Assessing Forest Succession Along Environment, Trait, and Composition Gradients in the Brazilian Atlantic Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
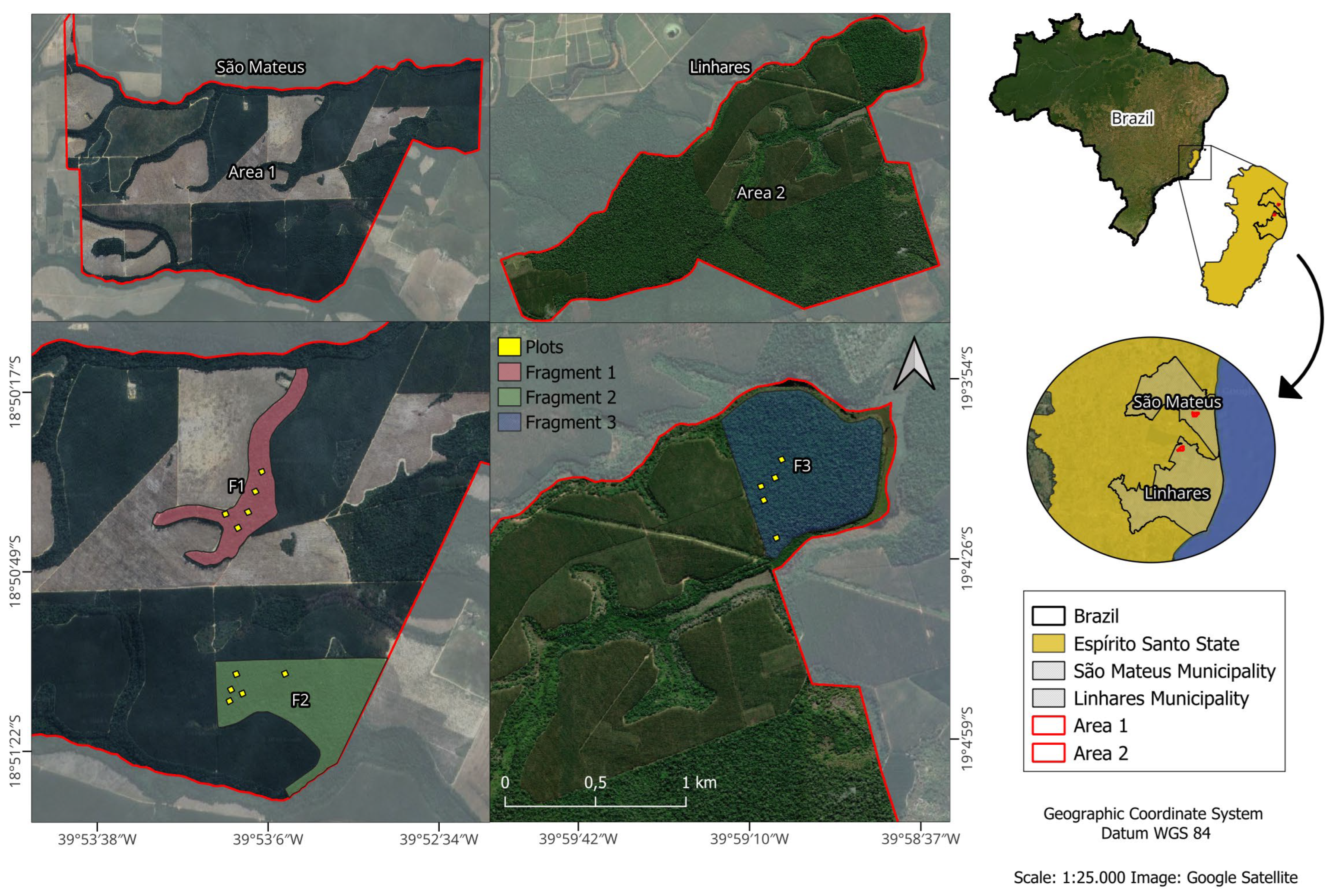

2.2. Data Collection in the Tree and Regenerating Strata
2.3. Canopy Openness Assessment
2.4. Soil Physical-Chemical Attributes
2.5. Data Analysis
2.6. Vegetation-Environment Relationship
2.6.1. Species Abundance Matrix
2.6.2. Environmental and Successional Data Matrix
3. Results
