon Storage through Increasing Urban Vegetation. J. Environ. Manag. 2022, 312, 114922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Pan, Y.; Chen, Y. Impact of LUCC on Carbon Storage and Its Components in Hangzhou from 2000 to 2030. Environ. Sci. Technol. 2024, 47, 22–34. [Google Scholar] [CrossRef]
- Bherwani, H.; Banerji, T.; Menon, R. Role and Value of Urban Forests in Carbon Sequestration: Review and Assessment in Indian Context. Environ. Dev. Sustain. 2024, 26, 603–626. [Google Scholar] [CrossRef]
- Edmondson, J.L.; Davies, Z.G.; McHugh, N.; Gaston, K.J.; Leake, J.R. Organic Carbon Hidden in Urban Ecosystems. Sci. Rep. 2012, 2, 963. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Su, Y.; Wan, W.; Liu, W.; Zhang, H.; Sun, X.; Ouyang, Z.; Wang, X. Urban Plant Diversity in Relation to Land Use Types in Built-up Areas of Beijing. Chin. Geogr. Sci. 2018, 28, 100–110. [Google Scholar] [CrossRef]
- Wang, C.; Peng, Z.; Tao, K. Characteristics and development of urban forest in China. Chin. J. Ecol. 2004, 23, 88–92. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Zhang, Q.; Jia, S.; Xu, G.; Ye, B.; Han, B. Soil geochemical characteristics and influencing factors in Beijing Plain. Geophys. Geochem. Explor. 2021, 45, 502–516. [Google Scholar]
- Xue, Y.; Nan, X.; Li, X.; Yu, M.; Ma, B.; Xu, C. Effects of neighborhood competition on visual morphological traits of coniferous trees in fine-scale landscapes of urban forests. Acta Ecol. Sin. 2024, 44, 4758–4769. [Google Scholar] [CrossRef]
- Wang, L.; Fu, H.; Yang, L. Distribution of Soil pH in Beijing Urban Area. Chin. J. Soil Sci. 2006, 2, 2398–2400. [Google Scholar] [CrossRef]
- Ma, X. Study on the characteristics of pH, soluble salts and porosity in greenbelt soil in Beijing. For. Ecol. Sci. 2013, 28, 384–387. [Google Scholar]
- Luo, S.; Mao, Q.; Ma, K.; Wu, J. Spatial distribution of soil carbon and nitrogen in urban greenspace of Beijing. Acta Ecol. Sin. 2014, 34, 6011–6019. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Yang, S. Spatial variability of soil nutrients in the core area of Beijing. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2018, 47, 580–586. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, Z.; Lu, J.; Liu, J. Estimation of Forest Biomass in Beijing (China) Using Multisource Remote Sensing and Forest Inventory Data. Forests 2020, 11, 163. [Google Scholar] [CrossRef]
