Abstract
Monsoon evergreen broad-leaved forests (MEBFs) represent one of the most species-rich and structurally complex vegetation types, and one of the most widely distributed forests in Yunnan Province, Southwest China. However, they have yet to undergo a comprehensive analysis on their community diversity, spatial differentiation patterns, and underlying drivers across Yunnan. Based on extensive field surveys during 2021–2024 with 548 MEBF plots, this study employed the Unweighted Pair Group Method for forest community classification and Non-metric Multidimensional Scaling for ordination and interpretation of community–environment association. A total of 3517 vascular plant species were recorded in the plots, including 1137 tree species, 1161 shrubs, and 1219 herbs. Numerical classification divided the plots into 3 alliance groups and 24 alliances: (1) Castanopsis–Schima (Lithocarpus) Forest Alliance Group (16 alliances), predominantly distributed west of 102°E in central-south and southwest Yunnan; (2) Castanopsis–Machilus (Beilschmiedia) Forest Alliance Group (6 alliances), concentrated east of 101°E in southeast Yunnan with limited latitudinal range; (3) Castanopsis–Camellia Forest Alliance Group (2 alliances), restricted to higher-elevation mountainous areas within 103–104° E and 22.5–23° N. Climatic variation accounted for 81.1% of the species compositional variation among alliance groups, with contributions of 83.5%, 57.6%, and 62.1% to alliance-level differentiation within alliance groups 1, 2, and 3, respectively. Precipitation days in the driest quarter (PDDQ) and precipitation seasonality (PS) emerged as the strongest predictors of community differentiation at both alliance group and alliance levels. Topography and soil features significantly influenced alliance differentiation in Groups 2 and 3. Collectively, the interaction between the monsoon climate and topography dominate the spatial differentiation of MEBF communities in Yunnan.
1. Introduction
The monsoon evergreen broad-leaved forest (MEBF), recognized as the climatic zonal vegetation in the subtropical regions of southern China [1], is primarily distributed across mountainous areas at elevations of 800–1300 m. These include the southern section of Yushan Mountain in Taiwan, the south aspect of Daiyun Mountain in Fujian, and the Nanling Mountain Ranges, extending westward to southern Guizhou, central-southern Yunnan, and the eastern Himalaya in Tibet [2,3]. MEBF communities are dominated by evergreen broad-leaved species from the Fagaceae (Castanopsis Spach, Lithocarpus Blume, Cyclobalanopsis Oerst.), Lauraceae (Beilschmiedia Nees, Machilus Nees), Theaceae (Schima Reinw. ex Blume, Camellia L.), and Magnoliaceae (Manglietia Blume) [2,3,4], with significant contributions from tropical floristic elements (Fabaceae, Rubiaceae, Moraceae) [5]. MEBF represents the most structurally complex and species-rich forest types in China, exhibiting pronounced spatial heterogeneity in species composition [4,6], in responding to the regional differentiation of climatic and topographic conditions, as well as temporal dynamics driven by natural and human disturbances.
Quantitative classification and ordination serve as fundamental approaches for exploring plant community diversity, deciphering geographic differentiation patterns of vegetation, and identifying key environmental drivers. With the exponential growth of vegetation survey datasets and advancements in quantitative analytical techniques, plant community classification has been implemented using a suite of methods [7,8]. Beyond the conventional numerical classification methods employed in early studies, the Two-Way Indicator Species Analysis (TWINSPAN) was one of the first widely used methods for quantitative vegetation classification [9]. The Multivariate Regression Tree (MRT) was first introduced to plant community classification by De’Ath in 2002 [10], and was widely accepted due to its multivariate partitioning of community data using environmental variables, generating groups with high homogeneity in species composition or abundance. The Unweighted Pair Group Method with Arithmetic Mean (UPGMA), a hierarchical clustering algorithm widely applied in ecology, systematics, and taxonomy [11], was initially implemented for community classification of Isoetes-dominated wetland vegetation by Molina (2005) [12]. UPGMA is characterized by its computational simplicity, intuitive interpretability, and robustness against outliers and local variability, ensuring stable and consistent clustering outcomes [13]. These attributes have driven its widespread adoption in more recent plant community classification studies [14,15].
The vegetation classification systems and nomenclature vary significantly across different schools of science [16,17,18]. Due to the complexity of vegetation typology, no universally authoritative nomenclature code is generally accepted [19,20]. Fang et al. (2020) [18] proposed a framework synthesizing Anglo-American approaches with China’s foundational. The Vegetation of China (1980), adopting intermediate units (Alliance and Alliance Group) and basic units (Association and Association Group) [3,18]. Building on this, Wang et al. (2020) [19] established formal nomenclature conventions: Alliance Groups combine genus names with qualifiers (e.g., Quercus Forest Alliance Group, Castanopsis–Lithocarpus Evergreen Broadleaf Forest Alliance Group), while Alliances use dominant/codominant canopy species with qualifiers (e.g., Picea schrenkiana Forest Alliance, Quercus variabilis + Quercus acutissima Deciduous Broadleaf Forest Alliance). However, for the nomenclature within the same vegetation type, qualifiers already specified in the vegetation type designation (e.g., Needleleaf, Broadleaf) can be omitted.
As a methodology for gradient analysis and attribution of plant community composition, ordination has evolved concurrently with clustering techniques and been often applied complementarily to clustering in vegetation analysis [8,21]. Regarding the environmental interpretation of community differentiation, two categories of ordination methods have been developed: direct and indirect gradient analyses [22,23]. Direct gradient analysis derives ordination axes solely from species composition data to visualize the distribution patterns of plot communities in the ordination space. The ecological significance of these axes is subsequently interpreted through regressions of axis scores against environmental variables [24,25]. In contrast, indirect gradient analysis incorporates environmental constraints by embedding regression steps between the ordination axes and environmental factors during iterative calculations, thereby explicitly linking ordination axes to environmental drivers [26,27]. Among diverse ordination methods, Non-metric Multidimensional Scaling (NMDS) is highly versatile for ecological datasets [28]. It transforms between-group dissimilarities into low-dimensional distances along the ordination axes, effectively visualizing compositional gradients. NMDS further allows environmental variables to be projected onto the ordination space, facilitating the identification of key factors driving species distributions and community differentiation [29]. This robustness has established NMDS as one of the most widely used vegetation ordination tools. For instance, Cabido et al. (2018) integrated NMDS with UPGMA clustering in a study of woody vegetation in central Argentina, successfully capturing disturbance-induced successional dynamics and compositional heterogeneity [30]. Similarly, Joelson et al. (2025) demonstrated in North Patagonian Andean forests that UPGMA dendrograms provided discrete classification boundaries, while NMDS ordination revealed transitional features within communities, underscoring the synergistic application of both methods to comprehensively unravel vegetation structural complexity and eco-environmental responses [31].
MEBF represents one of the dominant vegetation types in Yunnan Province, distributed across central-southern mountainous regions south of 30° N latitude. Its range extends westward to the border areas between China and Myanmar, eastward connecting to MEBF in Guangxi Province of China, with a potential distribution area of 69,323 km2 [32]. However, due to natural disturbances (e.g., wildfires) and anthropogenic activities (e.g., land use change, artificial forest management), Yunnan’s MEBF has experienced severe fragmentation and contraction, with its extant area reduced to approximately 20,000 km2 [33,34]. The extensive geographic distribution, coupled with complex topography and pronounced climatic heterogeneity within the region, drives significant intra-biome variation in community composition [35].
The Vegetation of Yunnan (1987) classified MEBF into four alliance groups and six alliances: (a) Castanopsis, Lithocarpus Forest Alliance Group, including Ca. hystrix, Ca. indica Forest Alliance, Ca. fleuryi, L. truncatus Forest Alliance, and Ca. fabri, L. truncatus Forest Alliance; (b) Machilus, Ca. Forest Alliance Group, including M. kurzii, Ca. fargesii Forest Alliance; (c) Quercus, Podocarpus Forest Alliance Group, including Q. utilis Forest Alliance; (d) Cyclobalanopsis, Manglietia Forest Alliance Group, including Cy. blakei Forest Alliance [2]. This classification system, however, requires revision given expanded vegetation surveys and accumulated datasets over recent decades [1,36,37]. In the past 40 years, research on Yunnan’s MEBF has predominantly implemented at local scale (e.g., forest dynamics plots) or on an individual alliance [38,39]. For instance, Shi and Zhu (2003) investigated community structure and species–area relationships in Xishuangbanna’s montane MEBF using five 25 m × 20 m plots, identifying 1500 m2 as the minimum representative area for MEBF communities [40]. Li et al. (2020) conducted a quantitative classification of associations within a 30-hectare MEBF plot in Pu’er region, revealing micro-environmental and compositional divergence among associations [1]. Recently, Chen et al. (2024) demonstrated that seedling community assembly in a 30 ha forest plot is jointly driven by stochastic dispersal, habitat filtering, and limiting similarity (5.7%–27.2%) through analyses of species composition, dominant species partitioning, and functional traits [41]. However, the comprehensive studies on MEBF differentiation in Yunnan remain scarce, with localized findings yet to be synthesized. Consequently, the macroecological patterns of community diversity, species composition shifts, and environmental drivers within this critical subtropical vegetation type in western China remain poorly understood.
Between 2021 and 2024, we conducted extensive field surveys across Yunnan’s MEBF, compiling the most comprehensive and spatially representative plot-based dataset for this vegetation type to date. Leveraging these data, this study integrates quantitative classification, ordination, and environmental interpretation to elucidate the species composition, community diversity, and spatial differentiation patterns of Yunnan’s MEBF. Specifically, we address two core scientific questions: (1) How many distinct community types exist within Yunnan’s MEBF, and what are their geographic distribution patterns and structural attributes? (2) What are the dominant mechanisms driving the spatial differentiation of Yunnan’s MEBF communities across environmental gradients?
2. Materials and Methods
2.1. Study Site and Field Survey
2.1.1. Study Site
The study area encompasses Yunnan’s MEBF across its southeastern, central-southern, and southwestern regions, spanning longitudinal ranges of 97° E–107° E, latitudinal gradients of 21° N–25° N, and elevational zones from 700 to 2200 m. Climatically, the region is dominated by a southern subtropical monsoon climate and tropical montane climate, characterized by mean annual precipitation of 900–1700 mm, mean annual temperatures of 17–21 °C, and coldest-month temperatures averaging 6–14 °C, with extreme minima near 0 °C. Meteorological station data reveal a pronounced seasonal regime, with a distinct dry season (November–April) and wet season (May–October), the latter contributing over 80% of annual precipitation, while the winter (December–February) is the driest period, accounting for merely ~6% of annual precipitation. The geomorphology of the study area transits sharply between karst hills and mountains east of 103° E, and north-south low mountain–valley systems west of 103° E. Soil types exhibit marked spatial heterogeneity: acidic montane ferralic acrisols and plinthic ferralsols dominate the central-western regions, whereas, neutral to weakly alkaline calcic, cambisols prevail in the eastern karst zones, reflecting lithological controls [2].
2.1.2. Field Surveys of Plant Communities
From 2021 to 2024, we conducted extensive vegetation surveys across the study area following the protocols outlined in Vegetation of China [19]. Each 600 m2 plot (20 m × 30 m) was subdivided into six 100 m2 subplots (Figure 1c). In subplots A–F, a full census of the tree layer was performed, recording species identity, diameter at breast height (DBH ≥ 3 cm), height, and crown width for all woody individuals. Shrub layer surveys were conducted in subplots S1 and S2, documenting species identity, mean basal diameter, mean height, stem density, and coverage, while only species presence was recorded in the remaining four 100 m2 subplots. Herb layer surveys were carried out in six 1 m × 1 m quadrats (H1–H6), measuring species identity, coverage, mean height, and abundance; beyond these quadrats, only herbaceous species presence was recorded. A total of 548 forest community plots were surveyed (Figure 1a).
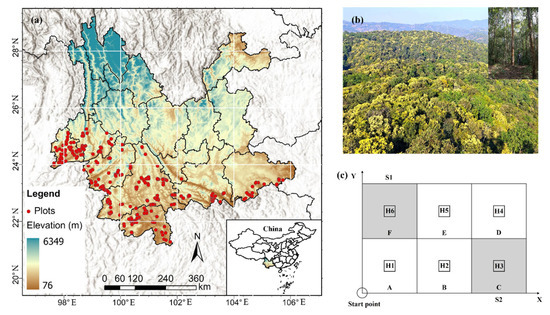
Figure 1.
