Abstract
Sustainable Forest Management (SFM) requires the building of relationships among diameter increment, shape, and size (ISS), and increment–age variables to identify critical changes in forest structure and dynamics. This understanding is essential for maintaining forest productivity, structural and species diversity, stability, and sustainability. This study focused on measuring, reporting, and modeling these relationships for Araucaria angustifolia (Bertol.) Kuntze, across various diameters and three stands, located at different rural properties in southern Brazil. A random sample of 186 individual trees was acquired; the trees were measured for multiple dendrometric variables, and several morphometric indices were calculated. Additionally, two cores were extracted from each tree using an increment borer, enabling the measurement of growth rings and annual diameter increments. These were modeled using generalized linear models to assess the relationships among them and to quantify changes in forest structure and dynamics. The results revealed the dominance of A. angustifolia and a decline in the increment rate with increasing age, shape, and size in both old and young trees, indicating potential risks to the structure and dynamics of these unmanaged forests. Therefore, the models constructed in this study can guide conservation-by-use efforts and ensure the long-term continuity and productivity of forest remnants at selected rural properties, where A. angustifolia trees are predominant.
1. Introduction
The mixed ombrophilous forest (MOF) is maintained by Brazilian and state of Santa Catarina legislation under a preservation regime, that is, without the application of forest management. In the past, these forests were exploited without a management system for converting forest areas into agricultural land, driven by economic, food (edible seeds), and demographic interests. The methodology adopted was to cut trees with a diameter at breast height ≥ 40 cm. Although this is not a management model, during this period, the removal of trees favored the availability of resources and space for the growth of the remaining trees, promoting the regeneration and diversity of the forest.
Continued exploitation contributed to the reduction in and fragmentation of this forest ecosystem, associated with migratory processes, increased population density, and the intensive use of forest resources (agricultural and pasture land), mainly for socioeconomic progress and development. Exploitation was a process stimulated by the Land Law of 1850, which led to the creation of private property and economic power, with forests considered unproductive from a Eurocentric perspective [1,2,3]. The opposite of this exploitation and progress was the prohibitive legislation for MOF management, as outlined in IBAMA Normative Regulation 37 [4] and CONAMA Resolution 278 [5], which led to increased taxes, a higher occurrence of environmental crimes, a lack of interest in species conservation, and reduced or stagnated growth, ultimately resulting in a loss of diversity. Thus, with the implementation of a policy to preserve forest resources for multiple uses, the Araucaria angustifolia (Bertol.) Kuntze (araucaria) and other valuable species of this forest typology have not been considered as alternatives for the socioeconomic and cultural development of rural landowners [6].
Recently, after four decades of regime preservation (unmanaged), the remaining fragments of the MOF have been verified, but these are not a guarantee of the continuity of the structure and dynamics and the maintenance of the diversity of forest resources. These concepts are the main factors and processes that determine the development of a forest stand, which can be evaluated by the relationship between increment, shape, and size (ISS), allowing managers to assess the conditions for species continuity. Disturbances (natural or anthropogenic) maintain the dynamics and structure of forest ecosystems. Structural variations alter the dynamics, which in turn modify the growth, shape, size, recruitment, mortality, and composition of species [7,8]. Through the measurement of these elements, adaptive management is formulated to conserve species, forest stands, and their multiple benefits. It is important to note that when there are no changes in this relationship, this can be characterized as stress and a lack of structural complexity in the forest stand.
The increment, changes in shape and size (ISS), and disturbances (anthropogenic or natural) are the primary evidence of forest dynamics [9]. The study of forest dynamics focuses on changes in the structure and composition of forests over time, including their behavior in response to both anthropogenic and natural disturbances [10]. This information has two primary focuses and applications: management and ecology. In management, it is used to determine production, cutting cycles, biological rotation, management models, maintenance, and the continuity of structure, as well as the adequacy of density and the availability of resources and space. In ecology, management encompasses the relationships between gross primary production and net primary production, as well as the costs associated with respiration, leaf, seed, wood, and root production, recycling, autecology of species, and succession [11].
The maintenance of forest ecosystem resources is a crucial aspect of Sustainable Forest Management (SFM), which aims to utilize resources in an ecologically, socially, and economically sustainable manner. In this sense, Bergseng et al. [12] conclude that only conservation use implies a reduction in the income from wood production for society and forest owners, as well as an increase in the costs of not using the resource. They evaluate the economic impacts, noting a 10%–45% reduction in value compared to forests without use restrictions and a 50% increase in rotation.
Forest ecosystems and their adaptive conservation management should inform policies aimed at mitigating climate change, maintaining biodiversity, promoting socioeconomic development, ensuring human welfare and needs, and maintaining the continuity of production. In the current literature, there are two distinct views, or a dichotomy, namely, that environmental changes or the degradation of natural resources are caused anthropogenically by the use of resources and that a lack of management causes damage to structure, dynamics, growth, and production, restricts environmental goods and services, energy, and carbon sequestration, and reduces diversity [13,14,15,16]. Researchers highlight that reductions or changes in species composition can result in a 15% to 25% decrease in the function of ecosystem services. Therefore, it is necessary to adapt management strategies and silvicultural practices as a preemptive measure to ensure the functioning of forests, minimizing excessive exploitative uses or inadequate management [17,18].
The problem verified and measured in this study and other studies of the pure conservation proposition concerns the structure, shape, and growth of the forest, which resembles a normal and regular distribution, with the dominance of one species, Araucaria angustifolia (Bertol.) Kuntze. We have noted the standardization of shape and size, competition, and a reduction in the rate of growth (stagnation), primarily affecting young trees, as well as a decrease in diversity. For the araucaria species, there are few publications on the ISS relationship; thus, in this study, these indicators were evaluated and modeled by the increment, shape, and size (ISS) relationship. These indicate past and current changes in the structure and dynamics of the forest, making it possible to plan the need for silvicultural interventions, thus adapting the availability of resources and spaces to the continuity of ecosystem functions and services.
In addition to shifts in forest productive capacity, changes in the use of biomass with higher added value, such as textiles and chemicals, should be considered, which would favor new markets for forest products and address other social demands. Management strategies address changes in species composition (currently a regular MOF structure), rotation duration, and thinning intensity. One proposal aimed to generate models based on processes, dynamics, and structure, identifying the impacts of non-management as negative for growth and favoring the forestry sector [19]. Thus, SFM is crucial for ensuring changes in the growth rate of trees and the structure and dynamics of forest ecosystems. Trees change shape and size as they compete for light, nutrients, water, and space during their development. This is part of forest successional processes, affecting both dominant and nondominant trees [20]. Forests undergoing successional processes are often considered sustainable. However, dominant species tend to persist for more extended periods, even as their growth rates decline. This persistence may limit the ability of nondominant trees to access essential resources [21].
Dominance closes the canopy and reduces overall forest growth. Even after the development stage, dominant trees become less efficient in utilizing resources as they enter a phase of physiological decline, as dominance is associated with an age-related decline [19]. This process can be assessed using models that link growth with variables such as age, size, shape, and tree density [20,21,22]. Such models may provide valuable information to underpin SFM operations.
Thus, managing and guiding the growth of trees within a forest remnant hold both economic and ecological significance. Growth typically has a predictable relationship with age, increasing in young forests as crowns develop and decreasing once crowns and leaf areas are fully developed. Multiple interactions, including resource availability, competition for space and nutrients, and the efficiency of resource utilization, influence this process. These dynamics significantly influence forest structure, with dominant trees often exhibiting greater growth rates while subordinate trees grow more slowly. This highlights the need for interventions in forest structure, particularly because long-lived dominant trees tend to maintain a closed canopy even after their growth rates stagnate, thereby hindering the recycling of forest composition and structure [23].
Management approaches target trees that have reached their maximum growth support capacity, are of larger sizes or older ages, or are dominated and under competition, thereby maintaining forest structure. However, validating this strategy requires measuring and modeling variable indices of shape, size, and growth to understand the ecological processes of forest structure and dynamics. Therefore, managing dynamics becomes sustainable as processes such as mortality, competition, growth, recruitment, and regeneration are logical and inevitable. This ensures the sustainable use of forest resources and socioeconomic benefits, preventing the loss or waste of vital forest resources [24,25,26,27,28].
