Phenotypic Variability of Juglans neotropica Diels from Different Provenances During Nursery and Plantation Stages in Southern Ecuador
Abstract
1. Introduction
2. Materials and Methods
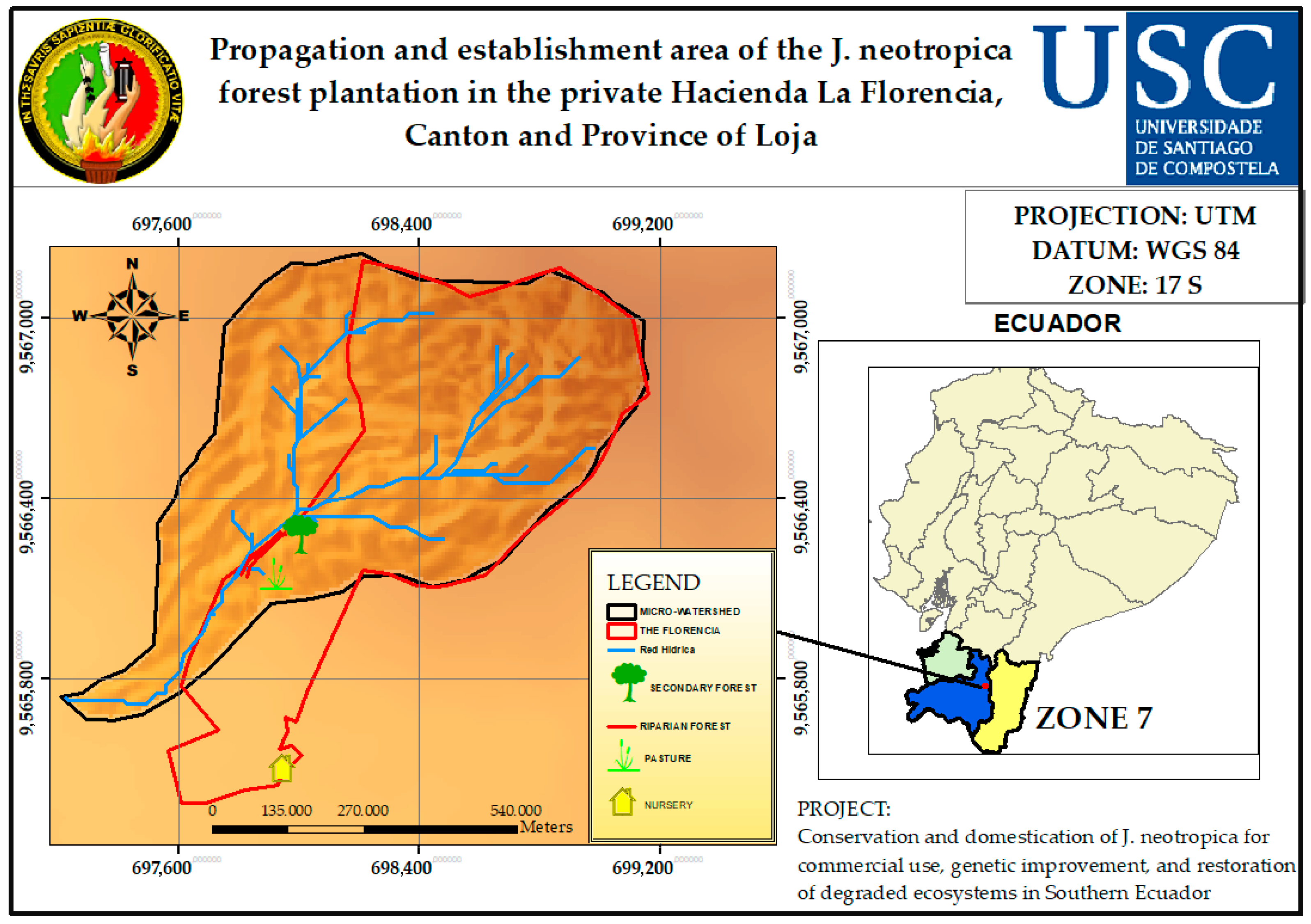
2.1. Study Area
2.2. Study Description
2.3. First Phase: At the Nursery Level
2.3.1. Quality Parameters of J. neotropica Seeds
- Purity Percentage: To evaluate seed purity, a comprehensive physical analysis was conducted. First, a physical test was performed, involving the meticulous separation of seeds from possible impurities such as sand, soil, stones, plant debris, and other waste. This process aimed to ensure sample cleanliness and quality by removing any undesired elements. Subsequently, the genetic purification stage was carried out, with the objective of separating the analyzed seeds from other crops or weeds present. This phase is crucial to ensure sample homogeneity and obtain precise results regarding seed purity. Purity was quantified as the percentage by weight of pure seeds in relation to the total sample analyzed. This comprehensive methodology guaranteed an exhaustive evaluation of seed purity, which is essential for quality and performance in agroforestry projects. To determine seed purity, five seed lots of one kilogram each were randomly selected, containing impurities. The following formula was applied for the assessment (Figure 2).
- The number of seeds per kilogram was determined by randomly selecting seeds until reaching one kilogram. This process was repeated five times, and an average number of seeds per kilogram was subsequently calculated (Figure 3). To ensure consistency across all samples, seeds were weighed using a digital precision scale (Camry Electronic Scale Model EK5350, Zhongshan, Guangdong, China).
- Moisture Content Percentage (% MC): For the determination of moisture content in J. neotropica seeds, 25 seeds were selected as samples for each locality. Subsequently, the initial weight of each seed was recorded before subjecting them to a storage process. Since these are recalcitrant seeds, they have a hard appearance but are delicate and lose their viability quickly after being extracted from the fruit. This type of seed typically requires specific conditions and special care for storage and preservation. For this reason, they were stored for a period of six months at an ambient temperature of 18 °C. Gradual weighing was carried out at the beginning (time 0) and six months after collection to determine the percentage of moisture lost during the established period (Figure 4).
- 4.
