Histological and Histochemical Analysis of Austrocedrus chilensis Trees Healthy and Infected with Phytophthora austrocedri
Abstract
1. Introduction
2. Materials and Methods
2.1. Saplings Inoculation
2.2. Naturally Infected Adult Trees
2.3. Sample Processing
2.4. Light-Microscopy Examination
- Safranin-Fast Green Staining: This method was applied following [29], with some modifications. The process begins by decolorizing the samples in sodium hypochlorite (0.4 g·L−1) for 3 min, followed by three rinses with distilled water. The samples are then dehydrated in 60% ethanol for 5 min, after which they are stained in a saturated solution of safranin in 80% ethanol for 5 min. The dehydration continues in 96% ethanol for another 5 min, and the samples are subsequently stained with a saturated solution of Fast Green in 96% ethanol for 10 s. Immediately after this, the samples are transferred to absolute ethanol (100%) for 10 s, completing the dehydration with xylene. Finally, the sections are mounted with clear acrylic and allowed to dry for 24 h at room temperature.
- Cotton Blue Staining: The slices are first decolorized using a 30% hydrogen peroxide solution for 1.5 h. They are then rinsed with distilled water 3 to 4 times before being immersed in a solution of Cotton Blue (0.05%) in lactophenol for 24 h. After staining, the slices are rinsed again with distilled water 3 to 4 times and subsequently mounted in lacto glycerol.
- Water Mounting: Mounted in distilled water without the use of any stain.
2.5. Histochemical Analyses
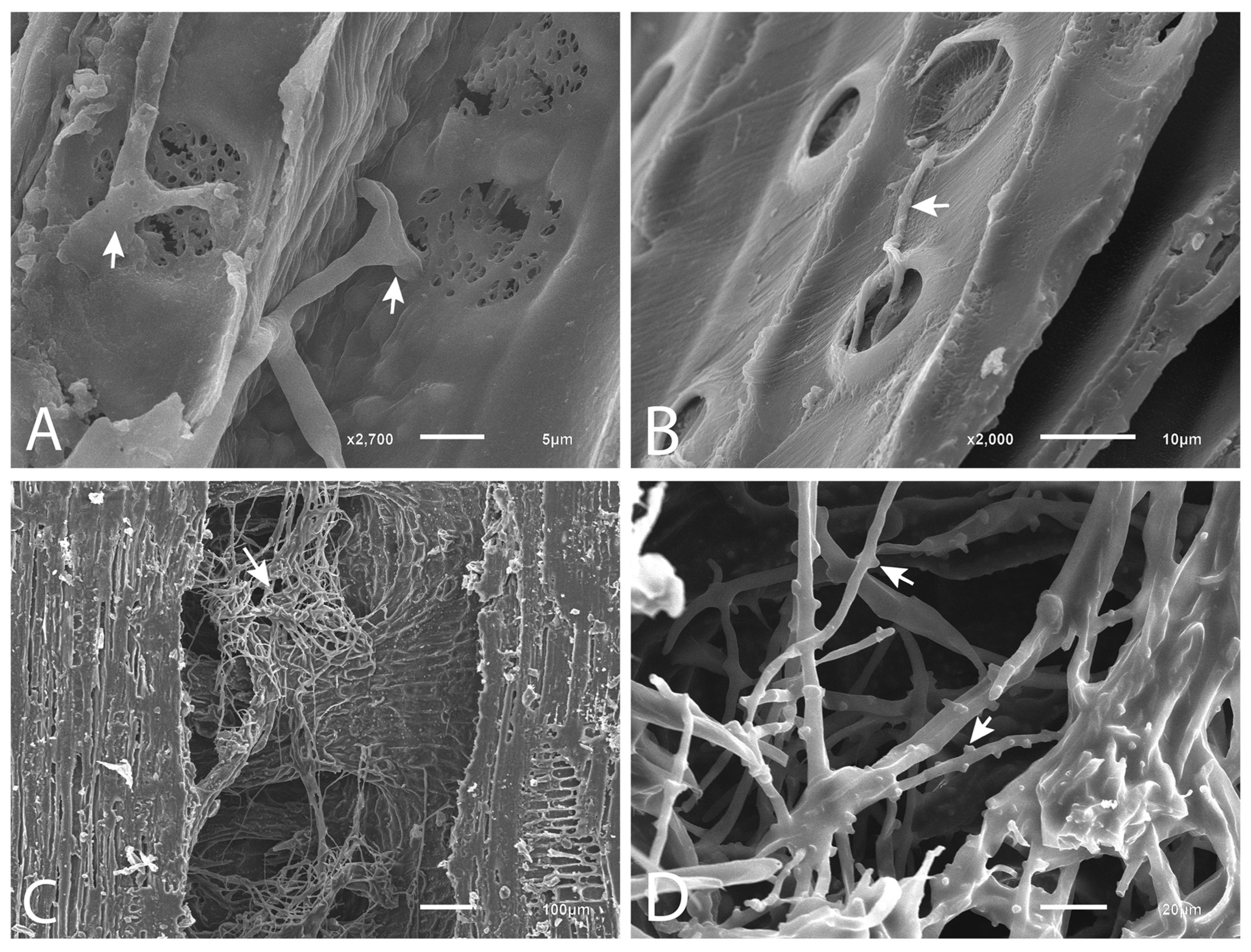
2.6. Scanning Electron Microscopy (SEM)
3. Results
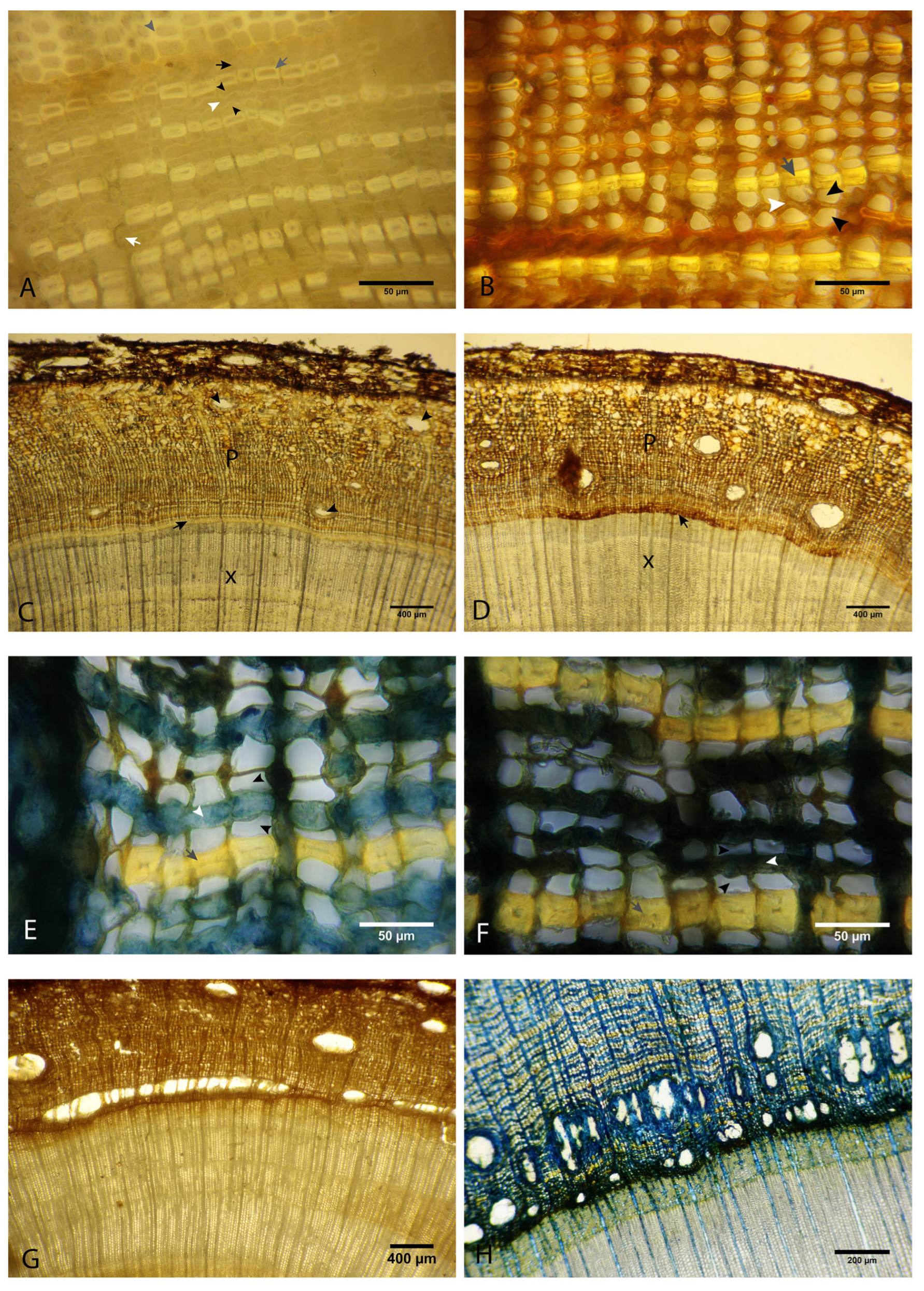
3.1. Histological Description of Healthy Tissues
