Characterization and Mapping of Conservation Hotspots for the Climate-Vulnerable Conifers Abies nephrolepis and Picea jezoensis in Northeast Asia
Abstract
1. Introduction
2. Materials and Methods
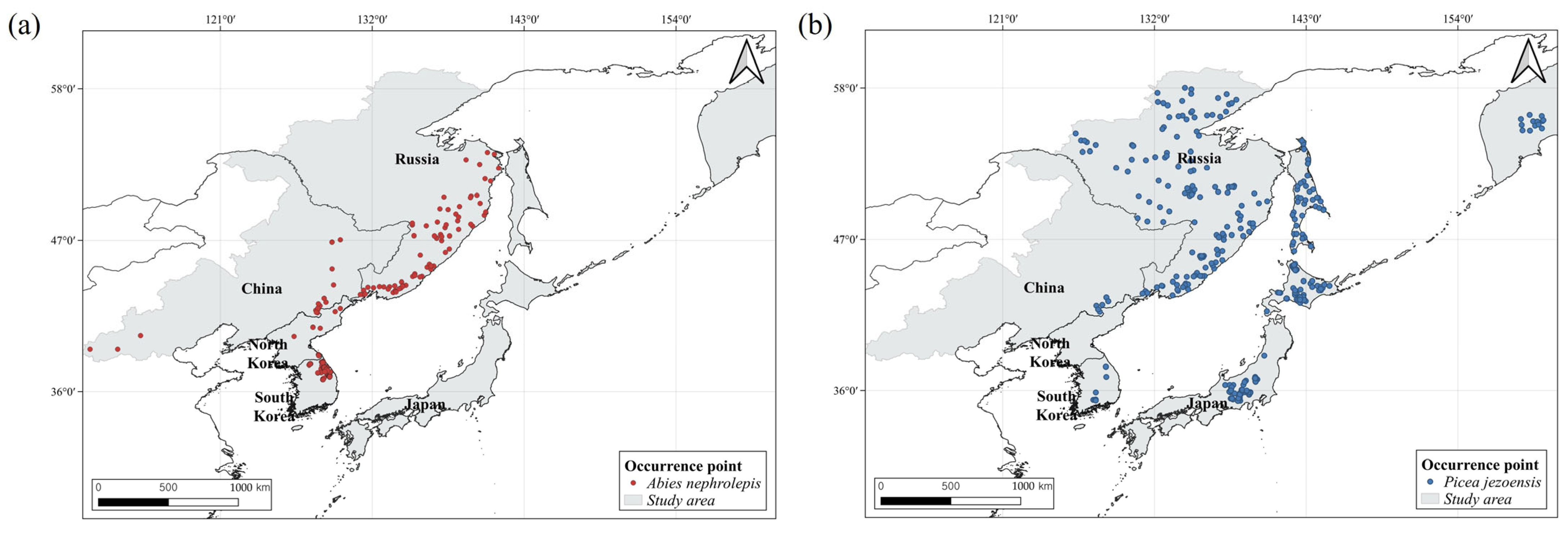
2.1. Species Occurrence Data
2.2. Environmental Data
2.3. Model Development
2.4. Model Evaluation
2.5. Analysis of Potential Hot Spot Areas for Species Conservation
3. Results
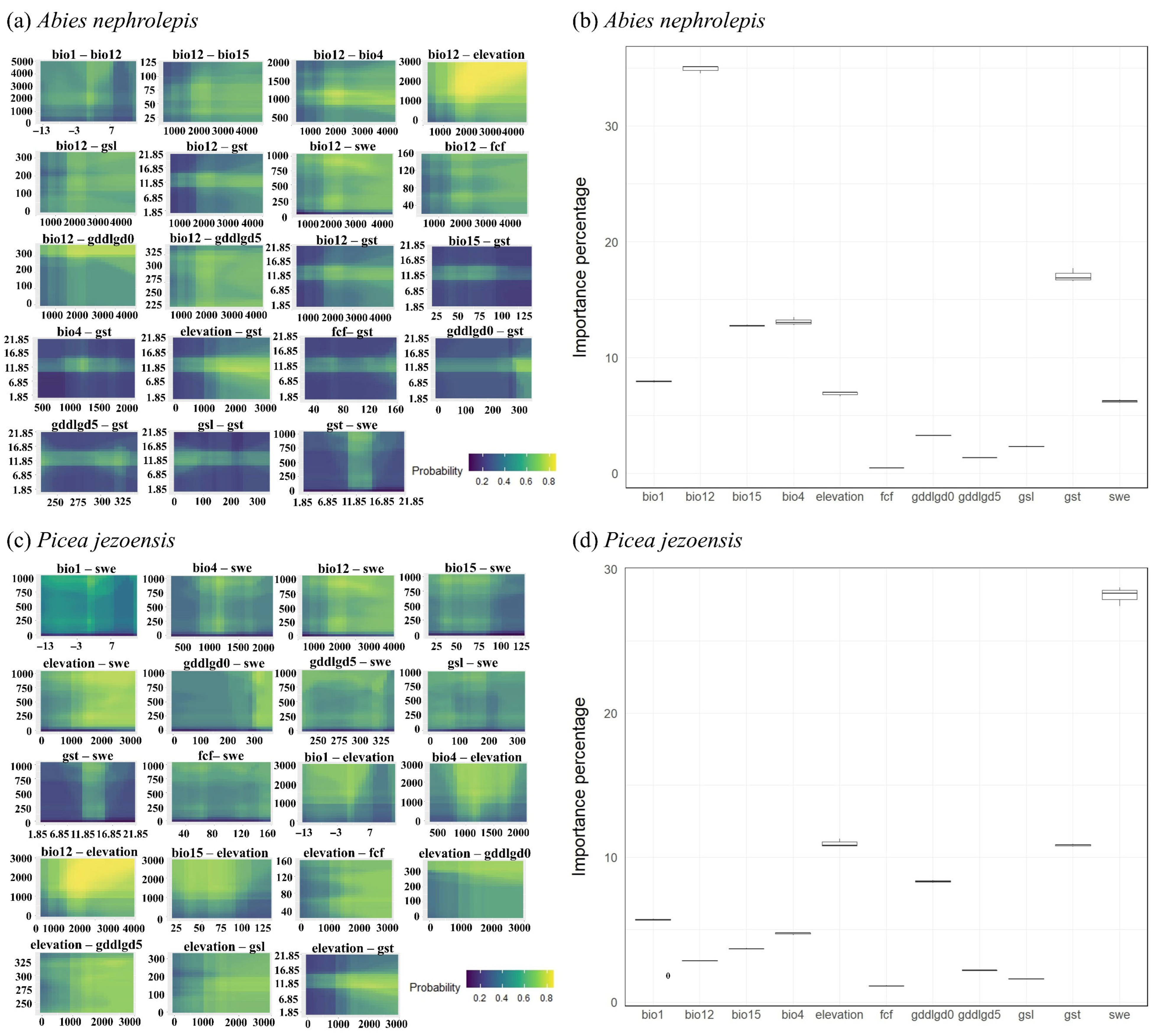
3.1. Model Evaluation and Importance of Factors
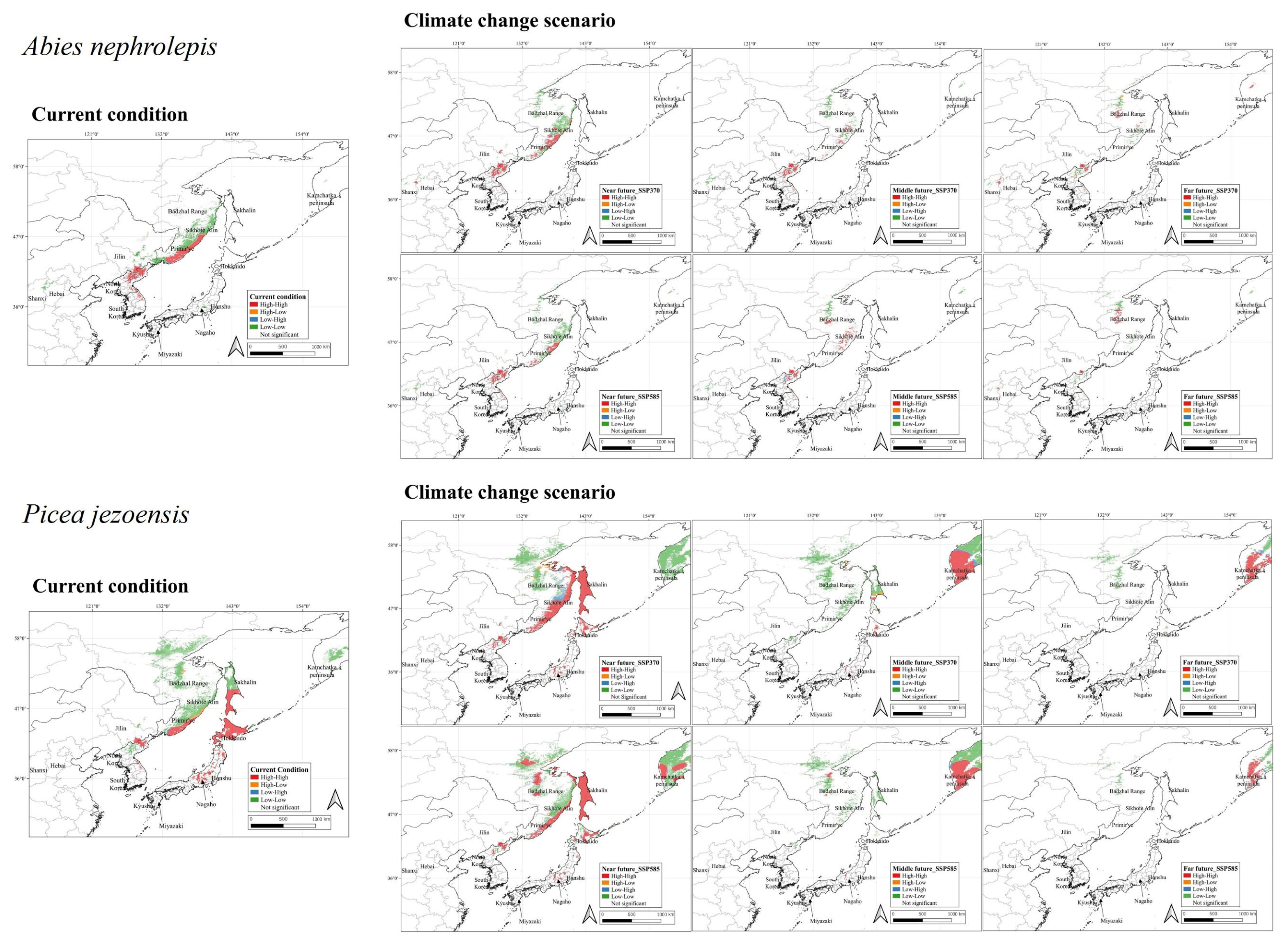
3.2. Potential Distribution of A. nephrolepis and P. jezoensis Under Current Climate
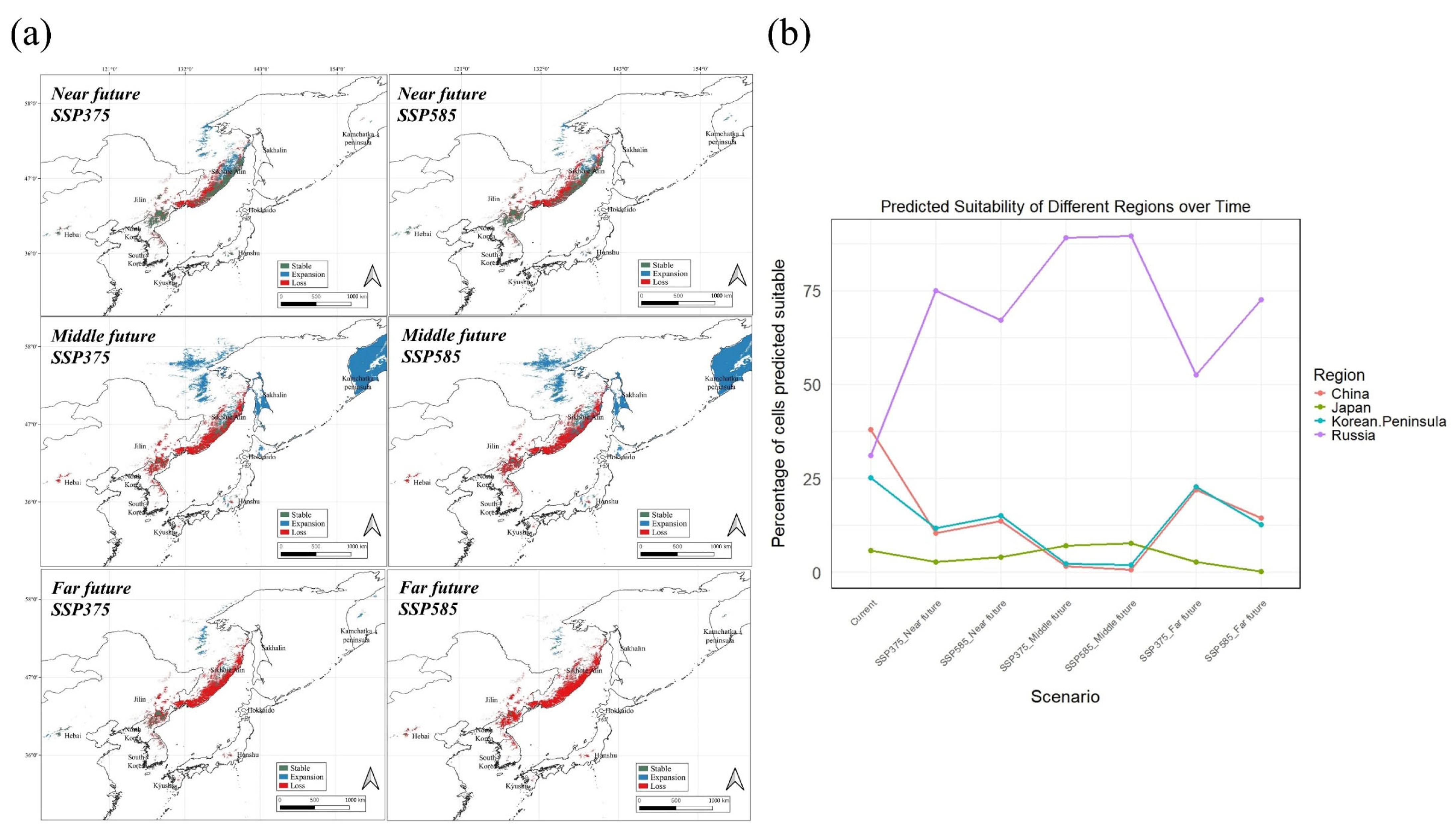
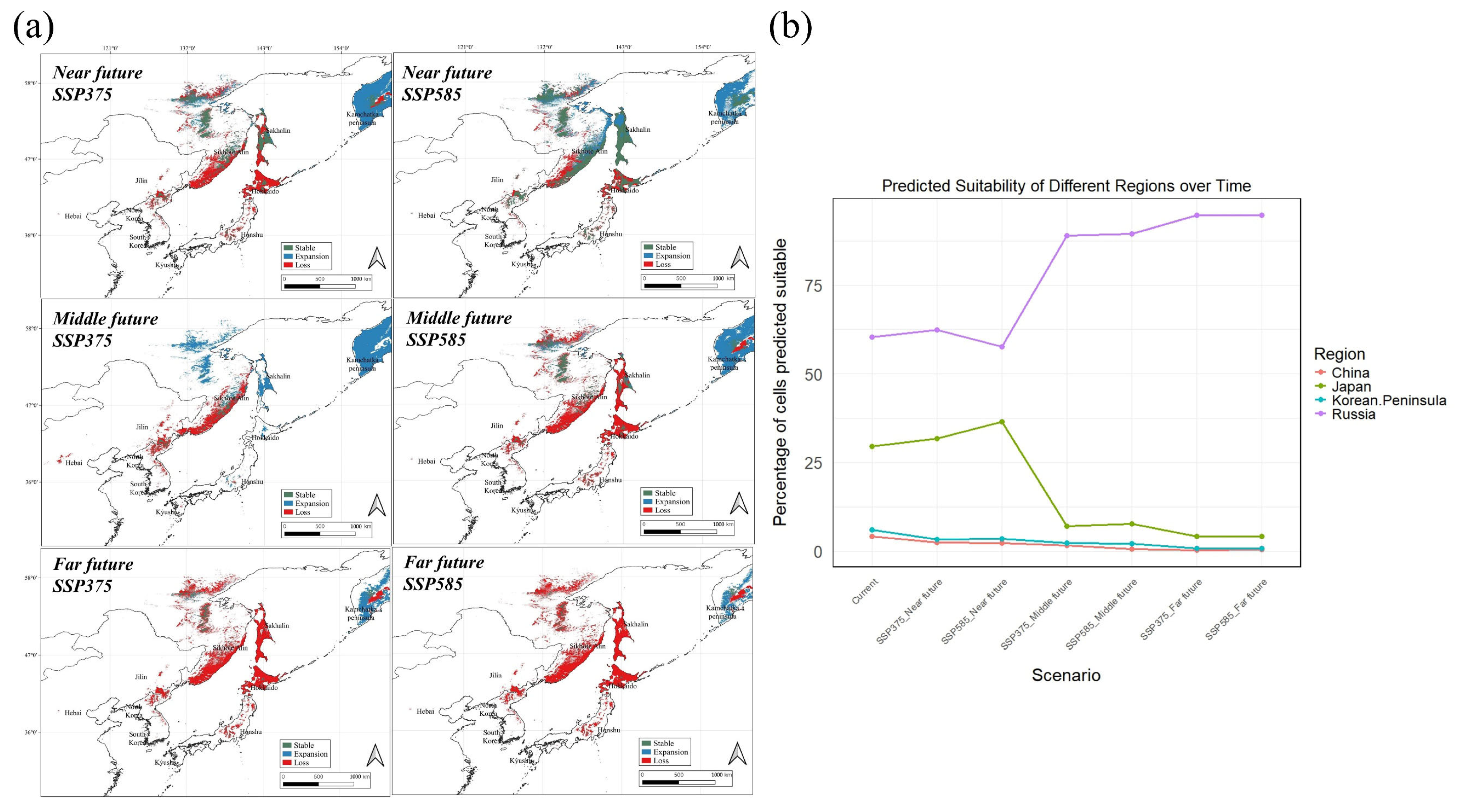
3.3. Potential Distribution of A. nephrolepis and P. jezoensis Under Future Climate
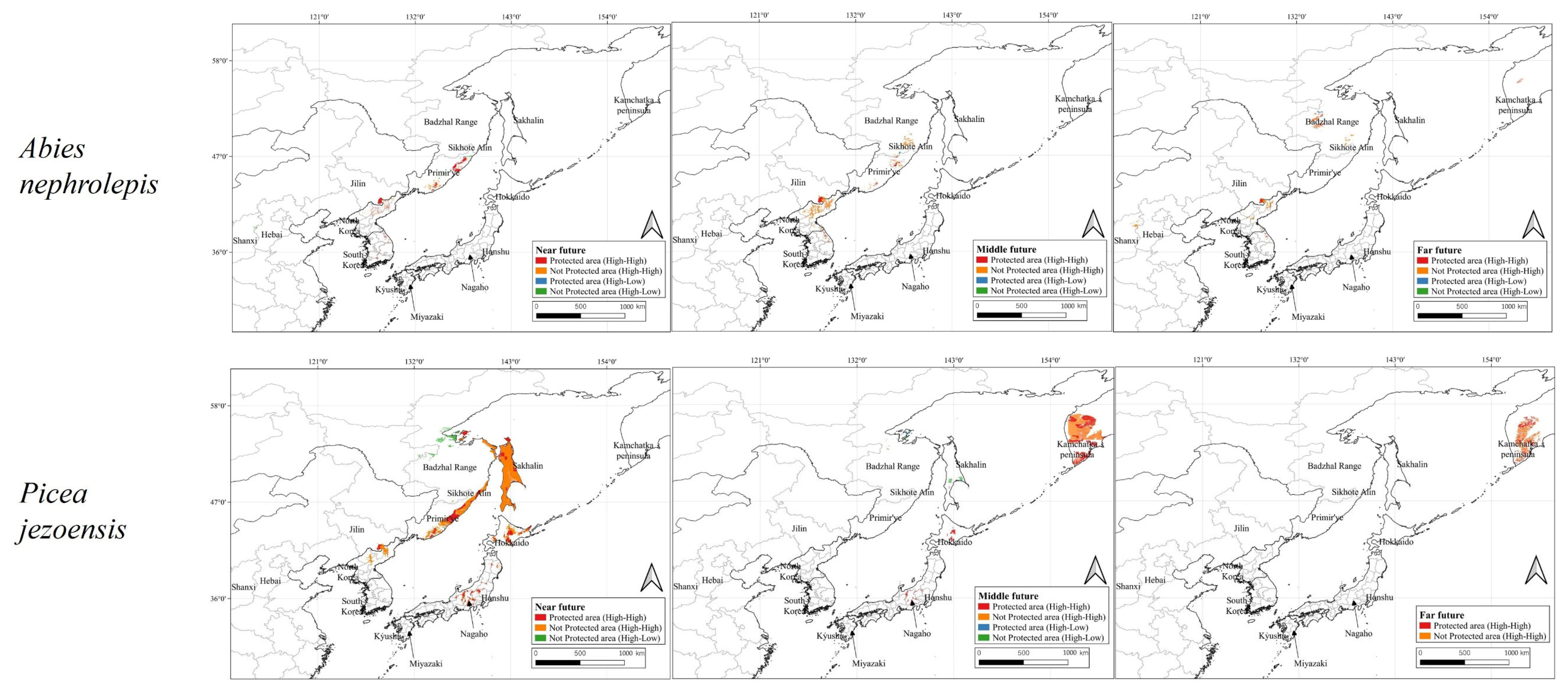
3.4. Hotspot Identification for Conservation
4. Discussion
4.1. Model Evaluation and Key Environmental Factors Affecting A. nephrolepis and P. jezoensis Distribution
4.2. Exploring Changes in Distribution According to Climate Change Scenarios
4.3. Conservation Priority Areas Strategy Proposals
4.4. Importance and Challenges of Ecological Corridors Between Protected Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dixon, R.K.; Solomon, A.; Brown, S.; Houghton, R.; Trexier, M.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Schaub, M.; Matyssek, R.; Wieser, G.; Augustaitis, A.; Bastrup-Birk, A.; Bytnerowicz, A.; Günthardt-Goerg, M.; Müller-Starck, G.; Serengil, Y. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environ. Pollut. 2010, 158, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. A matter of tree longevity. Science 2017, 355, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.J.; Fu, Q.; Randel, W.J.; Reichler, T.J. Widening of the tropical belt in a changing climate. Nat. Geosci. 2008, 1, 21–24. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Miller, A.D.; Mohan, J.E.; Hudiburg, T.W.; Duval, B.D.; DeLucia, E.H. Altered dynamics of forest recovery under a changing climate. Glob. Change Biol. 2013, 19, 2001–2021. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef] [PubMed]
- Forzieri, G.; Dakos, V.; McDowell, N.G.; Ramdane, A.; Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 2022, 608, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Bose, A.K.; Gessler, A.; Bolte, A.; Bottero, A.; Buras, A.; Cailleret, M.; Camarero, J.J.; Haeni, M.; Hereş, A.M.; Hevia, A.; et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Change Biol. 2020, 26, 4521–4537. [Google Scholar] [CrossRef] [PubMed]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Change Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhou, X.; van Groenigen, K.J.; Zhou, G.; Zhou, H.; Zhou, L.; Lu, M.; Xia, J.; Jiang, L.; Hungate, B.A.; et al. Warming effects on grassland productivity depend on plant diversity. Glob. Ecol. Biogeogr. 2022, 31, 588–598. [Google Scholar] [CrossRef]