- Germination Percentage: The methodology for determining the germination percentage, following the standards of the International Seed Testing Association, adhered to a standardized procedure that ensured both accuracy and reproducibility of results. For this study, a representative sample of seeds was selected based on criteria of uniformity in size, maturity, and physiological state. These seeds were stored under controlled conditions, maintaining an average temperature of 18 °C and a relative humidity of 70%, parameters defined from previous studies and adjusted to the physiological requirements of J. neotropica in its natural habitat. During the storage period, systematic monitoring of viability was conducted, recording variations in moisture and physiological state to minimize the influence of external environmental factors. These conditions allowed for the collection of precise data on conservation and contributed to promoting germination. Subsequently, the germinated seeds were evaluated and classified according to defined criteria, such as normal or abnormal development. The germination percentage was calculated by dividing the number of normal seedlings by the total number of seeds sown and multiplying the result by 100, thus providing a reliable indicator of seed quality. This internationally recognized and adopted method ensured uniformity in assessing seed viability on a global scale.
- Mechanical treatment: The seeds were placed on a solid surface and struck with a hammer until they cracked.
- Hot water imbibition: The seeds were placed in a container with hot water at 100 °C, at a ratio of 4 to 5 times their volume, and removed after two minutes of immersion in the heat source.
- Cold water imbibition and sun exposure: To remove inhibitors, the seeds were exposed to sunlight during the day and soaked in water at night. This process was repeated for three consecutive days and nights.
- Control treatment: This method served as a baseline for comparing the effectiveness of the other treatments.
2.3.2. Phenotypic Characteristics of J. neotropica
- Seed size and weight: To determine the size of J. neotropica seeds, a detailed methodology was implemented. Seed samples from different progenies were collected from various geographical localities. Subsequently, precise measurements of each seed’s size were taken using a digital caliper (Mitutoyo, Kawasaki, Japan). This process was systematically repeated for each locality, where seed samples consisting of 100 units were evaluated. These experimental samples, referred to as seed lots, allowed for the quantification of inherent variability within each sampled population. The applied methodology ensured rigorous statistical representation, facilitating the generation of consistent and reproducible data on germination performance under diverse environmental conditions (Figure 5).
- Seedling height at the nursery level (Th, cm): This morphological parameter is related to the plant’s photosynthetic capacity and transpiration surface. It corresponds to the length from the root collar to the apex of the main stem, measured in cm. It was measured for all living plants in the experimental unit six months after germination for all localities.
- Basal diameter of seedlings (Bd, mm): The basal diameter is a key indicator of the plant’s ability to transport water to the aerial parts, as well as its structural resistance and relative tolerance to high-temperature conditions. This parameter was measured at the height of the root collar, at the transition point between the root system and the base of the stem, in all living plants of the experimental unit. Measurements were conducted six months after germination, covering all the localities included in the study.
- Number of leaves per treatment: This morphological parameter was evaluated in all living individuals of the experimental unit propagated in the nursery under the different treatments. Measurements were conducted six months after germination, coinciding with the scheduled evaluations of the other morphological variables and covering all localities included in the study. In each case, the number of leaves was recorded using the same assessment framework, allowing for comparative analysis of foliar development among the different origin localities [11,12] (Figure 6).
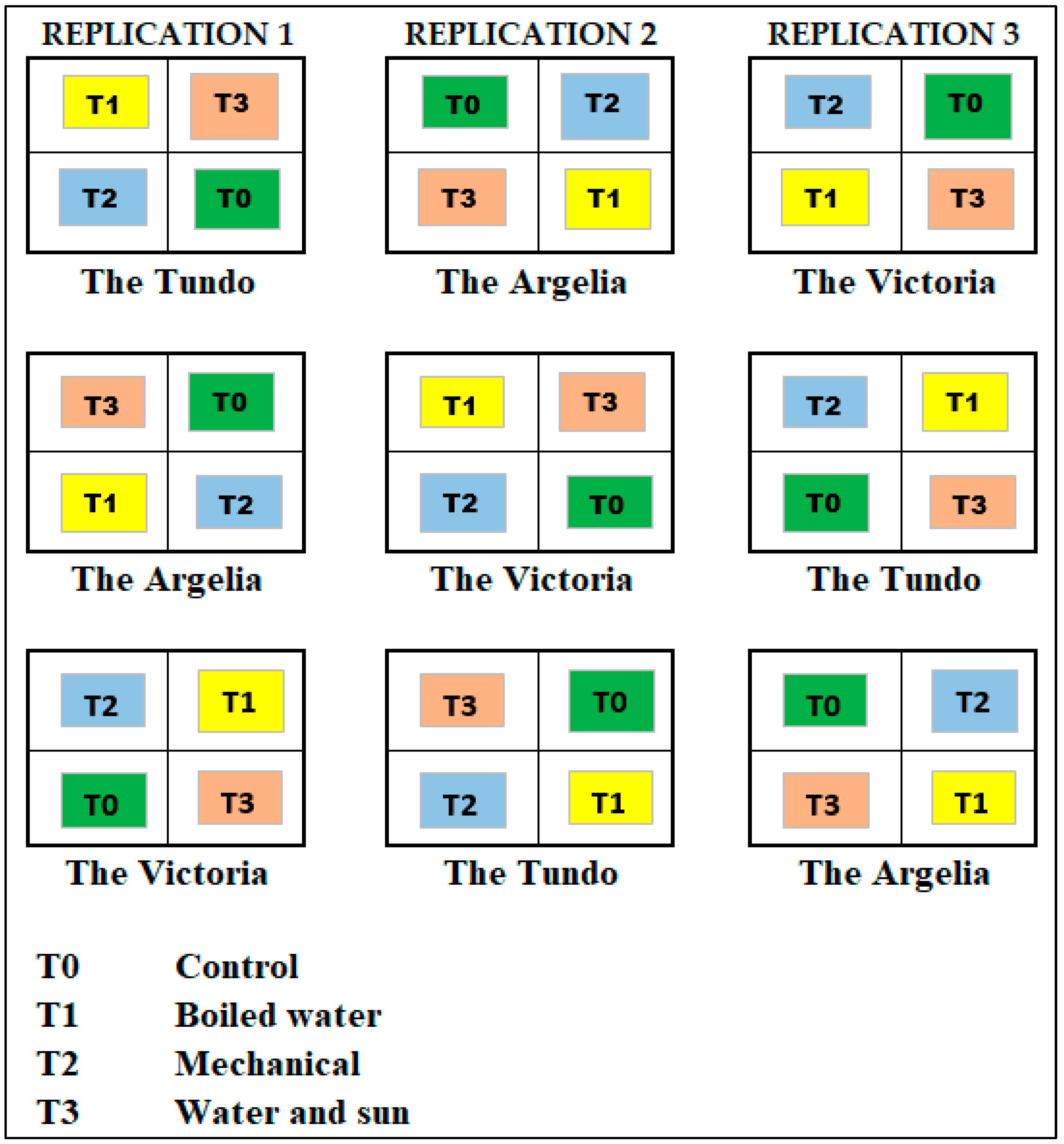
2.3.3. Experimental Design at Nursery Level
- Functional analysis: The coefficient of variation was calculated to assess data dispersion. Additionally, mean separation was analyzed using Tukey’s test at a 5% significance level to identify statistical differences among treatments and localities.