3.1. Ecological Indicators of the Successional Trajectories of the Tree and Regenerating Strata
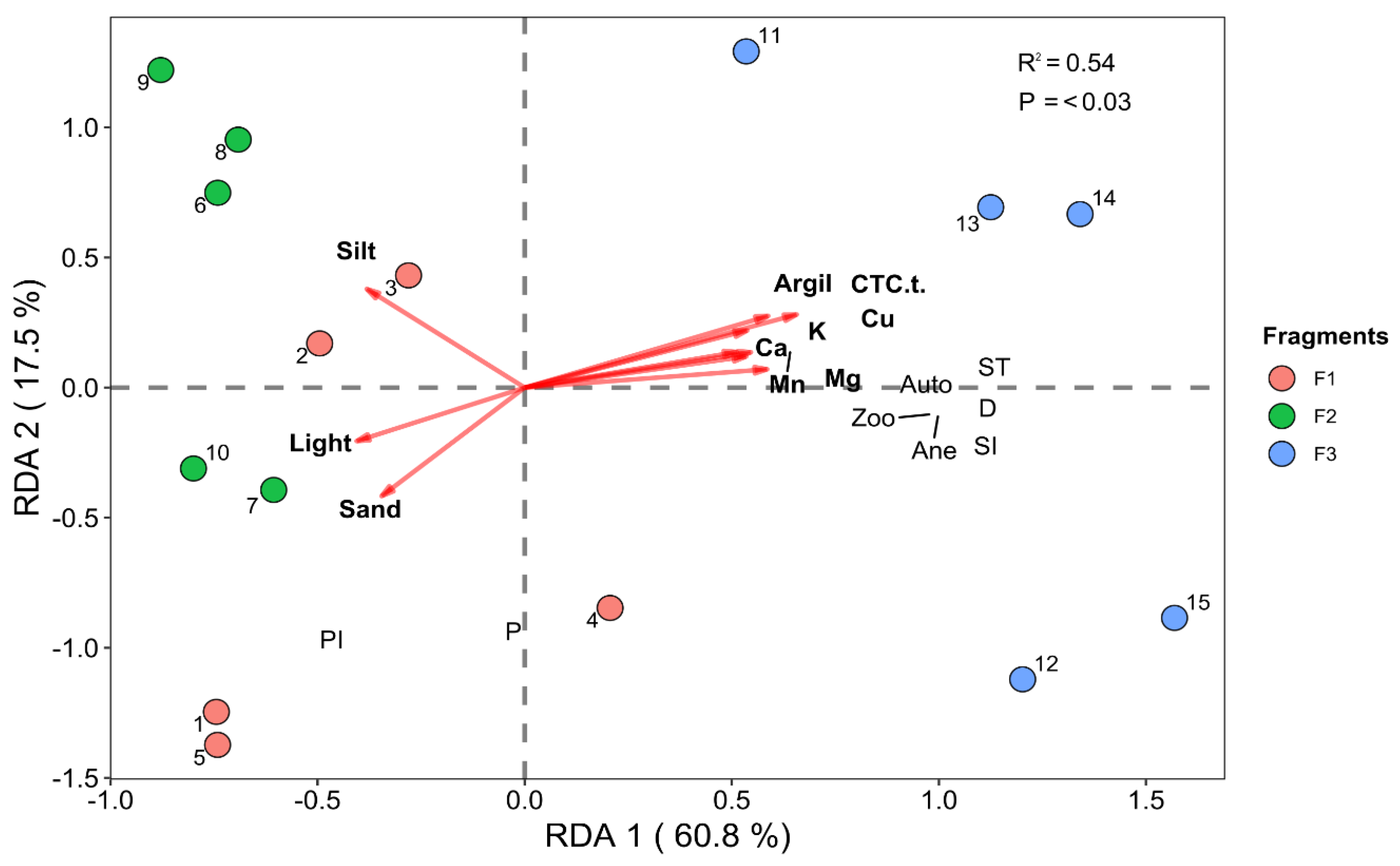
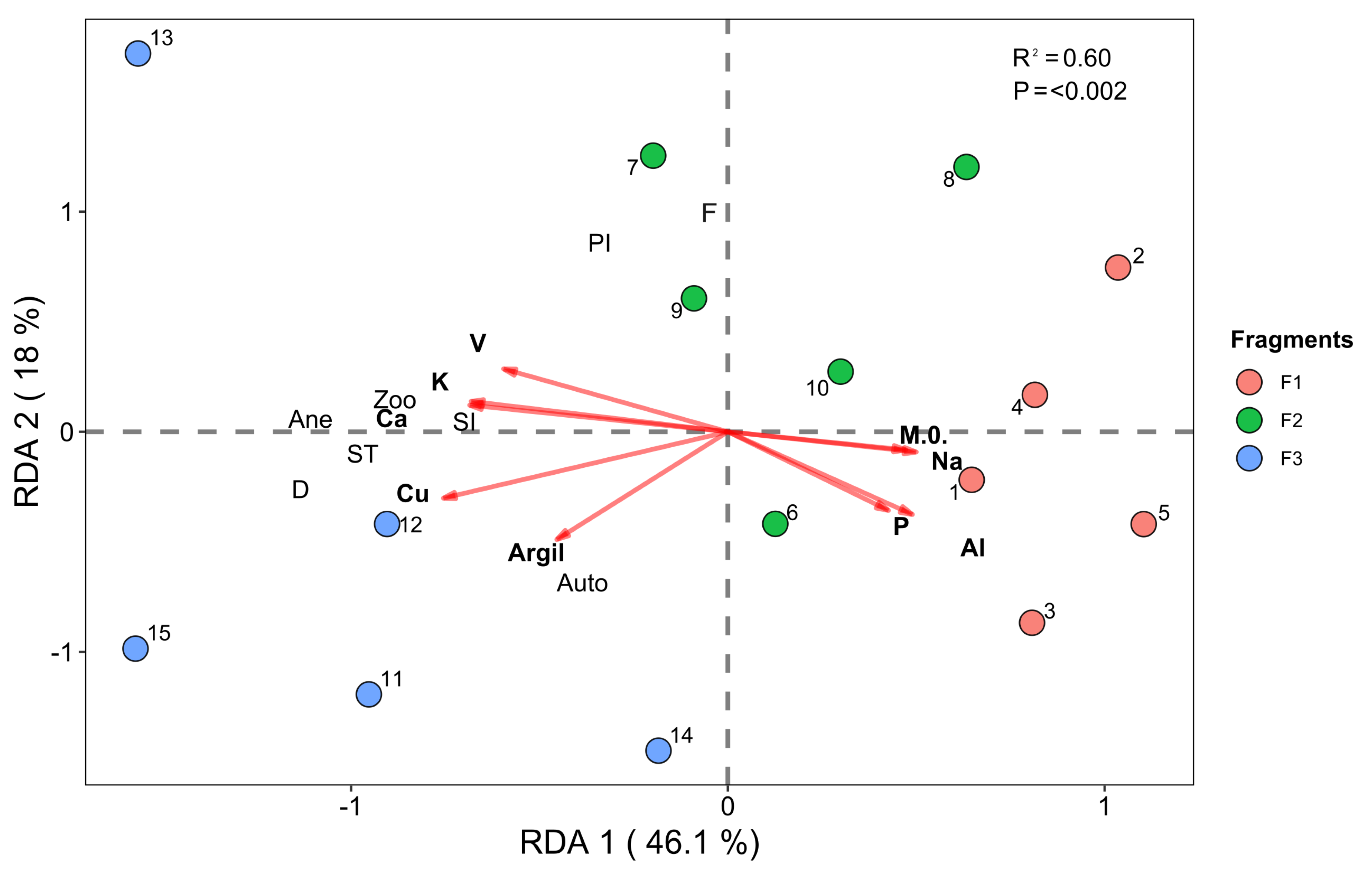
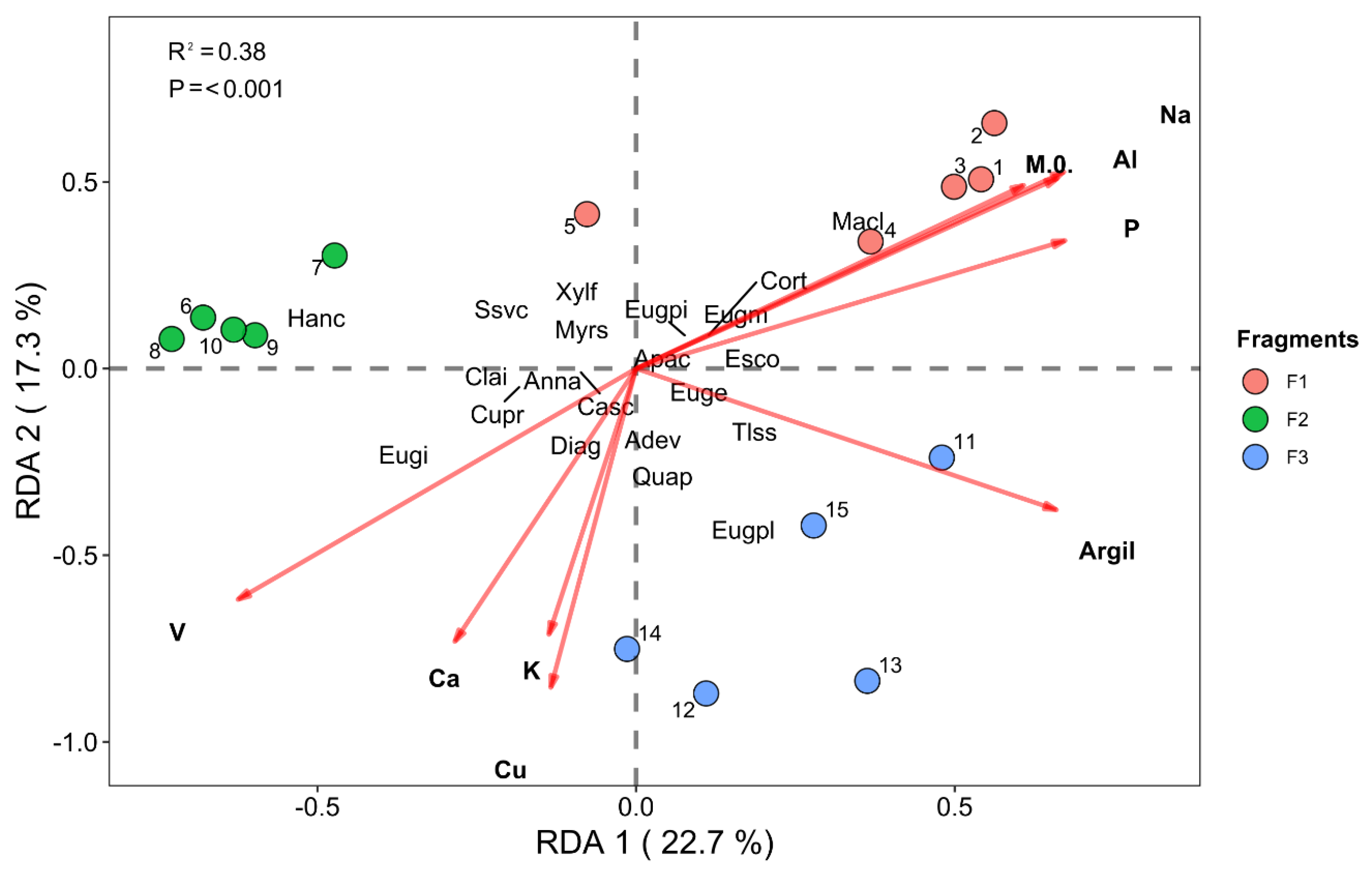
3.2. Redundancy Analysis—RDA
3.2.1. Vegetation–Environment Interactions (Environmental and Successional Data)
3.2.2. Vegetation–Environment Interactions (Environmental and Species Composition Data)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass Resilience of Neotropical Secondary Forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- MapBiomas. Destaque Do Mapeamento Anual Da Cobertura e Uso Do Terra No Brasil de 1985 a 2021—Mata Atlântica; MapBiomas: São Paulo, Brazil, 2022. [Google Scholar]
- Fundação SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais. Atlas Dos Remanescentes Florestais Da Mata Atlântica Período 2022–2023; Fundação SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais: São Paulo, Brazil, 2024.