- Liu, J.; Yue, C.; Pei, C.; Li, X.; Zhang, Q. Prediction of Regional Forest Biomass Using Machine Learning: A Case Study of Beijing, China. Forests 2023, 14, 1008. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 30–35. ISBN 978-7-109-06644-1. [Google Scholar]
- Kan, Z.-R.; Liu, Q.-Y.; Virk, A.L.; He, C.; Qi, J.-Y.; Dang, Y.P.; Zhao, X.; Zhang, H.-L. Effects of Experiment Duration on Carbon Mineralization and Accumulation under No-Till. Soil Tillage Res. 2021, 209, 104939. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 978-94-015-7358-0. [Google Scholar]
- Magurran, A.E.; Phillip, D.A.T. Implications of Species Loss in Freshwater Fish Assemblages. Ecography 2001, 24, 645–650. [Google Scholar] [CrossRef]
- Meng, X.Y. Dendrology, 3rd ed.; China Forestry Publishing House: Beijing, China, 2006; ISBN 978-7-5038-4169-9. [Google Scholar]
- Ali, A.; Yan, E.-R.; Chen, H.Y.H.; Chang, S.X.; Zhao, Y.-T.; Yang, X.-D.; Xu, M.-S. Stand Structural Diversity Rather than Species Diversity Enhances Aboveground Carbon Storage in Secondary Subtropical Forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef]
- Steinparzer, M.; Schaubmayr, J.; Godbold, D.L.; Rewald, B. Particulate Matter Accumulation by Tree Foliage Is Driven by Leaf Habit Types, Urbanization- and Pollution Levels. Environ. Pollut. 2023, 335, 122289. [Google Scholar] [CrossRef] [PubMed]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, D.; Wang, Z.; Harrison, M.T.; Liu, K.; Song, Z.; Chen, F.; Yin, X. Challenges and Strategies in Estimating Soil Organic Carbon for Multi-Cropping Systems: A Review. Carbon Footpr. 2024, 3, 19. [Google Scholar] [CrossRef]
- Jandl, R.; Rodeghiero, M.; Martinez, C.; Cotrufo, M.F.; Bampa, F.; van Wesemael, B.; Harrison, R.B.; Guerrini, I.A.; Richter, D.D.; Rustad, L.; et al. Current Status, Uncertainty and Future Needs in Soil Organic Carbon Monitoring. Sci. Total Environ. 2014, 468–469, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Canedoli, C.; Ferrè, C.; Abu El Khair, D.; Comolli, R.; Liga, C.; Mazzucchelli, F.; Proietto, A.; Rota, N.; Colombo, G.; Bassano, B.; et al. Evaluation of Ecosystem Services in a Protected Mountain Area: Soil Organic Carbon Stock and Biodiversity in Alpine Forests and Grasslands. Ecosyst. Serv. 2020, 44, 101135. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is Tree Species Diversity or Species Identity the More Important Driver of Soil Carbon Stocks, C/N Ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Birdsey, R.A.; Pan, Y. Biomass and NPP Estimation for the Mid-Atlantic Region (USA) Using Plot-Level Forest Inventory Data. Ecol. Appl. 2001, 11, 1174–1193. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. National-Scale Biomass Estimators for United States Tree Species. For. Sci. 2003, 49, 12–35. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon Allocation in Forest Ecosystems. Glob. Change Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root Effects on Soil Organic Carbon: A Double-Edged Sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Frangi, P.; Comin, S.; Vigevani, I.; Rettori, A.A.; Brunetti, C.; Moura, B.B.; Ferrini, F. Effects of Pavements on Established Urban Trees: Growth, Physiology, Ecosystem Services and Disservices. Landsc. Urban Plan. 2022, 226, 104501. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Yao, L. Soil Microbial Community and Physicochemical Properties Together Drive Soil Organic Carbon in Cunninghamia Lanceolata Plantations of Different Stand Ages. PeerJ 2022, 10, e13873. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, X.; Zhou, S.; Liu, X.; Lie, Z.; Carlos Ramos Aguila, L.; Xu, W.; Liu, J. Soil Organic Carbon Sources Exhibit Different Patterns with Stand Age in Rhizosphere and Non-Rhizosphere Soils. CATENA 2025, 248, 108579. [Google Scholar] [CrossRef]
- Ma, J.; Jia, B. Diversity and Structural Characteristics of Woody Plants in the Greenbelt Attached to Urban Roads in the Sixth Ring Road of Beijing. Sci. Silvae Sin. 2019, 55, 13–21. [Google Scholar] [CrossRef]
- Wei, Y.; Xiong, X.; Ryo, M.; Badgery, W.B.; Bi, Y.; Yang, G.; Zhang, Y.; Liu, N. Repeated Litter Inputs Promoted Stable Soil Organic Carbon Formation by Increasing Fungal Dominance and Carbon Use Efficiency. Biol. Fertil. Soils 2022, 58, 619–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.; You, Y.; Guo, X.; Wu, C.; Liu, S.; Sun, O.J. Differential Effects of Forest-Floor Litter and Roots on Soil Organic Carbon Formation in a Temperate Oak Forest. Soil Biol. Biochem. 2023, 180, 109017. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil Carbon Sequestration—An Interplay between Soil Microbial Community and Soil Organic Matter Dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Sokol, N.W.; Van Groenigen, K.J.; Bradford, M.A.; Ji, D.; Crowther, T.W.; Liang, C.; Luo, Y.; Kuzyakov, Y.; Wang, J.; et al. A Stoichiometric Approach to Estimate Sources of Mineral-associated Soil Organic Matter. Glob. Change Biol. 2024, 30, e17092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, S.; Jiang, W.; Tan, W.; Mao, R. Shrub Encroachment Balances Soil Organic Carbon Pool by Increasing Carbon Recalcitrance in a Temperate Herbaceous Wetland. Plant Soil 2021, 464, 347–357. [Google Scholar] [CrossRef]