3.2. Histological Analysis of Affected Stem Tissues of Saplings and Adult Trees
3.2.1. Pathogen Recovery from Affected Tissues
3.2.2. Saplings
3.2.3. Adult Trees
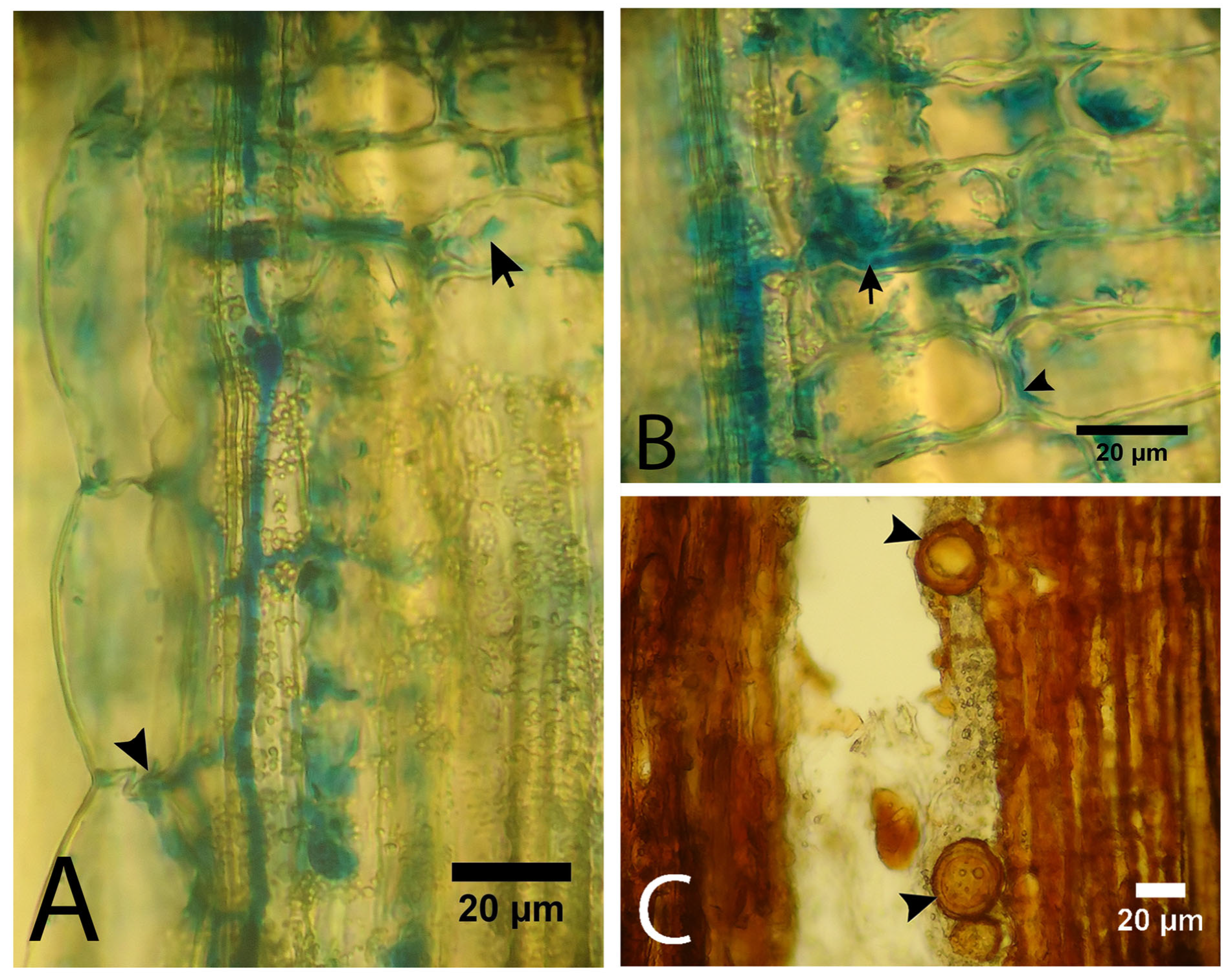
3.3. Presence of the Pathogen in the Tissues
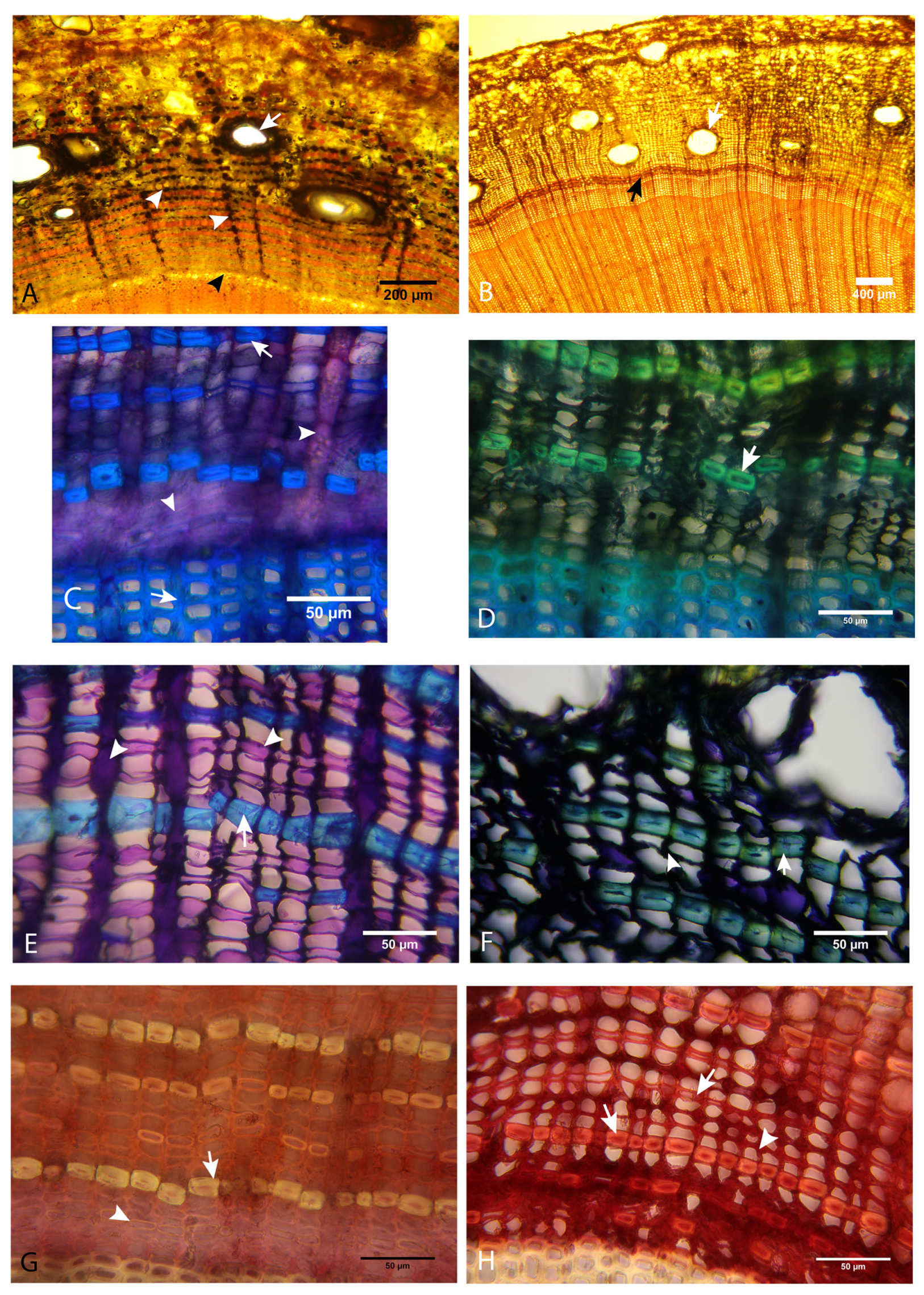
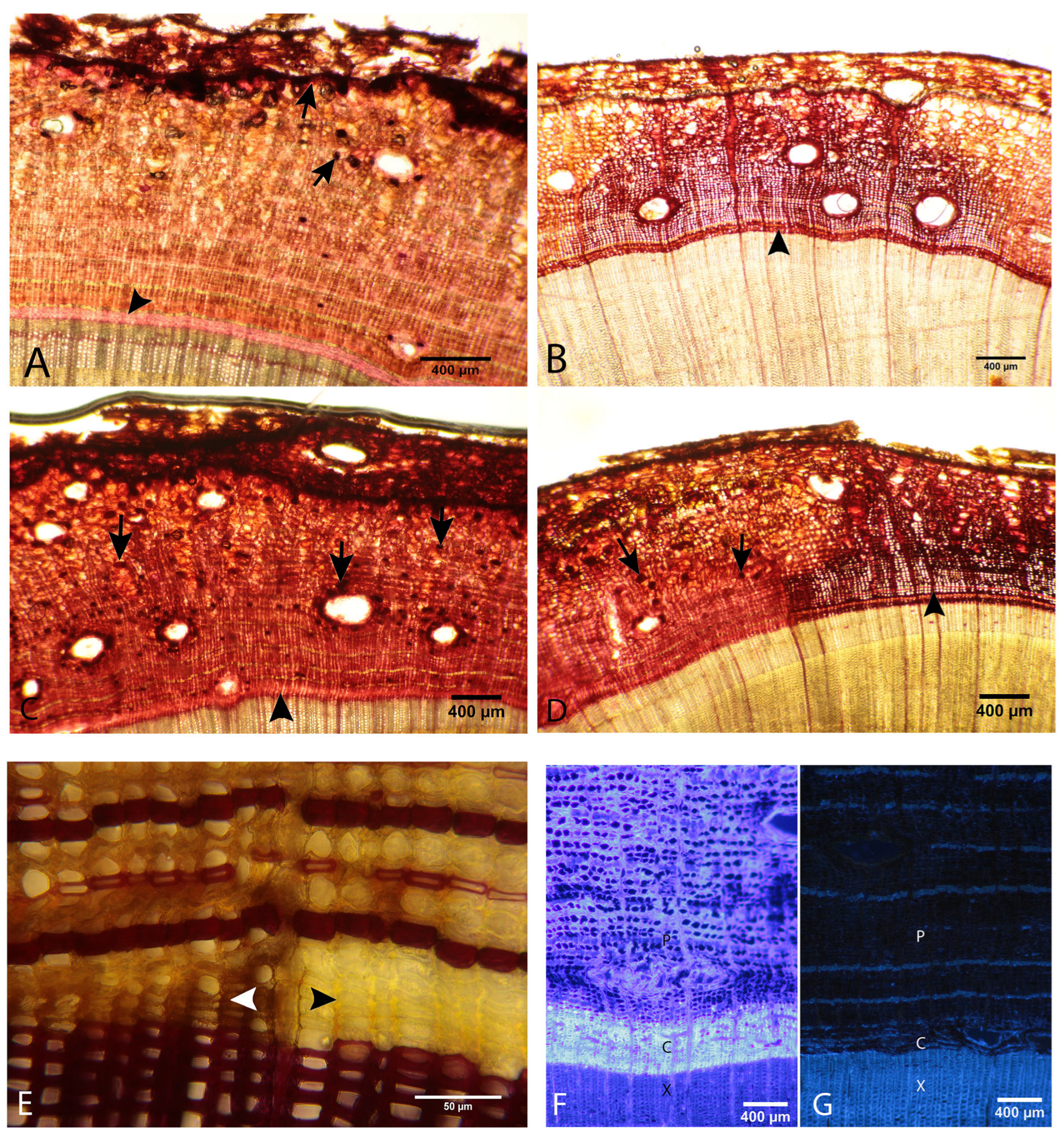
3.4. Histochemical Analysis
3.4.1. Evaluation of the Presence of Starch
3.4.2. Evaluation of the Presence of Phenolic Compounds
3.4.3. Evaluation of the Presence of Tannins
3.4.4. Evaluation of the Presence of Lignin
3.4.5. Histochemical Analysis with Epifluorescence