- Dias, P.B.; Moreira, L.N.; Da Silva, G.F.; Pezzopane, J.E.M.E.; Dias, H.M. Richness, Structure and Environmental Relations in a National Forest in Southeast Brazil. Rev. Bras. Cienc. Agrar. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Liu, Y.; He, H.; Kou, Y.; Liu, Q. Dominant Plant Species and Soil Properties Drive Differential Responses of Fungal Communities and Functions in the Soils and Roots during Secondary Forest Succession in the Subalpine Region. Rhizosphere 2022, 21, 100483. [Google Scholar] [CrossRef]
- Poorter, L.; Craven, D.; Jakovac, C.C.; van der Sande, M.T.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A.; et al. Multidimensional Tropical Forest Recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Norden, N.; Angarita, H.A.; Bongers, F.; Martínez-Ramos, M.; Granzow-de la Cerda, I.; van Breugel, M.; Lebrija-Trejos, E.; Meave, J.A.; Vandermeer, J.; Williamson, G.B.; et al. Successional Dynamics in Neotropical Forests Are as Uncertain as They Are Predictable. Proc. Natl. Acad. Sci. USA 2015, 112, 8013–8018. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.L. Reading the Landscape: Primary vs. Secondary Forests. Arnoldia 1995, 55, 2–10. [Google Scholar] [CrossRef]
- Woodall, C.W.; Kamoske, A.G.; Hayward, G.D.; Schuler, T.M.; Hiemstra, C.A.; Palmer, M.; Gray, A.N. Classifying Mature Federal Forests in the United States: The Forest Inventory Growth Stage System. For. Ecol. Manag. 2023, 546, 121361. [Google Scholar] [CrossRef]
- Martin, P.; Jung, M.; Brearley, F.Q.; Ribbons, R.R.; Lines, E.R.; Jacob, A.L. Can We Set a Global Threshold Age to Define Mature Forests? PeerJ 2016, 4, e1595. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.N.; Pelz, K.; Hayward, G.D.; Schuler, T.; Salverson, W.; Palmer, M.; Schumacher, C.; Woodall, C.W. Perspectives: The Wicked Problem of Defining and Inventorying Mature and Old-Growth Forests. For. Ecol. Manag. 2023, 546, 121350. [Google Scholar] [CrossRef]
- Safar, N.V.H.; van der Sande, M.; Carlos, C.E.; Luiz, L.F.; Martins, S.V.; Simonelli, M.; Poorter, L. Landscape Openness Has Different Effects on the Structure, Diversity and Functional Composition of Brazilian Rainforests. For. Ecol. Manag. 2022, 520, 120395. [Google Scholar] [CrossRef]
- Magnago, L.F.S.; Simonelli, M.; Martins, S.V.; Matos, F.A.R.; Demuner, V.G. Variações Estruturais e Características Edáficas Em Diferentes Estádios Sucessionais de Floresta Ciliar de Tabuleiro, ES. Rev. Árvore 2011, 35, 445–456. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Species Abundance and Diversity; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat Fragmentation, Variable Edge Effects, and the Landscape-Divergence Hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar] [CrossRef] [PubMed]
- Pyles, M.V.; Magnago, L.F.S.; Borges, E.R.; van den Berg, E.; Carvalho, F.A. Land Use History Drives Differences in Functional Composition and Losses in Functional Diversity and Stability of Neotropical Urban Forests. Urban For. Urban Green. 2020, 49, 126608. [Google Scholar] [CrossRef]
- Poorter, L.; Rozendaal, D.M.A.; Bongers, F.; de Jarcilene, S.A.; Àlvarez, F.S.; Luìs Andrade, J.; Arreola Villa, L.F.; Becknell, J.M.; Bhaskar, R.; Boukili, V.; et al. Functional Recovery of Secondary Tropical Forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2003405118. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.C.M.; Zwiener, V.P.; Ramos, F.M.; Borgo, M.; Marques, R. Forest Structure and Species Composition along a Successional Gradient of Lowland Atlantic Forest in Southern Brazil. Biota Neotrop. 2014, 14, e20140094. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L.; Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties Oxford University Press; Oxford University Press: Oxford, UK, 2016. [Google Scholar][Green Version]
- Letcher, S.G.; Lasky, J.R.; Chazdon, R.L.; Norden, N.; Wright, S.J.; Meave, J.A.; Pérez-García, E.A.; Muñoz, R.; Romero-Pérez, E.; Andrade, A.; et al. Environmental Gradients and the Evolution of Successional Habitat Specialization: A Test Case with 14 Neotropical Forest Sites. J. Ecol. 2015, 103, 1276–1290. [Google Scholar] [CrossRef]
- Knapp, S.; Stadler, J.; Harpke, A.; Klotz, S. Dispersal Traits as Indicators of Vegetation Dynamics in Long-Term Old-Field Succession. Ecol. Indic. 2016, 65, 44–54. [Google Scholar] [CrossRef]
- Huanca-Nuñez, N.; Chazdon, R.L.; Russo, S.E. Determinism and Stochasticity in Seed Dispersal-Successional Feedbacks. bioRxiv 2019, 791988. [Google Scholar] [CrossRef]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Chazdon, R.L.; Guariguata, M.R. Natural Regeneration as a Tool for Large-Scale Forest Restoration in the Tropics: Prospects and Challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The Potential for Species Conservation in Tropical Secondary Forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental Changes during Secondary Succession in a Tropical Dry Forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef]
- Callegaro, R.M.; Longhi, S.J.; Andrzejewski, C.; Araujo, M.M. Regeneração Natural de Espécies Arbóreas Em Diferentes Comunidades de Um Remanescente de Floresta Ombrófila Mista. Ciência Rural 2015, 45, 1795–1801. [Google Scholar] [CrossRef]
- Ministerio do Meio Ambiente; Conservação Internacional; Fundação SOS Mata Atlântica. O Corredor Central Da Mata Atlantica. Uma Nova Escala de Conservacao Da Biodiversidade. 2006. Available online: https://www.conservation.org/docs/default-source/brasil/corredorcentraldamataatlantica.pdf?sfvrsn=8e09808_2 (accessed on 15 November 2024).
- IBGE (Fundação Instituto Brasileiro de Geografia e Estatística). Rio Doce: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial Da Terra. Projeto Radambrasil, Volume SF.34; IBGE: Rio de Janeiro, Brazil, 1987.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Manual Técnico Da Vegetação Brasileira. Manuais Técnicos Em Geociências; IBGE: Rio de Janeiro, Brazil, 2012.
- Rizzini, C.T. Tratado de Fitogeografia Do Brasil—Aspectos Ecológicos, Sociológicos e Florísticos, 2nd ed.; Âmbito Cultural Edições Ltda.: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- ICMBIO Reserva Biológica de Sooretama—Aspectos Físicos e Biológicos. Available online: https://www.icmbio.gov.br (accessed on 4 May 2022).
- Magnago, L.F.S.; Edwards, D.P.; Edwards, F.A.; Magrach, A.; Martins, S.V.; Laurance, W.F. Functional Attributes Change But Functional Richness Is Unchanged After Fragmentation of Brazilian Atlantic Forests. J. Ecol. 2014, 102, 475–485. [Google Scholar] [CrossRef]
- MapBiomas. Projeto MapBiomas—Coleção 7.0 Da Série Anual de Mapas de Cobertura e Uso de Solo Do Brasil. Available online: https://plataforma.brasil.mapbiomas.org (accessed on 25 May 2023).