- Lee, S.-J.; Shin, D.-B.; Byeon, J.-G.; Oh, S.-H. Climate Change Vulnerability Assessment and ecological characteristics study of Abies nephrolepis in South Korea. Forests 2023, 14, 855. [Google Scholar] [CrossRef]
- Delcourt, H.R.; Delcourt, P.A.; Webb, T., III. Dynamic plant ecology: The spectrum of vegetational change in space and time. Quat. Sci. Rev. 1982, 1, 153–175. [Google Scholar] [CrossRef]
- Williams, J.W.; Shuman, B.N.; Webb, T., III. Dissimilarity analyses of late-Quaternary vegetation and climate in eastern North America. Ecology 2001, 82, 3346–3362. [Google Scholar] [CrossRef]
- Kong, W. Species composition and distribution of native Korean conifers. J. Geol. Soc. 2004, 39, 528–543. [Google Scholar]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.P.; Lenoir, J.; Mai, G.S.; Kuo, H.C.; Chen, I.C.; Shen, S.F. Climate velocities and species tracking in global mountain regions. Nature 2024, 629, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, J.A.; Comte, L.; Grenouillet, G.; Lenoir, J.; Baecher, J.A.; Bandara, R.M.W.J.; Sunday, J. Mechanisms, detection and impacts of species redistributions under climate change. Nat. Rev. Earth Environ. 2024, 5, 351–368. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Leonelli, G.; Pelfini, M.; Morra di Cella, U.; Garavaglia, V. Climate warming and the recent treeline shift in the European Alps: The role of geomorphological factors in high-altitude sites. Ambio 2011, 40, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Krestov, P.V. Coniferous forests of the temperate zone of Asia. Conifer. For. Ser. Ecosyst. World 2005, 6, 163–220. [Google Scholar]
- Petropavlovskiy, B.; Chavtur, N.; Dochevaia, N. Antropogenniye izmenenia lesnogo pokrova Primorskogo kraya [Antropogenic changes in the forests of Primorskiy region]. In Dinamika Rastitelnosti Yuga Dalnego Vostoka [Dynamics of Vegetation of the Southern Far East]; Dalnauka: Vladivostok, Russia, 1985; pp. 44–51. [Google Scholar]
- Venier, L.A.; Thompson, I.D.; Fleming, R.; Malcolm, J.; Aubin, I.; Trofymow, J.A.; Langor, D.; Sturrock, R.; Patry, C.; Outerbridge, R.; et al. Effects of natural resource development on the terrestrial biodiversity of Canadian boreal forests. Environ. Rev. 2014, 22, 457–490. [Google Scholar] [CrossRef]
