Summer Cafe: In Vitro Case Study of Biological Repellents Against the Large Pine Weevil
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Kim, S.I.; Yoon, J.S.; Baeck, S.J.; Lee, S.H.; Ahn, Y.J.; Kwon, H.W. Toxicity and synergic repellency of plant essential oil mixtures with vanillin against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Netherer, S.; Schopf, A. Potential effects of climate change on insect herbivores in European forests—General aspects and the pine processionary moth as specific example. For. Ecol. Manag. 2010, 259, 831–838. [Google Scholar] [CrossRef]
- Day, K.R.; Nordlander, G.; Kenis, M.; Halldorson, G. General biology and life cycles of bark weevils. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 331–349. [Google Scholar]
- Mason, W.L.; Alía, R. Current and future status of Scots pine (Pinus sylvestris L.) forests in Europe. For. Syst. 2000, 9, 317–335. [Google Scholar] [CrossRef]
- Results of Forest Resource Monitoring. Meža Resursu Monitoringa Rezultāti, Latvian State Forest Research Institute Silava. Available online: https://www.silava.lv/images/Petijumi/Nacionalais-meza-monitorings/MRM-rezultati/2024-MRM-publiskais-zi%C5%86ojums.pdf (accessed on 1 April 2025).
- Björkman, C.; Bylund, H.; Nilsson, U.; Nordlander, G.; Schroeder, M. Effects of new forest management on insect damage risk in a changing climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI: Wallingford, UK, 2015; pp. 248–266. Available online: https://www.cabidigitallibrary.org/doi/10.1079/9781780643786.0248 (accessed on 1 July 2025).
- Lalík, M.; Galko, J.; Kunca, A.; Nikolov, C.; Rell, S.; Zúbrik, M.; Dubec, M.; Vakula, J.; Gubka, A.; Leontovyč, R.; et al. Ecology, management and damage by the large pine weevil (Coleoptera: Curculionidae) in coniferous forests within Europe. Cent. Eur. For. J. 2021, 67, 91–107. [Google Scholar] [CrossRef]
- Skrzecz, I.; Sukovata, L.; Jabłoński, T.; Sowińska, A.; Szmidla, H. Spatio-temporal distribution of Hylobius abietis in Scots pine stands–implications for pest monitoring. J. Pest Sci. 2021, 94, 1393–1404. [Google Scholar] [CrossRef]
- Toivonen, R.; Viiri, H. Adult large pine weevils Hylobius abietis feed on silver birch Betula pendula even in the presence of conifer seedlings. Agric. For. Entomol. 2006, 8, 121–128. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Inward, D.J.G.; Wainhouse, D.; Peace, A. The effect on temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and potential impacts of climate change. Agric. For. Entomol. 2012, 14, 348–357. [Google Scholar] [CrossRef]
- Leather, S.R.; Day, K.R.; Salisbury, A.N. The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): A problem of dispersal? Bull. Entomol. Res. 1999, 89, 3–16. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Venäläinen, A.; Lehtonen, I.; Laapas, M.; Ruosteenoja, K.; Tikkanen, O.P.; Viiri, H.; Ikonen, V.P.; Peltola, H. Climate change induces multiple risks to boreal forests and forestry in Finland: A literature review. Glob. Change Biol. 2020, 26, 4178–4196. [Google Scholar] [CrossRef]
- Wainhouse, D.; Inward, D.J.G.; Morgan, G. Modelling geographical variation in voltinism of Hylobius abietis under climate change and implications for management. Agric. For. Entomol. 2014, 16, 136–146. [Google Scholar] [CrossRef]
- Nordlander, G.; Mason, E.G.; Hjelm, K.; Nordenhem, H.; Hellqvist, C. Influence of climate and forest management on damage risk by the pine weevil Hylobius abietis in northern Sweden. Silva Fenn. 2017, 1, 7751. [Google Scholar] [CrossRef]
- Tan, J.Y.; Wainhouse, D.; Day, K.R.; Morgan, G. Flight ability and reproductive development in newly-emerged pine weevil Hylobius abietis and the potential effects of climate change. Agric. For. Entomol. 2010, 12, 427–434. [Google Scholar] [CrossRef]