Natural or anthropogenic disturbances drive changes in the structure and resource conditions that affect the growth of forest species. Disturbances regulate the growth cycle by varying light conditions, soil nutrients, and space, accommodating developments or increases in tree shape and size [29]. Management (disturbances) alters the shape and size (diameter, crown, canopy) of trees and renews species composition, forest structure, growth, photosynthesis, mortality rates, productivity, and diversity. In contrast, unmanaged forests often exhibit increased competition, reduced crown sizes in both young and old trees, greater mortality rates, and reduced ingrowth of trees across diameter classes [30,31,32]. Managed forests, on the other hand, promote growth by providing space and nutrients, thereby enabling more efficient use of resources [33].
For the forest ecosystem, four decades of forest conservation without silvicultural interventions in southern Brazil have affected the structure, dynamics, diversity, growth, reproduction success, and productivity of these forests, as well as the maintenance of their ecosystem functions and services [34,35,36,37,38,39]. The problem lies in the assumption that continued beneficial practices (such as preservation and conservation without use), that is, without changes in forest structure and management practices, will continue to produce better results. For forest management, assessing the structure of the ISS relationship—whether static or dynamic—within the forest is crucial for maintaining the forest ecosystem. This depends on the plasticity of species and competition for light, space, and nutrients. The shape of the crown, its size, and that of the stem, as well as density and growth, define the structure of a forest. It is necessary to study the changes that occur in these variables to understand the dynamics of the forest [39,40].
Based on this context, two questions were raised: (1) Does a lack of management impact the increment, shape, and size of the species? (2) How do these elements affect the structure, dynamics, and conservation of forest stands?
In sum, our review reveals two critical research gaps. First, there is a lack of integrated modeling approaches that simultaneously consider tree increment, shape, and size as indicators of structural and functional forest health. Second, there is a lack of empirical studies applying these metrics to A. angustifolia populations within long-preserved forest remnants in southern Brazil.
By addressing these gaps, the present study advances both the theoretical understanding of forest dynamics and the practical application of SFM in native forests that have been under exclusive preservation for decades [31,39,40]. It also provides an analytical framework to support future policies that balance biodiversity conservation with the sustainable use of forest resources.
2. Materials and Methods
2.1. Description of the Study Areas
The study areas are remnants of the mixed ombrophilous forest (MOF), with the occurrence of the A. angustifolia species, located in three regions of southern Brazil (Figure 1). The regions are designated as follows: the São Joaquim (SJQ) site, with a total area of 73.5 ha and a sampling area of 1.9 ha; the Urupema (URU) site, with a total area of 18 ha and a sampling area of 1.2 ha; and the Painel (PNL) site, with a total area of 50.5 ha and a sampling area of 1.3 ha (Figure 1). The selection of these study areas was based on logistical considerations, spatial representativeness, and the cooperation of rural landowners, who granted access to their properties for conducting fieldwork.
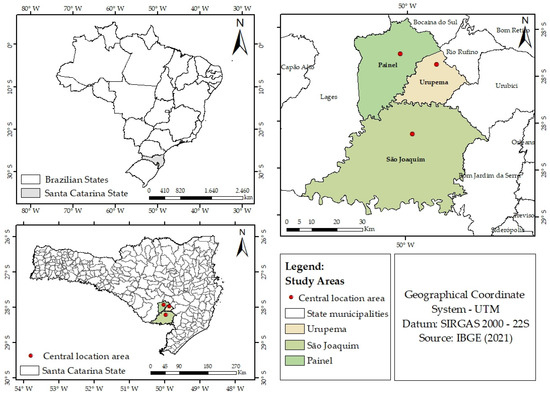
Figure 1.
Location of the three study areas within national, state, and municipality contexts. The red dots show the central location of the three selected study areas.
At all sites, samples were taken from individual trees representing the distribution of diameter classes at breast height (d), including trees with d ≥ 10 cm. Diameter at breast height (d) was used as a variable to determine sampling sufficiency, indicating the need for 135 trees across all sites. This value is the minimum number of trees to be measured in the forest inventory based on the size of the area of each site. However, a total of 186 trees of different diameter classes were sampled to ensure the measurement of the variation in the relationship between the variables: increment, age, shape, and dimension. Trees were selected to ensure sufficient spatial separation and to reduce potential spatial autocorrelation.
According to the Köppen classification, the region has a Cfb climate characterized as temperate and consistently humid, with no distinct dry season [41]. The SJQ site is 1352 m above sea level, with an average annual temperature of 14 °C and an average yearly precipitation of 1683 mm. The URU site is 1324 m above sea level, with an average annual temperature of 14.1 °C and a recorded average yearly precipitation of 1634 mm. The PNL site is located at an elevation of 1123 m, with an average annual temperature of 15.3 °C and an average yearly precipitation of 1543 mm.
All sites exhibit the dominance and formation of a uniform (regular) forest of the species A. angustifolia, as illustrated in Figure 2. This species is predominant because it occupies all strata of the forest, including the canopy domain, unmanaged forest, and longest-lived trees. Despite a decrease in the increment rate, the species compromises structure renewal, natural regeneration, diversity, production, and reproductive success, thereby damaging ecosystem functions, services, and fauna. Given the dominance of and closure of the canopy by this species, both old and young trees exhibit reduced growth rates, increased species mortality, and a concentration of trees in a specific diameter class range (30–60 cm). The structure has remained almost static, compromising the formation of future structure and biodiversity sustainability. This situation is observed across all chosen sites.

Figure 2.
Aerial and ground pictures of the study areas showing the dominance of A. angustifolia in the studied forest remnants.
2.2. Measurements and Statistical Analysis
For all 186 trees sampled at the SJQ, URU, and PNL sites, d, total height (h), crown base height (cbh), and four crown radii (cr) were measured. In addition, two increment cores were taken at d from each tree to determine the annual periodic increment in d, considering a 10- and 5-year timespan. The geographic coordinates for each tree were also measured. Increment cores were glued, dried, and sanded, and growth rings were counted and measured using the Lintab 6®, Heidelberg, Germany, measurement table (with an accuracy of 0.0001 mm) and evaluated using the Time Series Analysis Program—TSAPTM-Win software (Version 4.89) with cross-dating.
Through the use of the increment data, the annual periodic increment in diameter (APId) was estimated for 10- and 5-year periods. The percentage rate of reduction in increment over these years was calculated by comparing the two periods. This rate of reduction in increment was used to assess the reduction in crown surface area, which indicates an increase or decrease in photosynthetic capacity for the two periods, associated with changes in resource availability, growth space, resource use efficiency, and/or the stabilization of tree form. This formulation was adapted from [42], which defined growth efficiency as the stem volume per unit leaf area, a measure of tree vigor.
With the dataset, morphometric indices (Equations (1)–(6)), crown efficiency (Equation (7)), and annual periodic increments were calculated for two periods: 10 and 5 years (Equation (8)). Models linking ISS and increment–age were then fitted (Equations (9)–(14)).
where is the mean crown ratio in m, cd is the crown diameter in m, si is the saliency index, cl is the crown length in m, cf is the crown form, csa is the crown surface area in m2, ce is the crown efficiency in cm year−1.m2, APId is the annual periodic increment in diameter for the periods of ten and five years, d is the diameter at breast height in cm in the final period, dt is the diameter in cm at the beginning of the period, t the period considered, id is the increment in d, and are the model parameters to be estimated, and is the random residual term.
The models were fitted using maximum likelihood estimators assuming generalized linear models (GLMs) with a gamma residual distribution and either the identity or logarithmic link functions. The statistical significance of model parameters was evaluated using Wald’s chi-squared test at a significance level of Pr > 0.0001.
The choice of regression analysis using GLMs is due to the problems in modeling in several areas of knowledge, which include the characteristics of heteroscedasticity and autocorrelation (in the case of models involving time series), as these violate the basic assumptions of linear and nonlinear models. In some cases, models may exhibit these two characteristics, indicating that the residuals do not follow a normal distribution and, therefore, impair the estimation of the response variable according to the predictor variable.