- Kim, E.-S.; Lee, J.-S.; Park, G.-E.; Lim, J.-H. Change of subalpine coniferous forest area over the last 20 years. J. Korean Soc. 2019, 108, 10–20. [Google Scholar]
- Park, G.E.; Kim, E.-S.; Jung, S.-C.; Yun, C.-w.; Kim, J.-s.; Kim, J.-d.; Kim, J.; Lim, J.-H. Distribution and Stand Dynamics of Subalpine Conifer Species (Abies nephrolepis, A. koreana, and Picea jezoensis) in Baekdudaegan Protected Area. J. Korean Soc. 2022, 111, 61–71. [Google Scholar]
- Rodrigues, A.S.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Cowling, R.M.; Fishpool, L.D.; Da Fonseca, G.A.; Gaston, K.J.; Hoffmann, M.; et al. Effectiveness of the global protected area network in representing species diversity. Nature 2004, 428, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Geldmann, J.; Manica, A.; Burgess, N.D.; Coad, L.; Balmford, A. A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. USA 2019, 116, 23209–23215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; de Knegt, H.J.; Hof, A.R. The effectiveness of a large protected area to conserve a global endemism hotspot may vanish in the face of climate and land-use changes. Front. Ecol. Evol. 2022, 10, 984842. [Google Scholar] [CrossRef]
- Hannah, L.; Midgley, G.; Andelman, S.; Araújo, M.; Hughes, G.; Martinez-Meyer, E.; Pearson, R.; Williams, P. Protected area needs in a changing climate. Front. Ecol. Environ. 2007, 5, 131–138. [Google Scholar] [CrossRef]
- Stein, B.A.; Staudt, A.; Cross, M.S.; Dubois, N.S.; Enquist, C.; Griffis, R.; Hansen, L.J.; Hellmann, J.J.; Lawler, J.J.; Nelson, E.J.; et al. Preparing for and managing change: Climate adaptation for biodiversity and ecosystems. Front. Ecol. Environ. 2013, 11, 502–510. [Google Scholar] [CrossRef]
- Tingley, M.W.; Darling, E.S.; Wilcove, D.S. Fine-and coarse-filter conservation strategies in a time of climate change. Ann. N. Y. Acad. Sci. 2014, 1322, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, P.; Yue, W.; Huang, J.; Li, D.; Tian, Z. Assessing polycentric urban development in mountainous cities: The case of Chongqing Metropolitan Area, China. Sustainability 2019, 11, 2790. [Google Scholar] [CrossRef]
- Cerioni, M.; Brabec, M.; Bače, R.; Bāders, E.; Bončina, A.; Brůna, J.; Chećko, E.; Cordonnier, T.; de Koning, J.H.; Diaci, J.; et al. Recovery and resilience of European temperate forests after large and severe disturbances. Glob. Change Biol. 2024, 30, e17159. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yin, Q.; Sang, Z.; Zhu, Z.; Jia, Z.; Ma, L. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 1077. [Google Scholar] [CrossRef]
- Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- Farashi, A.; Shariati, M. Biodiversity hotspots and conservation gaps in Iran. J. Nat. Conserv. 2017, 39, 37–57. [Google Scholar] [CrossRef]
- Lanzas, M.; Hermoso, V.; de-Miguel, S.; Bota, G.; Brotons, L. Designing a network of green infrastructure to enhance the conservation value of protected areas and maintain ecosystem services. Sci. Total Environ. 2019, 651, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zeng, W.; Xie, Y.; Wang, Z.; Hu, G.; Li, Q.; Cao, R.; Zhuo, Y.; Zhang, T. Boundary delineation and grading functional zoning of Sanjiangyuan National Park based on biodiversity importance evaluations. Sci. Total Environ. 2022, 825, 154068. [Google Scholar] [CrossRef] [PubMed]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Kim, H.T.B. Lee Herbarium Vascular Plant Collection. Version 1.21. TB Lee Herbarium. 2021. Occurrence Dataset. Available online: https://doi.org/10.15468/3lchvw (accessed on 5 January 2024).
- Seregin, A. Moscow University Herbarium (MW). Version 1.312. Lomonosov Moscow State University. 2023. Occurrence Dataset. Available online: https://doi.org/10.15468/cpnhcc (accessed on 2 January 2024).
- Yang, L.; Xu, Z. 2023 Contributions of Plant Specimen Data inside China. Chinese Academy of Sciences (CAS). 2023. Occurrence Dataset. Available online: https://doi.org/10.15468/9us6fb (accessed on 3 January 2024).
- Tojo, K. Herbarium of Shinshu University. Version 1.6. National Museum of Nature and Science, Japan. 2023. Occurrence Dataset. Available online: https://doi.org/10.15468/6docrj (accessed on 5 January 2024).
- Seregin, A.; Stepanova, N. MHA Herbarium: Collections of vascular plants. Version 1.243. Tsitsin Main Botanical Garden Russian Academy of Sciences. 2024. Occurrence Dataset. Available online: https://doi.org/10.15468/827lk2 (accessed on 5 January 2024).
- Ebihara, A. Vascular plant specimens of National Museum of Nature and Science (TNS). National Museum of Nature and Science, Japan. 2023. Occurrence Dataset. Available online: https://doi.org/10.15468/6rld6e (accessed on 5 January 2024).
- Betts, M.G.; Diamond, A.; Forbes, G.; Villard, M.-A.; Gunn, J. The importance of spatial autocorrelation, extent and resolution in predicting forest bird occurrence. Ecol. Modell. 2006, 191, 197–224. [Google Scholar] [CrossRef]
- Segurado, P.; Araujo, M.B.; Kunin, W. Consequences of spatial autocorrelation for niche-based models. J. Appl. Ecol. 2006, 43, 433–444. [Google Scholar] [CrossRef]