- Örlander, G.; Nilsson, U.; Nordlander, G. Pine weevil abundance on clear-cuttings of different ages: A 6-year study using pitfall traps. Scand. J. For. Res. 1997, 12, 225–240. [Google Scholar] [CrossRef]
- Jactel, H.; Nicoll, B.C.; Branco, M.; Gonzalez-Olabarria, J.R.; Grodzki, W.; Långström, B.; Moreira, F.; Netherer, S.; Orazio, C.; Piou, D.; et al. The influences of forest stand management on biotic and abiotic risks of damage. Ann. For. Sci. 2009, 66, 701. [Google Scholar] [CrossRef]
- Nordlander, G.; Hellqvist, C.; Hjelm, K. Replanting conifer seedlings after pine weevil emigration in spring decreases feeding damage and seedling mortality. Scandinavian J. For. Res. 2017, 32, 60–67. [Google Scholar] [CrossRef]
- Nordlander, G.; Hellqvist, C.; Johansson, K.; Nordenhem, H. Regeneration of European boreal forests: Effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. For. Ecol. Manag. 2011, 262, 2354–2363. [Google Scholar] [CrossRef]
- von Sydow, F. Abundance of pine weevils (Hylobius abietis) and damage to conifer seedlings in relation to silvicultural practices. Scand. J. For. Res. 1997, 12, 157–167. [Google Scholar] [CrossRef]
- Kolmodin-Hedman, B.; Åkerblom, M.; Flato, S.; Alex, G. Symptoms in forest workers handling conifer plants treated with permethrin. Bull. Environ. Contam. Toxicol. 1995, 55, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mian, L.S.; Mulla, M.S. Effects of pyrethroid insecticides on nontarget invertebrates in aquatic ecosystems. J. Agric. Entomol. 1992, 9, 73–98. [Google Scholar]
- Pisa, L.; Goulson, D.; Yang, E.C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef] [PubMed]
- Galko, J.; Lalík, M.; Rell, S.; Nikolov, C.; Barta, M.; Pittner, J.; Hyblerová, S.; Zúbrik, M.; Kunca, A.; Vakula, J.; et al. Comprehensive comparison of treatments for controlling the large pine weevil (Hylobius abietis) in Central Europe. Sci. Rep. 2022, 12, 9673. [Google Scholar] [CrossRef]
- Hardy, C.; Sayyed, I.; Leslie, A.D.; Dittrich, A.D. Effectiveness of insecticides, physical barriers and size of planting stock against damage by the pine weevil (Hylobius abietis). Crop Prot. 2020, 137, 105307. [Google Scholar] [CrossRef]
- Thomas, H.; Nisbet, T.R.; Willoughby, I.H. Acetamiprid used to prevent Hylobius abietis damaging young trees poses a very low risk of causing water contamination provided good forestry practices are followed. Forestry 2022, 96, 207–216. [Google Scholar] [CrossRef]
- Luoranen, J.; Laine, T.; Saksa, T. Field performance of sand-coated (Conniflex®) Norway spruce seedlings planted in mounds made by continuously advancing mounder and in undisturbed soil. For. Ecol. Manag. 2022, 517, 120259. [Google Scholar] [CrossRef]
- Nordlander, G.; Nordenhem, H.; Hellqvist, C. A flexible sand coating (Conniflex) for the protection of conifer seedlings against damage by the pine weevil Hylobius abietis. Agric. For. Entomol. 2009, 11, 91–100. [Google Scholar] [CrossRef]
- Sikström, U.; Hjelm, K.; Hanssen, K.H.; Saksa, T.; Wallertz, K. Influence of mechanical site preparation on regeneration success of planted conifers in clearcuts in Fennoscandia–A review. Silva Fennica. 2020, 54, 10172. [Google Scholar] [CrossRef]
- Azeem, M.; Iqbal, Z.; Emami, S.N.; Nordlander, G.; Nordenhem, H.; Mozūratis, R.; El-Seedi, H.R.; Borg-Karlson, A.K. Chemical composition and antifeedant activity of some aromatic plants against pine weevil (Hylobius abietis). Ann. Appl. Biol. 2020, 177, 121–131. [Google Scholar] [CrossRef]
- Willoughby, I.H.; Moore, R.; Moffat, A.J.; Forster, J.; Sayyed, I.; Leslie, K. Are there viable chemical and non-chemical alternatives to the use of conventional insecticides for the protection of young trees from damage by the large pine weevil Hylobius abietis L. in UK forestry? For. Int. J. For. Res. 2020, 93, 694–712. [Google Scholar] [CrossRef]