Distribution of plots (a), structural and physiognomic characteristics of MEBF (b), and field plot configuration (c).
2.2. Environmental Factors
2.2.1. Climate Data
Meteorological data were obtained from the National Meteorological Data Centre of China (NMDC, https://data.cma.cn, accessed on 29 November 2024). Following the methodology of Xiahou et al. (2024) [32], we extracted temperature and precipitation records (1990–2022) from 60 meteorological stations within and adjacent to Yunnan’s MEBF distribution area, and then generated climate raster layers for the study region using a thin plate smoothing spline interpolation [42], and subsequently extracted climatic variables at a 100 m resolution for each vegetation plot based on geographic coordinates. Variable collinearity was assessed via the Variable Importance in Projection (VIP) method [43], resulting in the selection of three precipitation factors—mean annual precipitation (MAP), precipitation seasonality (PS), and precipitation days in the driest quarter (PDDQ)—and three temperature factors: mean annual temperature (MAT), temperature seasonality (TS), and annual temperature range (ATR) (Table 1).

Table 1.
Environmental factors and indices used for interpretation of MEBF ordinations.
2.2.2. Topography and Soil Data
Topographic factors included slope (SLO) and transformed aspect (ASP) (Table 1). SLO was measured in situ using a compass clinometer. ASP was derived from the trigonometric transformation of the original azimuth values (ASPorig, 0–360°) using the formula
resulting in values ranging from –1 to 1. This transformation assigns north-facing slopes (0° or 360°) an ASP value of −1 and south-facing slopes (180°) a value of 1, with higher ASP values indicating sun-exposed (southward) slopes and lower values corresponding to shaded (northward) slopes.
Soil factors were derived from the Soilgrids Database (250 m resolution) [44], with three key surface soil (0–5 cm) indicators—pH, soil organic carbon content (SOC), and clay content (CLAY, reflecting soil texture)—extracted using plot coordinates.
2.3. Community Classification
2.3.1. Important Value of Species
We calculated the Important Value (IV) for each tree species within the tree layer at the 20 m × 30 m plot level. The IV for a species in each plot was determined [45] as
where the components are defined as follows:
In the RA (Relative Abundance), Ni represents the number of individuals of species i, and Ntotal denotes the total number of individuals of all species within a plot, reflecting the numerical dominance of a species in a plot. In the RF (Relative Frequency), Fi indicates the frequency of species i across six 10 m × 10 m subplots, while Ftotal is the summed frequency of all species within a plot, and quantifies a species’ spatial uniformity and commonness in the sampling area. In the RP (Relative Prominence), BAi is the sectional area at breast height (i.e., 1.3 m) for species i, and BAtotal signifies the aggregate area of all species in a plot, evaluating a species’ spatial occupancy capacity and structural dominance in a community.
2.3.2. Quantitative Classification and Ordination
Community classification of the 548 MEBF plots was conducted based on tree layer IV data. Given the pronounced ecological gradients in Yunnan’s MEBF distribution area, we employed chord distance over Bray–Curtis distance to better reflect these gradients when calculating pairwise dissimilarities between plots [46,47], and performed hierarchical clustering via the UPGMA [48]. To enhance clustering precision, a two-step approach was implemented: initial clustering of all 548 plots excluded branches with total area <1500 m2 (minimum representative area) [40] or <3 plots (26 plots removed); followed by secondary clustering of the remaining 522 plots, with three outlier plots merged into nearest branches based on field validation. Building on this approach, we calculated species frequency (%) and fidelity values (φ) across groups, with φ-value significance determined via Fisher’s exact test (p < 0.05) to identify constant species (frequency ≥ 60%) and diagnostic species (φ ≥ 0.25) per group. Dominant species were defined as those with Importance Value (IV) > 10%. Following Fang et al. (2020) [18], plots sharing dominant/diagnostic species from congeneric taxa were classified as the same alliance group, while ploys sharing identical dominant/diagnostic species constituted the same alliance. The nomenclature for alliance groups and alliances subsequently applied standardized rules of Wang et al. (2020) [18,19,49].
Plant community ordination was performed using NMDS combined with environmental variable fitting (via the envfit function), generating NMDS axis scores for all plots and species [50,51]. Environmental variables were fitted to the ordination space by calculating multiple regression models between each NMDS axis and multivariate environmental data using envfit, with their axis scores projected onto NMDS ordination diagrams [29]. Two- or three-dimensional visual ordination diagrams integrating plots, species, and environmental variables were constructed based on primary NMDS axes. The correlation coefficients (R2) between environmental factors and the ordination space, computed via envfit, were standardized as percentage contribution proportions to assess their influence on the differentiation of alliance groups and alliances.
All analyses were conducted in R 4.4.2, with the vegan package employed for calculating pairwise dissimilarities among plots and performing community ordination, the stats package for hierarchical clustering. Diagnostic species analysis was conducted using the indicspecies package and the envfit function within the vegan package to quantify the proportional contributions of environmental factors.
3. Results
3.1. Species Composition
A total of 3517 vascular plant species were recorded across the 548 MEBF plots, comprising 1137 tree species (including 44 woody lianas and 7 tree ferns), 1161 shrub species (246 woody lianas), and 1219 herb species (306 ferns and 108 herb lianas), representing 186 families and 968 genera (Table 2). Species richness among the three growth forms (tree, shrub, herb) was broadly comparable, with accumulation rates increasing proportionally to plot number, though herbaceous species exhibited a marginally higher accumulation rate (Figure 2). At the family level, the Lauraceae (139 species) and Fagaceae (85 species) dominated the tree layer, while the Rubiaceae (107 species) and Fabaceae (99 species) were most speciose in the shrub layer. The herb layer was characterized by the Orchidaceae (152 species) and Asteraceae (80 species) (see Appendix A Table A1).

Table 2.
Species composition across life forms in Yunnan’s MEBF.
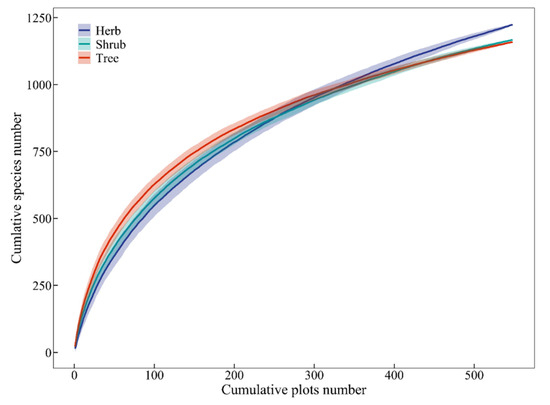
Figure 2.
Species accumulation curves across three growth forms in Yunnan’s MEBF.
3.2. Community Composition
UPGMA clustering of community plots initially identified 27 community types. After merging three branches containing a single plot (based on field validation), 24 MEBF alliances were ultimately delineated and subsequently categorized into three alliance groups (Figure 3):
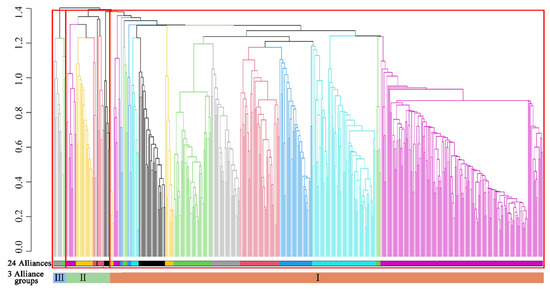
Figure 3.
UPGMA-derived hierarchical dendrogram of Yunnan’s MEBF communities.
(1) Castanopsis–Schima (Lithocarpus) Forest Alliance Group (AG1): Including 16 alliances and 462 plots.
(2) Castanopsis–Machilus (Beilschmiedia) Forest Alliance Group (AG2): Including 6 alliances and 46 plots.

Table 3.
Composition of alliance groups and alliances in Yunnan’s MEBF.
Synoptic table for alliance groups and alliances refer to Appendix A Table A6, Table A7, Table A8 and Table A9.
3.3. Distribution Patterns and Community Structure Among Alliance Groups
AG1 was primarily distributed across central-southern and southwestern Yunnan (west of 102° E, 21.5° N–25° N), with minor extensions into the southeastern region, exhibiting the most extensive distribution and highest compositional complexity among alliance groups (Table 2). AG2 occupied a narrow latitudinal belt in southeastern Yunnan (east of 101° E, 22.5° N–23.5° N), while AG3 was localized within 103° E–104° E and 22.5° N–23° N, overlapping longitudinally and latitudinally with AG1 and AG2 but occurring at significantly higher elevations (Figure 4).
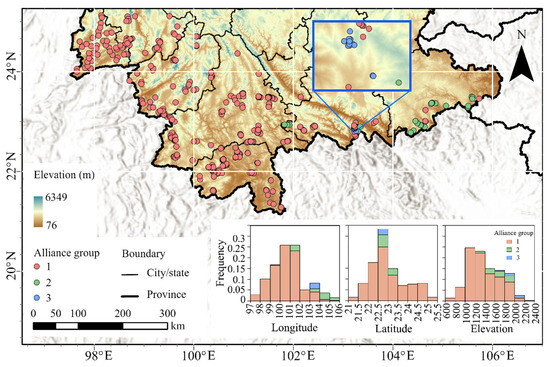
Figure 4.
Distribution patterns of alliance groups in Yunnan’s MEBF, with inset (lower right) showing frequency distributions of plot longitude, latitude, and elevation.
Significant structural divergence was observed among the three alliance groups (Table 4). AG3 exhibited significantly higher mean species richness than both AG2 and AG1 (p < 0.05), with AG2 also surpassing AG1 (p < 0.05). Mean tree density followed AG3 > AG2 > AG1, though differences were non-significant (p > 0.05). For mean DBH, AG3 had the largest values, followed by AG1 and then AG2, with significant differences between AG1 and AG2 (p < 0.05). Canopy height ranked AG2 > AG1 > AG3, showing significant contrasts between AG1 and AG2 (p < 0.05), while canopy was comparable across all groups. Notably, AG3 combined the largest mean DBH with the shortest canopy height, whereas AG2 displayed the smallest DBH but the tallest canopy height. Despite AG1 encompassing the highest number of plots, it demonstrated the lowest variability in all structural metrics among the groups.

Table 4.
Community structural differences (mean ± SE) among the three alliance groups in Yunnan’s MEBF.
3.4. Community Ordination and Environmental Drivers
3.4.1. NMDS Ordination of Alliance Groups
AG1 exhibited clear differentiation from AG2 and AG3 along NMDS Axis 1, which primarily reflected environmental gradients in dry-season precipitation evenness, specifically the number of precipitation days in the driest quarter (PDDQ). AG2 and AG3 occupied regions with higher PDDQ compared to AG1 (Figure 5a,b). Differentiation between AG2 and AG3 occurred along NMDS Axes 2 and 3, driven predominantly by precipitation seasonality (PS) and temperature seasonality (TS). AG2 was associated with areas of stronger PS and TS, while AG3 clustered in regions with weaker seasonal climatic fluctuations (Figure 5a–c). Additionally, soil organic carbon (SOC) and pH contributed to the divergence between AG2 and AG3: AG2 aligned with higher SOC and pH values, whereas AG3 corresponded to lower values (Figure 5b,c). Topographic factors played minimal roles in differentiating the three alliance groups. Collectively, climatic variables explained 81.1% of the alliance group differentiation (48.70% from precipitation, 32.39% from temperature), followed by soil factors (15.4%), with topographic variation contributing only 3.6% at local scales (Figure 5d).