- Dormann, C.F. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob. Ecol. Biogeogr. 2007, 16, 129–138. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Naimi, B.; Skidmore, A.K.; Groen, T.A.; Hamm, N.A. Spatial autocorrelation in predictors reduces the impact of positional uncertainty in occurrence data on species distribution modelling. J. Biogeogr. 2011, 38, 1497–1509. [Google Scholar] [CrossRef]
- Maria, B.; Udo, S. Why input matters: Selection of climate data sets for modelling the potential distribution of a treeline species in the Himalayan region. Ecol. Modell. 2017, 359, 92–102. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence–absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Samal, P.; Srivastava, J.; Singarasubramanian, S.; Saraf, P.N.; Charles, B. Ensemble modeling approach to predict the past and future climate suitability for two mangrove species along the coastal wetlands of peninsular India. Ecol. Inform. 2022, 72, 101819. [Google Scholar] [CrossRef]
- Samal, P.; Srivastava, J.; Charles, B.; Singarasubramanian, S. Species distribution models to predict the potential niche shift and priority conservation areas for mangroves (Rhizophora apiculata, R. mucronata) in response to climate and sea level fluctuations along coastal India. Ecol. Indic. 2023, 154, 110631. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Jaime, L.; Batllori, E.; Lloret, F. Bark beetle outbreaks in coniferous forests: A review of climate change effects. Eur. J. For. Res. 2024, 143, 1–17. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Marini, L.; Ayres, M.P.; Battisti, A.; Faccoli, M. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Change 2012, 115, 327–341. [Google Scholar] [CrossRef]
- Guisan, A.; Graham, C.H.; Elith, J.; Huettmann, F.; NCEAS Species Distribution Modelling Group. Sensitivity of predictive species distribution models to change in grain size. Divers. Disrib. 2007, 13, 332–340. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Ecol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 2015, 5, 424–430. [Google Scholar]
- Saatkamp, A.; Argagnon, O.; Noble, V.; Finocchiaro, M.; Meineri, E. Climate change impacts on Mediterranean vegetation are amplified at low altitudes. Glob. Ecol. Biogeogr. 2023, 32, 1113–1126. [Google Scholar] [CrossRef]
- Rather, Z.A.; Ahmad, R.; Khuroo, A.A. Ensemble modelling enables identification of suitable sites for habitat restoration of threatened biodiversity under climate change: A case study of Himalayan Trillium. Ecol. Eng. 2022, 176, 106534. [Google Scholar] [CrossRef]
- Lee, S.-J.; Byeon, J.-G.; Kim, J.-S.; Cho, J.-h.; Oh, S.-H. Modeling Habitat Suitability of the Climate-vulnerable Plant Thuja koraiensis in Response to Climate Change. Sens. Mater. 2024, 36, 1511–1523. [Google Scholar] [CrossRef]