- Brasil. Lei No 9.985, de 18 de Julho de 2000. Sistema Nacional de Unidades de Conservação Da Natureza; The Ministry of Environment and Climate Change: Brasilia, Brazil, 2000.
- Srbek-Araujo, A.C.; Kierulff, M.C.M. Mammals of Medium and Large Size of the Tabuleiro Forests in Northern Espírito Santo: Functional Groups and Main Threats. In Atlantic Forest of Tabuleiro: Diversity and Endemisms in the Vale Natural Reserve; Rolim, S.G., Menezes, L.F.T., Srbek-Araujo, A.C., Eds.; Rupestre: Belo Horizonte, Brazil, 2016; pp. 469–479. [Google Scholar]
- Peixoto, A.L.; De Jesus, R.M. Reserva Natural Vale: Memórias de 65 Anos de Conservação. In Floresta Atlântica de Tabuleiro: Diversidade e endemismos na Reserva Natural da Vale; Rupestre: Belo Horizonte, Brazil, 2016; pp. 21–30. ISBN 9788562805639. [Google Scholar]
- Valente, C.; Moura, C.; Hollunder, R.; Cabral, R.; Rodrigues, N.; Lobato, L.; Dias, H.; Da Silva, G. Diversity and Structure of Tree and Regenerating Strata in Fragments at Different Successional Stages in the Atlantic Forest of Brazil. Phytocoenologia 2024, 52, 71–85. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods Vegetation Ecology; J. Wiley: New York, NY, USA, 1974. [Google Scholar]
- Gandolfi, S.; Leitão Filho, H.d.F.; Bezerra, C.L.F. Floristic Survey and Successional Character of Shrub-Tree Species in a Semideciduous Mesophytic Forest in the Municipality of Guarulhos, SP. Bol. Mus. Biol. Mello Leitão 1995, 55, 753–767. [Google Scholar]
- Pijl, V.D.L. Principles of Dispersal in Higher Plants; Springer: New York, NY, USA, 1982; 215p. [Google Scholar]
- Tichý, L. Field Test of Canopy Cover Estimation by Hemispherical Photographs Taken with a Smartphone. J. Veg. Sci. 2016, 27, 427–435. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; ISBN 9788570357717. [Google Scholar]
- Magurran, A.E. Medindo a Diversidade Biológica; UFPR: Curitiba, Brazil, 2013. [Google Scholar]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Core: Vienna, Austria, 2022. [Google Scholar]
- Braga, S.R.; Oliveira, M.L.R.; Gorgens, E.B. Forestmangr: Forest Mensuration and Management Package Version 0.9.6. Available online: https://cran.r-project.org/web/packages/forestmangr/index.html (accessed on 20 November 2022).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Wagner, H.; Vegan: Community Ecology Package. R Package Version 2.3-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 26 April 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Legendre, P.; Gallagher, E. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.A.; Inoue, T.T.; Neto, M.E.; Muniz, A.S. Princípios de Fertilidade Do Solo, Adubação e Nutrição Mineral. In Hortaliças-fruto; Eduem, Ed.; EUDEM: Maringá, Brazil, 2018; pp. 113–162. [Google Scholar]
- Yuan, Z.; Ali, A.; Ruiz-Benito, P.; Jucker, T.; Mori, A.S.; Wang, S.; Zhang, X.; Li, H.; Hao, Z.; Wang, X.; et al. Above-and below-Ground Biodiversity Jointly Regulate Temperate Forest Multifunctionality along a Local-Scale Environmental Gradient. J. Ecol. 2020, 108, 2012–2024. [Google Scholar] [CrossRef]
- Furey, G.N.; Tilman, D. Plant Biodiversity and the Regeneration of Soil Fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2111321118. [Google Scholar] [CrossRef] [PubMed]
- Rolim, S.G.; Machado, R.E.; Pillar, V.D. Divergence in a Neotropical Forest during 33 Years of Succession Following Clear-Cutting. J. Veg. Sci. 2017, 28, 495–503. [Google Scholar] [CrossRef]
- Saiter, F.Z.; Rolim, S.G.; Oliveira-Filho, A.T. A Floresta de Linhares No Contexto Fitogeográfico Do Leste Do Brasil. In Floresta Atlântica Tabuleiro: Diversidade e Endemismos na Reserva Natural Vale; Rolim, S.G., Menezes, L.F.T., Srbek-Araujo, A.C., Eds.; Rupestre: Belo Horizonte, Brazil, 2016; pp. 61–69. [Google Scholar]
- Souza, C.R.; Maia, V.A.; de Aguiar-Campos, N.; Santos, A.B.M.; Rodrigues, A.F.; Farrapo, C.L.; Gianasi, F.M.; de Paula, G.G.P.; Fagundes, N.C.A.; Silva, W.B.; et al. Long-Term Ecological Trends of Small Secondary Forests of the Atlantic Forest Hotspot: A 30-Year Study Case. For. Ecol. Manag. 2021, 489, 119043. [Google Scholar] [CrossRef]
- Ronquim, C.C. Conceitos de Fertilidade Do Solo e Manejo Adequado Para as Regiões Tropicais; Campinas, EMBRAPA: Brasilia, Brazil, 2010. [Google Scholar]
- Pinotti, B.T.; Pagotto, C.P.; Pardini, R. Habitat Structure and Food Resources for Wildlife across Successional Stages in a Tropical Forest. For. Ecol. Manag. 2012, 283, 119–127. [Google Scholar] [CrossRef]
- Purschke, O.; Schmid, B.C.; Sykes, M.T.; Poschlod, P.; Michalski, S.G.; Durka, W.; Kühn, I.; Winter, M.; Prentice, H.C. Contrasting Changes in Taxonomic, Phylogenetic and Functional Diversity during a Long-Term Succession: Insights into Assembly Processes. J. Ecol. 2013, 101, 857–866. [Google Scholar] [CrossRef]
- Bhaskar, R.; Dawson, T.E.; Balvanera, P. Community Assembly and Functional Diversity along Succession Post-Management. Funct. Ecol. 2014, 28, 1256–1265. [Google Scholar] [CrossRef]