- Xu, X.; Jin, Y.; Xu, J.; Zhang, Y.; Yang, J. Effects of Herbaceous Plant Encroachment on the Soil Carbon Pool in the Shrub Tundra of the Changbai Mountains. Forests 2025, 16, 197. [Google Scholar] [CrossRef]
- Chien, S.-C.; Krumins, J.A. Natural versus Urban Global Soil Organic Carbon Stocks: A Meta-Analysis. Sci. Total Environ. 2022, 807, 150999. [Google Scholar] [CrossRef] [PubMed]
- Vasenev, V.; Kuzyakov, Y. Urban Soils as Hot Spots of Anthropogenic Carbon Accumulation: Review of Stocks, Mechanisms and Driving Factors. Land Degrad. Dev. 2018, 29, 1607–1622. [Google Scholar] [CrossRef]
- Douglas, O.; Lennon, M.; Scott, M. Green Space Benefits for Health and Well-Being: A Life-Course Approach for Urban Planning, Design and Management. Cities 2017, 66, 53–62. [Google Scholar] [CrossRef]
- Daniels, B.; Zaunbrecher, B.S.; Paas, B.; Ottermanns, R.; Ziefle, M.; Roß-Nickoll, M. Assessment of Urban Green Space Structures and Their Quality from a Multidimensional Perspective. Sci. Total Environ. 2018, 615, 1364–1378. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.O. The Ecology of Tree Roots and the Practical Significance Thereof. Arboric. Urban For. (AUF) 1982, 8, 197–211. [Google Scholar] [CrossRef]

| Indicator Name | Model | Explaining | References |
|---|---|---|---|
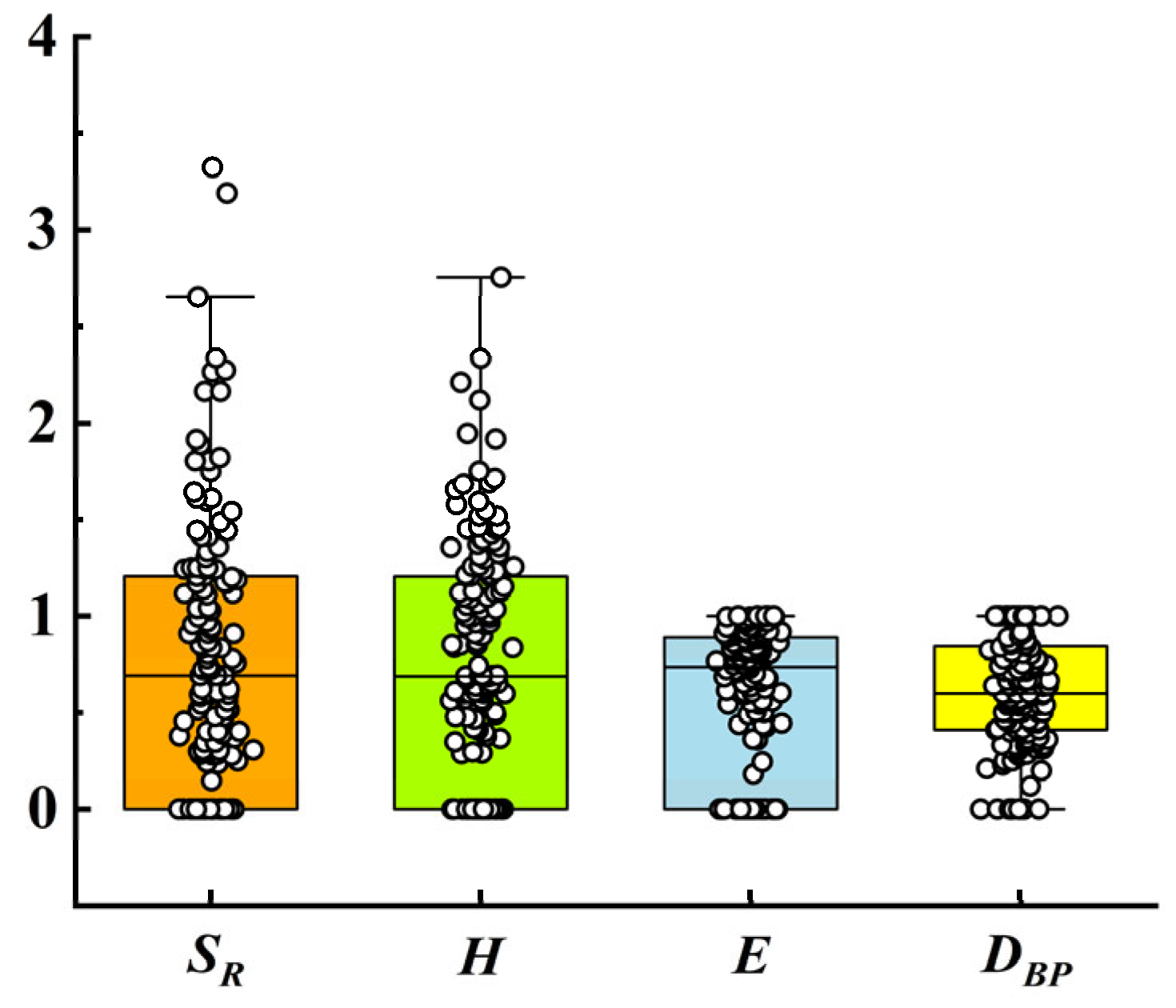
| Margalef richness Index (SR) | s is the number of species; N is the total number of individuals of all species. | [52] (page 11) | |
| Shannon–Wiener Index (H) | pi is the proportion of species i to all of trees. s is the total number of species | [52] (page 35) | |
| Pielou evenness (E) | S is the number of all species; H is the Shannon–Wiener Diversity Index. | [52] (page 37) | |
| Berger–Parker dominance (DBP) | Nmax is the abundance of the species with the highest relative abundance. NT is the total abundance. | [53] | |
| Basal area density (Total basal area per hectare) (G) | gi represents the basal area (m2) of the ith tree at breast height; N represents the total number of trees in the stand; A represents the area of the inventory plot (hm2). We used G instead of number of trees per hectare to express tree density. | [54] (page 113) | |