- Factors under study:
- Factor 1 (pre-germination treatments):
- T0 = Control (seeds without any treatment);
- T1 = Immersion in boiling water at 100 °C (2 min);
- T2 = Mechanical (cracked with a hammer);
- T3 = Immersion in water and exposure to sunlight (three days in ambient water and three days in the sun).
- Factor 2 (localities):
- L1 = The Tundo;
- L2 = The Victoria;
- L3 = The Argelia.
- Treatments under study: The combination of the factors under study resulted in 12 treatments, which are detailed below (Table 1).
- Experimental field specifications:
- Number of treatments: 12;
- Number of replications: 3;
- Total number of units: 36;
- Total trial area: 40 m2;
- Total number of seeds per plot: 100;
- Total number of seeds per treatment: 25.
- Dependent variables used in the statistical analysis:
- Mathematical model for the statistical design:
2.4. Second Phase: At the Level of Forest Plantation
2.4.1. Selection of the Area to Be Planted
2.4.2. Selection and Field Establishment of Plants
2.4.3. Fertilization
2.4.4. Management
2.4.5. Plant Response to Plantation Conditions
- Survival rate (%): The survival rate (%) was determined based on an evaluation conducted 60 days after the trial establishment. To achieve this, the number of established plants in each experimental unit was quantified and calculated as the ratio of surviving individuals to the total number of planted trees. This calculation was performed using the following equation: (number of established plants/total number of planted trees) × 100. The evaluation was carried out under standardized conditions, ensuring uniformity in the application of establishment criteria, which allows for the interpretation of the plants’ adaptability to the planting site and their response to the environmental conditions of the study area.
- Stem straightness at 12 months: To carry out the phenotypic development assessment, a methodology based on shape codes was implemented, following the guidelines proposed by [14,15]. The individuals under study were evaluated at twelve months to determine their physical characteristics. These categories provided a comprehensive framework for quantitatively describing stem shape, allowing for a more precise evaluation of phenotypic characteristics across four categories (Table 2).
2.4.6. Phenotypic Traits Based on Dendrometric Variables
- Basal diameter (BD, cm)
- 2.
- Total height (m)
2.4.7. Experimental Design at Plantation Level
- 1.
- Functional analysis: The coefficient of variation was determined, and the mean separation was analyzed using Tukey’s test at a 5% error level with a 95% confidence interval.
- 2.
- Factors under study: Two study factors were analyzed for each of the three strata (secondary forest, riparian forest, and pasture), as detailed below.
- Factor A: Localities
- L1 = The Tundo;
- L2 = The Victoria.
- Factor B: Fertilizers
- F0 = Control;
- F1 = Urea;
- F2 = NPK.
- 3.
- Treatments under study: The combination of the factors under study resulted in 8 treatments, which are detailed below (Table 3).
- 4.
- Experimental unit: A 50 × 50 m (2500 m2) plot was used in each stratum, with four rectangular subplots of 25 × 25 m (625 m2). Two subplots corresponded to the forest site of The Tundo and the other two to The Victoria. Each subplot contained five experimental units measuring 5 × 25 m (125 m2), with each unit including five plants, where each plant was considered a replicate. This resulted in a subtotal of 10 experimental units per forest site and a total of 20 experimental units across both sites.
- 5.
- Localities: Two localities were evaluated, one from the Sozoranga canton and the other from the Macará canton, both belonging to the Loja province in southern Ecuador.
- L1 = The Tundo
- L2 = The Victoria
- 6.
- Mathematical model for the statistical design: To statistically analyze and determine whether there are significant differences among the treatments tested in J. neotropica, an ANOVA (analysis of variance) was applied.the dependent variable;µ: the global mean of all treatments;(alpha) factor 1 under study;: the levels of factor;(beta) factor 2 under study;the levels of factor 2;the interaction between the i-th level of the locality of origin and the j-th level of the fertilizer;random effect of replication;the experimental error of the ijk observation.
- 7.
- Data analysis
3. Results
3.1. Phase 1: At the Nursery Level
3.1.1. Quality Parameters of Juglans neotropica Diels Seeds
- Purity percentage
- 2.
- Number of seeds per kilogram (kg)
- 3.
- Percentage of moisture content (MC %)
- 4.
- Germination percentage
3.1.2. Germination Percentage Among Treatments and Localities
- 5.