- Jang, D.-H. The Excavation of Geomorphological Landscape Resources and Assessment of Value for Designating Ecological and Landscape Conservation Area in Mt. Ilwol. J. Assoc. Korean Geogr. 2012, 1, 205–216. [Google Scholar]
- Oh, H.-K.; Son, B.-Y.; You, J.-H. Vascular Plants and Characteristics by Type in Mt. Ilwolsan (Yeongyang, Gyeongbuk) for Designating an Ecological and Landscape Conservation Area. J. Korean Soc. Environ. Restor. Technol. 2015, 18, 43–62. [Google Scholar] [CrossRef][Green Version]
- Pisarenko, O.Y.; Fedosov, V.; Korznikov, K.A.; Shkurko, A.V.; Ignatova, E. The moss flora of the Badzhal Mountain Range (Khabarovsk Territory, Russian Far East). Bot. Pac. 2022, 11, 98–114. [Google Scholar] [CrossRef]
- Eichhorn, M.P. Boreal forests of Kamchatka: Structure and composition. Forests 2010, 1, 154–176. [Google Scholar] [CrossRef]
- IUCN. World Heritage Nomination IUCN Technical Evaluation: Volcanoes of Kamchatka (Russian Federation)—Extension to include Kluchevskoy Nature Park; UNESCO World Heritage Centre: Paris, France, 2001; 4p. [Google Scholar]
- Semerikov, V.L.; Semerikova, S.A. Genetic variation and population history of three related fir species Abies sachalinensis, A. nephrolepis and A. gracilis (Pinaceae) revealed by nuclear microsatellites. Bot. Pac. 2023, 12, 145–154. [Google Scholar] [CrossRef]
- Krestov, P.V.; Omelko, A.M.; Nakamura, Y. Vegetation and natural habitats of Kamchatka. Ber. Reinh.-Tüxen-Ges. 2008, 20, 195–218. [Google Scholar]
- Lee, S.-J.; Lee, A.-R.; Byeon, J.-G.; Oh, S.-H. Pre-drought effects on northern temperate trees and vine invasion in forest gaps hindering regeneration. Sci. Total Environ. 2024, 951, 175707. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Yoshimaru, H.; Saito, H.; Katsuki, T.; Kawahara, T.; Kitamura, K.; Shi, F.; Kaji, M. Phylogeography of a northeast Asian spruce, Picea jezoensis, inferred from genetic variation observed in organelle DNA markers. Mol. Ecol. Resour. 2007, 16, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Rammer, W.; Scheller, R.M.; Spies, T.A. An individual-based process model to simulate landscape-scale forest ecosystem dynamics. Ecol. Modell. 2012, 231, 87–100. [Google Scholar] [CrossRef]
- Buma, B.; Wessman, C. Disturbance interactions can impact resilience mechanisms of forests. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Buma, B. Disturbance interactions: Characterization, prediction, and the potential for cascading effects. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Harvey, I.F.; Corbet, P.S. Territorial behaviour of larvae enhances mating success of male dragonflies. Anim. Behav. 1985, 33, 561–565. [Google Scholar] [CrossRef]