| Average DBH (DA) | Di is the diameter at breast height (DBH) of the ith tree. n is the number of trees. | [54] (page 50) | |
| Average tree height () | is the arithmetic average height of trees of the ith diameter class; Gi is cross area at breast height of trees in each diameter class, and k is the number of diameter classes. | [54] (page 51) | |
| Diversity of DBH (DDBH) | qj is the proportion of jth diameter class to all of trees. m is total number of diameter classes. We divided the diameter class by a 2 cm interval. DDBH mainly reflects horizontal spatial heterogeneity of trees in urban forests. | [55] | |
| Diversity of tree height (DTH) | rl is the proportion of lth height class to all of trees. t is total number of height classes. We divided the height class by 2 m intervals. DTH mainly reflects vertical spatial heterogeneity of trees in urban forests. | [55] |
| Predictor Groups | |||
|---|---|---|---|
| 4 Species Diversity Indicators | 5 Structure Indicators | All 9 Indicators | |
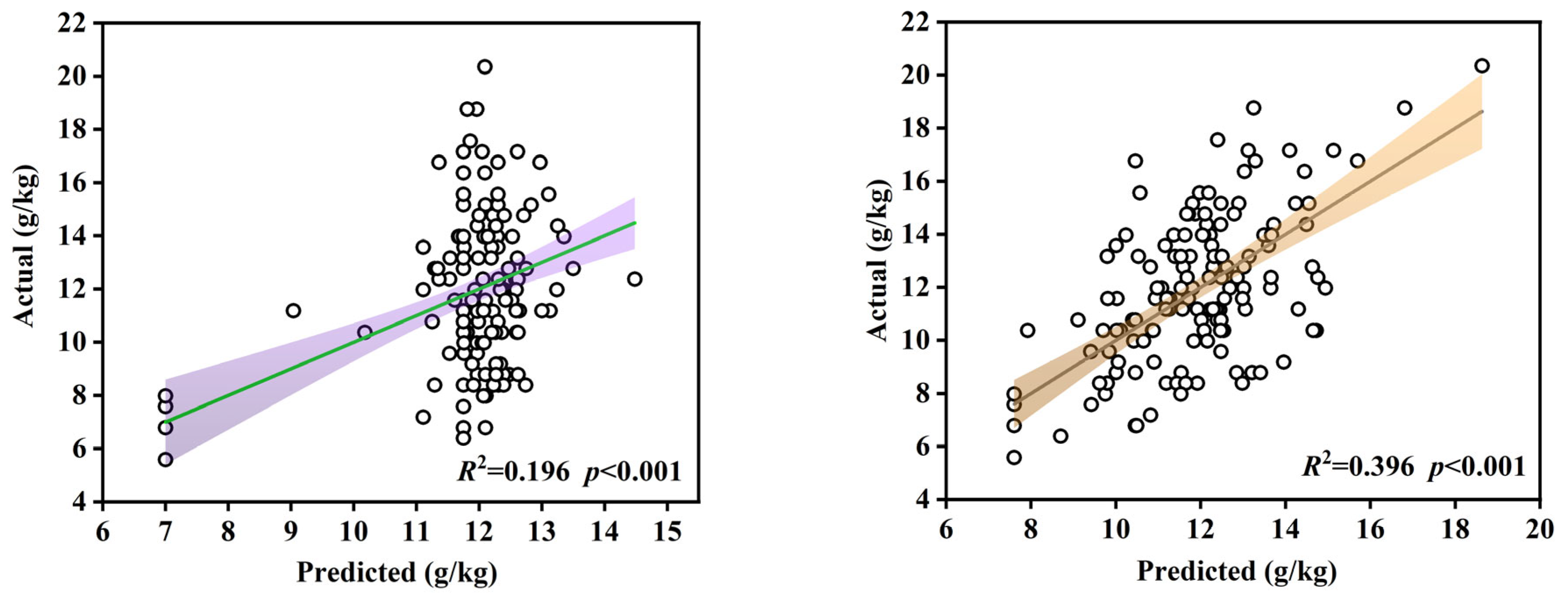
| Adjusted R2 | 0.196 | 0.396 | 0.396 |
| RMSE | 2.66 | 2.31 | 2.31 |
| Indicators selected by SLR | H (<0.001) DBP (<0.001) | DDBH (0.003) G (0.004) (0.010) | DDBH (0.003) G (0.004) (0.010) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Shah, S.M.H.; Xu, C.; Li, X.; Li, S.; Ma, B. Positive Relationships Between Soil Organic Carbon and Tree Physical Structure Highlights Significant Carbon Co-Benefits of Beijing’s Urban Forests. Forests 2025, 16, 1206. https://doi.org/10.3390/f16081206
Xie R, Shah SMH, Xu C, Li X, Li S, Ma B. Positive Relationships Between Soil Organic Carbon and Tree Physical Structure Highlights Significant Carbon Co-Benefits of Beijing’s Urban Forests. Forests. 2025; 16(8):1206. https://doi.org/10.3390/f16081206
Chicago/Turabian StyleXie, Rentian, Syed M. H. Shah, Chengyang Xu, Xianwen Li, Suyan Li, and Bingqian Ma. 2025. "Positive Relationships Between Soil Organic Carbon and Tree Physical Structure Highlights Significant Carbon Co-Benefits of Beijing’s Urban Forests" Forests 16, no. 8: 1206. https://doi.org/10.3390/f16081206
APA StyleXie, R., Shah, S. M. H., Xu, C., Li, X., Li, S., & Ma, B. (2025). Positive Relationships Between Soil Organic Carbon and Tree Physical Structure Highlights Significant Carbon Co-Benefits of Beijing’s Urban Forests. Forests, 16(8), 1206. https://doi.org/10.3390/f16081206