- Fan, K.; Tao, J.; Zang, L.; Yao, J.; Huang, J.; Lu, X.; Ding, Y.; Xu, Y.; Zang, R. Changes in Plant Functional Groups During Secondary Succession in a Tropical Montane Rain Forest. Forests 2019, 10, 1134. [Google Scholar] [CrossRef]
- Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes-García, C.; Paz, H.; Jackson, P.C. Functional Diversity of Small and Large Trees Along Secondary Succession in a Tropical Dry Forest. Forests 2016, 7, 163. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How Much Is Left, and How Is the Remaining Forest Distributed? Implications for Conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Bovo, A.A.A.; Ferraz, K.M.P.M.B.; Magioli, M.; Alexandrino, E.R.; Hasui, É.; Ribeiro, M.C.; Tobias, J.A. Habitat Fragmentation Narrows the Distribution of Avian Functional Traits Associated with Seed Dispersal in Tropical Forest. Perspect. Ecol. Conserv. 2018, 16, 90–96. [Google Scholar] [CrossRef]
- Wendt, A.L.; Chazdon, R.L.; Vargas Ramirez, O. Successional Trajectories of Seed Dispersal Mode and Seed Size of Canopy Tree Species in Wet Tropical Forests. Front. For. Glob. Change 2022, 5, 946541. [Google Scholar] [CrossRef]
- Medeiros, A.d.S.; Pereira, M.G.; Braz, D.M. Estrutura e Conservação de Um Trecho de Floresta Estacional Em Piraí, RJ. Floresta Ambiente 2016, 23, 330–339. [Google Scholar] [CrossRef]
- Da Rocha, M.J.R.; Cupertino-Eisenlohr, M.A.; Leoni, L.S.; Gomes Da Silva, A.; Nappo, M.E. Floristic and Ecological Attributes of a Seasonal Semideciduous Atlantic Forest in a Key Area for Conservation of the Zona Da Mata Region of Minas Gerais State, Brazil. Hoehnea 2017, 44, 29–43. [Google Scholar] [CrossRef]
- Lima, P.I.; Nogueira, R.M.; Monteiro, R.L.; Peracchi, A.L. Frugivoria e Dispersão de Sementes Por Morcegos Na Reserva Natural Vale, Sudeste Do Brasil. In Floresta Atlântica de Tabuleiro: Diversidade e endemismos na Reserva Natural da Vale; Rolim, S.G., de Menezes, L.F.T., Srbek-Araujo, A.C., Eds.; Rupestre: Belo Horizonte, Brazil, 2016; pp. 433–452. [Google Scholar]
- Fundação SOS Mata Atlântica; Instituto Nacional de Pesquisas Espaciais. Relatório Técnico: Atlas Dos Remanescentes Florestais Da Mata Atlântica Período 2021-2022; Fundação SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais—INPE: São Paulo, Brazil, 2023.
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From Hotspot to Hopespot: An Opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Aslan, C.; Beckman, N.G.; Rogers, H.S.; Bronstein, J.; Zurell, D.; Hartig, F.; Shea, K.; Pejchar, L.; Neubert, M.; Poulsen, J.; et al. Employing Plant Functional Groups to Advance Seed Dispersal Ecology and Conservation. AoB Plants 2019, 11, plz006. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R. Regeneração de Florestas Tropicais Tropical Forest Regeneration. Bol. Mus. Para. Emílio Goeldi-Ciências Nat. 2012, 7, 195–218. [Google Scholar] [CrossRef]
- Carvalho, P.E.R. Cupiúva: Tapirira guianensis. In Espécies Arbóreas Brasileiras; Embrapa Informação Tecnológica: Brasília, Brazil; Embrapa Florestas: Colombo, Brazil, 2006; pp. 189–198. [Google Scholar]
- León, M.L.V. Macrolobium latifolium (Fabaceae). In Lista Vermelha Da Flora Brasileira; Centro Nacional de Conservação Da Flora; Instituto de Pesquisas Jardim Botânico Do Rio de Janeiro: Rio de Janeiro, Brazil, 2020. Available online: https://proflora.jbrj.gov.br/html/Macrolobium latifolium_2020.html (accessed on 19 May 2022).
- Seng, V.; Bell, R.W.; Willett, I.R. Effect of Lime and Flooding on Phosphorus Availability and Rice Growth on Two Acidic Lowland Soils. Commun. Soil Sci. Plant Anal. 2006, 37, 313–336. [Google Scholar] [CrossRef]
- Martins, S.V. Potencial de Regeneração Natural de Florestas Nativas Nas Diferentes Regiões do Estado do Espírito Santo; Vitória, CEDAGRO: Vitória, Brazil, 2014.
- Barbosa, M.R.; Souza, L.M.; de Souza, R.A.; Houllou, L.M. Aspectos Aspects of the In Vitro Establishment of Handroanthus Chrysotrichus (Bignoniaceae) for the Production of Seedlings. Braz. J. Dev. 2020, 6, 2830–2840. [Google Scholar] [CrossRef]
- Costa, M.B. Sucessão Ecológica Pós-Fogo Em Fragmentos de Mata Atlântica Sobre Tabuleiros Costeiros No Sudeste Do Brasil. Master’s Thesis, Universidade Federal do Espírito Santo, São Mateus, Brazil, 2014; p. 111. [Google Scholar]
- Rolim, S.G.; Peixoto, A.L.; Pereira, O.J.; de Araujo, D.S.D.; Siqueira, G.; de Menezes, L.F.T. Angiospermas da Reserva Natural Vale, na Floresta Atlântica do Norte do Espírito Santo. In Floresta Atlântica de Tabuleiro: Diversidade e Endemismos na Reserva Natural Vale; Rolim, S.G., de Menezes, L.F.T., Srbek-Araujo, A.C., Eds.; Rupestre: Belo Horizonte, Brazil, 2016; pp. 167–230. [Google Scholar]
- Archanjo, K.M.P.d.A.; da Silva, G.F.; Chichorro, J.F.; Soares, C.P.B. Structure of the Arboreal Component of Cafundó Natural Heritage Private Reserve, Cachoeiro de Itapemirim, Espírito Santo, Brazil. Floresta 2012, 42, 145–160. [Google Scholar] [CrossRef]
- Jardim Botânico do Rio de Janeiro Quararibea in Flora Do Brasil 2020 Em Construção. Available online: https://floradobrasil2020.jbrj.gov.br/FB16495 (accessed on 14 March 2023).