- Phenotypic Characteristics of J. neotropica Seeds
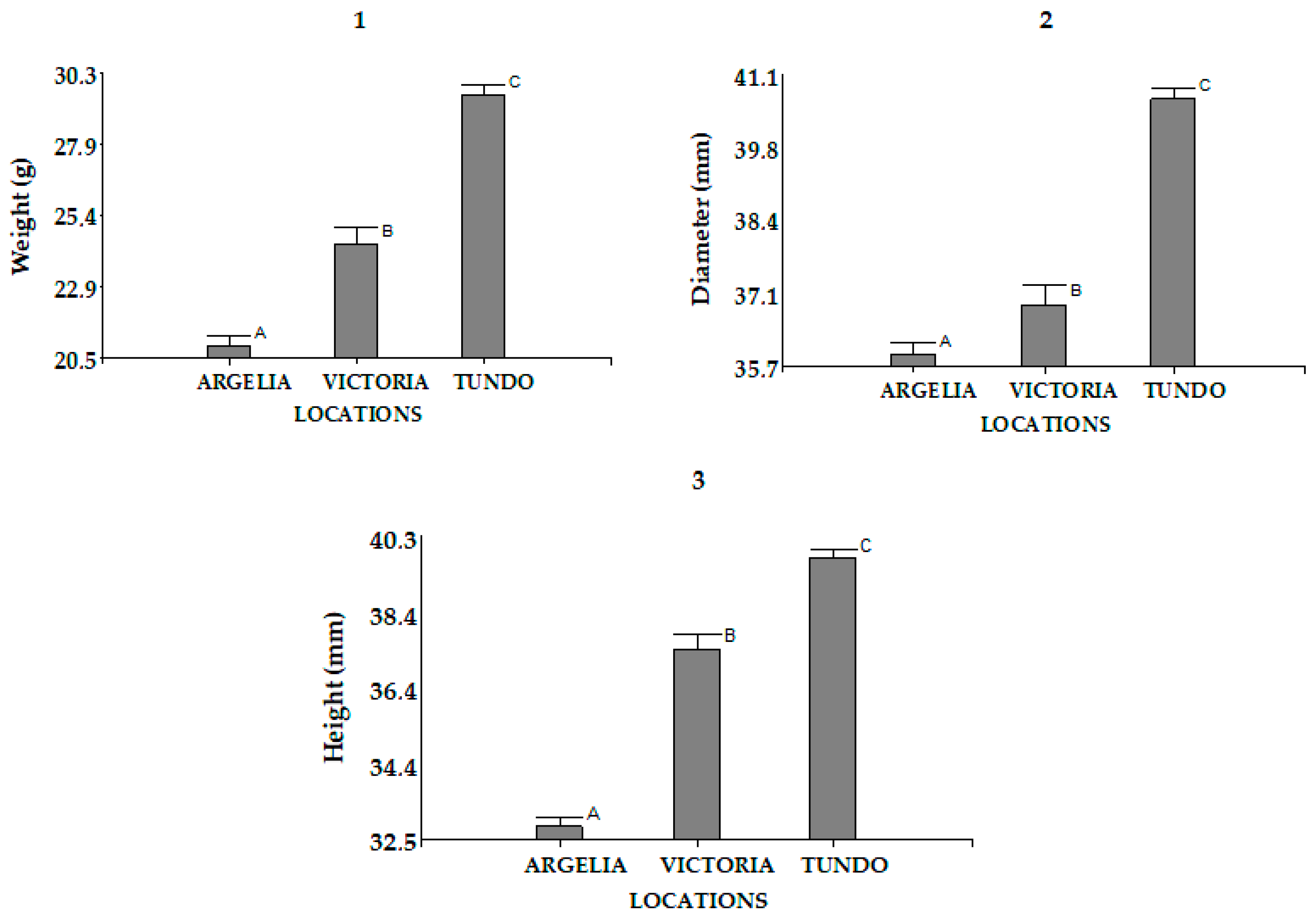
3.1.3. Size and Weight of the Seeds
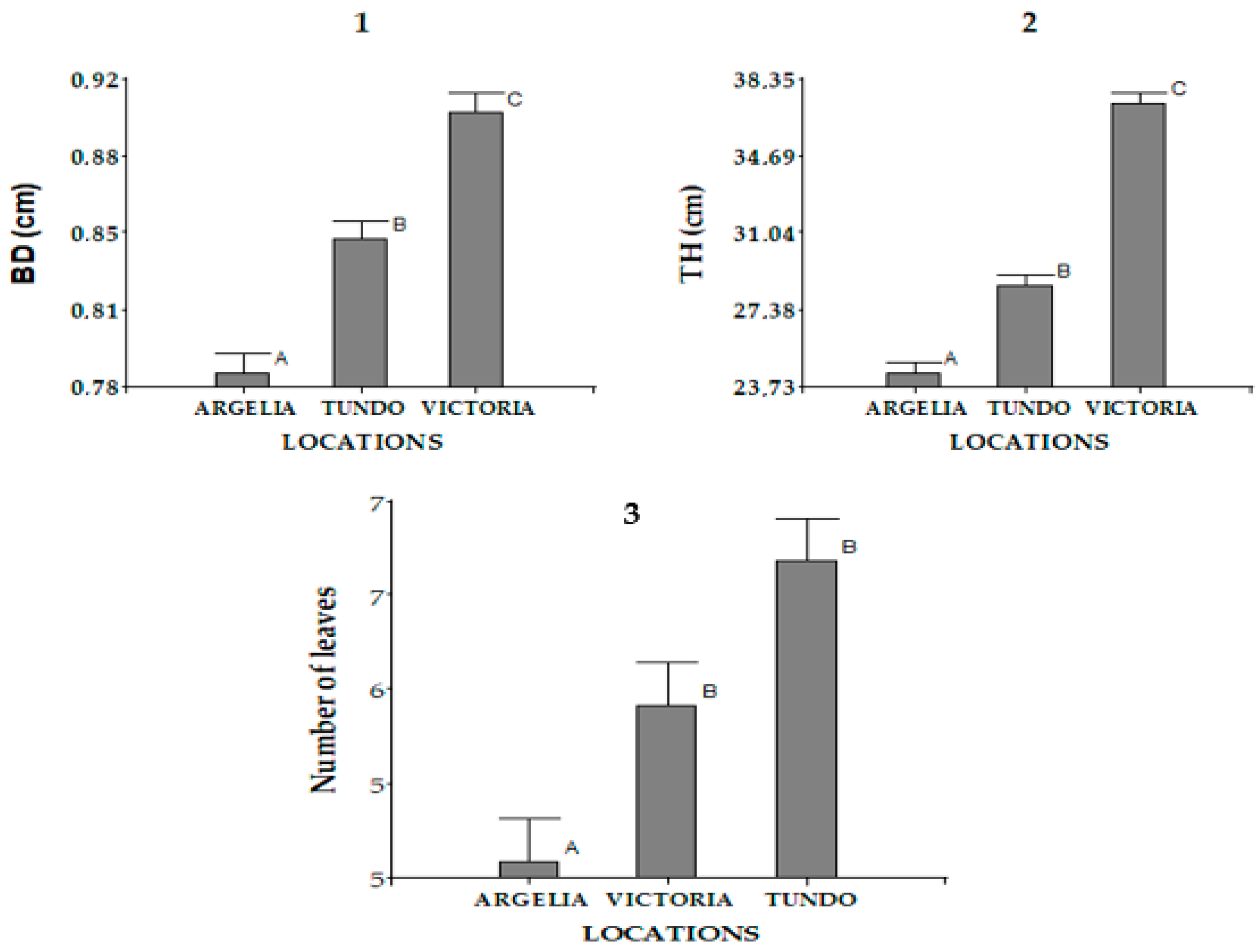
3.1.4. Basal Diameter, Total Height, and Number of Leaves of the Nursery Plants
3.2. Phase II: At the Level of Forest Plantation
3.2.1. Adaptability Parameters
- Survival %. During the first year of establishment, J. neotropica exhibited a high overall survival rate of 92% across the plantation trial. However, stratified and locality-based analyses revealed important differences that highlight environmental influences on early survival.