- Luik, A.; Sibul, I.; Voolma, K. Influence of some plant extracts and neem preparations on the maturation feeding of the large pine weevil, Hylobius abietis L. Balt. For. 2000, 6, 53–58. [Google Scholar]
- Harlin, C.; Eriksson, S. Mekaniska Plantskydd Mot Snytbaggeskador, Anlagt 2007; Report 1; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2010. [Google Scholar]
- Egigu, M.C.; Ibrahim, M.A.; Yahya, A.; Holopainen, J.K. Cordeauxia edulis and Rhododendron tomentosum extracts disturb orientation and feeding behavior of Hylobius abietis and Phyllodecta laticollis. Entomol. Exp. Et Appl. 2011, 138, 162–174. [Google Scholar] [CrossRef]
- Sibul, I.; Ploomi, A.; Voolma, K. Influence of neem oil on the large pine weevil, Hylobius abietis L.(Coleoptera, Curculionidae). Balt. For. 2009, 15, 255–261. [Google Scholar]
- Eriksson, C.; Mansson, P.E.; Sjoedin, K.; Schlyter, F. Antifeedants and feeding stimulants in bark extracts of ten woody non-host species of the pine weevil. Hylobius abietis. J. Chem. Ecol. 2008, 34, 1290–1297. [Google Scholar] [CrossRef]
- Lalík, M.; Galko, J.; Nikolov, C.; Rell, S.; Kunca, A.; Modlinger, R.; Holuša, J. Non-pesticide alternatives for reducing feeding damage caused by the large pine weevil (Hylobius abietis L.). Ann. Appl. Biol. 2020, 177, 132–142. [Google Scholar] [CrossRef]
- Moore, R.; Willoughby, I.H.; Moffat, A.J.; Forster, J. Acetamiprid, chlorantraniliprole, and in some situations the physical barriers MultiPro® or Kvaae® wax, can be alternatives to traditional synthetic pyrethroid insecticides for the protection of young conifers from damage by the large pine weevil Hylobius abietis L. Scand. J. For. Res. 2021, 36, 230–248. [Google Scholar]
- Christensen, R.H.B. Cumulative link models for ordinal regression with the R package ordinal. J. Stat. Softw. 2018, 35, 1–46. [Google Scholar]
- R. Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2025. Available online: https://www.R-project.org/ (accessed on 7 March 2025).
- Christensen, R. Ordinal-Regression Models for Ordinal Data; R Package Version 2023.12-4.1. 2023. Available online: https://CRAN.R-project.org/package=ordinal (accessed on 1 July 2025).
- Kuhns, E.H.; Martini, X.; Hoyte, A.; Stelinski, L.L. Repellent activity of botanical oils against Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2016, 7, 35. [Google Scholar] [CrossRef]
- Møller, A.; Jennions, M.D. How much variance can be explained by ecologists and evolutionary biologists? Oecologia 2002, 132, 492–500. [Google Scholar] [CrossRef]
- Jansons, Ā.; Zeltiņš, P.; Donis, J.; Neimane, U. Long-term effect of Lophodermium needle cast on the growth of Scots pine and implications for financial outcomes. Forests 2020, 11, 718. [Google Scholar] [CrossRef]
- Mumm, R.; Hilker, M. Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci. 2006, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zas, R.; Björklund, N.; Sampedro, L.; Hellqvist, C.; Karlsson, B.; Jansson, S.; Nordlander, G. Genetic variation in resistance of Norway spruce seedlings to damage by the pine weevil Hylobius abietis. Tree Genet. Genomes 2017, 13, 111. [Google Scholar] [CrossRef]
- Holmes, S.B.; MacQuarrie, C.J. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef]
- Sarwar, M. Inorganic insecticides used in landscape settings and insect pests. Chem. Res. J. 2016, 1, 50–57. [Google Scholar]
- An, H.; Tak, J.H. Miticidal and repellent activity of thirty essential oils and their synergistic interaction with vanillin against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2022, 182, 114872. [Google Scholar] [CrossRef]
- BVL. Nicht Verkehrsfähige Pflanzenstärkungsmittel. 2025. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/PflStM_nicht_verkehrsfaehig.pdf?__blob=publicationFile&v=32 (accessed on 31 March 2025).