Thus, the use of GLMs expands the range of options for the distribution of the response variable, allowing it to belong to the exponential family of distributions (gamma, Poisson, exponential, Log-normal) without having to consider only the normal distribution. In the present study, the residuals did not meet the regression conditions for a normal distribution. Lower values for the standard error (deviance) and homogeneous residuals for the gamma distribution were present.
The model prediction accuracy was assessed using the deviance criteria, the Akaike Information Criterion (AIC), and the Bayesian Information Criterion (BIC), as well as graphical representations of the residual distribution. The models were initially fitted using data from all sites and were subsequently subjected to analysis of covariance (ANCOVA) to test for differences in slopes and regression line levels among the sites; for this purpose, “site” was set as a categorical variable [43]. The ANCOVAs revealed differences in the levels and slopes of the regression lines, as well as the interaction between continuous and categorical variables (p < 0.0001), indicating the need for site-specific models. All statistical analyses were performed using the Statistical Analysis System (SAS 9.3).
3. Results
3.1. Analysis of Descriptive Statistics and Models Fitted
Table 1 presents the descriptive statistics of the ISS variables for the three study areas, and Figure 3 shows the frequency distribution of trees by diameter class. These statistics describe the current structure, growth, and dynamics of the forest. The results indicate that the forest has increased in size and shape and, over time, has reduced its growth rate, suggesting the need for intervention to maintain its structure.


Table 1.
Descriptive statistics for the shape–size–increment variables in the three study areas.
Table 1.
Descriptive statistics for the shape–size–increment variables in the three study areas.
| SJQ | ||||
|---|---|---|---|---|
| Variable | Mean | Max | Min | Std |
| d | 41.3 | 60.2 | 20.1 | 9.8 |
| cd | 9.7 | 16.6 | 4.2 | 2.7 |
| si | 0.23 | 0.31 | 0.16 | 0.03 |
| cl | 5.1 | 10.9 | 1.6 | 1.8 |
| csa10 | 80.1 | 216.4 | 13.9 | 42.2 |
| csa5 | 63.5 | 174.3 | 8.5 | 33.8 |
| cf | 2.3 | 6.1 | 0.8 | 1.3 |
| ce | 0.0043 | 0.018 | 0.0007 | 0.0034 |
| id | 0.7 | 4.1 | 0.09 | 0.4 |
| APId10 | 0.4 | 0.8 | 0.2 | 0.15 |
| APId5 | 0.3 | 0.7 | 0.1 | 0.15 |
| URU | ||||
| d | 36.6 | 70.1 | 18.8 | 11.1 |
| cd | 8.2 | 13.5 | 4.1 | 2.2 |
| si | 0.23 | 0.31 | 0.1 | 0.04 |
| cl | 5.3 | 13.1 | 0.3 | 2.3 |
| csa10 | 56.4 | 142.1 | 13.2 | 30.3 |
| csa5 | 49.7 | 134.5 | 9.8 | 28.6 |
| cf | 2.5 | 38.7 | 0.8 | 4.7 |
| ce | 0.0032 | 0.0088 | 0.0008 | 0.0018 |
| id | 0.7 | 3.2 | 0.1 | 0.4 |
| APId10 | 0.24 | 0.65 | 0.1 | 0.1 |
| APId5 | 0.21 | 0.69 | 0.08 | 0.11 |
| PNL | ||||
| d | 37.5 | 62.7 | 23.6 | 6.7 |
| cd | 4.5 | 8.8 | 1.3 | 1.7 |
| si | 0.12 | 0.22 | 0.03 | 0.04 |
| cl | 6.3 | 12.0 | 1.0 | 2.3 |
| csa10 | 20.2 | 60.8 | 1.2 | 14.3 |
| csa5 | 10.5 | 29.6 | 1.3 | 11.3 |
| cf | 0.9 | 4.6 | 0.2 | 0.6 |
| ce | 0.013 | 0.0526 | 0.0026 | 0.0097 |
| id | 0.9 | 3.9 | 0.002 | 0.6 |
| APId10 | 0.53 | 0.88 | 0.25 | 0.18 |
| APId5 | 0.41 | 0.66 | 0.09 | 0.14 |
SJQ, São Joaquim; URU, Urupema; PNL, Painel; Max, maximum value; Min, minimum value; Std, standard deviation; d, diameter at breast height in cm; cd, crown diameter in m; si, saliency index; cl, crown length in m; csa, crown surface area in m2 for 10- and 5-year periods; cf, crown form; ce, crown efficiency in cm. year−1.m−2; id, diameter increment; APId, annual periodic increment in diameter in cm for 10- and 5-year periods.
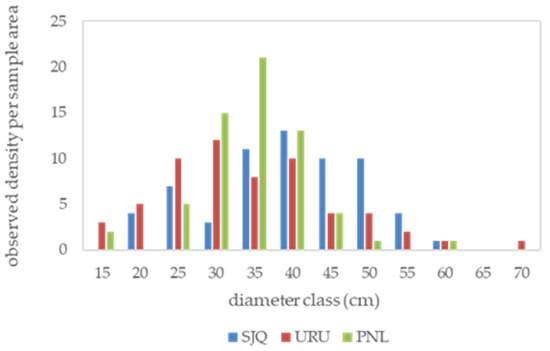
Figure 3.
Frequency distribution of Araucaria angustifolia trees by diameter class. SJQ—São Joaquim; URU—Urupema; PNL—Painel.
The density of trees by diameter class presents a normal distribution, which is not characteristic of mixed forests. This indicates the dominance of a single species, the formation of a regular forest, and the compromising of the structure and dynamics of the ecosystem. These characteristics are caused by the lack of management disturbances, which have been absent for four decades in this typology, resulting in a homogeneity in species composition. The relevance of management (low-intensity disturbance) lies in generating a heterogeneous structure while maintaining a balance between mortality and regeneration, as well as the distribution of trees with different diameters [8].
The descriptive statistics reveal interesting information about the forest structure, including areas with a minimum diameter at breast height (d ≥ 20 cm). This represents the compromising of the maintenance of dynamics, as there is no ingress of trees with smaller d (unbalanced structure). It is worth noting that ingress and growth occur more slowly than mortality and aging [44]. The SJQ and URU sites have trees with greater age, crown area, crown diameter, lateral expansion, and crown form, lower ce, and a greater reduction in the increment rate compared to PNL. These morphometric variables indicate that these trees have wider and shorter crowns, reduced photosynthetic capacity, and, consequently, a greater reduction in crown growth rate and efficiency (Table 1).
The PNL site has a greater tree density, as indicated by the smaller crown diameter (cd) and narrow, elongated crowns. This is reflected in a smaller crown form and greater photosynthetic capacity, which increases over time, as well as greater crown efficiency. Tree density per hectare is obtained by dividing 10,000 (m2) by the crown surface area (m2). Consequently, the SJQ and URU areas have lower tree densities compared to PNL. These results indicate the formation of a regular horizontal structure, with trees exhibiting a concentration of diameter distribution in the 35 and 70 cm classes. This structure formation resembles a normal distribution, indicating that unmanaged forests reduce diversity and highlighting the need for managed forest resources in this forest domain. The diameter concentration presented in the results is worrying for the renewal of the forest structure, as it indicates that there is no variation in the size of the trees.
APId10 showed a reduction of 39% in SJQ, 69% in URU, and 41% in PNL, suggesting that trees may have reached their maximum growth capacity. The models presented in Figure 3 have negative coefficients (Table 2), which corroborate the descriptive statistics of the ISS relationship: older trees have smaller crown efficiencies and reduced growth capacities.

Table 2.
Estimated coefficients and accuracy metrics of the models for shape–dimension–increment relationships.
The dataset enabled the modeling of the ISS relationship, yielding results on the current and future dynamics of structure for an unmanaged forest. It is worth noting that the analysis of covariance was significant (p < 0.0001), indicating that there are differences in the growth rate and changes in shape and size across the areas.