- 2.
- Stem straightness at 12 months. It is important to note that the bifurcation caused by the borer in the planted individuals is not considered a genetic phenotypic trait of the species J. neotropico (Table 9).
| Category | No. of Individuals | Sinous (%) | Inclined (%) | Forked (%) | Straight (%) |
|---|---|---|---|---|---|
| Strata | |||||
| Secondary Forest | 100 | 0.00 | 0.00 | 0.00 | 100.00 |
| Riparian Forest | 99 | 0.00 | 0.00 | 0.00 | 99.00 |
| Pasture | 100 | 0.00 | 0.00 | 0.00 | 100.00 |
| Localities | |||||
| The Tundo | 150 | 0.00 | 0.00 | 0.00 | 100.00 |
| The Victoria | 149 | 0.00 | 0.00 | 0.67 | 99.33 |
3.2.2. Phenotypic Characteristics Based on Dasometric Variables
4. Discussion
4.1. Phase I: Influence of Seed Provenance on Early Performance of Juglans neotropica Diels in the Nursery
4.2. Phase II: Influence of Ecosystem and Provenance on the Establishment of J. neotropica in Field Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raurau, M. Caracterización de Fuentes Semilleras para uso Sostenible y Conservación de Recursos Forestales de los Bosques Andinos de Loja, Ecuador. Master’s Thesis, CATIE, Turrialba, Costa Rica, 2012; 147p. [Google Scholar]
- Geilfus, F.; Bailon, M. Manual de Agroforestería Para el Desarrollo Rural; CATIE: Turrialba, Costa Rica, 1994. [Google Scholar]
- Ponce, G.; Morales, D. Estudio de Procesos de Elaboración de Tintes Naturales con dos Especies Vegetales: Nogal (Juglans neotropica) y Guarango (Caesalpinia spinosa). 2011. Available online: https://agris.fao.org/search/en/providers/124703/records/6705145bb1dfe472e14575f3 (accessed on 7 July 2025).
- Roldán, E.R.S.; Garde, J.J. Biotecnología de la Reproducción y Conservación de Especies en Peligro de Extinción; Museo Nacional de Ciencias Naturales (CSIC): Madrid, Spain, 2018. [Google Scholar]
- Boeri, P.A.; Dalzotto, D.C. Propagación de especies en peligro de extinción. In Biotecnología y biodiversidad: Diálogo de Saberes; Sharry, S., Trujillo, I., Eds.; EDULP: La Plata, Argentina, 2018; Capítulo 6; p. 308. [Google Scholar]
- López Alfonsín, M.; Bucetto, M.S. Las especies en peligro de extinción y los mecanismos para la recuperación y conservación de la biodiversidad. LEX-Rev. De La Fac. De Derecho Y Cienc. Políticas 2019, 17, 297. [Google Scholar]
- Palacios-Herrera, B.; Pereira-Lorenzo, S.; Pucha-Cofrep, D. Natural and Artificial Occurrence, Structure, and Abundance of Juglans neotropica Diels in Southern Ecuador. Agronomy 2023, 13, 2531. [Google Scholar] [CrossRef]
- Ordóñez, L. Sitios de Recolección de Semillas Forestales Andinas del Ecuador; Editorial Abya Yala: Quito, Ecuador, 2001. [Google Scholar]
- Trujillo, E. Guía de Reforestación; El Semillero: Bogotá, Colombia, 2013. [Google Scholar]
- International Seed Testing Association. Chapter 2: Sampling. In International Rules for Seed Testing, 2025th ed.; ISTA: Zürich, Switzerland, 2025; pp. 1–48. Available online: https://www.seedtest.org/api/rm/97C62HX4T55R346/ista-rules-2025-02-sampling-web.pdf (accessed on 1 June 2025).