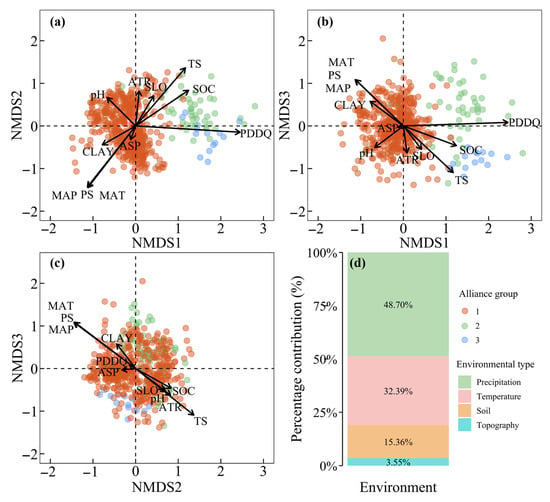
Figure 5.
NMDS ordination of Yunnan’s MEBF alliance groups (a–c) and proportional contributions of environmental factors to ordination patterns (d).
3.4.2. NMDS Ordination of Alliances
NMDS ordination of alliances within AG1 revealed that spatial differentiation was primarily driven by precipitation seasonality (PS) and temperature seasonality (TS), with additional influence from precipitation days in the driest quarter (PDDQ). Along NMDS Axis 1, alliance 1_1 clustered in regions with stronger PS and higher PDDQ, while alliances 1_3, 1_5, 1_6, 1_7, and 1_8 occupied areas with weaker PS and fewer PDDQ (Figure 6a,b). Along NMDS Axis 2, alliances 1_2, 1_1, and 1_4 exhibited sequential increases in both PS and TS intensity (Figure 6a,c). NMDS Axis 3 differentiation correlated with soil clay content (CLAY) and pH: Alliances 1_2, 1_3, and 1_7 associated with higher CLAY values, whereas alliances 1_4 and 1_5 aligned with lower pH values (Figure 6b,c). Environmental contributions to AG1 alliance ordination patterns were dominated by precipitation and temperature (83.5%), followed by soil properties (14.3%), with negligible direct topographic effects (2.2%) (Figure 6d).
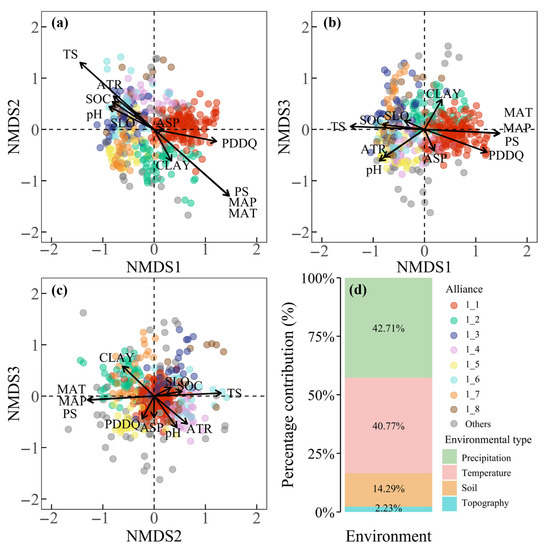
Figure 6.
NMDS ordination of alliances within AG1 (a–c) and proportional contributions of environmental factors to ordination patterns of AG1 alliances (d).
NMDS ordination of alliances within AG2 revealed distinct differentiation patterns across axes. Along NMDS Axis 1, alliances 2_1, 2_2, 2_3, and 2_4 were segregated primarily by precipitation days in the driest quarter (PDDQ) and precipitation seasonality (PS). Alliance 2_2 occupied regions with the highest PDDQ, followed by 2_1 and then 2_4, while PS and temperature seasonality (TS) progressively decreased from 2_2 to 2_1 and 2_3 (Figure 7a,b). NMDS Axis 2 differentiated alliances 2_1, 2_2, and 2_3 based on aspect (ASP), with alliance 2_1 associated with sun-exposed slopes (higher ASP values) and alliances 2_2 and 2_3 clustered on shaded slopes (lower ASP values) (Figure 7a,c). Along NMDS Axis 3, alliances 2_5 and 2_6 diverged due to soil clay content (CLAY), where alliance 2_6 inhabited CLAY-rich habitats and 2_5 occurred in CLAY-poor ones, with the remaining four alliances exhibiting intermediate CLAY values (Figure 7b,c). Environmental contributions to AG2 ordination patterns were dominated by precipitation and temperature (57.6%), followed by topographic factors (23.71%, slightly exceeding temperature’s 23.65%) and soil properties (18.7%) (Figure 7d).
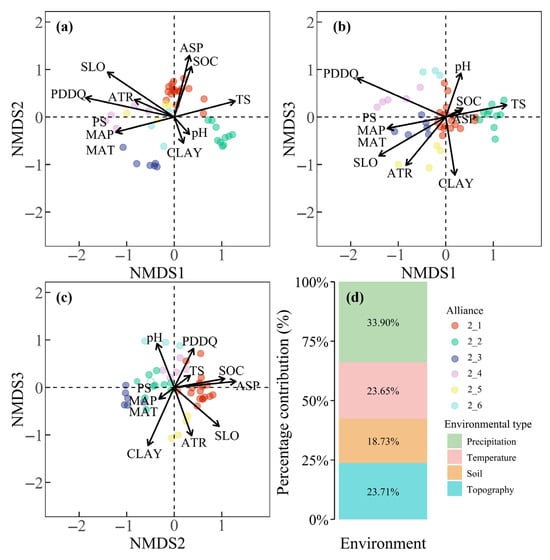
Figure 7.
NMDS ordination of alliances within AG2 (a–c) and proportional contributions of environmental factors to ordination patterns of AG2 alliances (d).
For alliances within AG3, NMDS Axis 1 effectively differentiated alliance 3_1 from 3_2, with precipitation seasonality (PS) and temperature seasonality (TS) serving as the dominant drivers. Alliance 3_1 clustered in regions with stronger PS and TS, while alliance 3_2 occupied areas with weaker seasonal climatic fluctuations (Figure 8a,b). Differentiation along NMDS Axes 2 and 3 was negligible for both alliances (Figure 8a–c). Environmental contributions to AG3 alliance differentiation were dominated by climatic factors (precipitation and temperature: 62.1%), followed by soil properties (24.8%) and topographic variables (13.2%) (Figure 8d).
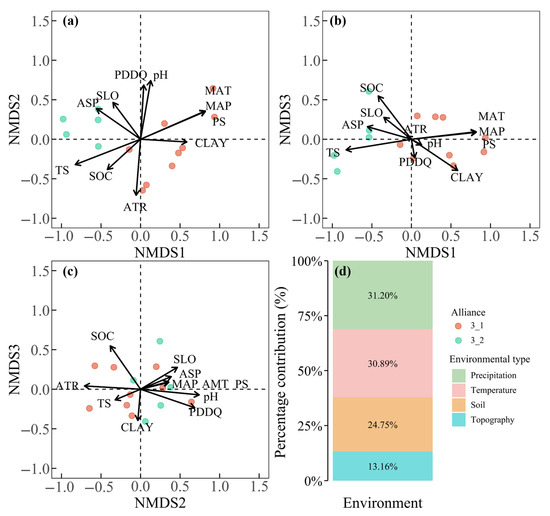
Figure 8.
NMDS ordination of alliances within AG3 (a–c) and proportional contributions of environmental factors to ordination patterns of AG3 alliances (d).
In summary, the seasonal distribution of moisture—specifically, precipitation days in the driest quarter (PDDQ) and precipitation seasonality (PS)—was identified as the core driver of differentiation among MEBF communities in Yunnan, with precipitation factors contributing the highest proportion to this divergence at both alliance group and alliance levels. Soil and topographic factors played a minor role in alliance group differentiation and within AG1 alliances, but their contributions increased significantly for alliances in AG2 and AG3. In AG2, topographic influence slightly exceeded that of temperature and was markedly stronger than soil effects, while in AG3, soil factors exerted substantially greater influence than topography.
4. Discussion
4.1. Comparison of Species Composition in Yunnan’s MEBF
The MEBF represents one of the most species-diverse and structurally complex vegetation types among evergreen broad-leaved forests [4,6,52]. Our findings demonstrate that the species richness of tree, shrub, and herb layers in Yunnan’s MEBF remains consistently comparable across plot-to-regional scales (Table 2, Figure 2), a distinct feature contrasting with other evergreen broad-leaved forest types in Yunnan. For instance, semi-humid evergreen broad-leaved forests in the region exhibit marked disparities in richness among life forms, with trees, shrubs, and herbs accounting for 9.5%, 28.5%, and 62.0% of total species, respectively [53]. Zhu (2021) also mentioned that monsoon evergreen broad-leaved forests show minimal differences in species counts among phanerophytes (predominantly trees), chamaephytes (shrubs), and hemicryptophytes/cryptophytes (herbs), unlike semi-humid and middle-montane wet evergreen broad-leaved forests [4]. Yunnan’s MEBF also diverges from its eastern Chinese counterparts: for example, the canopy layer of Dinghushan’s MEBF harbors obviously fewer species (100) compared to its shrub and sub-canopy layers (164 combined) [54], while the MEBF in Fujian’s Huboliao Reserve displays skewed richness (48 tree, 118 shrub, and 47 herb species) [55]. In contrast, Yunnan’s MEBF exhibits substantially higher tree species richness. Furthermore, significant differences in plot-scale species richness exist among alliance groups, following AG3 (102.43 ± 4.09) > AG2 (73.13 ± 4.06) > AG1 (55.56 ± 1.38). Although the entire MEBF distribution falls under a subtropical monsoon climate, regional climatic divergence—driven by Indian Ocean monsoon influences in southwestern/southern Yunnan versus Pacific monsoon impacts in the southeast—manifests most prominently in precipitation seasonality [56,57]. AG3’s distribution areas, despite slightly lower annual precipitation and temperature, experience twice the precipitation days in the driest quarter compared to AG1 (Table 5). This results in stronger winter–spring drought stress in AG1’s warmer and drier habitats, likely explaining its reduced species richness relative to AG2 and AG3.

Table 5.
Climatic features of three alliance groups in Yunnan’s MEBF (mean ± SE).
4.2. Community Diversity of Yunnan’s MEBF
Community classification of evergreen broad-leaved forests has long been a focal yet challenging task in vegetation ecology [18,20]. Studies on Yunnan’s MEBF reveal substantial discrepancies in classification outcomes. The Vegetation of China (1980) divided Chinese MEBF into two alliance groups and eight alliances, with Yunnan’s MEBF classified as a single “Castanopsis, Schima Forest Alliance Group” containing four alliances [3]. The Vegetation of Yunnan (1987) further partitioned Yunnan’s MEBF into four alliance groups and six alliances [2], while Zeng (2018) delineated thirty-nine forest types (equivalent to alliances) [37]. These divergent results largely stem from historical data integration constrained by incomplete or unrepresentative datasets, leading to inconsistencies in reported community diversity. Leveraging comprehensive field survey data and adhering to the hierarchical classification system of Vegetation Records of China [18], this study identified 3 alliance groups and 24 alliances for Yunnan’s MEBF (Table 3). The “Castanopsis, Schima Forest Alliance Group” from The Vegetation of China (1980) and the “Castanopsis, Lithocarpus Forest Alliance Group” from The Vegetation of Yunnan (1987), which exhibit significant floristic overlap, were consolidated into AG1 (Castanopsis–Schima (Lithocarpus) Forest Alliance Group) to reflect compositional coherence and species continuity. AG2 (Castanopsis–Machilus (Beilschmiedia) Forest Alliance Group) corresponds to the “Machilus, Castanopsis Forest Alliance Group” in The Vegetation of Yunnan (1987), and AG3 (Castanopsis–Camellia Forest Alliance Group) aligns with the “Cyclobalanopsis, Manglietia Forest Alliance Group”. Yunnan’s MEBF demonstrates greater complexity at alliance group and alliance levels compared to its eastern Chinese counterparts, where the “Castanopsis, Cryptocarya Forest Alliance Group” predominates [2,3,20]. AG1, the most widespread and diverse alliance group in Yunnan, contrasts sharply with this pattern. Notably, the “Quercus, Podocarpus Forest Alliance Group” reported in The Vegetation of Yunnan (1987) was absent in our study, as field surveys within its documented distribution range failed to validate this community type, and no subsequent studies have confirmed its existence since 1987.