The models demonstrated accuracy for the deviation, AIC, and BIC criteria. Equations (9)–(11) and (13) present negative coefficient b1, characterizing an inverse relationship in the ISS, indicating a decrease in growth over time (stagnation) and size and form stabilization without the continuity of dynamics (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8) (Appendix A). The models present homogenized residues, which contribute to the reduction in variability not explained by the study’s response variables. Residues with greater deviation (outliers) were not removed from the figures, as they indicate the characteristic variation in the species’ ontogeny, the environment, and natural or anthropic interference in the ecosystem. In this study, it was decided to present the observed value together with the model adjustment; in this way, it is possible to visualize the variation in the quality of the adjustment for each tree in its class distribution, increment, shape, and dimension. The pattern of the ISS relationship depends on age, species, structural variation, and the availability of environmental resources [45].
3.2. Assessment of the Structure, Dynamics, and Growth of the Forest Ecosystem
The age of the trees varies between sites. In SJQ, the trees have a larger diameter and age and, consequently, a smaller, more minor increase. In URU and PNL, the trees have a smaller diameter, are older, and exhibit a higher average growth rate. The age range between sites was 16 to 78 years, with averages of 51 years for SJQ, 36 years for URU, and 30 years for PNL. It should be noted that this is not the real age of the trees, but the average age obtained from the increment borer taken from the diameter at breast height, as at this point there is a loss in the number of rings. Figure 4 illustrates that trees exhibit a decline in growth over time, affecting both young and mature trees.
According to Binkley et al. [45], this reversed growth pattern has historically contributed to dominance growth. The greater growth of larger or dominant trees over time results from their enhanced capture of light, belowground resources, and space compared to smaller trees, accentuating differences in tree size and shape and, consequently, in forest structure and dynamics.
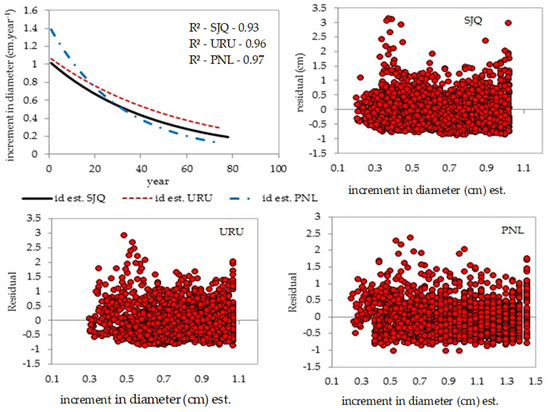

Figure 4.
Negative exponential relationship between annual periodic increment and age, which shows the stagnation of the growth of individual trees in the forest and the distribution of the residuals of the adjustment for the areas. SJQ—São Joaquim; URU—Urupema; PNL—Painel.
Figure 5 shows a reduction in APId10 as the salience index (si) increases, indicating that the trees stabilized their crown diameter (cd) and diameter (d) growth. The trees are older or in competition and have experienced a decline in photosynthetic efficiency over the past decade. The (si) is an indicator of how many times dc is greater than d and can be used as an element based on which to propose silvicultural intervention.
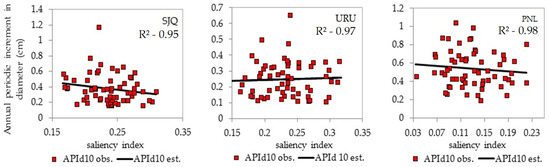

Figure 5.
Characteristics of the relationship between the annual periodic increment (cm) and salience index for A. angustifolia in unmanaged forests at the study sites. SJQ—São Joaquim; URU—Urupema; PNL—Painel. APId10—annual periodic increment in diameter (cm) for a 10-year period.
The results show the ontogenetic characteristics and crown plasticity of the species, with increases with age in crown diameter but decreases in crown length; that is, adults of the species have a short and long crown, while the young have a long and narrow crown and, consequently, a reduced crown form and greater photosynthetic and growth capacity (Figure 6). Both indices corroborate the assumptions of the hypotheses: the MOF, specifically the studied sites, present ISS stagnation, and there is a need for adaptive management to maintain the structure and dynamics of the forest.
These indices assess crown plasticity, as they explain the species’ ability to adjust to forest structure and receive light, and the influences on growth efficiency and survival. In summary, forest management involves information on age, species specificity, density, spatial distribution, resource availability, and competition.
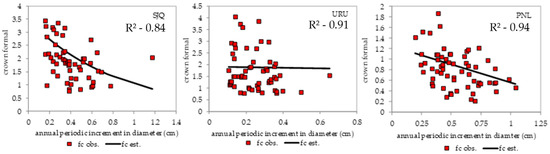

Figure 6.
Importance of the relationship between crown form and annual periodic increase in diameter (cm), indicating that the smaller the crown form, the greater the increment, and the greater the photosynthetic capacity and production. SJQ—São Joaquim; URU—Urupema; PNL–Painel.
The results suggest that crown efficiency, which is synonymous with growth vigor, can be used as a metric to quantify the effects of shape–size–increment relationships. This measure also highlights the contribution of trees of different sizes to overall production, aiding in effective management planning (Figure 7 and Figure 8). Across all sites, a positive correlation exists between diameter increment and efficiency: as diameter increases, efficiency also increases. However, a substantial portion of trees fail to achieve significant efficiency values. As trees attain larger crown areas, their growth tends to decrease, reflecting stability in crown growth and increment rates characteristic of old-growth forests.
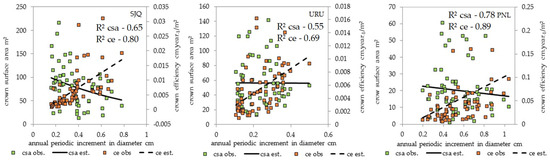

Figure 7.
Relationships among crown surface area, crown efficiency, and annual periodic increment in diameter for A. angustifolia at the three study sites. The crown efficiency as a function of the increment presents a directly proportional relationship, while the crown surface area shows an inversely proportional relationship. SJQ: São Joaquim; URU: Urupema; PNL: Painel site; csa: crown surface area; ce: crown efficiency.
In general, Figure 8 illustrates the decline in growth efficiency and the development of crown surface area. However, a positive relationship was observed between crown surface area and annual periodic increment in diameter over the last five years in URU. Because most sampled trees have large diameters, averaging 40 cm (Table 1), they contribute less to the overall population growth. The adjusted models emphasize that a lack of management does not prevent structural dynamics; the structure self-regulates, albeit at a slow pace, which leads to a reduction in structure through reduced growth rates of dominant trees and decreased resource use efficiency (the law of diminishing returns). These processes interrupt forest regeneration and renewal and reduce the growth, diversity, and productivity of the forest.
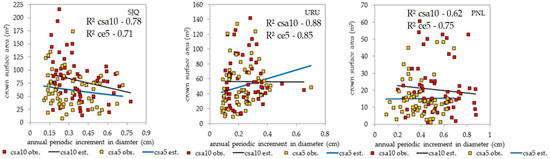

Figure 8.
Models for the dynamics of shape–growth relationships considering 10- and 5-year periods for the three study sites. SJQ: São Joaquim; URU: Urupema; PNL: Painel.
The results highlight the importance of assessing the ISS relationship, as it indicates the quantity of suitable trees during changes in the forest structure’s dynamics, including shape, size, and growth partitioning, and is applicable in managing competition throughout the forest stand’s rotation. The dynamics alter the patterns of the ISS relationship that is linked to competition between trees of different sizes and forest development. Planning management with human intervention emphasizes the importance of monitoring the ISS relationship, which demonstrates the development of the forest population with resilience control, as opposed to relying on self-regulation.
The adjusted models reveal that forests require adaptive management interventions for certain diameter classes and by region, which will influence changes in growth, shape, and dimension, as well as the production, stability, and current diversity of this forest typology (MOF).
4. Discussion
Tree growth, shape, and size are highly variable with changes in resource and space availability (density). They depend on numerous factors, making them challenging to model in mixed forests [46]. Tree growth is a dynamic process that brings about changes in a tree’s shape and size [47]. Data on tree growth and a better understanding of its drivers are needed for many forests [48] to predict future stand composition and density [49], calibrate dynamic models [50], assess carbon sequestration [51], and develop decision-support tools to guide forest management [52].