- Farjon, G.; Itzhaky, Y.; Khoroshevsky, F.; Bar-Hillel, A. Leaf counting: Fusing network components for improved accuracy. Front. Plant Sci. 2021, 12, 575751. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-L.; Lin, W.-Y.; Lin, Y.-C. Toward automatic plant phenotyping: Starting from leaf counting. Multimed. Tools Appl. 2022, 81, 11865–11879. [Google Scholar] [CrossRef]
- Ladrach, W.E. Manejo Práctico de Plantaciones Forestales en el Trópico y Subtrópico; Editorial Tecnológica de Costa Rica: Cartago, Costa Rica, 2010. [Google Scholar]
- Martínez, H.B.; Hernández, J.V. Variación fenotípica y selección de árboles en una plantación de melina (Gmelina arborea Linn. Roxb.) de tres años de edad. Rev. Chapingo Ser. Cienc. For. Amb. 2004, 10, 13–19. [Google Scholar]
- Daetz, C. Evaluación del Crecimiento de Plantaciones de Eucalipto en Lanquín, Alta Verapaz. Bachelor’s Thesis, Universidad Rafael Landívar Facultad de Ciencias Ambientales y Agrícolas, Quetzaltenango, Guatemala, 2015. [Google Scholar]
- Poulsen, K.A.R.E.N.; Stubsgaard, F.; CATIE. Tres métodos de escarificación mecánica de semillas de testa dura. Ser. Técnica Man. Técnico 2000, 36, 35. [Google Scholar]
- Di Rienzo, J.; Balzarini, M.; González, L.; Casanoves, F.; Tablada, M.; Robledo, C.W. InfoStat: Software Para Análisis Estadístico; Grupo InfoStat, Universidad Nacional de Córdoba: Córdoba, Argentina, 2010. [Google Scholar]
- Cevallos, D.F.F.; Díaz, E.F.P.; Toledo, D.D.S.; Benavides, J.G.C.; Molina, E.M.V.; Flores, B.H.G. Análisis de tratamientos pregerminativos químicos en semillas de Juglans neotropica Diels de procedencia de San Blas, Cantón Urcuquí, Imbabura–Ecuador. Cienc. Lat. Rev. Cienc. Multidiscip. 2023, 7, 8428–8441. [Google Scholar]
- Toro Vanegas, E.; Roldán Rojas, I.C. Estado del arte, propagación y conservación de Juglans neotropica Diels en zonas andinas. Madera Bosques 2019, 24, e2411560. [Google Scholar] [CrossRef]
- Haase, D.L.; Pinto, J.R.; Davis, A.S. The high cost of the low-cost polybag system: A review of nursery seedling production systems. Forests 2021, 12, 443. [Google Scholar] [CrossRef]
- Camarena-Yupanqui, H.; Álvarez-Cordero, E.; Mostacero-León, M. Seedling production of Retrophyllum rospigliosii in nurseries and potential reforestation areas using modeling techniques. Forests 2024, 15, 67. [Google Scholar] [CrossRef]
- Longjam, R.; Kotiyal, M. Effect of Different Pre-Germination Treatments on Seed Germination of Terminalia bellerica (Gaertn.) Roxb. Forests 2024, 15, 122. [Google Scholar] [CrossRef]
- Espitia-Camacho, M.; Cardona-Ayala, C.; Araméndiz-Tatis, H. Morfología y viabilidad de semillas de Bombacopsis quinata y Anacardium excelsum. Cultiv. Trop. 2017, 38, 75–83. [Google Scholar]
- Sosa, J.L.R.; Espinosa, C.A. Morphological structure, germination and vigor of Juglans jamaicensis C. DC. seeds. Rev. Cuba. Cienc. For. 2019, 7, 297–304. [Google Scholar]
- Jiménez Cueva, T.P.; Palacios Herrera, B.G. Establecimiento de una plantación de nueve especies forestales con fines de rehabilitación de suelos degradados en la Hacienda La Florencia, Cantón y provincia de Loja. Cienc. Lat. Rev. Cienc. Multidiscip. 2023, 7, 2036–2051. [Google Scholar] [CrossRef]
- Veintimilla, R.A.R.; Vélez, R.V.; Olmedo, J.E.G.; Veintimilla, M.R.R. Biomasa anhidra en plántulas de Juglans neotropica Diels en la etapa de vivero. Alfa Publicaciones 2022, 4, 97–114. [Google Scholar] [CrossRef]
- Schwartz, F.W.; Zhang, H. Fundamentals of Groundwater, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Hansen, T.F.; Pélabon, C. Evolvability: A quantitative-genetics perspective. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 153–175. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities; CABI Publishing: Wallingford, UK, 2000; pp. 31–57. [Google Scholar]
- Mediavilla Mediavilla, J.S. Crecimiento inicial de Juglans neotropica Diels con fertilización química y orgánica en el campus Yuyucocha. Bachelor’s Thesis, Universidad Técnica del Norte, Ibarra, Ecuador, 2023. [Google Scholar]
- Leibing, C.; Signer, J.; Van Zonneveld, M.; Jarvis, A.; Dvorak, W. Selection of provenances to adapt tropical pine forestry to climate change on the basis of climate analogs. Forests 2013, 4, 155–178. [Google Scholar] [CrossRef]
- Barner, H.; Ditlevsen, B.; Olesen, K. Introducción al Mejoramiento Genético Forestal; Danida Forest Seed Centre: Humlebæk, Dinamarca, 1992. [Google Scholar]