4.3. Community Differentiation of Yunnan’s MEBF
Ordination analyses reveal that climatic seasonality—particularly precipitation seasonality—dominates community differentiation in Yunnan’s MEBF. Among these factors, the temporal evenness of precipitation in the driest quarter (PDDQ, rather than total precipitation) emerged as the strongest control on alliance group differentiation (R2 = 0.339, p < 0.001) (Figure 5, Appendix A Table A2). Esquivel-Muelbert et al. (2016) similarly demonstrated that dry-season precipitation timing and distribution exert significant negative impacts on plant diversity in tropical forests [58]. Thus, seasonal precipitation allocation not only constrains vegetation growth [2,59] but also critically shapes community structure [60,61] and biodiversity maintenance [60,62], thereby driving ecological differentiation across MEBF communities.
At the alliance level, climatic seasonality (PS, TS, PDDQ) also dominated differentiation within AG1 (Figure 6, Appendix A Table A3). In AG1, Castanopsis species formed distinct co-constructive species combinations with taxa from other families, resulting in alliances adapted to varying climatic regimes. For instance, Lithocarpus fenestratus + Schima wallichii Forest Alliance (1_3), S. wallichii + Aporosa dioica Forest Alliance (1_5), and L. truncatus + S. wallichii Forest Alliance (1_8)—where L. fenestratus, L. truncatus, and S. wallichii serve as co-constructive species—were associated with regions experiencing fewer dry-season precipitation days and stronger temperature seasonality (Figure 6). Similarly, in AG2, despite occupying areas with more evenly distributed dry-season precipitation, PDDQ (R2 = 0.478, p < 0.001) remained the strongest driver of alliance differentiation (Figure 5 and Figure 7; Appendix A Table A4). Topographic and soil factors exerted greater influence on alliance divergence in AG2 and AG3 than in AG1. For example, alliance 2_2 (Beilschmiedia glauca var. glaucoides + Helicia pyrrhobotrya Forest Alliance) predominantly occurred on steep shaded slopes with higher clay content (Figure 7), while alliance 3_2 (Cas. lamontii + Camellia sp. Forest Alliance) favored gentler shaded slopes with lower clay content (Figure 8). These patterns reflect the preference of Lauraceae and Magnoliaceae species for humid ravine habitats [18], a habitat affinity also observed in Southeast Asian monsoon forests [63].
4.4. Limitation and Perspective for a Vegetation Classification System of Yunnan’s MEBF
A vegetation classification system generally comprises a hierarchical structure, and is defined according to the floristic composition of common characteristic species in lower units, and the physiognomy of species and habitats in higher units [3,6,16,18], e.g., alliance, following the phytosociological syntaxonomic nomenclature. Given the extensive range of community investigation and a considerable sample size of plots included in this study, we are able to identify the higher vegetation units (i.e., alliance group and alliance) of MEBF in Yunnan. However, a formal vegetation classification system of MEBF with complete lower units in this region requires a more complete field sampling and adequate samples for each basic unit (i.e., association), and further comprehensive analysis following the phytosociological methodology.
5. Conclusions
Based on data from 548 plots spanning the core distribution range of Yunnan’s MEBF, this study provides the first comprehensive characterization of community differentiation, geographic distribution, and environmental interpretations of this vegetation type. Our findings identify three alliance groups and twenty-four alliances. AG1 predominates in central- and southwestern Yunnan (west of 102° E), AG2 occupies a narrow latitudinal belt in southeastern Yunnan (east of 101° E), and AG3 is restricted to high-elevation mountainous zones between 103° E–104° E and 22.5° N–23° N. Yunnan’s MEBF consistently displays balanced species allocation across tree, shrub, and herb layers (approximately 1:1:1 ratio), with habitats characterized by similar seasonal climates of alternating dry–wet cycles. However, AG1 experiences fewer rainy days in the driest quarter than AG2 and AG3, while AG3 is located at sites with stronger temperature seasonality than AG2. The intensity of moisture and temperature seasonality—particularly the temporal evenness of dry-season precipitation—emerges as the core driver of differentiation among alliance groups and alliances in the MEBF.
Based on the differentiation patterns observed in Yunnan’s MEBF, forest conservation and management strategies under intensifying climate change should prioritize the impact of precipitation seasonality on forest distribution and community differentiation. Furthermore, special attention must be given to protecting forest communities dominated by endangered species—such as Trigonobalanus doichangensis Forman and Castanopsis concinna A.DC.—through targeted conservation efforts.
Author Contributions
Conceptualization, Z.S.; Data curation, S.L., R.L., F.D., J.S. and Z.S.; Formal analysis, T.Y., X.W. and J.R.; Funding acquisition, Z.S.; Investigation, T.Y., X.W., J.R., S.L., S.L., F.D., C.Z., X.T., W.L., J.D. and H.Y.; Visualization, Z.S.; Writing—original draft, T.Y.; Writing—review and editing, Z.S. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Yunnan Fundamental Research Projects (the funder is Yunnan Province Science and Technology Department, and the funding number is 202302d4040076), and the National Key R&D Program of China (the funder is Ministry of Science and Technology of the People’s Republic of China, and the funding number is 2024YFF1308101).
Data Availability Statement
Original data cannot be shared under the project’s confidentiality agreements.
Conflicts of Interest
All authors declare no competing interests.
Appendix A

Table A1.
The top ten families and genera of different growth forms of plants in Yunnan’s MEBF.
Table A1.
The top ten families and genera of different growth forms of plants in Yunnan’s MEBF.
| Growth Form | The Top Ten Families and Genera | |||
|---|---|---|---|---|
| Family | No. of Species | Genus | No. of Species | |
| All species | Fabaceae | 194 | Ficus | 56 |
| Lauraceae | 157 | Ilex | 46 | |
| Rubiaceae | 157 | Smilax | 37 | |
| Orchidaceae | 156 | Symplocos | 34 | |
| Poaceae | 116 | Lithocarpus | 33 | |
| Liliaceae | 91 | Litsea | 32 | |
| Asteraceae | 90 | Rubus | 31 | |
| Fagaceae | 85 | Ardisia | 28 | |
| Euphorbiaceae | 83 | Castanopsis | 28 | |
| Rosaceae | 79 | Machilus | 26 | |
| Tree | Lauraceae | 139 | Ilex | 45 |
| Fagaceae | 85 | Lithocarpus | 33 | |
| Fabaceae | 73 | Ficus | 30 | |
| Aquifoliaceae | 45 | Castanopsis | 28 | |
| Euphorbiaceae | 43 | Machilus | 25 | |
| Moraceae | 41 | Symplocos | 25 | |
| Rosaceae | 40 | Elaeocarpus | 24 | |
| Theaceae | 39 | Litsea | 23 | |
| Magnoliaceae | 37 | Cinnamomum | 20 | |
| Araliaceae | 29 | Lindera | 19 | |
| —— | —— | Syzygium | 19 | |
| Shrub | Rubiaceae | 107 | Smilax | 37 |
| Fabaceae | 99 | Rubus | 30 | |
| Myrsinaceae | 60 | Ardisia | 27 | |
| Vitaceae | 41 | Ficus | 26 | |
| Poaceae | 40 | Lasianthus | 25 | |
| Liliaceae | 39 | Jasminum | 23 | |
| Euphorbiaceae | 38 | Tetrastigma | 23 | |
| Rosaceae | 36 | Embelia | 16 | |
| Verbenaceae | 35 | Maesa | 16 | |
| Moraceae | 33 | Vaccinium | 15 | |
| Herb | Orchidaceae | 152 | Dryopteris | 22 |
| Asteraceae | 80 | Dioscorea | 21 | |
| Poaceae | 76 | Lepisorus | 21 | |
| Polypodiaceae | 62 | Carex | 20 | |
| Zingiberaceae | 59 | Dendrobium | 20 | |
| Dryopteridaceae | 54 | Bulbophyllum | 19 | |
| Liliaceae | 52 | Piper | 19 | |
| Cyperaceae | 38 | Alpinia | 17 | |
| Labiatae | 36 | Pteris | 17 | |
| Urticaceae | 36 | Clematis | 16 | |
| —— | —— | Pilea | 16 | |
| —— | —— | Polygonum | 16 | |
| —— | —— | Polystichum | 16 | |
| Fern | Polypodiaceae | 62 | Dryopteris | 22 |
| Dryopteridaceae | 54 | Lepisorus | 21 | |
| Athyriaceae | 34 | Pteris | 17 | |
| Thelypteridaceae | 27 | Polystichum | 16 | |
| Pteridaceae | 17 | Athyrium | 14 | |
| Aspleniaceae | 13 | Allantodia | 12 | |
| Selaginellaceae | 11 | Arachniodes | 12 | |
| Hypolepidaceae | 9 | Asplenium | 12 | |
| Cyatheaeceae | 7 | Cyclosorus | 11 | |
| Davalliaceae | 7 | Selaginella | 11 | |
| Lygodiaceae | 7 | —— | —— | |
| liana | Fabaceae | 59 | Tetrastigma | 24 |
| Vitaceae | 39 | Dioscorea | 21 | |
| Menispermaceae | 25 | Piper | 17 | |
| Dioscoreaceae | 21 | Clematis | 16 | |
| Apocynaceae | 17 | Embelia | 11 | |
| Asclepiadaceae | 17 | Jasminum | 11 | |
| Piperaceae | 17 | Dalbergia | 8 | |
| Ranunculaceae | 16 | Millettia | 8 | |
| Cucurbitaceae | 13 | Schisandra | 8 | |
| Magnoliaceae | 13 | Stephania | 8 | |

Table A2.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliance groups.
Table A2.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliance groups.
| NMDS1 | NMDS2 | NMDS3 | R2 | Sig. | |
|---|---|---|---|---|---|
| SLO | 0.42666 | −0.74026 | 0.5196 | 0.0555 | *** |
| ASP | −0.37318 | 0.9276 | −0.0172 | 0.0066 | |
| pH | −0.62133 | −0.60665 | 0.49591 | 0.0638 | *** |
| SOC | 0.78589 | −0.5409 | 0.29968 | 0.1433 | *** |
| CLAY | −0.70942 | 0.43705 | −0.55291 | 0.0683 | *** |
| MAP | −0.54291 | 0.65618 | −0.5241 | 0.2529 | *** |
| PS | −0.54955 | 0.64886 | −0.52629 | 0.2519 | *** |
| PDDQ | 0.9985 | 0.03482 | −0.04221 | 0.339 | *** |
| MAT | −0.54291 | 0.65618 | −0.5241 | 0.2529 | *** |
| TS | 0.57428 | −0.62104 | 0.5334 | 0.247 | *** |
| ATR | 0.05566 | −0.83101 | 0.55346 | 0.0727 | *** |
Significance level: “***” p < 0.001.

Table A3.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG1.
Table A3.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG1.
| NMDS1 | NMDS2 | NMDS3 | R2 | Sig. | |
|---|---|---|---|---|---|
| SLO | −0.76683 | 0.59566 | 0.2391 | 0.0151 | |
| ASP | 0.43146 | −0.00711 | −0.9021 | 0.0113 | |
| pH | −0.75107 | 0.38142 | −0.5389 | 0.0755 | *** |
| SOC | −0.82969 | 0.55289 | 0.07702 | 0.0546 | *** |
| CLAY | 0.36213 | −0.65509 | 0.66311 | 0.0471 | *** |
| MAP | 0.74656 | −0.66485 | −0.025 | 0.2172 | *** |
| PS | 0.74467 | −0.66706 | −0.02217 | 0.2169 | *** |
| PDDQ | 0.91827 | −0.19537 | −0.34439 | 0.0985 | *** |
| MAT | 0.74656 | −0.66485 | −0.025 | 0.2172 | *** |
| TS | −0.73912 | 0.67342 | 0.01429 | 0.2147 | *** |
| ATR | −0.67807 | 0.53506 | −0.50392 | 0.076 | *** |
Significance level: “***” p < 0.001.

Table A4.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG2.