The structural complexity of a forest can be defined as all dimensional, architectural, and distributional patterns of plant individuals and their organs in a given space at a given point in time [52,53]. Thus, forest management aims to understand variables that maintain structural complexity, dynamics, and growth for different periods. This constitutes one of the main paradigms of modern forestry [54]. As a premise, most managed forests are less complex in structure than primary forests [55,56,57,58]. In contrast, some have been impacted in terms of their composition and horizontal and vertical structure, and have maintained structural complexity [57,58].
However, the key question of the present study was the following: what happens to the arrangement of structure, dynamics, and growth in unmanaged forests, such as the MOF in southern Brazil? The forest has been unmanaged for four decades, and the following question remains: are forest preservation and self-regulation better than forest management? It was possible to answer these questions by modeling the ISS relationships under the current conditions of structure, by evaluating the increment and shape of trees of the araucaria species of different sizes, and by gathering information about the dynamics of the growth and development of the stand population [59,60,61]. When a species, or group of species, reaches its maximum development and there is no management, the results verify stagnation in growth, shape, size, and, consequently, in the spatial arrangement of the structure.
The answer to the previous question is that forests are dynamic systems characterized by interactions between the elements in the system and the environment. These interactions are related to the crown, which adjusts to capture light, which influences the growth and size of the trees, all of which are governed by competence [62]. The crown ensures that trees maintain photosynthetic efficiency, a strategy for their survival and growth. It impacts photosynthesis, with assimilated carbon allocated to the woody tissue, and the relationship between the crown structure and radial growth. The shape and arrangement of the crowns that comprise the canopy structure control all the processes and factors within a forest, including light, microclimate, atmospheric exchanges, and biogeochemical cycles [63].
This study confirmed the dominance of A. angustifolia in the three selected study areas, characterized mostly by trees of a larger diameter and a smaller number of smaller-sized trees, stabilized tree shape and forest structure, and reduced growth rates. Although they contribute to a substantial portion of forest biomass, large trees typically exhibit the lowest growth rates and inhibit the growth of codominant and dominated individuals. Furthermore, as A. angustifolia demands light for increased growth, dominant trees may also inhibit the natural regeneration of this species, often resulting in left-skewed diameter distributions [64,65,66]. Under no silvicultural interventions, A. angustifolia requires large canopy openings to regenerate successfully, which typically occurs after large trees die and fall to the ground. This process, however, can take several hundred years, as suggested by the “lozenge model” [67].
The reduction in growth rates is attributed to factors such as age, competition, less efficient resource utilization, and reduced photosynthetic capacity. This configuration has a negative impact on ecosystem functions and services, including factors and processes such as precipitation, light, temperature, growth, resources, structural complexity, and dynamics. In this sense, this study contributes to the understanding of the relationship between ISS in unmanaged forests, which showed a reduction in growth, both in young and adult trees, and stabilization in shape and size. Thus, the ISS relationship informs us of the need for management to increase heterogeneity and structural complexity precisely because the MOF is a mixed forest. The current structure is worse than the structure would be if there were management; at least there would be specific structures according to a management goal [55].
A conservation regime with no active forest management, such as that observed in the three study areas, contributes to the simplification of forest dynamics, structure, species diversity, productivity, and regeneration in the Araucaria Forest. Trees with declining growth rates increase the respiratory costs needed to sustain forest biomass, a characteristic of old-growth forests, which in turn reduces their carbon dioxide absorption capacity [51,68]. This study demonstrates that ISS relationships are a valuable tool for identifying the current state of forest structure and the efficiency of tree crowns in utilizing resources for enhanced growth (Figure 5 and Figure 6).
Understanding the relationships among ISS variables helps forest managers evaluate which silvicultural treatments would be the most effective for a given forest stand. The results highlight the need for silvicultural interventions in tree diameter distribution, as the evaluated variables indicate stabilized and stagnant development. As suggested by Finger et al. [23], improvement in forest structure, in the sense that forest regeneration is promoted and shape–size–increment relationships are less homogeneous, is only possible with management interventions and canopy opening. Such controlled disturbances enhance forest growth by creating variations in light intensity, nutrient availability, and space. Post-intervention monitoring and control of the effects of disturbances on forest regeneration, growth, and structure are essential. The current ISS relationships indicate stress on the balance between growth and mortality, potentially compromising the resilience and continuity of A. angustifolia’s reproduction. Such relationships provide an effective means of assessing factors that affect the growth and development of individual trees and the overall system.
Another key component is the crown area, which indicates that larger areas are associated with smaller diameter increments, while smaller crown areas correspond to greater diameter growth (Figure 7 and Figure 8). At the three selected sites where large trees dominate, the growth of small and large trees is suppressed. This situation calls for management interventions to induce the differentiation of tree sizes, what has been described as “structural diversity” [69]. The observed relationship is influenced by crown shape, where trees with larger crown areas typically have short, wide crowns, resulting in reduced growth rates and lower crown efficiency [38].
Growth efficiency declines as crown area increases, influenced by factors such as total leaf area, the position of trees in the vertical layers, competition intensity, past suppression, and tree age (Figure 7). These relationships are crucial in understanding individual tree vigor and the interaction between growth and forest structure. The observed form–dimension–increment relationships suggest that the evaluated mixed forest stands lack distinct strata and are currently dominated by a single species, A. angustifolia, due to the absence of active management. Although not formally assessed, it was evident that there was an almost complete absence of A. angustifolia seedlings and young trees across all three study areas. The legal restrictions imposed in the past appear to have been effective in preventing deforestation. Still, they have led to a state of declining productivity and reduced structural diversity in forests. The lack of regeneration of A. angustifolia and other associated tree species is further exacerbated by the use of understory areas for cattle grazing, as noted by Oliveira and Vibrans [70].
The crown and growth efficiency of A. angustifolia is shaped by the tree’s ability to absorb and utilize limited resources within the context of the given forest ecosystem. However, great tree density and/or monospecific dominance may negatively affect overall growth. The models fitted in this study can serve as tools for the sustainable management of A. angustifolia, providing decision-makers with insights into the processes that affect forest dynamics. They emphasize the importance of conservation-by-use approaches to enhance structural diversity, prevent a decline in A. angustifolia populations, and stimulate landowners’ interest in increasing their forest remnants. Such a win–win approach would benefit landowners and enhance structural and species diversity.
Thus, the ISS relationship is an expression of density, age, site, and the management of forest resources, as a lack of management or poor management of the population affects smaller trees and species that require light (such as A. angustifolia), which is influenced by density and competition. These two factors modify the dimension, size, and growth of the crown, while shape and size affect the amount of light interception, water absorption, and nutrient availability. In this sense, variables related to the dimension of the crown, stem, growth, and morphometrics can be used to plan interventions in the forest and understand its development conditions. This study, in general, showed that both the size of the stem and the crown shape and size can be used to express growth.
Forest management must create and sustain conditions that foster an ecological, social, and economic balance to ensure forest conservation, regeneration, and the maintenance of ecosystem functions and services. Given the challenges of forest fragmentation, strategies such as community-based management, along with silvicultural treatments, can make SFM more feasible. However, these efforts require targeted actions from the government. Management interventions are crucial for ensuring the survival of A. angustifolia and the availability of forest resources, particularly in the face of a changing climate. We argue that a pragmatic approach can effectively navigate the complexities of different conservation regimes and SFM approaches at rural properties, as suggested by Caliman et al. [71] in their study of forest remnants in other related ecosystems within the Atlantic Forest domain.
The results and modeling of the ISS relationship confirm the hypotheses tested—a lack of management impacts the increment, shape, and size of the species—as they revealed a regular forest pattern, growth stagnation, and standardization (without dynamics) of the shape and size of the trees, which together impact the productivity and diversity of the MOF. This information confirms the second hypothesis, demonstrating how these elements affect the structure, dynamics, and conservation of forest stands, specifically in terms of structural homogeneity, competition for resources and space, lower efficiency, smaller leaf area, reduced photosynthesis and production, and reduced social and economic benefits, as well as adaptation to climate change and carbon use.
Guignabert et al. [72] confirm that non-management has reduced forest resilience and that tree-level process-based management models are a tool to mitigate global changes, with management alternatives based on functional diversity. With the occurrence of global changes, there is a need for adaptive forest management practices to enhance the resilience of forests, maintain the provision of their services, and preserve their composition and structure. The authors’ conclusion [72] leaves the question of the extent to which unmanaged forests will be adapted to face global changes in abiotic and biotic elements. How will structure, regeneration, and diversity be affected?