- Ektvedt, T.M.; Evans, M.N.; Falk, D.A.; Sheppard, P.R. Dendrochronology and isotope chronology of Juglans neotropica and its response to El Niño-related rainfall events in tropical highlands of Piura, northern Peru. Plants 2025, 14, 1704. [Google Scholar] [CrossRef] [PubMed]








| N° Treatments | Code | Description |
|---|---|---|
| 1 | T0L1 | Control—The Tundo |
| 2 | T0L2 | Control—The Victoria |
| 3 | T0L3 | Control—The Argelia |
| 4 | T1L1 | Boiling water—The Tundo |
| 5 | T1L2 | Boiling water—The Victoria |
| 6 | T1L3 | Boiling water—The Argelia |
| 7 | T2L1 | Mechanical—The Tundo |
| 8 | T2L2 | Mechanical—The Victoria |
| 9 | T2L3 | Mechanical—The Argelia |
| 10 | T3L1 | Water and sun—The Tundo |
| 11 | T3L2 | Water and sun—The Victoria |
| 12 | T3L3 | Water and sun—The Argelia |
| Category | Interpretation | Illustration |
|---|---|---|
| Sinuous | Twisted, tortuous, or crooked, with curved and serpentine shapes |  |
| Inclined | Indicates an inclination or slope |  |
| Bifurcated | Divided into two branches or parts |  |
| Straight | A line or surface without curves or angles |  |
| FACTORS | Levels | Treatment | |
|---|---|---|---|
| F1: Location | F2: Fertilizer | ||
| L1: The Tundo | Fert 0: | Control | T0 |
| Fert 1: NPK (13-40-13) | NPK 1: 100 gr | T1 | |
| NPK 2: 150 gr | T2 | ||
| NPK 3: 200 gr | T3 | ||
| NPK 4: 250 gr | T4 | ||
| Fert 2: Urea | Urea: 100 gr | T5 | |
| Urea: 150 gr | T6 | ||
| Urea: 200 gr | T7 | ||
| Urea: 250 gr | T8 | ||
| L2: The Victoria | Fert 0: 0 | Control | T0 |
| Fert 1: NPK (13-40-13) | NPK 1: 100 gr | T1 | |
| NPK 2: 150 gr | T2 | ||
| NPK 3: 200 gr | T3 | ||
| NPK 4: 250 gr | T4 | ||
| Fert 2: Urea | Urea: 100 gr | T5 | |
| Urea: 150 gr | T6 | ||
| Urea: 200 gr | T7 | ||
| Urea: 250 gr | T8 | ||
| Locality | Impurity (%) ± SD | Seeds kg−1 ± SD | CV Seeds (%) |
|---|---|---|---|
| The Tundo | 1.08 ± 0.22 | 30.0 ± 1.87 | 6.23 |
| The Victoria | 0.88 ± 0.22 | 32.0 ± 2.24 | 7.00 |
| The Argelia | 1.04 ± 0.29 | 35.0 ± 1.87 | 5.34 |
| Sampling Time | Locality | N° Seeds | Wws (g) | Dws (g) | Mc (%) | Viability |
|---|---|---|---|---|---|---|
| Measurement 1 (starting month) | The Tundo | 25 | 31.3 ± 0.2 | 31.1 ± 0.2 | 0.64 ± 0.03 | Viable |
| The Victoria | 25 | 28.7 ± 0.3 | 28.5 ± 0.3 | 0.73 ± 0.04 | Viable | |
| The Argelia | 25 | 25.6 ± 0.2 | 25.4 ± 0.2 | 0.51 ± 0.02 | Viable | |
| Measurement 2 (3 months) | The Tundo | 25 | 31.3 ± 0.2 | 30.9 ± 0.2 | 1.34 ± 0.05 | Viable |
| The Victoria | 25 | 28.7 ± 0.3 | 28.3 ± 0.3 | 1.46 ± 0.06 | Viable | |
| The Argelia | 25 | 25.6 ± 0.2 | 25.2 ± 0.2 | 1.37 ± 0.05 | Viable | |
| Measurement 3 (6 months) | The Tundo | 25 | 31.3 ± 0.2 | 30.6 ± 0.3 | 2.17 ± 0.07 | Not viable |
| The Victoria | 25 | 28.7 ± 0.3 | 28.0 ± 0.3 | 2.37 ± 0.08 | Not viable | |
| The Argelia | 25 | 25.6 ± 0.2 | 24.9 ± 0.2 | 2.58 ± 0.09 | Not viable |
| Locality | Variable | n | Mean ± SD | Min–Max |
|---|---|---|---|---|
| The Argelia | Weight (g) | 331 | 20.93 ± 6.06 | (8.00–40.00) |
| Diameter (mm) | 331 | 35.99 ± 3.88 | (25.70–46.70) | |
| Height (mm) | 331 | 32.81 ± 4.47 | (24.90–79.10) | |
| The Tundo | Weight (g) | 333 | 29.55 ± 6.15 | (14.00–46.00) |
| Diameter (mm) | 333 | 40.70 ± 3.30 | (29.80–48.50) | |
| Height (mm) | 333 | 39.75 ± 3.68 | (25.30–49.20) | |
| The Victoria | Weight (g) | 101 | 24.39 ± 4.67 | (13.00–38.00) |
| Diameter (mm) | 101 | 36.88 ± 3.01 | (30.40–58.20) | |
| Height (mm) | 101 | 37.40 ± 3.23 | (27.20–45.20) |
| Stratum | Locality | Planted | Living | Survival (%) |
|---|---|---|---|---|
| Secondary Forest | The Tundo | 50 | 50 | 100 |
| The Victoria | 50 | 50 | 100 | |
| Subtotal | 100 | 100 | 100 | |
| Riparian Forest | The Tundo | 50 | 48 | 96 |
| The Victoria | 50 | 44 | 88 | |
| Subtotal | 100 | 92 | 92 | |
| Pasture | The Tundo | 50 | 45 | 90 |
| The Victoria | 50 | 39 | 78 | |
| Subtotal | 100 | 84 | 84 | |
| General Total | 300 | 276 | 92 |
| F1: Localities | F2: Fertilizer | Levels | Treatment | Survival % | ||
|---|---|---|---|---|---|---|
| SF | RF | P | ||||
| L1: The Tundo | Fert 0: 0 | L1 | T0 | 100 | 70 | 56 |
| Fert 1: NPK (13-40-13) | NPK 1: 100 gr | T1 | 100 | 96 | 95 | |
| NPK 2: 150 gr | T2 | 100 | 100 | 93 | ||
| NPK 3: 200 gr | T3 | 100 | 100 | 98 | ||
| NPK 4: 250 gr | T4 | 100 | 100 | 99 | ||
| Fert 2 = Urea | Urea: 100 gr | T5 | 100 | 95 | 65 | |
| Urea: 150 gr | T6 | 100 | 100 | 100 | ||
| Urea: 200 gr | T7 | 100 | 100 | 100 | ||
| Urea: 250 gr | T8 | 100 | 100 | 100 | ||
| L2: The Victoria | Fert 0: 0 | L2 | T0 | 100 | 67 | 39 |
| Fert 1: NPK (13-40-13) | NPK 1: 100 gr | T1 | 100 | 92 | 90 | |