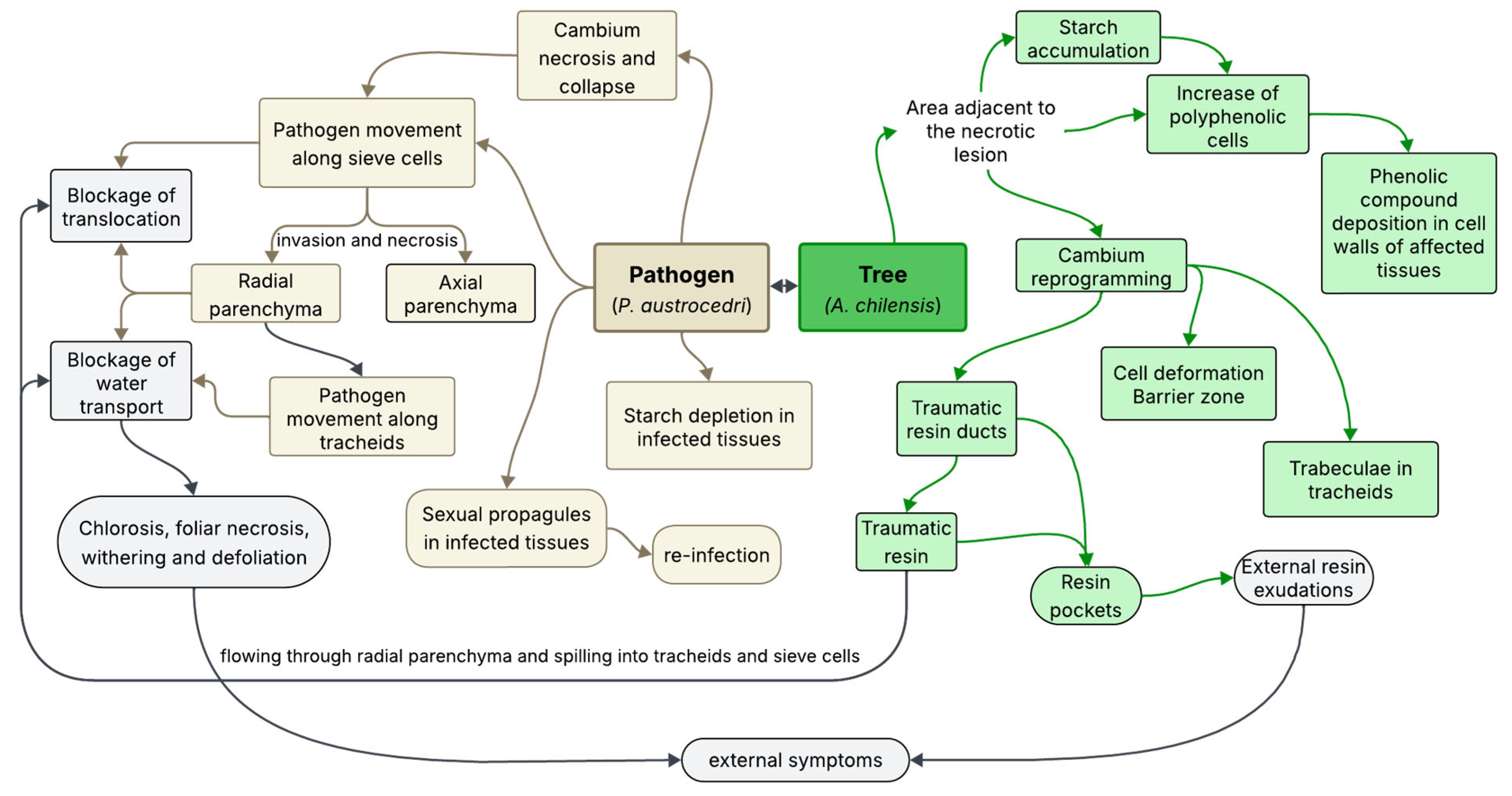
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greslebin, A.G.; Hansen, E.M.; Sutton, W. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia. Mycol. Res. 2007, 111, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Greslebin, A.G.; Hansen, E.M. Pathogenicity of Phytophthora austrocedrae on Austrocedrus chilensis and its relation with mal del ciprés in Patagonia. Plant Pathol. 2010, 59, 604–612. [Google Scholar] [CrossRef]
- Taccari, L.E.; Greslebin, A.G.; Salomón, M.E.S.; Vélez, M.L.; Santini, A. Two conifer species native to Patagonia threatened by Phytophthora austrocedri. For. Pathol. 2019, 49, e12496. [Google Scholar] [CrossRef]
- Green, S.; Elliot, M.; Armstrong, A.; Hendry, S.J. Phytophthora austrocedrae emerges as a serious threat to juniper (Juniperus communis) in Britain. Plant Pathol. 2015, 64, 456–466. [Google Scholar] [CrossRef]
- Werres, S.; Elliot, M.; Greslebin, A. Phytophthora austrocedrae Gresl. & E.M. Hansen. JKI Datasheets Plant Diseases and Diagnosis. 2014. Available online: https://forestphytophthoras.org/sites/default/files/educational_materials/3008-12007-1-PB.pdf (accessed on 20 June 2022).