In summary, the restrictions imposed over the last four decades have yielded unsatisfactory results so far, leading to a lack of interest among landowners in recognizing their forest remnants as a viable financial asset. This disinterest has demotivated them from expanding forest areas, especially on less favorable lands previously designated for agriculture and cattle ranching. Currently, the only income derived from A. angustifolia is through the collection of its nut-like seeds. However, this practice requires further study, as the overexploitation of seeds threatens the food supply for fauna, affecting both seed dispersal and natural regeneration.
5. Conclusions
This study has provided valuable insights into the current structure and relationships among ISS variables across the three study areas. These findings underscore stabilization in forest development, a reduction in growth rates, the dominance of A. angustifolia, and structural impoverishment that could potentially be found at other rural properties in southern Brazil.
This study reveals that low management intensities promote the growth of trees in smaller- and medium-diameter classes, thereby enhancing the dynamics and structural complexity of the forest, reducing production loss, increasing diversity, and improving ecosystem benefits. Likewise, preservation or a lack of management altered the forest’s structure, leading to homogenization in the diameter distribution, shape, and size of the trees, thereby putting at risk their capacity to adapt to temporal changes in climate, resources, resilience, and regenerative power.
Our findings highlight that unmanaged remnants of A. angustifolia forests, under a strict conservation regime, show signs of structural stagnation and limited regeneration. This study advocates the implementation of socially inclusive initiatives that promote the adoption of SFM practices at rural properties, particularly those with A. angustifolia as a predominant species. Such practices are crucial to support both the conservation and natural regeneration of the MOF. We argue that passive conservation alone has not effectively ensured the ecological functionality or long-term viability of these ecosystems. To advance both conservation and rural development goals, future research should focus on experimentally assessing SFM interventions across different forest types and regions. Moreover, it is essential to acknowledge the limitations of this study, including its observational nature, the lack of controlled sites for comparison, and the geographical restriction to a few specific areas in southern Brazil.
Author Contributions
Conceptualization, methodology, and formal analysis, A.F.H., L.D., V.L. and P.d.C.B.; software and validation, A.F.H. and L.D.; investigation, A.F.H. and L.D.; resources and data curation, A.F.H.; writing—original draft preparation, A.F.H., L.D. and P.d.C.B.; writing—review and editing, A.F.H., L.D., V.L., L.Z.O., M.O.A.A., E.A.C. and P.d.C.B.; visualization, A.F.H., V.L., L.D., L.Z.O., M.O.A.A., E.A.C. and P.d.C.B.; supervision, A.F.H., V.L. and P.d.C.B.; project administration, A.F.H.; funding acquisition, A.F.H., V.L. and P.d.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the FAPESC (Foundation for Research Support of the Santa Catarina State) for financial assistance for research groups (FAPESC 868/2023).
Data Availability Statement
All data are available upon reasonable request.
Acknowledgments
The authors are grateful for the support of Santa Catarina State University, the Department of Forest Engineering, the Forest Management and Growth Laboratory, and its Graduate Program. We also thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001) and the rural property owners for assuring access to their forest remnants. V.L. is supported by CNPq.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
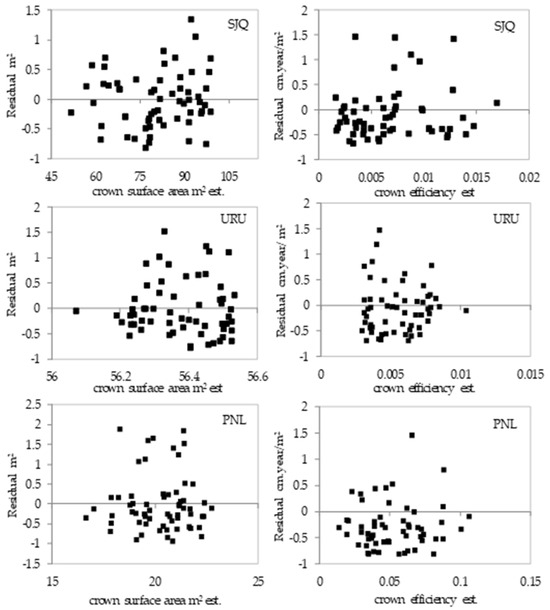
Figure A1.
Distribution of residuals from the adjustment of models 12 and 14 and Figure 7 representing the adjustment of the crown surface area and crown efficiency as a function of the annual periodic increment in diameter for the 10-year period.
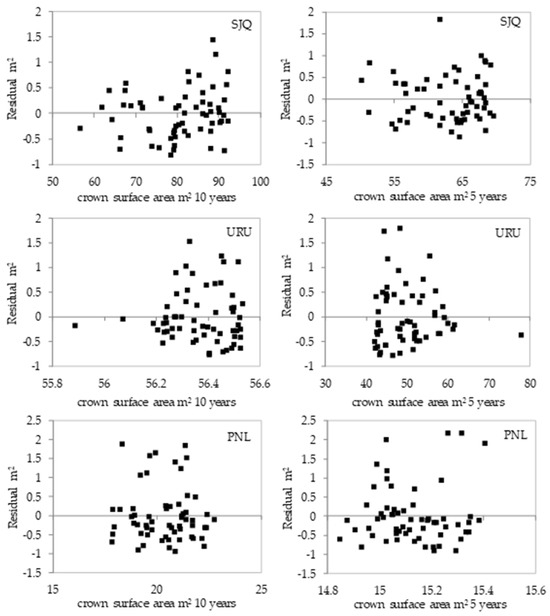
Figure A2.
Distribution of residuals from the adjustment of models 13 and 14 and Figure 8 representing the adjustment of the crown surface area as a function of the annual periodic increment in diameter for the 10- and 5-year periods.
References
- Randin, J.C. Um olhar sobre a colonização da fronteira sul. In História da Fronteira Sul; Radin, J.C., Valentini, D., Zarth, P., Eds.; Federal University of the Southern Border: Chapecó, Brazil, 2016; pp. 146–166. [Google Scholar]
- Salini, A.M. Colonização e meio Ambiente: A Transformação da Paisagem do Oeste Catarinense (1930 a 1970). Master’s Thesis, Federal University of the Southern Border, Chapecó, Brazil, 2018; 150p. [Google Scholar]
- De Sá, D.N.; Nodari, E.S.; Gerhardt, M. Colonização e transformação de paisagens na floresta com araucárias no século XX. Estud. Hist. 2023, 36, 518–541. [Google Scholar] [CrossRef]
- Ordinance n° 37 of the Brazilian Institute of Environment and Natural Resources. In Recognizes the Official List of Species of Brazilian Flora Threatened with Extinction; Brazilian Institute of Environment and Natural Resources: Brasília, Brazil, 1992.
- Conama Resolution n° 278. In Provides for the Cutting and Exploitation of Endangered Species of Flora; National Environment Council (CONAMA): Brasília, Brazil, 2001.