| NPK 2: 150 gr | T2 | 100 | 80 | 85 | ||
| NPK 3: 200 gr | T3 | 100 | 90 | 90 | ||
| NPK 4: 250 gr | T4 | 100 | 100 | 89 | ||
| Fert 2 = Urea | Urea: 100 gr | T5 | 100 | 100 | 55 | |
| Urea: 150 gr | T6 | 100 | 66 | 58 | ||
| Urea: 200 gr | T7 | 100 | 100 | 100 | ||
| Urea: 250 gr | T8 | 100 | 100 | 100 | ||
| Localities | Strata | Variable | Min | Mean | Max | SD | CV (%) |
|---|---|---|---|---|---|---|---|
| The Tundo | Secondary Forest | Diameter (mm) | 6.39 | 11.47 | 20.75 | 2.87 | 15.04 |
| Height (cm) | 32.00 | 54.74 | 101.00 | 15.04 | 27.48 | ||
| Riparian Forest | Diameter (mm) | 6.83 | 8.81 | 11.08 | 1.12 | 12.71 | |
| Height (cm) | 17.00 | 49.25 | 67.00 | 11.00 | 22.34 | ||
| Pasture | Diameter (mm) | 6.24 | 12.32 | 20.67 | 2.68 | 21.75 | |
| Height (cm) | 14.00 | 41.32 | 74.00 | 14.25 | 34.49 | ||
| The Victoria | Secondary Forest | Diameter (mm) | 6.12 | 12.08 | 19.77 | 3.66 | 30.30 |
| Height (cm) | 28.00 | 54.89 | 108.00 | 19.97 | 36.38 | ||
| Riparian Forest | Diameter (mm) | 5.70 | 9.57 | 16.25 | 2.15 | 22.47 | |
| Height (cm) | 20.00 | 47.60 | 87.00 | 14.80 | 31.09 | ||
| Pasture | Diameter (mm) | 5.85 | 13.86 | 20.45 | 3.20 | 23.09 | |
| Height (cm) | 20.00 | 44.58 | 76.00 | 14.29 | 32.05 |
| Category | Factor | Diameter (mm) | SE (mm) | CV Diameter (%) | p-Value | Height (cm) | SE (cm) | CV Height (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Locality | The Tundo | 11.80 | 0.27 | 49.29 | 0.2587 | 49.29 | 1.38 | 2.8 | 0.5681 |
| The Victoria | 11.35 | 0.29 | 48.14 | 48.14 | 1.46 | 3.03 | |||
| Stratum | Secondary Forest | 11.78 | 0.29 | 54.81 | 0.0001 | 54.81 | 1.55 | 2.83 | 0.0001 |
| Riparian Forest | 9.33 | 0.35 | 48.13 | 48.13 | 1.88 | 3.91 | |||
| Pasture | 13.02 | 0.30 | 42.8 | 42.80 | 1.59 | 3.71 | |||
| Treatment | T0 (Control) | 10.89 | 0.44 | 46.32 | 0.1692 | 46.32 | 2.27 | 4.9 | 0.7183 |
| T1 (100 g) | 10.91 | 0.59 | 49.78 | 49.78 | 3.04 | 6.11 | |||
| T2 (150 g) | 11.79 | 0.61 | 49.85 | 49.85 | 3.15 | 6.32 | |||
| T3 (200 g) | 12.54 | 0.61 | 50.15 | 50.15 | 3.15 | 6.28 | |||
| T4 (250 g) | 11.68 | 0.67 | 50.22 | 50.22 | 3.41 | 6.79 | |||
| T5 (100 g) | 10.62 | 0.63 | 44.37 | 44.37 | 3.21 | 7.23 | |||
| T6 (150 g) | 12.09 | 0.60 | 48.61 | 48.61 | 3.09 | 6.36 | |||
| T7 (200 g) | 12.19 | 0.63 | 52.94 | 52.94 | 3.21 | 6.06 | |||
| T8 (250 g) | 12.34 | 0.64 | 48.88 | 48.88 | 3.27 | 6.69 |
| Code | Locality | Treatment | Stratum | BD (mm) | SE | Tukey Group (BD) | TH (cm) | SE | Tukey Group (TH) |
|---|---|---|---|---|---|---|---|---|---|
| L2 T3–RF | L2 | T3 | Riparian forest | 7.58 | 1.56 | A | 47.00 | 8.73 | A–B |
| L1 T6–RF | L1 | T6 | Riparian forest | 7.74 | 2.69 | A | 51.00 | 15.10 | A–B |
| L1 T1–RF | L1 | T1 | Riparian forest | 8.11 | 1.91 | A–B | 26.00 | 10.67 | A |
| L2 T5–P | L2 | T5 | Pasture | 16.66 | 1.21 | C | 45.80 | 6.75 | A–B |
| L2 T0–P | L2 | T0 | Pasture | 15.89 | 1.35 | B–C | 60.75 | 7.55 | A–B |
| L1 T0–SF | L1 | T0 | Secondary forest | 13.07 | 1.21 | A–B–C | 72.56 | 6.75 | B |
| L2 T8–SF | L2 | T8 | Secondary forest | 13.14 | 0.85 | A–B–C | 52.60 | 4.77 | A–B |
| L1 T1–SF | L1 | T1 | Secondary forest | 11.07 | 1.21 | A–B–C | 66.40 | 6.75 | A–B |
| L2 T3–SF | L2 | T3 | Secondary forest | 13.31 | 1.21 | A–B–C | 69.40 | 6.75 | A–B |
| L2 T4–SF | L2 | T4 | Secondary forest | 9.23 | 1.21 | A–B–C | 42.20 | 6.75 | A–B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Herrera, B.; Pereira-Lorenzo, S.; Pucha-Cofrep, D. Phenotypic Variability of Juglans neotropica Diels from Different Provenances During Nursery and Plantation Stages in Southern Ecuador. Forests 2025, 16, 1141. https://doi.org/10.3390/f16071141
Palacios-Herrera B, Pereira-Lorenzo S, Pucha-Cofrep D. Phenotypic Variability of Juglans neotropica Diels from Different Provenances During Nursery and Plantation Stages in Southern Ecuador. Forests. 2025; 16(7):1141. https://doi.org/10.3390/f16071141
Chicago/Turabian StylePalacios-Herrera, Byron, Santiago Pereira-Lorenzo, and Darwin Pucha-Cofrep. 2025. "Phenotypic Variability of Juglans neotropica Diels from Different Provenances During Nursery and Plantation Stages in Southern Ecuador" Forests 16, no. 7: 1141. https://doi.org/10.3390/f16071141
APA StylePalacios-Herrera, B., Pereira-Lorenzo, S., & Pucha-Cofrep, D. (2025). Phenotypic Variability of Juglans neotropica Diels from Different Provenances During Nursery and Plantation Stages in Southern Ecuador. Forests, 16(7), 1141. https://doi.org/10.3390/f16071141