Table A4.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG2.
| NMDS1 | NMDS2 | NMDS3 | R2 | Sig. | |
|---|---|---|---|---|---|
| SLO | −0.74704 | 0.50272 | −0.43498 | 0.3924 | *** |
| ASP | 0.23851 | 0.9665 | 0.09485 | 0.1997 | * |
| pH | 0.30578 | −0.3475 | 0.88642 | 0.1208 | |
| SOC | 0.3167 | 0.93433 | 0.16345 | 0.1435 | |
| CLAY | 0.14245 | −0.40642 | −0.90251 | 0.2033 | * |
| MAP | −0.95143 | −0.24625 | −0.18479 | 0.1819 | * |
| PS | −0.95119 | −0.24656 | −0.18561 | 0.1863 | * |
| PDDQ | −0.89765 | 0.19586 | 0.39479 | 0.4783 | *** |
| MAT | −0.95143 | −0.24625 | −0.18479 | 0.1819 | * |
| TS | 0.95027 | 0.24927 | 0.1867 | 0.2018 | * |
| ATR | −0.61199 | 0.26856 | −0.74388 | 0.2069 | * |
Significance level: “***” p < 0.001; “*” p < 0.05.

Table A5.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG3.
Table A5.
Results of environmental factor fitting (envfit) in NMDS ordination space about alliances in AG3.
| NMDS1 | NMDS2 | NMDS3 | R2 | Sig. | |
|---|---|---|---|---|---|
| SLO | −0.54039 | 0.72322 | 0.43003 | 0.4149 | |
| ASP | −0.79849 | 0.55701 | 0.22835 | 0.4858 | |
| pH | 0.17027 | 0.98087 | −0.09433 | 0.5722 | * |
| SOC | −0.53162 | −0.48507 | 0.69432 | 0.6225 | * |
| CLAY | 0.82789 | −0.04841 | −0.5588 | 0.5 | |
| MAP | 0.91181 | 0.39878 | 0.09785 | 0.8046 | ** |
| PS | 0.91366 | 0.39246 | 0.10585 | 0.8042 | ** |
| PDDQ | 0.05629 | 0.94495 | −0.32234 | 0.527 | |
| MAT | 0.91181 | 0.39878 | 0.09785 | 0.8046 | ** |
| TS | −0.92148 | −0.35821 | −0.1502 | 0.8029 | ** |
| ATR | −0.07841 | −0.99523 | 0.05796 | 0.5077 |
Significance level: “**” p < 0.01; “*” p < 0.05.

Table A6.
Synoptic table for alliance groups.
Table A6.
Synoptic table for alliance groups.
| Alliance Group Number | 1 | 2 | 3 |
|---|---|---|---|
| Number of plots | 462 | 46 | 14 |
| Schima wallichii | 87 | 20 | 7 |
| Camellia crassicolumna | 1 | 9 | 86 |
| Castanopsis remotidenticulata | 0 | 4 | 86 |
| Laurocerasus phaeosticta | 1 | 4 | 79 |
| Rehderodendron macrocarpum | 2 | 11 | 79 |
| Acer sinense | 0 | 7 | 71 |
| Camellia tsaii | 2 | 4 | 71 |
| Castanopsis ceratacantha | 4 | 67 | 0 |
| Laurocerasus undulata | 2 | 7 | 57 |
| Castanopsis echidnocarpa | 57 | 2 | 0 |
| Lithocarpus fenestratus | 54 | 22 | 14 |
| Camellia tsingpienensis var. tsingpienensis | 0 | 0 | 50 |
| Litsea honghoensis | 0 | 0 | 50 |
| Manglietia insignis | 1 | 2 | 50 |
| Viburnum trabeculosum | 0 | 2 | 50 |
| Anneslea fragrans | 45 | 2 | 0 |
| Eurya groffii | 43 | 17 | 50 |
| Alcimandra cathcartii | 2 | 4 | 43 |
| Beilschmiedia fasciata | 1 | 9 | 43 |
| Euonymus laxiflorus | 1 | 2 | 43 |
| Lithocarpus fordianus | 1 | 0 | 43 |
| Michelia floribunda | 5 | 4 | 43 |
| Styrax grandiflorus | 1 | 4 | 43 |
| Lithocarpus truncatus | 41 | 9 | 0 |
| Castanopsis fissa | 0 | 41 | 0 |
| Castanopsis calathiformis | 39 | 11 | 0 |
| Lithocarpus hancei | 2 | 37 | 57 |
| Castanopsis lamontii | 0 | 0 | 36 |
| Erythroxylum sinensis | 1 | 13 | 36 |
| Schefflera hoi | 2 | 4 | 36 |
| Aporusa villosa | 34 | 0 | 0 |
| Rapanea neriifolia | 32 | 0 | 0 |
| Castanopsis hystrix | 31 | 4 | 0 |
| Lindera metcalfiana | 10 | 30 | 7 |
| Machilus rufipes | 17 | 30 | 29 |
| Cerasus pseudocerasus | 0 | 2 | 29 |
| Elaeocarpus decipiens | 4 | 11 | 29 |
| Elaeocarpus limitaneus | 1 | 7 | 29 |
| Eurya loquaiana | 1 | 7 | 29 |
| Rubus paniculatus | 0 | 7 | 29 |
| Symplocos spectabilis | 0 | 0 | 29 |
| Symplocos sumuntia | 1 | 11 | 29 |
| Betula alnoides | 28 | 11 | 0 |
| Manglietia fordiana | 0 | 28 | 0 |
| Beilschmiedia glauca var. glaucoides | 1 | 26 | 0 |
| Eurya tetragonoclada | 0 | 26 | 7 |
Note: Data represent species frequency (%). Species are ordered by descending fidelity value (φ, p < 0.05). Diagnostic species for the Alliance Group are highlighted: light gray background (0.25 ≤ φ < 0.5) and dark gray background (φ ≥ 0.5). Constant species (frequency ≥ 60%) are marked; only species with φ ≥ 0.25 are displayed due to space constraints.

Table A7.
Synoptic table for AG1.
Table A7.
Synoptic table for AG1.
| Alliance Number | 1_1 | 1_2 | 1_3 | 1_4 | 1_5 | 1_6 | 1_7 | 1_8 | 1_9 | 1_10 | 1_11 | 1_12 | 1_13 | 1_14 | 1_15 | 1_16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of plots | 173 | 51 | 42 | 40 | 33 | 31 | 28 | 19 | 9 | 8 | 7 | 5 | 5 | 4 | 4 | 3 |
| Anneslea fragrans | 62 | 37 | 24 | 70 | 27 | 32 | 25 | 32 | 0 | 25 | 0 | 100 | 20 | 0 | 100 | 0 |
| Aporusa villosa | 53 | 41 | 7 | 20 | 15 | 19 | 29 | 11 | 0 | 0 | 0 | 80 | 40 | 75 | 100 | 0 |
| Castanopsis calathiformis | 39 | 25 | 31 | 63 | 18 | 100 | 54 | 11 | 0 | 50 | 0 | 40 | 0 | 0 | 0 | 0 |
| Castanopsis echidnocarpa | 100 | 41 | 26 | 50 | 33 | 32 | 14 | 11 | 33 | 25 | 0 | 60 | 0 | 25 | 0 | 33 |
| Castanopsis ferox | 3 | 2 | 2 | 3 | 9 | 13 | 29 | 0 | 0 | 100 | 0 | 0 | 0 | 25 | 0 | 0 |
| Castanopsis fleuryi | 15 | 8 | 24 | 100 | 15 | 35 | 0 | 32 | 33 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Castanopsis hystrix | 44 | 100 | 2 | 8 | 0 | 3 | 7 | 0 | 44 | 13 | 0 | 0 | 20 | 0 | 0 | 33 |
| Castanopsis mekongensis | 20 | 2 | 5 | 0 | 0 | 10 | 100 | 0 | 0 | 38 | 0 | 0 | 0 | 0 | 0 | 0 |
| Castanopsis wattii | 4 | 16 | 0 | 0 | 21 | 3 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclobalanopsis kerrii | 6 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| Cyclobalanopsis myrsinaefolia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Lithocarpus elegans | 10 | 10 | 10 | 0 | 9 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 20 | 100 | 0 | 0 |
| Lithocarpus truncatus | 44 | 41 | 31 | 60 | 18 | 42 | 39 | 100 | 0 | 13 | 0 | 80 | 20 | 25 | 25 | 0 |
| Phyllanthus emblica | 12 | 16 | 17 | 18 | 9 | 3 | 29 | 11 | 0 | 13 | 0 | 100 | 20 | 25 | 50 | 33 |
| Schima wallichii | 83 | 90 | 86 | 100 | 100 | 84 | 96 | 68 | 89 | 75 | 71 | 60 | 80 | 100 | 50 | 100 |
| Styrax tonkinensis | 21 | 29 | 7 | 3 | 0 | 6 | 29 | 0 | 11 | 38 | 0 | 0 | 100 | 25 | 25 | 0 |
| Trigonobalanus doichangensis | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Vaccinium exaristatum | 25 | 20 | 5 | 8 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 100 | 20 | 0 | 0 | 0 |
| Wendlandia tinctoria ssp. intermedia | 30 | 10 | 12 | 25 | 15 | 19 | 21 | 0 | 0 | 0 | 0 | 100 | 40 | 25 | 75 | 67 |
| Wendlandia uvariifolia ssp. uvariifolia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Lithocarpus fenestratus | 51 | 55 | 98 | 55 | 24 | 61 | 64 | 53 | 0 | 75 | 14 | 60 | 20 | 50 | 25 | 0 |
| Castanopsis tcheponensis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 0 | 0 | 0 | 0 | 0 |
| Engelhardtia spicata | 16 | 12 | 14 | 15 | 9 | 6 | 18 | 16 | 0 | 0 | 86 | 60 | 0 | 25 | 25 | 0 |
| Aporusa villosa | 53 | 41 | 7 | 20 | 15 | 19 | 29 | 11 | 0 | 0 | 0 | 80 | 40 | 75 | 100 | 0 |
| Castanopsis indica | 9 | 14 | 2 | 0 | 18 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | 80 | 0 | 0 | 0 |
| Glochidion lanceolarium | 16 | 16 | 5 | 30 | 12 | 19 | 18 | 5 | 0 | 0 | 0 | 80 | 0 | 25 | 25 | 0 |
| Syzygium szemaoense | 14 | 10 | 2 | 3 | 6 | 10 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 75 | 0 |
| Wendlandia tinctoria ssp. intermedia | 30 | 10 | 12 | 25 | 15 | 19 | 21 | 0 | 0 | 0 | 0 | 100 | 40 | 25 | 75 | 67 |
| Betula alnoides | 25 | 22 | 38 | 28 | 55 | 39 | 14 | 21 | 44 | 13 | 71 | 0 | 0 | 25 | 0 | 33 |
| Lindera caudata | 12 | 6 | 12 | 18 | 9 | 19 | 11 | 16 | 11 | 0 | 71 | 0 | 0 | 50 | 25 | 0 |
| Litsea lancifolia | 1 | 2 | 0 | 0 | 3 | 0 | 0 | 16 | 0 | 0 | 71 | 0 | 0 | 0 | 0 | 0 |
| Eurya groffii | 42 | 25 | 36 | 55 | 55 | 71 | 36 | 68 | 33 | 50 | 29 | 40 | 0 | 50 | 25 | 0 |
| Anneslea fragrans | 62 | 37 | 24 | 70 | 27 | 32 | 25 | 32 | 0 | 25 | 0 | 100 | 20 | 0 | 100 | 0 |
| Eurya groffii | 42 | 25 | 36 | 55 | 55 | 71 | 36 | 68 | 33 | 50 | 29 | 40 | 0 | 50 | 25 | 0 |
| Anneslea fragrans var. fragrans | 2 | 0 | 2 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 67 |
| Diospyros kaki var. sylvestris | 8 | 2 | 24 | 5 | 12 | 16 | 4 | 11 | 33 | 13 | 0 | 0 | 0 | 0 | 0 | 67 |
| Itea macrophylla | 15 | 4 | 5 | 5 | 12 | 10 | 7 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 67 |
| Machilus pingii | 3 | 2 | 0 | 3 | 6 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 67 |
| Syzygium leptanthum | 0 | 2 | 7 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 67 |
| Toxicodendron succedaneum | 19 | 18 | 17 | 13 | 21 | 13 | 4 | 0 | 67 | 38 | 0 | 20 | 0 | 50 | 0 | 0 |
| Wendlandia pendula | 0 | 0 | 5 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 67 |
| Castanopsis calathiformis | 39 | 25 | 31 | 63 | 18 | 100 | 54 | 11 | 0 | 50 | 0 | 40 | 0 | 0 | 0 | 0 |
| Phoebe puwenensis | 23 | 12 | 12 | 0 | 15 | 23 | 57 | 21 | 22 | 63 | 14 | 0 | 20 | 50 | 0 | 33 |
| Anneslea fragrans | 62 | 37 | 24 | 70 | 27 | 32 | 25 | 32 | 0 | 25 | 0 | 100 | 20 | 0 | 100 | 0 |
| Aporusa yunnanensis | 23 | 31 | 7 | 3 | 6 | 0 | 21 | 0 | 0 | 25 | 0 | 60 | 0 | 25 | 0 | 0 |
| Artocarpus tonkinensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 |
| Canarium subulatum | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 60 | 0 | 0 | 0 |
| Choerospondias axillaris | 1 | 4 | 2 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 |
| Craibiodendron stellatum | 8 | 4 | 5 | 23 | 0 | 3 | 4 | 16 | 0 | 0 | 0 | 60 | 20 | 0 | 25 | 0 |
| Dalbergia fusca | 6 | 10 | 5 | 8 | 6 | 0 | 4 | 0 | 0 | 0 | 0 | 60 | 40 | 25 | 0 | 0 |
| Engelhardtia serrata | 18 | 6 | 5 | 10 | 9 | 3 | 7 | 11 | 0 | 0 | 0 | 60 | 0 | 0 | 25 | 33 |
| Engelhardtia spicata | 16 | 12 | 14 | 15 | 9 | 6 | 18 | 16 | 0 | 0 | 86 | 60 | 0 | 25 | 25 | 0 |
| Ficus racemosa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 25 | 0 | 0 |
| Lithocarpus truncatus | 44 | 41 | 31 | 60 | 18 | 42 | 39 | 100 | 0 | 13 | 0 | 80 | 20 | 25 | 25 | 0 |
| Spondias pinnat | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 |
| Myrica esculenta | 12 | 8 | 40 | 58 | 15 | 29 | 7 | 53 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Allomorphia balansae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 |
| Phoebe puwenensis | 23 | 12 | 12 | 0 | 15 | 23 | 57 | 21 | 22 | 63 | 14 | 0 | 20 | 50 | 0 | 33 |
| Betula alnoides | 25 | 22 | 38 | 28 | 55 | 39 | 14 | 21 | 44 | 13 | 71 | 0 | 0 | 25 | 0 | 33 |
| Myrica esculenta | 12 | 8 | 40 | 58 | 15 | 29 | 7 | 53 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aporusa villosa | 53 | 41 | 7 | 20 | 15 | 19 | 29 | 11 | 0 | 0 | 0 | 80 | 40 | 75 | 100 | 0 |
| Rapanea neriifolia | 52 | 31 | 26 | 30 | 15 | 13 | 11 | 26 | 0 | 0 | 0 | 20 | 40 | 25 | 0 | 0 |