- Eisfeld, R.L. Research, Legislation, Planting and Management of Araucaria angustifolia (Bertol.) O. Kuntze: Perspectives and Solutions. Ph.D. Thesis, Federal University of Parana, Curitiba, Brazil, 2020; p. 261. [Google Scholar]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Rodrigo, R.M.; Pettit, J.L.; Janda, P.; Pavlin, J.; Ralhan, D.; Kozak, D.; Matula, R.; Marchand, W.; Bače, R.; Dušátko, M.; et al. Past disturbance shape present tree size distribution in European temperate primary beech-dominated forests. For. Ecol. Manag. 2024, 574, 122364. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Brienen, R.J.W.; Gloor, E.; Phillips, O.L.; Prior, L.D. Detecting trends in tree growth: Not so simple. Trends Plant Sci. 2013, 18, 11–17. [Google Scholar] [CrossRef]
- Stepka, T.F.; de Mattos, P.P.; Filho, A.F.; Machado, S.A.; Braz, E.M. How are diameter growth, forest structure and carrying related in tree populations? Eco. Model. 2025, 506, 111141. [Google Scholar] [CrossRef]
- Grebner, D.L.; Bettinger, P.; Siry, J.P. Introduction to Forestry and Natural Resources; Academic Press: New York, NY, USA, 2013; p. 508. [Google Scholar]
- Bergseng, E.; Ask, J.A.; Framstad, E.; Gobakken, T.; Solberg, B.; Hoen, H.F. Biodiversity protection and economics in long term boreal forest management—A detailed case for the valuation of protection measures. For. Pol. Econ. 2012, 15, 12–21. [Google Scholar] [CrossRef]
- Eyvindson, K.; Repo, A.; Mönkkönen, M. Mitigating Forest biodiversity and ecosystem service losses in the era of bio-based economy. For. Pol. Econ. 2018, 92, 119–127. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Thom, D.; Seidl, R. Trade-offs between temporal stability and level of forest ecosystem services provisioning under climate change. Ecol. Appl. 2018, 28, 1884–1896. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Change 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626–1710. [Google Scholar] [CrossRef]
- Mauri, A.; Girardello, M.; Forzieri, G.; Manca, F.; Beck, P.S.A.; Cescatti, A.; Strona, G. Assisted tree migration can reduce but not avert the decline of forest ecosystem services in Europe. Glob. Environ. Change 2023, 80, 102676. [Google Scholar] [CrossRef]
- Santopuoli, G.; Temperli, C.; Alberdi, I.; Barbeito, I.; Bosela, M.; Bottero, A.; Klopčič, M.; Lesinski, J.; Panzacchi, P.; Tognetti, R.; et al. Pan-European sustainable forest management indicators for assessing Cli-mate-Smart Forestry in Europe. Can. J. For. Res. 2021, 51, 1741–1750. [Google Scholar] [CrossRef]
- Binkley, D. A hypothesis about the interaction of tree dominance and stand production through stand development. For. Ecol. Manag. 2004, 190, 265–271. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G.; Barnard, H.R.; Fownes, J. Age-related decline in forest ecosystem growth: An individual-tree, stand-structure hypothesis. Ecosystems 2002, 5, 58–67. [Google Scholar] [CrossRef]
- Weiskittel, A.R.; Maguire, D.A.; Monserud, R.A. Modeling crown structural responses to competing vegetation control, thinning, fertilization, and Swiss needle cast in coastal Douglas-fir of the Pacific Northwest, USA. For. Ecol. Manag. 2007, 245, 96–109. [Google Scholar] [CrossRef]
- Wang, B.; Bu, Y.; Tao, G.; Yan, C.; Zhou, X.; Li, W.; Zhao, P.; Yang, Y.; Gou, R. Quantifying the effect of crown vertical position on individual tree competition: Total overlap index and its application in sustainable forest management. Sustainability 2020, 12, 7498. [Google Scholar] [CrossRef]
- Finger, C.A.G.; Costa, E.A.; Hess, A.F.; Liesenberg, V.; Bispo, P.C. Simulating sustainable forest management practices using crown attributes: Insights for Araucaria angustifolia trees in southern Brazil. Forests 2023, 14, 1285. [Google Scholar] [CrossRef]
- Ford, E.D.; Sorrensen, K.A. Theory and models of inter-plant competition as a spatial process. In Individual-Based Models and Approaches in Ecology: Populations, Communities, and Ecosystems; Taylor & Francis: Abingdon, UK, 1992; pp. 363–407. [Google Scholar]
- Fujimori, T. Ecological and Silvicultural Strategies for Sustainable Forest Management; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A., Jr.; Vanclay, J.K. Forest Growth and Yield Modeling; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zhang, J.; Huang, S.; He, F. Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proc. Natl. Acad. Sci. USA 2015, 112, 4009–4014. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesiński, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2020, 482, 118866. [Google Scholar] [CrossRef]
- Nogueira, A.C. Reação do Crescimento Radial da Araucaria angustifolia (Bert.) Kuntze em Florestas Naturais que Sofreram Corte Seletivo. Master’s Thesis, Federal University of Parana, Curitiba, Brazil, 1989. [Google Scholar]
- Costa, E.A.; Finger, C.A.G.; Fleig, F.D.; Hess, A.F.; Marangon, G.P. Density management diagram for araucaria uneven aged forest. Floresta 2016, 46, 173–184. [Google Scholar] [CrossRef]
- Hess, A.F.; Atanazio, K.A.; Borsoi, G.A.; Schorr, L.P.B.; Souza, I.A.; Costa, E.A.; Klein, D.R.; Krefta, S.M.; Stepka, T.F.; Abatti, R.; et al. Crown efficiency and pine cones production for Brazilian pine (Araucaria angustifolia (Bertol.) Kuntze) in south Brazil. J. Agric. Sci. 2019, 11, 247–259. [Google Scholar] [CrossRef]
- Lockhart, B.R.; Weih, R.C., Jr.; Smith, K.M. Crown Radius and Diameter at Breast Height Relationships for Six Bottomland Hardwood Species. J. Ark. Acad. Sci. 2005, 59, 110–115. [Google Scholar]
- Beckert, S.M.; Rosot, M.A.D.; Rosot, N.C. Crescimento e dinâmica de Araucaria angustifolia (Bert.) O. Ktze. em fragmento de Floresta Ombrófila Mista. Sci. For. 2014, 42, 209–218. [Google Scholar]
- Costa, E.A.; Finger, C.A.G.; Schneider, P.R.; Hess, A.F. The crown efficiency of Parana-Pine. Aust. J. Basic Appl. Sci. 2017, 11, 86–92. [Google Scholar] [CrossRef]
- Hess, A.F.; Minatti, M.; Liesenberg, V.; Mattos, P.P.; Braz, E.M.; Costa, E.A. Brazilian pine diameter at breast height and growth in mixed Ombrophilous forest in Southern Brazil. Aust. J. Crop Sci. 2018, 12, 770–777. [Google Scholar] [CrossRef]
- Schorr, L.B.P. Dinâmica e Relações Alométricas para Espécies Arbóreas em Floresta Ombrófila Mista sob Regime de não Manejo no sul do Brasil. Master’s Thesis, Santa Catarina State University, Florianópolis, Brazil, 2019. [Google Scholar]
- Demétrio, L.; Hess, A.F.; Sousa, A.N.; Costa, E.A.; Liesenberg, V.; Freisleben, M.J.; Schimalski, M.B.; Finger, C.A.G.; Hofico, N.S.A.; Bispo, P.C. Can we predict male strobili production in Araucaria angustifolia trees with dendrometric and morphometric atributes? Forests 2022, 13, 2074. [Google Scholar] [CrossRef]
- Silva, V.V.; Nicoletti, M.F.; Dobner, M., Jr.; Vaz, D.R.; Oliveira, G.S. Fragmentos de floresta ombrófila mista em diferentes estágios sucessionais: Caracterização dendrométrica e determinação da biomassa e carbono. Rev. Ciências Agrovet. 2023, 22, 695–704. [Google Scholar] [CrossRef]
- Atanazio, K.A.; Hess, A.F.; Krefta, S.M.; Schorr, L.P.B.; Souza, I.A.; Domiciano, C.A.R.; Cuchi, T.; Moraes, G.C. Modelagem das relações morfométricas com a produção de pinhas de Araucaria angustifolia (Bertol.) Kuntze no sul do Brasil. Ciência Florest. 2022, 32, 1247–1267. [Google Scholar] [CrossRef]