- Mantzoukas, S.; Ntoukas, A.; Lagogiannis, I.; Kalyvas, N.; Eliopoulos, P.; Poulas, K. Larvicidal action of cannabidiol oil and neem oil against three stored product insect pests: Effect on survival time and in progeny. Biology 2020, 9, 321. [Google Scholar] [CrossRef]

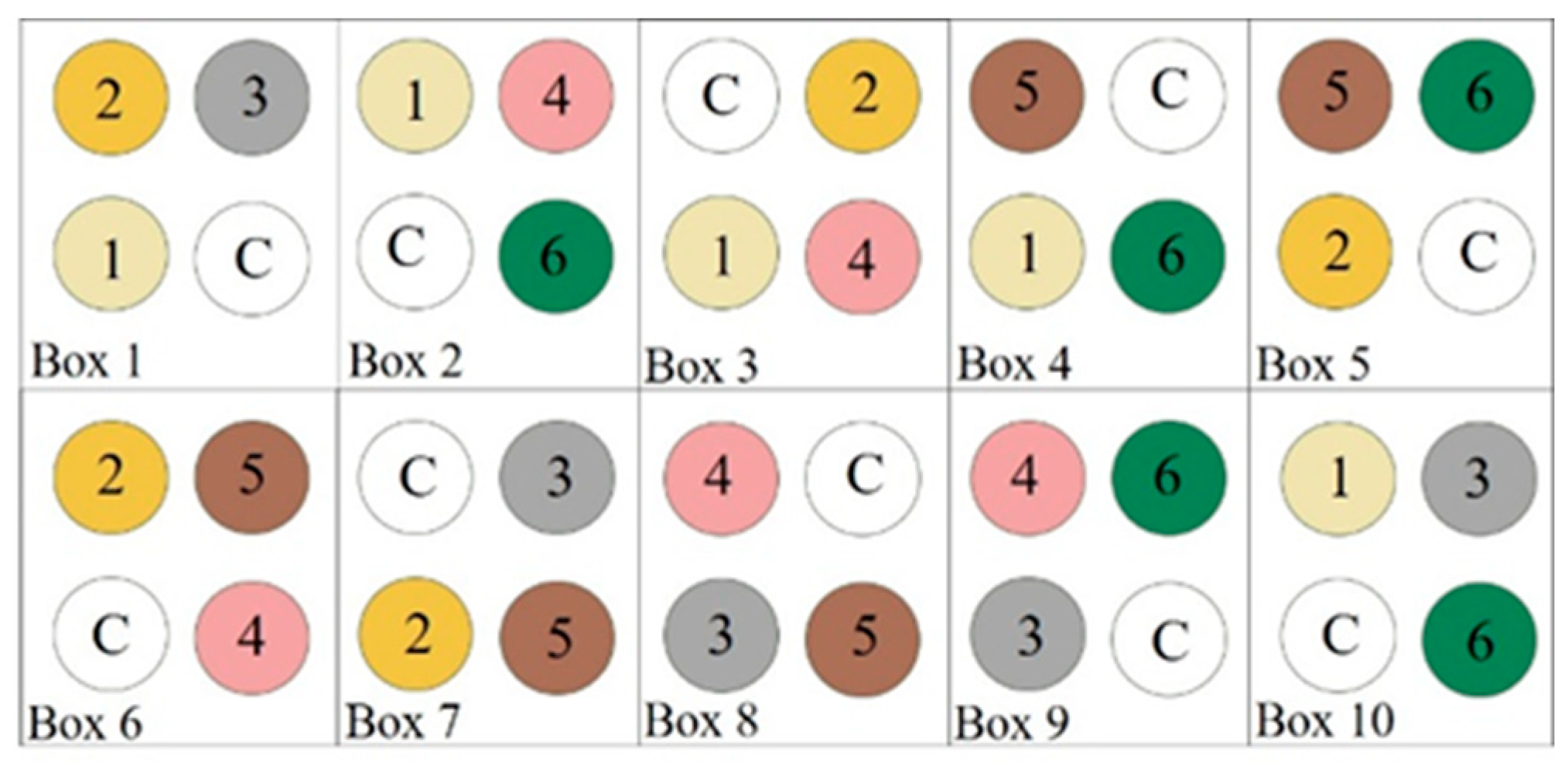
| Repellent No. | Description |
|---|---|
| 1 | Base * + calcium carbonate (chalk, fine flour, 20%) |
| 2 | Base * + beeswax (<15%) |
| 3 | Base * + vanillin (<15%) |
| 4 | More concentrated base * (higher share of epoxy (50%), no water; more viscous) |
| 5 | Base * |
| 6 | Base * with hemp oil (20%) as a substitute for canola oil |
| C (control) | Norfort LDW 115 |
| The Extent of Damage | Location of Damage | |
|---|---|---|
| Grade | Description | Description |
| 0 | no damage | no damage |
| 1 | 1–2 small damages; growth was not affected | damage to the untreated part |