| Albizia kalkora | 3 | 10 | 10 | 3 | 3 | 3 | 11 | 5 | 11 | 0 | 0 | 0 | 20 | 50 | 0 | 0 |
| Cinnamomum bejolghota | 5 | 10 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 25 | 14 | 0 | 0 | 50 | 0 | 0 |
| Craspedolobium schochii | 17 | 27 | 2 | 20 | 9 | 3 | 50 | 0 | 0 | 13 | 0 | 0 | 20 | 0 | 0 | 0 |
| Dalbergia polyadelpha | 3 | 4 | 0 | 8 | 6 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 50 | 0 |
| Diospyros kaki | 3 | 4 | 7 | 5 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 |
| Gnetum montanum | 14 | 6 | 2 | 0 | 6 | 0 | 4 | 0 | 0 | 0 | 14 | 20 | 20 | 25 | 50 | 0 |
| Helicia nilagirica | 15 | 6 | 31 | 50 | 9 | 42 | 14 | 47 | 0 | 13 | 14 | 0 | 0 | 0 | 0 | 67 |
| Macaranga denticulata | 4 | 2 | 5 | 3 | 15 | 6 | 4 | 5 | 0 | 0 | 0 | 0 | 20 | 50 | 0 | 0 |
| Machilus robusta | 10 | 8 | 7 | 13 | 12 | 13 | 32 | 5 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mallotus philippensis | 5 | 6 | 7 | 0 | 6 | 6 | 21 | 0 | 0 | 13 | 0 | 40 | 20 | 50 | 0 | 0 |
| Micromelum integerrimum | 1 | 0 | 0 | 0 | 3 | 0 | 7 | 0 | 0 | 13 | 0 | 0 | 0 | 50 | 0 | 0 |
| Phoebe lanceolata | 12 | 4 | 2 | 5 | 0 | 0 | 11 | 5 | 0 | 0 | 0 | 0 | 20 | 50 | 0 | 0 |
| Ternstroemia simaoensis | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 50 | 0 |
| Helicia nilagirica | 15 | 6 | 31 | 50 | 9 | 42 | 14 | 47 | 0 | 13 | 14 | 0 | 0 | 0 | 0 | 67 |
| Lindera metcalfiana | 13 | 6 | 5 | 3 | 12 | 6 | 18 | 0 | 44 | 0 | 0 | 20 | 0 | 0 | 0 | 0 |
| Castanopsis hystrix | 44 | 100 | 2 | 8 | 0 | 3 | 7 | 0 | 44 | 13 | 0 | 0 | 20 | 0 | 0 | 33 |
| Alniphyllum fortunei | 1 | 0 | 0 | 3 | 6 | 3 | 0 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Ilex chapaensis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Itea indochinensis var. pubinervia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Macaranga tanarius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Schefflera hoi | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 16 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Helicia nilagirica | 15 | 6 | 31 | 50 | 9 | 42 | 14 | 47 | 0 | 13 | 14 | 0 | 0 | 0 | 0 | 67 |
| Myrica esculenta | 12 | 8 | 40 | 58 | 15 | 29 | 7 | 53 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bauhinia acuminata | 1 | 0 | 5 | 0 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Dalbergia fusca | 6 | 10 | 5 | 8 | 6 | 0 | 4 | 0 | 0 | 0 | 0 | 60 | 40 | 25 | 0 | 0 |
| Dalbergia pinnata | 2 | 2 | 0 | 3 | 9 | 10 | 4 | 5 | 11 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Elaeocarpus rugosus | 3 | 6 | 0 | 0 | 3 | 3 | 0 | 0 | 11 | 13 | 0 | 40 | 0 | 25 | 0 | 0 |
| Erythrina variegata | 1 | 0 | 2 | 0 | 3 | 0 | 4 | 0 | 0 | 13 | 0 | 0 | 40 | 0 | 0 | 0 |
| Machilus fasciculata | 8 | 2 | 0 | 0 | 0 | 3 | 7 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Mallotus philippensis | 5 | 6 | 7 | 0 | 6 | 6 | 21 | 0 | 0 | 13 | 0 | 40 | 20 | 50 | 0 | 0 |
| Millettia velutina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Radermachera microcalyx | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Syzygium leptanthum | 0 | 2 | 7 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 67 |
| Castanopsis delavayi | 0 | 0 | 38 | 3 | 6 | 10 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lyonia doyonensis | 3 | 0 | 14 | 38 | 0 | 13 | 0 | 16 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lyonia ovalifolia | 5 | 4 | 14 | 38 | 6 | 6 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Schefflera delavayi | 6 | 2 | 5 | 3 | 3 | 0 | 7 | 11 | 0 | 38 | 0 | 0 | 0 | 0 | 0 | 0 |
| Actinodaphne henryi | 12 | 29 | 2 | 0 | 9 | 3 | 36 | 5 | 0 | 13 | 0 | 20 | 0 | 0 | 0 | 0 |
| Vernonia volkameriifolia | 6 | 20 | 12 | 10 | 6 | 10 | 36 | 11 | 0 | 25 | 29 | 0 | 0 | 0 | 0 | 33 |
| Canthium horridum | 21 | 35 | 7 | 3 | 9 | 6 | 32 | 0 | 0 | 25 | 0 | 20 | 20 | 0 | 0 | 0 |
| Arthraxon nudus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Castanopsis argyrophylla | 1 | 2 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 33 |
| Diospyros kaki var. sylvestris | 8 | 2 | 24 | 5 | 12 | 16 | 4 | 11 | 33 | 13 | 0 | 0 | 0 | 0 | 0 | 67 |
| Lindera kariensis f. glabrescens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Olea laxiflora | 1 | 0 | 5 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Symplocos racemosa | 1 | 0 | 0 | 3 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Tarennoidea wallichii | 27 | 33 | 14 | 5 | 12 | 0 | 21 | 11 | 0 | 13 | 14 | 0 | 20 | 0 | 25 | 0 |
| Canthium horridum | 21 | 35 | 7 | 3 | 9 | 6 | 32 | 0 | 0 | 25 | 0 | 20 | 20 | 0 | 0 | 0 |
| Idesia polycarpa | 3 | 6 | 0 | 0 | 3 | 10 | 32 | 0 | 0 | 13 | 0 | 20 | 0 | 0 | 0 | 0 |
| Machilus robusta | 10 | 8 | 7 | 13 | 12 | 13 | 32 | 5 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phoebe tavoyana | 5 | 27 | 2 | 0 | 6 | 3 | 32 | 5 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Castanopsis delavayi | 0 | 0 | 38 | 3 | 6 | 10 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aporusa yunnanensis | 23 | 31 | 7 | 3 | 6 | 0 | 21 | 0 | 0 | 25 | 0 | 60 | 0 | 25 | 0 | 0 |
| Aporusa dioica | 18 | 10 | 5 | 20 | 30 | 10 | 4 | 0 | 22 | 13 | 0 | 20 | 0 | 0 | 0 | 33 |
| Wendlandia tinctoria ssp. intermedia | 30 | 10 | 12 | 25 | 15 | 19 | 21 | 0 | 0 | 0 | 0 | 100 | 40 | 25 | 75 | 67 |
| Glochidion lanceolarium | 16 | 16 | 5 | 30 | 12 | 19 | 18 | 5 | 0 | 0 | 0 | 80 | 0 | 25 | 25 | 0 |
| Wendlandia uvariifolia | 14 | 10 | 14 | 30 | 3 | 13 | 7 | 26 | 0 | 0 | 0 | 20 | 0 | 0 | 25 | 0 |
| Actinodaphne henryi | 12 | 29 | 2 | 0 | 9 | 3 | 36 | 5 | 0 | 13 | 0 | 20 | 0 | 0 | 0 | 0 |
| Styrax tonkinensis | 21 | 29 | 7 | 3 | 0 | 6 | 29 | 0 | 11 | 38 | 0 | 0 | 100 | 25 | 25 | 0 |
| Aralia armata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 |
| Castanopsis ferox | 3 | 2 | 2 | 3 | 9 | 13 | 29 | 0 | 0 | 100 | 0 | 0 | 0 | 25 | 0 | 0 |
| Cyclobalanopsis rex | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 |
| Evodia lepta | 3 | 2 | 2 | 0 | 9 | 6 | 14 | 0 | 0 | 13 | 29 | 0 | 0 | 0 | 0 | 0 |
| Maesa perlarius | 2 | 4 | 0 | 0 | 0 | 0 | 29 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phoebe macrocarpa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 |
| Phyllanthus emblica | 12 | 16 | 17 | 18 | 9 | 3 | 29 | 11 | 0 | 13 | 0 | 100 | 20 | 25 | 50 | 33 |
| Wendlandia bouvardioides | 5 | 8 | 0 | 0 | 3 | 6 | 29 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Litsea garrettii | 28 | 16 | 2 | 10 | 12 | 0 | 11 | 0 | 0 | 25 | 0 | 0 | 20 | 0 | 0 | 33 |
| Paramichelia baillonii | 28 | 22 | 5 | 8 | 9 | 10 | 11 | 16 | 0 | 13 | 0 | 20 | 20 | 25 | 0 | 0 |
| Vaccinium mandarinorum | 10 | 10 | 14 | 28 | 0 | 16 | 4 | 16 | 0 | 13 | 0 | 0 | 0 | 0 | 25 | 0 |
| Craspedolobium schochii | 17 | 27 | 2 | 20 | 9 | 3 | 50 | 0 | 0 | 13 | 0 | 0 | 20 | 0 | 0 | 0 |
| Phoebe tavoyana | 5 | 27 | 2 | 0 | 6 | 3 | 32 | 5 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lindera metcalfiana var. dictyophylla | 7 | 4 | 12 | 15 | 27 | 19 | 0 | 16 | 22 | 13 | 0 | 0 | 0 | 0 | 0 | 33 |
| Tarennoidea wallichii | 27 | 33 | 14 | 5 | 12 | 0 | 21 | 11 | 0 | 13 | 14 | 0 | 20 | 0 | 25 | 0 |
| Vaccinium exaristatum | 25 | 20 | 5 | 8 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 100 | 20 | 0 | 0 | 0 |
| Acrocarpus fraxinifolius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Actinodaphne forrestii | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Albizia julibrissin | 2 | 0 | 2 | 0 | 3 | 3 | 18 | 5 | 0 | 25 | 0 | 20 | 20 | 0 | 0 | 0 |
| Artocarpus chama | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Arytera littoralis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Beilschmiedia glauca var. glaucoides | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bischofia javanica | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 11 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Cinnamomum bejolghota | 5 | 10 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 25 | 14 | 0 | 0 | 50 | 0 | 0 |
| Cinnamomum subavenium | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Clausena excavata | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Cyclobalanopsis gambleana | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 |
| Diplospora dubia | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Diplospora mollissima | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 25 | 0 | 0 |
| Duabanga grandiflora | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Eberhardtia aurata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 25 | 0 | 0 |
| Elaeocarpus braceanus | 2 | 0 | 0 | 5 | 6 | 3 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ficus auriculata | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Ficus cyrtophylla | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 25 | 0 | 0 |
| Ficus henryi | 1 | 0 | 2 | 0 | 0 | 0 | 25 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ficus racemosa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 25 | 0 | 0 |
| Fissistigma minuticalyx | 3 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Magnolia henryi | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Mallotus tetracoccus | 1 | 0 | 5 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Osyris wightiana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 |
| Pterospermum acerifolium | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Pygeum topengii | 2 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saurauia napaulensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Spatholobus suberectus | 8 | 4 | 5 | 0 | 0 | 3 | 25 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streblus indicus | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 25 | 0 | 0 |
| Symplocos cochinchinensis | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetrastigma cauliflorum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Toddalia asiatica | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 25 | 0 | 0 |
| Ulmus lanceaefolia | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| Vitex quinata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 25 | 0 | 0 |
| Xylosma longifolium | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 25 | 0 | 0 |
Note: Data represent species frequency (%). Species are ordered by descending fidelity value (φ, p < 0.05). Diagnostic species for the Alliance are highlighted: light gray background (0.25 ≤ φ < 0.5) and dark gray background (φ ≥ 0.5). Constant species (frequency ≥ 60%) are marked; only species with φ ≥ 0.25 are displayed due to space constraints.