- Olivier, M.D.; Schneider, R.; Fournier, A.R. Responde of sugar maple (Acer saccharum, Marsh.) tree crown structure to competition in pure versus mixed stands. For. Ecol. Manag. 2016, 374, 20–32. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparoveck, G. Köppen climate classification map for Brazil. Meteor. Zeitsc. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Waring, R.H.; Theis, W.G.; Muscato, D. Stem growth per unit of leaf area-a measure of tree vigor. For. Sci. 1980, 26, 112–117. [Google Scholar]
- Kaps, M.; Lamberson, W.R. Biostatitistics for Animal Science; CABI Publishing: London, UK, 2004. [Google Scholar]
- Chambers, J.Q.; Negron-Juarez, R.I.; Marra, D.M.; Vittorio, A.D.; Tews, J.; Roberts, D.; Ribeiro, G.H.P.M.; Trumbore, S.E.; Higuchi, N. The steady-state mosaic of disturbance and succession across old-growth Central Amazon forest landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 3949–3954. [Google Scholar] [CrossRef]
- Binkley, D.; Kashian, D.M.; Boyden, S.; Kaye, M.W.; Bradford, J.B.; Arthur, M.A.; Fornwalt, P.J.; Ryan, M.G. Patterns of growth dominance in forests of the Rocky Mountains, USA. For. Ecol. Manag. 2006, 236, 193–201. [Google Scholar] [CrossRef]
- Ncutirakiza, J.B.N.; Goulert-Fleury, S.; Lejeune, P.; Bry, X.; Trottier, C.; Mortier, F.; Fayolle, A.; Habiyaremye, F.M.; Mianda-Bungi, L.N.; Ligot, G. Using high-resolution images to analyze the importance of crown size and competition for the growth of tropical tress. For. Ecol. Manag. 2024, 552, 121553. [Google Scholar] [CrossRef]
- Guerra-Hernández, J.; Gonzáles-Ferreiro, E.; Monléon, V.J.; Faias, S.P.; Tomé, M.; Diáz-Varela, R.A. Use of multi-temporal UAV-derived imagery for estimating individual tree growth in Pinus pinea stands. Forests 2017, 8, 300. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Phillips, O.L.; Lewis, S.L.; Affum-Baffoe, K.; Alvarez-Davila, E.; Andrade, A.; Aragão, L.E.O.C.; Araujo-Murakami, A.; Baker, T.R.; Bánki, O.; et al. Competition influences tree growth, but not mortality, across environmental gradients in Amazonia and tropical Africa. Ecology 2020, 101, e03052. [Google Scholar] [CrossRef]
- Rüger, N.; Berger, U.; Hubbel, B.; Nicolini, E.A.; Blanc, L. Contrasting above-ground biomass balance in a Neotropical rain forest. J. Veg. Sci. 2011, 21, 672–682. [Google Scholar] [CrossRef]
- Purves, D.; Pacala, S. Predictive models of forest dynamics. Science 2013, 342, 776. [Google Scholar] [CrossRef]
- Rutishauser, E.; Barthélémy, D.; Blanc, L.; Eric-André, N. Crown fragmentation assessment in tropical trees: Method, insights and perspectives. For. Ecol. Manag. 2010, 261, 400–407. [Google Scholar] [CrossRef]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2012; p. 457. [Google Scholar]
- McElhinny, C. Forest and Woodland Structure as an Index of Biodiversity: A Review; NSW National Parks and Wildfire Service: Parramatta, Australia, 2002.
- Seidel, D.; Annighöfer, P.; Ehbrecht, M.; Magdon, P.; Wöllauer, S.; Ammer, C. Deriving stand structural complexity from airborne laser scanning data-what does it tell us about a forest? Remote Sens. 2020, 12, 1854. [Google Scholar] [CrossRef]
- Seidel, D.; Ammer, C. Towards a casual understanding of the relationship between structural complexity, productivity, and adaptability of forests based on principles of thermodynamics. For. Ecol. Manag. 2023, 544, 121238. [Google Scholar] [CrossRef]
- Seidel, D.; Ehbrecht, M.; Puettmann, K. Assessing different components of three-dimensional forest structure with single-scan terrestrial laser scanning: A case study. For. Ecol. Manag. 2016, 381, 196–208. [Google Scholar] [CrossRef]
- Stiers, M.; Willim, K.; Seidel, D.; Ehbrecht, M.; Kabal, M.; Ammer, C.; Annighöfer, P. A quantitative comparison of the structural complexity of managed, lately unmanaged and primary European beech (Fagus sylvatica L.) forests. For. Ecol. Manag. 2018, 430, 357–365. [Google Scholar] [CrossRef]
- Stiers, M.; Annighöfer, P.; Seidel, D.; Willim, K.; Neudman, L.; Ammer, C. Quantifying the target state of forest stands managed with the continuous cover approach-revisiting Möller’s “Dauerwald” concept after 100 years. Trees For. People 2020, 1, 100004. [Google Scholar] [CrossRef]
- Camarretta, N.; Ehbrecht, M.; Seidel, D.; Wenzel, A.; Zuhdi, M.; Merk, M.S.; Schlund, M.; Erasmi, S.; Knohl, A. Using airborne laser scanning to characterize land-use systems in a tropical landscape based on vegetation structural metrics. Remote Sens. 2021, 13, 4794. [Google Scholar] [CrossRef]
- Helliwell, D.R. Dauerwald. For. Int. J. For. Res. 1997, 70, 375–379. [Google Scholar] [CrossRef]
- Looney, C.E.; D’Amato, A.W.; Jovan, S. Investigating linkages between the size-growth relationship and drought, nitrogen deposition, and structural complexity in western U.S. Forests. For. Ecol. Manag. 2021, 497, 119494. [Google Scholar] [CrossRef]
- Ahmed, S.; Hilmers, T.; Uhl, E.; Tupinambá-Simões, F.; Ordéñez, C.; Bravo, F.; del Río, M.; Peters, R.L.; Pretzsch, H. From suppressed to dominant: 3D crown shapes explain the “to grow or wait” growth behavior in close-to-nature forest. For. Ecol. Manag. 2025, 592, 122814. [Google Scholar] [CrossRef]
- Owen, H.J.F.; Lines, E.R. Common field measures and geometric assumptions of tree shape produce consistently biased estimates of tree and canopy structure in mixed Mediterranean forests. Ecol. Indicate. 2024, 165, 112219. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Lieffers, V.J. Inequality of size and size increment in Pinus banksiana in relation to stand dynamics and annual growth rate. Ann. Bot. 2007, 101, 561–571. [Google Scholar] [CrossRef]
- Masaki, T.; Mori, S.; Kajimoto, T.; Hitsuma, G.; Sawata, S.; Mori, M.; Osumi, K.; Sakurai, S.; Seki, T. Long-term growth analyses of Japanese cedar trees in a plantation: Neighborhood competition and persistence of initial growth deviations. J. For. Res. 2006, 11, 217–225. [Google Scholar] [CrossRef]
- Souza, A.F. Ecological interpretation of multiple population size structures in trees: The case of Araucaria angustifolia in south America. Austral Ecol. 2007, 32, 524–533. [Google Scholar] [CrossRef]
- Paludo, G.F.; Lauterjung, M.B.; dos Reis, M.S.; Mantovani, A. Inferring population trends Araucaria angustifolia (Araucariaceae) using a transition matrix model in an old-growth forest. S. For. A J. For. Sci. 2016, 78, 137–143. [Google Scholar] [CrossRef]
- Hess, A.F.; Demétrio, L.; de Sousa, A.N.; Costa, E.A.; Liesenberg, V.; Biffi, L.J.; Finger, C.A.G.; Borsoi, G.A.; Stepka, T.F.; de Ransoni, J.G.R.L.; et al. Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys. Sustainability 2024, 16, 3361. [Google Scholar] [CrossRef]
- McElhinny, C.; Gibbons, P.; Brack, C.; Bauhus, J. Forest and woodland stand structural complexity: Its definition and measurement. For. Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Oliveira, L.Z.; Vibrans, A.C. An approach to illustrate the naturalness of the Brazilian Araucaria forest. Can. J. For. Res. 2019, 50, 32–41. [Google Scholar] [CrossRef]
- Caliman, J.P.; Reis, G.G.; Reis, M.G.F.; Leite, H.G.; Torres, C.M.M.E.; Volpato, M.M.L.; Resende, R.T.; Monte, M.M. Temporal and spatial variability of the diameter distribution in a secondary Brazilian Atlantic forest suggest site-specific management pratices. Rev. Árvore 2020, 44, e4406. [Google Scholar] [CrossRef]
- Guignabert, A.; Jonard, M.; Messier, C.; André, F.; de Coligny, F.; Doyon, F.; Ponette, Q. Adaptive forest management improves stand-level resilience of temperate forest under multiple stressores. Sci. Total Environ. 2024, 948, 174168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).