| 2 | more than damages; growth was not affected | damage to the treated part |
| 3 | significant damage, barely surviving | damage on the untreated and treated parts |
| 4 | seedling is dead | |
| Repellent No. | Description |
|---|---|
| 1 | On applying, forms a thin layer, which remains flexible but smears a little. |
| 2 | Hard application. As the product dries, microcracks form, and within a week, it mostly falls off, forming uncovered areas. The residues of repellent remain sticky, partially immobilising H. abietis. |
| 3 | Does not dry and remains slightly sticky, thus immobilising H. abietis. |
| 4 | Repellent is layered and difficult to blend. When applied, forms a thick layer and does not dry completely. |
| 5 | After applying, forms a flexible coating and does not dry completely. |
| 6 | Hard application. Applies unevenly. Shortly after application, microcracks form, and the repellent appears to be dripping, thus forming uncovered areas. |
| C (control) | Easy application. Dries and holds on well. |
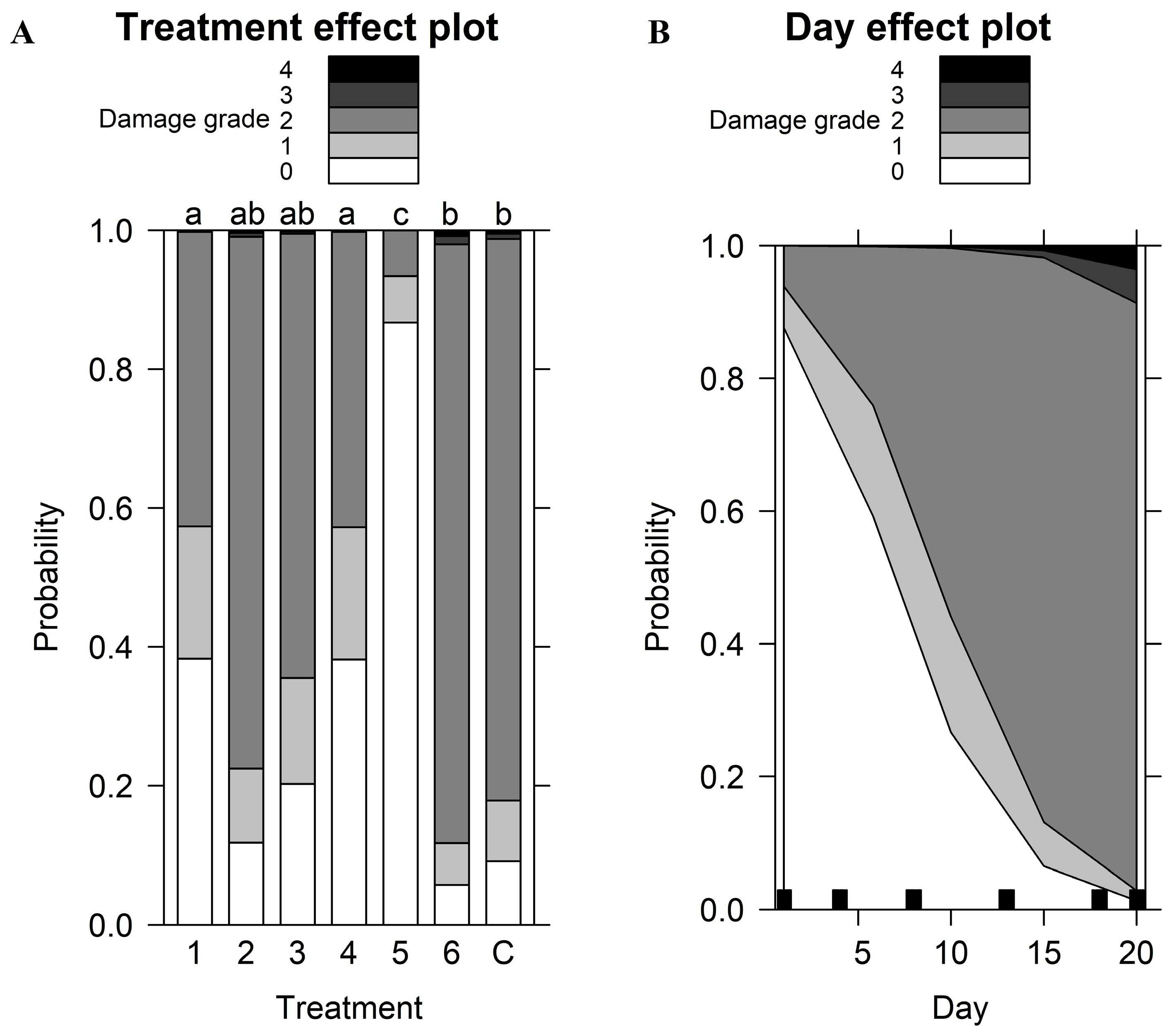
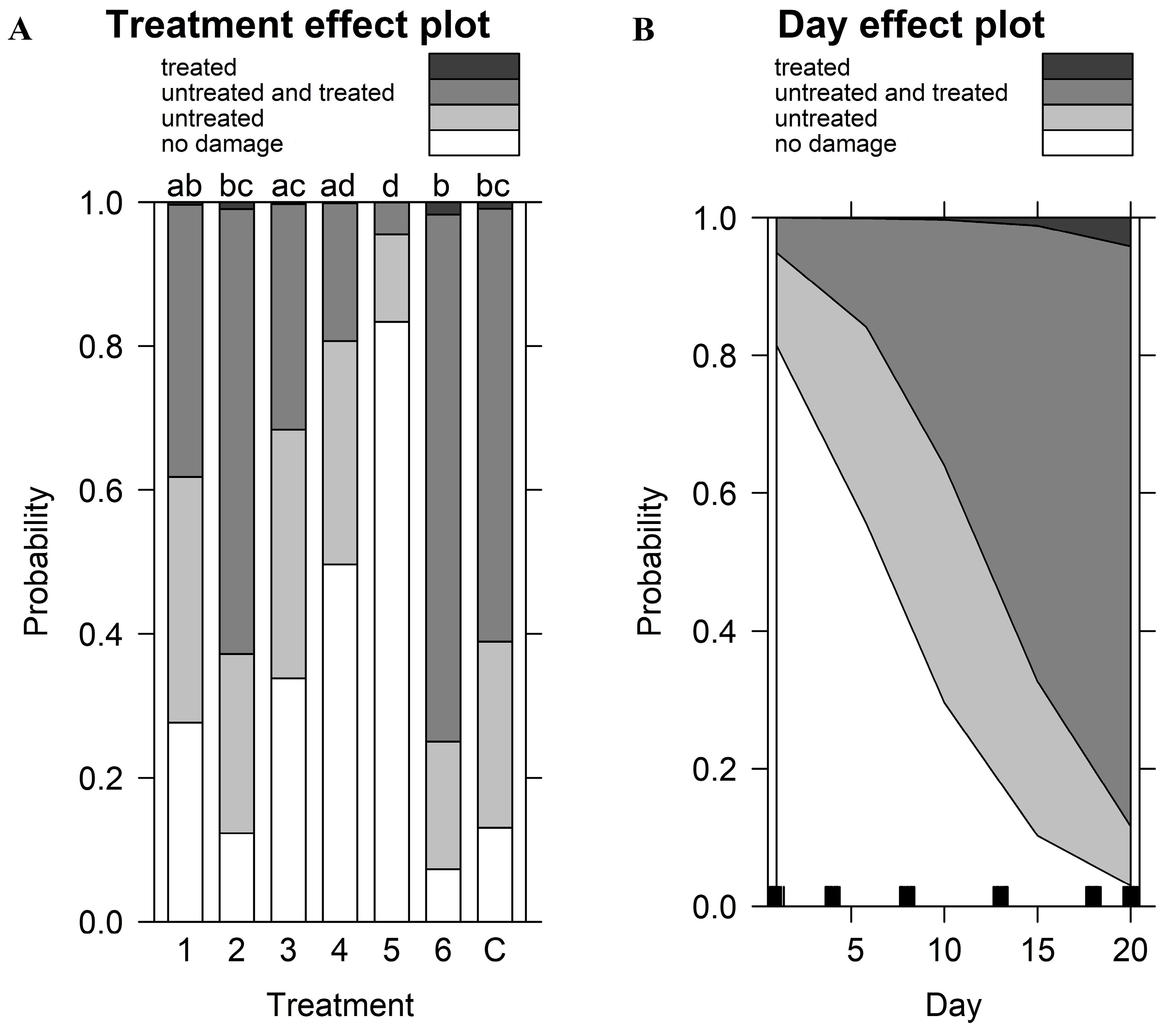
| Damage Grade | Location of Damage | |||
|---|---|---|---|---|
| Coefficients of the fixed effects | ||||
| Variable | χ2 | p-value | χ2 | p-value |
| Treatment (repellent) | 64.99 | <0.001 | 64.42 | <0.001 |
| Day | 159.54 | <0.001 | 129.27 | <0.001 |
| Random effects | ||||
| Variance | Variance | |||
| Box | 0.39 | 0.05 | ||
| Pseudo R2 (estimated against null model) | ||||
| Pseudo R2 | Pseudo R2 | |||
| Nagelkerke (Cragg and Uhler) | 0.62 | 0.56 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matisone, I.; Ozoliņš, K.; Matisons, R.; Spāde, M.; Grīnfelds, U.; Trukšs, R. Summer Cafe: In Vitro Case Study of Biological Repellents Against the Large Pine Weevil. Forests 2025, 16, 1139. https://doi.org/10.3390/f16071139
Matisone I, Ozoliņš K, Matisons R, Spāde M, Grīnfelds U, Trukšs R. Summer Cafe: In Vitro Case Study of Biological Repellents Against the Large Pine Weevil. Forests. 2025; 16(7):1139. https://doi.org/10.3390/f16071139
Chicago/Turabian StyleMatisone, Ilze, Kristaps Ozoliņš, Roberts Matisons, Mārtiņš Spāde, Uldis Grīnfelds, and Rinalds Trukšs. 2025. "Summer Cafe: In Vitro Case Study of Biological Repellents Against the Large Pine Weevil" Forests 16, no. 7: 1139. https://doi.org/10.3390/f16071139
APA StyleMatisone, I., Ozoliņš, K., Matisons, R., Spāde, M., Grīnfelds, U., & Trukšs, R. (2025). Summer Cafe: In Vitro Case Study of Biological Repellents Against the Large Pine Weevil. Forests, 16(7), 1139. https://doi.org/10.3390/f16071139