- Ribeiro, M.; Peixoto, A.L.; Pereira, O.J.; De Menezes, L.F.T. Tabuleiro Forest in Southeast Brazil: Exploring the Neglected Diversity of a Forest Fragment. Pesqui. Botânica 2022, 76, 149–191. [Google Scholar]
- Garbin, M.L.; Saiter, F.Z.; Carrijo, T.T.; Peixoto, A.L. Breve Histórico e Classificação Da Vegetação Capixaba. Rodriguésia 2017, 68, 1883–1894. [Google Scholar] [CrossRef]
- Barros, M.F.; Ribeiro, E.M.S.; Vanderlei, R.S.; de Paula, A.S.; Silva, A.B.; Wirth, R.; Cianciaruso, M.V.; Tabarelli, M. Resprouting Drives Successional Pathways and the Resilience of Caatinga Dry Forest in Human-Modified Landscapes. For. Ecol. Manag. 2021, 482, 118881. [Google Scholar] [CrossRef]
- Dente, D.H.; Estrada-Villegas, S. Uniting Niche Differentiation and Dispersal Limitation Predicts Tropical Forest Succession. Trends Ecol. Evol. 2021, 36, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.B.; Gomes, L.P.; Callegaro, R.M.; Carvalho, F.A.; Dias, H.M. Structural and Environmental Variability from the Edge to the Interior of an Atlantic Forest Remnant in Brazil. J. Trop. For. Sci. 2021, 33, 308–332. [Google Scholar] [CrossRef]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]



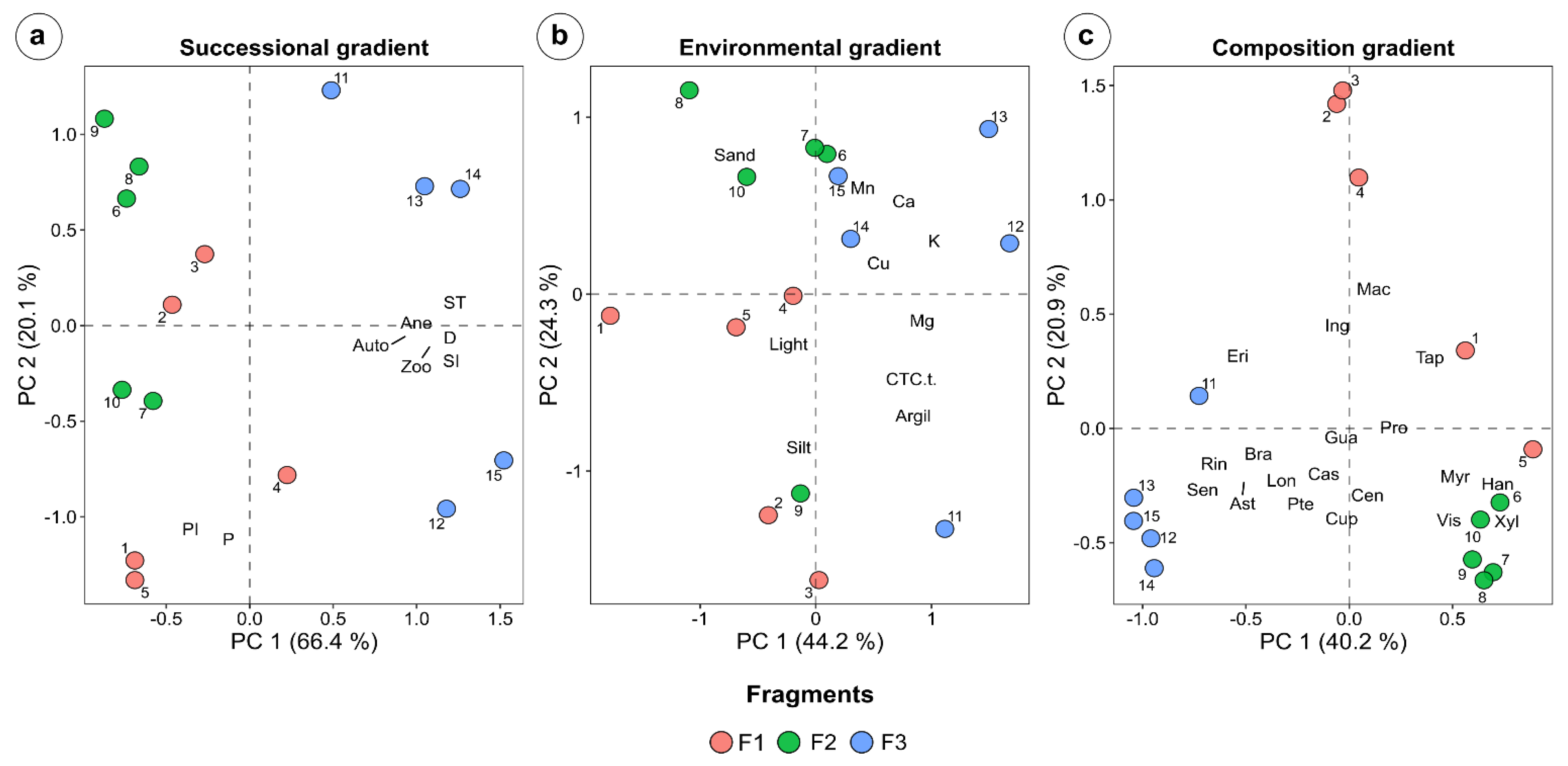
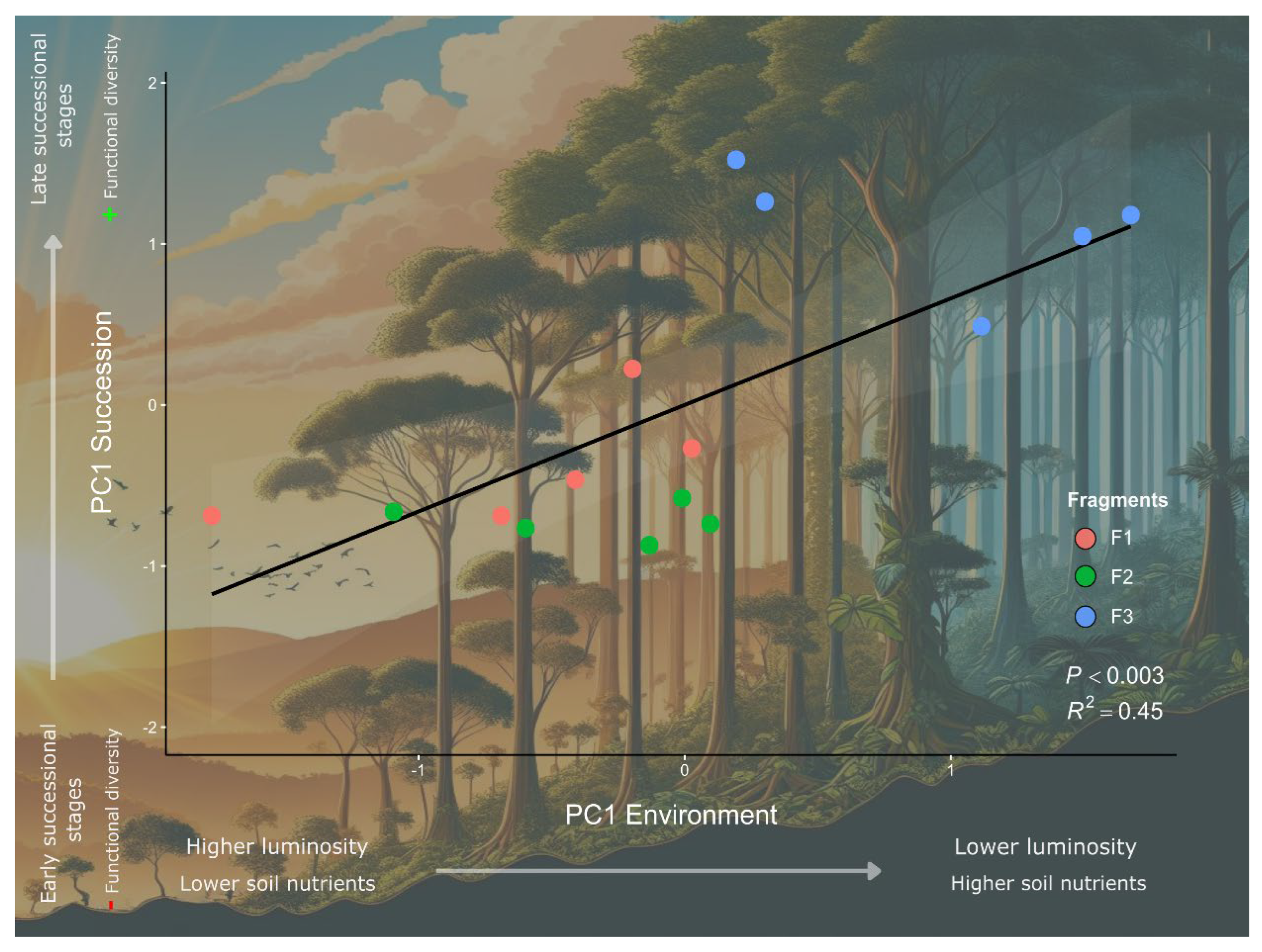

| Stratum | F1 | F2 | F3 |
|---|---|---|---|
| Tree | - | - | - |
| Total number of individuals | 545 | 710 | 495 |
| Shannon index | 3.63 | 2.68 | 4.49 |