- Haddad, N.M.; Tewksbury, J.J. Low-quality habitat corridors as movement conduits for two butterfly species. Ecol. Appl. 2005, 15, 250–257. [Google Scholar] [CrossRef]
- Kietzka, G.J.; Pryke, J.S.; Gaigher, R.; Samways, M.J. Webs of well-designed conservation corridors maintain river ecosystem integrity and biodiversity in plantation mosaics. Biol. Conserv. 2021, 254, 108965. [Google Scholar] [CrossRef]
- Chester, C.C. Yellowstone to Yukon: Transborder conservation across a vast international landscape. Environ. Sci. Policy 2015, 49, 75–84. [Google Scholar] [CrossRef]

| Variable Name | Unit | Description |
|---|---|---|
| bio1 | °C | Mean annual temperature |
| bio4 | °C/month × 100 | Temperature seasonality (standard deviation × 100) |
| bio12 | mm | Annual precipitation amount |
| bio15 | mm | Precipitation seasonality |
| fcf | count | Number of events in which tmin or tmax go above, or below 0 °C |
| gsl | number of days | Length of the growing season |
| gst | °C | Mean temperature of all growing season days based on TREELIM |
| swe | kg m−2 year−1 | Amount of liquid water if snow is melted |
| gdd1gd0 | – | Last day of the year above 0 °C |
| gdd1gd5 | – | Last day of the year above 5 °C |
| DEM | m | Digital elevation model |
| Indicators | Species | GLM | GAM | CTA | GBM | RF | XGBOOST | |
|---|---|---|---|---|---|---|---|---|
| AUC | Avg | A. nephrolepis | 0.967 | 0.957 | 0.928 | 0.975 | 0.975 | 0.969 |
| (S.D) | (0.010) | (0.019) | (0.027) | (0.008) | (0.009) | (0.011) | ||
| Max | 0.987 | 0.988 | 0.975 | 0.991 | 0.994 | 0.994 | ||
| Min | 0.941 | 0.877 | 0.851 | 0.954 | 0.948 | 0.943 | ||
| Avg | P. jezoensis | 0.872 | 0.897 | 0.857 | 0.912 | 0.912 | 0.905 | |
| (S.D) | (0.052) | (0.017) | (0.026) | (0.014) | (0.014) | (0.015) | ||
| Max | 0.930 | 0.933 | 0.905 | 0.945 | 0.943 | 0.942 | ||
| Min | 0.697 | 0.843 | 0.789 | 0.876 | 0.868 | 0.859 | ||
| TSS | Avg | A. nephrolepis | 0.804 | 0.777 | 0.788 | 0.817 | 0.814 | 0.803 |
| (S.D) | (0.037) | (0.047) | (0.047) | (0.033) | (0.039) | (0.044) | ||
| Max | 0.902 | 0.898 | 0.871 | 0.912 | 0.927 | 0.902 | ||
| Min | 0.727 | 0.658 | 0.632 | 0.742 | 0.732 | 0.681 | ||
| Avg | P. jezoensis | 0.570 | 0.614 | 0.591 | 0.644 | 0.624 | 0.601 | |
| (S.D) | (0.057) | (0.043) | (0.051) | (0.043) | (0.051) | (0.047) | ||
| Max | 0.720 | 0.729 | 0.734 | 0.744 | 0.757 | 0.714 | ||
| Min | 0.394 | 0.496 | 0.451 | 0.524 | 0.488 | 0.483 | ||
| Species | Scenario | China | Japan | Russia | Korean Peninsula | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| West | East | Kyushu | Honshu | Hokkaido | Primor’Ye–Khabarovsk | Kamchatka Peninsula | Sakhalin | Republic of Korea | North Korea | ||