Table A8.
Synoptic table for AG2.
Table A8.
Synoptic table for AG2.
| Alliance Number | 2_1 | 2_2 | 2_3 | 2_4 | 2_5 | 2_6 |
| Alliance number | 18 | 10 | 6 | 5 | 4 | 3 |
| Beilschmiedia glauca var. glaucoides | 11 | 100 | 0 | 0 | 0 | 0 |
| Castanopsis boisii | 0 | 0 | 100 | 40 | 0 | 33 |
| Castanopsis ceratacantha | 100 | 60 | 17 | 40 | 75 | 33 |
| Castanopsis fabri | 11 | 0 | 0 | 0 | 100 | 0 |
| Castanopsis fissa | 56 | 0 | 17 | 100 | 0 | 100 |
| Eurya tetragonoclada | 33 | 0 | 50 | 0 | 0 | 100 |
| Helicia pyrrhobotrya | 6 | 100 | 0 | 0 | 0 | 0 |
| Lithocarpus hancei | 39 | 60 | 0 | 20 | 0 | 100 |
| Parakmeria yunnanensis | 11 | 0 | 33 | 0 | 100 | 67 |
| Eberhardtia aurata | 6 | 90 | 0 | 0 | 0 | 0 |
| Manglietia fordiana | 22 | 90 | 0 | 0 | 0 | 0 |
| Elaeocarpus braceanus | 11 | 80 | 0 | 0 | 0 | 0 |
| Lindera caudata | 11 | 0 | 50 | 80 | 0 | 67 |
| Acanthopanax trifoliatus | 6 | 0 | 0 | 0 | 75 | 0 |
| Alniphyllum fortunei | 17 | 0 | 0 | 0 | 75 | 0 |
| Beilschmiedia purpurascens | 17 | 0 | 0 | 0 | 75 | 0 |
| Cyclobalanopsis augustinii | 17 | 0 | 0 | 0 | 75 | 0 |
| Elaeocarpus decipiens | 6 | 10 | 0 | 0 | 75 | 0 |
| Evodia glabrifolia | 0 | 0 | 0 | 0 | 75 | 0 |
| Sloanea leptocarpa | 22 | 0 | 33 | 20 | 75 | 33 |
| Castanopsis rockii | 6 | 70 | 0 | 0 | 0 | 0 |
| Celastrus monospermus | 6 | 70 | 0 | 0 | 0 | 0 |
| Laurocerasus jenkinsii | 6 | 70 | 0 | 0 | 0 | 0 |
| Sterculia lanceolata | 0 | 70 | 17 | 0 | 0 | 0 |
| Castanopsis concinna | 0 | 0 | 0 | 0 | 0 | 67 |
| Eberhardtia tonkinensis | 0 | 0 | 0 | 20 | 0 | 67 |
| Elaeocarpus sylvestris | 6 | 0 | 33 | 0 | 0 | 67 |
| Euonymus nitidus | 0 | 0 | 0 | 0 | 0 | 67 |
| Helicia cochinchinensis | 11 | 0 | 67 | 0 | 0 | 0 |
| Kadsura coccinea | 11 | 0 | 0 | 0 | 0 | 67 |
| Lithocarpus fenestratus | 22 | 0 | 67 | 40 | 0 | 0 |
| Lithocarpus truncatus | 11 | 0 | 0 | 0 | 0 | 67 |
| Neolitsea homilantha | 0 | 0 | 0 | 20 | 0 | 67 |
| Symplocos glandulifera | 17 | 0 | 0 | 20 | 0 | 67 |
| Bauhinia chalcophylla | 0 | 60 | 0 | 0 | 0 | 0 |
| Ilex corallina | 6 | 0 | 17 | 60 | 0 | 0 |
| Lithocarpus harlandii | 0 | 0 | 33 | 60 | 25 | 0 |
| Macaranga henryi | 11 | 0 | 33 | 60 | 0 | 0 |
| Machilus rufipes | 33 | 60 | 17 | 0 | 0 | 33 |
| Adinandra glischroloma | 6 | 0 | 0 | 0 | 50 | 0 |
| Bridelia insulana | 6 | 0 | 50 | 0 | 0 | 0 |
| Camellia crassicolumna | 6 | 0 | 50 | 0 | 0 | 0 |
| Cinnamomum iners | 0 | 0 | 50 | 0 | 0 | 0 |
| Cylindrokelupha balansae | 6 | 0 | 0 | 20 | 50 | 0 |
| Litsea chunii var. latifolia | 0 | 0 | 0 | 0 | 50 | 0 |
| Machilus verruculosa | 6 | 0 | 0 | 0 | 50 | 0 |
| Meliosma squamulata | 6 | 0 | 50 | 0 | 0 | 0 |
| Miliusa sinensis | 6 | 0 | 50 | 0 | 0 | 0 |
| Rhamnus davurica | 11 | 0 | 0 | 0 | 50 | 0 |
| Diplospora fruticosa | 0 | 40 | 0 | 0 | 0 | 0 |
| Fissistigma minuticalyx | 0 | 40 | 0 | 0 | 0 | 0 |
| Michelia figo | 0 | 0 | 0 | 40 | 0 | 0 |
| Schefflera chinensis | 6 | 40 | 0 | 0 | 0 | 0 |
| Styrax grandiflorus | 0 | 0 | 0 | 40 | 0 | 0 |
| Acer crassum | 0 | 0 | 33 | 0 | 0 | 0 |
| Acer sinense | 6 | 0 | 33 | 0 | 0 | 0 |
| Castanopsis jucunda | 0 | 0 | 33 | 0 | 0 | 0 |
| Cleyera japonica var. lipingensis | 0 | 0 | 33 | 0 | 0 | 0 |
| Cyclobalanopsis kontumensis | 0 | 0 | 33 | 0 | 0 | 0 |
| Elaeocarpus limitaneus | 0 | 0 | 33 | 20 | 0 | 0 |
| Exbucklandia populnea | 0 | 0 | 33 | 0 | 0 | 33 |
| Garcinia multiflora | 0 | 0 | 33 | 20 | 0 | 0 |
| Litsea monopetala | 0 | 10 | 33 | 0 | 0 | 0 |
| Vaccinium duclouxii | 0 | 0 | 33 | 0 | 0 | 0 |
| Vaccinium iteophyllum | 0 | 0 | 33 | 0 | 0 | 0 |
| Camellia cordifolia | 0 | 30 | 0 | 0 | 0 | 0 |
| Laurocerasus undulata | 0 | 30 | 0 | 0 | 0 | 0 |
| Lithocarpus pseudoreinwardii | 0 | 30 | 0 | 0 | 0 | 0 |
| Neolitsea levinei | 0 | 30 | 0 | 0 | 0 | 0 |
| Phoebe puwenensis | 6 | 30 | 0 | 0 | 0 | 0 |
Note: Data represent species frequency (%). Species are ordered by descending fidelity value (φ, p < 0.05). Diagnostic species for the Alliance are highlighted: dark gray background (φ ≥ 0.5). Constant species (frequency ≥ 60%) are marked; only species with φ ≥ 0.25 are displayed due to space constraints.

Table A9.
Synoptic table for AG3.
Table A9.
Synoptic table for AG3.
| Alliance Number | 3_1 | 3_2 |
|---|---|---|
| Alliance number | 9 | 5 |
| Castanopsis lamontii | 0 | 100 |
| Euonymus laxiflorus | 11 | 100 |
| Eurya groffii | 22 | 100 |
| Manglietia insignis | 22 | 100 |
| Viburnum trabeculosum | 22 | 100 |
| Erythroxylum sinensis | 11 | 80 |
| Eurya loquaiana | 0 | 80 |
| Alcimandra cathcartii | 67 | 0 |
Note: Data represent species frequency (%). Species are ordered by descending fidelity value (φ, p < 0.05). Diagnostic species for the Alliance are highlighted: dark gray background (φ ≥ 0.5). Constant species (frequency ≥ 60%) are marked; only species with φ ≥ 0.25 are displayed due to space constraints.