| Pielou’s evenness index | 0.80 | 0.66 | 0.89 |
| Total families | 29 | 36 | 42 |
| Total species | 84 | 55 | 149 |
| Regeneration | - | - | - |
| Total number of individuals | 137 | 448 | 282 |
| Shannon index | 3.45 | 2.75 | 4.07 |
| Pielou’s evenness index | 0.95 | 0.87 | 0.98 |
| Total families | 25 | 22 | 29 |
| Total species | 40 | 37 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, C.; Hollunder, R.; Moura, C.; Siqueira, G.; Dias, H.; da Silva, G. Assessing Forest Succession Along Environment, Trait, and Composition Gradients in the Brazilian Atlantic Forest. Forests 2025, 16, 1169. https://doi.org/10.3390/f16071169
Valente C, Hollunder R, Moura C, Siqueira G, Dias H, da Silva G. Assessing Forest Succession Along Environment, Trait, and Composition Gradients in the Brazilian Atlantic Forest. Forests. 2025; 16(7):1169. https://doi.org/10.3390/f16071169
Chicago/Turabian StyleValente, Carem, Renan Hollunder, Cristiane Moura, Geovane Siqueira, Henrique Dias, and Gilson da Silva. 2025. "Assessing Forest Succession Along Environment, Trait, and Composition Gradients in the Brazilian Atlantic Forest" Forests 16, no. 7: 1169. https://doi.org/10.3390/f16071169
APA StyleValente, C., Hollunder, R., Moura, C., Siqueira, G., Dias, H., & da Silva, G. (2025). Assessing Forest Succession Along Environment, Trait, and Composition Gradients in the Brazilian Atlantic Forest. Forests, 16(7), 1169. https://doi.org/10.3390/f16071169