| A. nephrolepis | Near370 | −13.03 | −64.67 | −81.25 | −22.14 | – | 254.42 | 8.31 | – | −60.27 | −27.60 |
| Near585 | 15.00 | −69.71 | −61.06 | −56.30 | −18.11 | −22.92 | 131.50 | – | −60.51 | −31.83 | |
| Middle370 | −99.48 | −91.26 | −99.79 | 77.14 | – | 288.95 | 9926.98 | – | −99.71 | −82.16 | |
| Middle585 | −99.07 | −97.75 | −99.79 | 33.92 | – | 156.06 | 9653.55 | – | −99.97 | −89.17 | |
| Far370 | 25.42 | −84.85 | −100.0 | −77.87 | – | −26.56 | 654.51 | – | −68.81 | −57.61 | |
| Far585 | −76.87 | −97.78 | −100.0 | −99.40 | – | −73.96 | 26.41 | – | −98.80 | −93.64 | |
| P. jezoensis | Near370 | −36.52 | −85.67 | −95.38 | −63.18 | −90.02 | −72.98 | −27.91 | −97.70 | −90.69 | −86.44 |
| Near585 | −36.59 | −86.06 | −95.14 | −58.37 | −85.92 | −72.22 | −55.57 | −96.56 | −90.19 | −84.95 | |
| Middle370 | −83.89 | −75.33 | −99.90 | −82.95 | −90.83 | −6.34 | 18.77 | −42.35 | −99.30 | −75.93 | |
| Middle585 | −71.32 | −93.66 | −99.90 | −87.11 | −93.84 | −38.34 | 15.53 | −72.10 | −99.93 | −85.39 | |
| Far370 | −84.86 | −97.73 | −100.0 | −93.88 | −97.86 | −77.65 | 354.73 | −96.31 | −100.0 | −95.49 | |
| Far585 | −96.13 | −98.44 | −100.0 | −96.66 | −98.81 | −91.57 | 189.87 | −99.22 | −100.0 | −97.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Shin, D.-B.; Byeon, J.-G.; Lee, S.-H.; Lee, D.-H.; Che, S.H.; Bae, K.H.; Oh, S.-H. Characterization and Mapping of Conservation Hotspots for the Climate-Vulnerable Conifers Abies nephrolepis and Picea jezoensis in Northeast Asia. Forests 2025, 16, 1183. https://doi.org/10.3390/f16071183
Lee S-J, Shin D-B, Byeon J-G, Lee S-H, Lee D-H, Che SH, Bae KH, Oh S-H. Characterization and Mapping of Conservation Hotspots for the Climate-Vulnerable Conifers Abies nephrolepis and Picea jezoensis in Northeast Asia. Forests. 2025; 16(7):1183. https://doi.org/10.3390/f16071183
Chicago/Turabian StyleLee, Seung-Jae, Dong-Bin Shin, Jun-Gi Byeon, Sang-Hyun Lee, Dong-Hyoung Lee, Sang Hoon Che, Kwan Ho Bae, and Seung-Hwan Oh. 2025. "Characterization and Mapping of Conservation Hotspots for the Climate-Vulnerable Conifers Abies nephrolepis and Picea jezoensis in Northeast Asia" Forests 16, no. 7: 1183. https://doi.org/10.3390/f16071183
APA StyleLee, S.-J., Shin, D.-B., Byeon, J.-G., Lee, S.-H., Lee, D.-H., Che, S. H., Bae, K. H., & Oh, S.-H. (2025). Characterization and Mapping of Conservation Hotspots for the Climate-Vulnerable Conifers Abies nephrolepis and Picea jezoensis in Northeast Asia. Forests, 16(7), 1183. https://doi.org/10.3390/f16071183