References
- Li, S.F.; Lang, X.D.; Huang, X.B.; Wang, Y.H.; Liu, W.D.; Xu, C.H.; Su, J.R. Association classification of a 30 hm2 dynamics plot in the monsoon broad-leaved evergreen forest in Pu’er, Yunnan, China. Chin. J. Plant Ecol. 2020, 44, 236–247. [Google Scholar] [CrossRef]
- The Editorial Committee of Vegetation of Yunnan. Vegetation of Yunnan; Science Press: Beijing, China, 1987; pp. 197–230. [Google Scholar]
- The Editorial Committee of Vegetation of China. Vegetation of China; Science Press: Beijing, China, 1980; pp. 306–355. [Google Scholar]
- Zhu, H. Vegetation geography of evergreen broad-leaved forests in Yunnan, southwestern China. Chin. J. Plant Ecol. 2021, 45, 224–241. [Google Scholar] [CrossRef]
- Zhu, H. Floristic divergence of the evergreen broad-leaved forests in Yunnan, southwestern China. Phytotara 2019, 393, 1–20. [Google Scholar] [CrossRef]
- Song, Y.C. Tentative classification scheme of evergreen broad-leaved forests of China. Acta Phytoecol. Sin. 2004, 28, 435–448. [Google Scholar] [CrossRef][Green Version]
- Whittaker, R.H. Classification of Plant Communities; Dr W. Junk bv Publishers: Hague, The Netherlands, 1978. [Google Scholar]
- Zhang, J.T. Quantitative Ecology; Science Press: Beijing, China, 2004. [Google Scholar]
- Hill, M.O. TWINSPAN: A FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes; Section of Ecology and Systematics, Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- De’Ath, G. Multivariate regression trees: A new technique for modeling species-environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Molina, J.A. The vegetation of temporary ponds with Isoetes in the Iberian Peninsula. Phytocoenologia 2005, 35, 219–230. [Google Scholar] [CrossRef]
- Moura, D.; Machado, A.S.; Mariano, F.S.; Fernandes, R.; Souza, D.; Rodrigo, C.; Mengez, L.; Carolina, U.; Fontes, L.; Aurélio, M. Mesoscale bird distribution pattern in montane phytophysiognomies along an ecotone between two hotspots. Acta Sci. Biol. Sci. 2021, 43, 56931. [Google Scholar] [CrossRef]
- Tomaselli, V.; Pietro, R.D.; Sciandrello, S. Plant communities structure and composition in three coastal wetlands in southern Apulia (Italy). Biologia 2011, 66, 1027–1043. [Google Scholar] [CrossRef]
- Myliwy, M.; Pesic, V. Tall Herb Fringe Vegetation on Banks of Montenegrin Rivers as a Habitat Type of European Importance. Water 2023, 15, 3684. [Google Scholar] [CrossRef]
- Weber, H.E.; Moravec, J.; Theurillat, J.P. International Code of Phytosociological Nomenclature. 3rd. J. Veg. Sci. 2000, 11, 739–768. [Google Scholar] [CrossRef]
- Rodwell, J.S.; Pigott, C.D.; Ratcliffe, D.A.; Malloch, A.J.C.; Birks, H.J.B.; Proctor, M.C.F.; Shimwell, D.W.; Huntley, J.P.; Radford, E.; Wigginton, M.J.; et al. British Plant Communities; Cambridge University Press: Cambridge, UK, 1991; Volume I. [Google Scholar]
- Fang, J.Y.; Guo, K.; Wang, G.H.; Tang, Z.Y.; Xie, Z.Q.; Shen, Z.H.; Wang, R.Q.; Qiang, S.; Liang, C.Z.; Da, L.J.; et al. Vegetation classification system and classification of vegetation types used for the compilation of vegetation of China. Chin. J. Plant Ecol. 2020, 44, 96–110. [Google Scholar] [CrossRef]
- Wang, G.H.; Fang, J.Y.; Guo, K.; Xie, Z.Q.; Tang, Z.Y.; Shen, Z.H.; Wang, R.Q.; Wang, X.P.; Wang, D.L.; Qiang, S.; et al. Contents and protocols for the classification and description of Vegetation Formations, Alliances and Associations in vegetation of China. Chin. J. Plant Ecol. 2020, 44, 128–178. [Google Scholar] [CrossRef]
- Song, Y.C.; Chen, X.Y.; Wang, X.H. Studies on Evergreen Broad-leaved Forests of China: A Retrospect and Prospect. J. East China Norm. Univ. 2005, 1, 1–8. [Google Scholar] [CrossRef]
- Gauch, H.G. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Whittaker, R.H. Ordination and Classification of Communities; Dr W. Junk bv Publishers: Hague, The Netherlands, 1973. [Google Scholar]
- Zhang, J.T. Quantitative Classification Methods in Plant Ecology; Science Press: Beijing, China, 1995. [Google Scholar]
- Austin, M.P.; Cunningham, R.B.; Fleming, P.M. New approaches to direct gradient analysis using environmental scalars and statistical curve-fitting procedures. Vegetatio 1984, 55, 11–27. [Google Scholar] [CrossRef]
- Brunet, J.; von Oheimb, G.; Diekmann, M. Factors influencing vegetation gradients across ancient-recent woodland borderlines in southern Sweden. J. Veg. Sci. 2000, 11, 515–524. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Dyakov, N.R. Gradient analysis of vegetation on the south slope of vitosha mountain, southwest bulgaria. Appl. Ecol. Env. Res. 2014, 12, 1003–1025. [Google Scholar] [CrossRef]
- Kenkel, N.C.; Orloci, L. Applying metric and nonmetric multidimensional-scaling to ecological-studies—Some new results. Ecology 1986, 67, 919–928. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cabido, M.; Zeballos, S.R.; Zak, M.; Carranza, M.L.; Giorgis, M.A.; Cantero, J.J.; Acosta, A.T.R. Native woody vegetation in central Argentina: Classification of Chaco and Espinal forests. Appl. Veg. Sci. 2018, 21, 298–311. [Google Scholar] [CrossRef]
- Joelson, N.Z.; Simon, A.; von Lampe, F.; Loguercio, G.A.; Zerbe, S.; Leuschner, C.; Walentowski, H. Floristic patterns in the Andes of northern Patagonia’s forests, Argentina: Towards integrating ecological responses with expert-based and unsupervised classification methods. Veg. Classif. Surv. 2025, 6, 37–56. [Google Scholar] [CrossRef]
- Xiahou, M.J.; Liu, Y.N.; Yang, T.; Shen, Z.H. Estimating potential vegetation distribution and restoration in a biodiversity hotspot region under future climate change. J. Geogr. Sci. 2024, 34, 2128–2144. [Google Scholar] [CrossRef]
- Xiahou, M.J.; Shen, Z.H.; Yang, T.; Duan, J.H.; Peng, M.C.; Wang, C.Y.; Ou, X.K. Biodiversity conservation and ecological restoration dominated vegetation dynamics during the 1980s–2010s in Yunnan, China. Biol. Conserv. 2024, 299, 110798. [Google Scholar] [CrossRef]
- Xiahou, M.J.; Peng, M.C.; Shen, Z.H.; Wen, Q.Z.; Wang, C.Y.; Liu, Y.N.; Zhang, Q.Y.; Peng, L.; Yu, C.Y.; Ou, X.Q.; et al. Vegetation mapping of Yunnan Province by integrating remote sensing, field observations, and models. Sci. China Earth Sci. 2025, 68, 836–849. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, K.F.; Schnitzer, S.A.; Fan, Z.X.; Zhang, J.L.; Bongers, F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 2014, 205, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y. Forest productivity in China and its response to global climate change. Chin. J. Plant Ecol. 2000, 05, 513–517. [Google Scholar]
- Zeng, J.M. The classification system of natural forests and its geographic distribution in Yunnan. J. Southwest For. Univ. 2018, 38, 1–18. [Google Scholar]
- Ye, W.H.; Cao, H.L.; Huang, Z.L.; Lian, J.Y.; Wang, Z.G.; Li, L.; Wei, S.G.; Wang, Z.M. Community structure of a 20 hm2 lower subtropical evergreen broad-leaved forest plot in Dinghushan, China. J. Plant Ecol. 2008, 32, 274286. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, T.F.; Deng, X.B.; Liu, J.Y. Vegetation numerical classification and ordination of a 20-hectare tropical forest plot in Xishuangbanna, Southwest Yunnan. Chin. J. Ecol. 2018, 37, 347–352. [Google Scholar] [CrossRef]
- Shi, J.P.; Zhu, H. A Community Ecology Study on the Monsoonal Evergreen Broad teaved Forest in Tropical Montane of Xishuangbanna. Acta Bot. Yunnanica 2003, 25, 513–520. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Wang, M.H.; Hu, Z.H.; Lang, X.D.; He, Y.Q.; Liu, W.D. Mechanisms of seedling community assembly in a monsoon evergreen broadleaf forest in Pu’er, Yunnan, China. Chin. J. Plant Ecol. 2024, 48, 68–79. [Google Scholar] [CrossRef]
- Hutchinson, M.F. Interpolating mean rainfall using thin plate smoothing splines. Int. J. Geogr. Inf. Syst. 1995, 9, 385–403. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Tomislav, H.; Jorge, M.D.J.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotic, A.; Shangguan, W.; Wright, M.N.; Geng, X.Y.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Sun, X.W.; Yang, Q.S.; Liu, H.M.; Wang, X.H. Classification of plant associations based on a 20 hm2 dynamics plot of evergreen broad-leaved forest in Mt. Tiantong, Zhejiang, China. Chin. J. Plant Ecol. 2018, 42, 550–561. [Google Scholar] [CrossRef]
- Orlóci, L. An agglomerative method for classification of plant communities. J. Ecol. 1967, 55, 193–206. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Belbin, L.; Mcdonald, C. Comparing 3 classification strategies for use in ecology. J. Veg. Sci. 1993, 4, 341–348. [Google Scholar] [CrossRef]
- Guo, K.; Fang, J.Y.; Wang, G.H.; Tang, Z.Y.; Xie, Z.Q.; Shen, Z.H.; Wang, R.Q.; Qiang, S.; Liang, C.Z.; Da, L.J.; et al. A revised scheme of vegetation classification system of China. Chin. J. Plant Ecol. 2020, 44, 111–127. [Google Scholar] [CrossRef]
- Kruskal, J. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G. Vegan: Community Ecology Package (Version 2.6-4). 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 December 2024).
- Tang, C.Q.; Chiou, C.R.; Lin, C.T.; Hsieh, C.F. Plant diversity patterns in subtropical evergreen broad-leaved forests of Yunnan and Taiwan. Ecol. Res. 2013, 28, 81–92. [Google Scholar] [CrossRef]
- Yang, T.; Shen, Z.H.; Wang, X.F.; Rao, J.S.; Liu, W.C.; Tian, X.; Chen, X.; Zhang, Q.Y.; Liu, Q.; Qian, H.J.; et al. Characteristics of plant community diversity in a subtropical semi-humid evergreen broad-leaved forest in the Central Yunnan Plateau. Biodivers. Sci. 2023, 31, 19–32. [Google Scholar] [CrossRef]
- Gui, X.J.; Lian, J.Y.; Zhang, R.Y.; Li, Y.P.; Shen, H.; Ni, Y.L.; Ye, W.H. Vertical structure and its biodiversity in a subtropical evergreen broad-leaved forest at Dinghushan in Guangdong Province, China. Biodivers. Sci. 2019, 27, 619–629. [Google Scholar] [CrossRef]
- Huang, Y.R.; Lin, J.; You, L.H.; Wu, W.J. Community Characteristics of South Subtropical Monsoon Forest in Exiandong, Huboliao Conservation Area. Prot. For. Sci. Technol. 2022, 2, 12–14. [Google Scholar]
- Shen, Z.H.; Ying, L.X. The imprints of Indian Ocean Monsoon and West Pacific Monsoon on the spatial and temporal patterns of forest fires in Yunnan, Southwest China. Gen. Assem. 2020, 17, 6135. [Google Scholar] [CrossRef]
- Zhu, H. Flora and vegetation of Yunnan are shaped by geological events and monsoon climate. Biodivers. Sci. 2023, 31, 38–56. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; ter Steege, H.; Lopez-Gonzalez, G.; Mendoza, A.M.; Brienen, R.; Feldpausch, T.R.; Pitman, N.; et al. Seasonal drought limits tree species across the Neotropics. Ecography 2016, 40, 618–629. [Google Scholar] [CrossRef]
- Rishmawi, K.; Prince, S.D.; Xue, Y.K. Vegetation Responses to Climate Variability in the Northern Arid to Sub-Humid Zones of Sub-Saharan Africa. Remote Sens. 2016, 8, 910. [Google Scholar] [CrossRef]
- Zhu, X.; He, H.S.; Zhang, S.X.; Dijak, W.D.; Fu, Y.Y. Interactive Effects of Climatic Factors on Seasonal Vegetation Dynamics in the Central Loess Plateau, China. Forests 2019, 10, 1071. [Google Scholar] [CrossRef]
- Xue, S.Y.; Wu, G.C. Sensitivities of Vegetation Gross Primary Production to Precipitation Frequency in the Northern Hemisphere from 1982 to 2015. Remote Sens. 2024, 16, 21. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, Y.M.; Xue, Y.K.; Piao, S.L. Climate Change Trends and Impacts on Vegetation Greening Over the Tibetan Plateau. J. Geophys. Res. Atmos. 2019, 124, 7540–7552. [Google Scholar] [CrossRef]
- Miettinen, J.; Shi, C.; Liew, S.C. Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob. Change Biol. 2011, 17, 1365–2486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).