- Mahdikhani, M.; Matinfar, M.; Aghaalikhani, A. First report of Phytophthora austrocedri causing phloem lesions and bronzing on Cupressus sempervirens in northern Iran. New Dis. Rep. 2017, 36, 10. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A Molecular Phylogeny of Phytophthora and Related Oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Olate, V.R.; Vélez, M.L.; Greslebin, A.; Schmeda-Hirschmann, G. Phytophthora austrocedri Elicitates Changes in Diterpene Profile of Austrocedrus chilensis. Molecules 2015, 20, 15084–15097. [Google Scholar] [CrossRef]
- Vélez, M.L.; Silva, P.V.; Troncoso, O.A.; Greslebin, A.G. Alteration of physiological parameters of Austrocedrus chilensis by the pathogen Phytophthora austrocedrae. Plant Pathol. 2012, 61, 877–888. [Google Scholar] [CrossRef]
- Gómez, F.J.R.; Navarro-Cerrillo, R.M.; Sánchez-Cuesta, R.; Pérez-De-Luque, A. Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathol. 2015, 64, 605–616. [Google Scholar] [CrossRef]
- Redondo, M.Á.; Pérez-Sierra, A.; Abad-Campos, P.; Torres, L.; Solla, A.; Reig-Armiñana, J.; García-Breijo, F. Histology of Quercus ilex roots during infection by Phytophthora cinnamomi. Trees 2015, 29, 1943–1957. [Google Scholar] [CrossRef]
- Fernandes, P.; Machado, H.; Silva, M.D.C.; Costa, R.L. A Histopathological Study Reveals New Insights Into Responses of Chestnut (Castanea spp.) to Root Infection by Phytophthora cinnamomi. Phytopathology 2021, 111, 345–355. [Google Scholar] [CrossRef]
- Nave, C.; Schwan, J.; Werres, S.; Riebesehl, J. Alnus glutinosa Threatened by Alder Phytophthora: A Histological Study of Roots. Pathogens 2021, 10, 977. [Google Scholar] [CrossRef]
- Vieites-Blanco, C.; Colangelo, M.; Camarero, J.J.; Caballol, M.; García Breijo, F.J.; Štraus, D.; Oliva, J. Pathogenicity of Phytophthora and Halophytophthora species on black alder and the host histological response. Mycol. Prog. 2023, 22, 71. [Google Scholar] [CrossRef]
- Brown, A.V.; Brasier, C.M. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathol. 2007, 56, 227–241. [Google Scholar] [CrossRef]
- Parke, J.L.; Oh, E.; Voelker, S.; Hansen, E.M.; Buckles, G.; Lachenbruch, B. Phytophthora ramorum Colonizes Tanoak Xylem and Is Associated with Reduced Stem Water Transport. Phytopathology 2007, 97, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, M.B.; Hansen, E.M.; Kitin, P. Histology of Phytophthora ramorum in Notholithocarpus densiflorus bark tissues. N. Z. J. For. Sci. 2011, 41, S89–S100. [Google Scholar]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Goodman, R.N.; Novacky, A.J. The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon; APS Press: St. Paul, MN, USA, 1994; 253p. [Google Scholar]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Jung, M.H.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant–Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Pieterse, C.M.; van Loon, L.C. Salicylic acid-independent plant defence pathways. Trends Plant Sci. 1999, 4, 52–58. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Franceschi, V.R. Methyl Jasmonate-Induced Ethylene Production Is Responsible for Conifer Phloem Defense Responses and Reprogramming of Stem Cambial Zone for Traumatic Resin Duct Formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P.; Nagy, N.E.; Solheim, H. Methyl jasmonate and oxalic acid treatment of Norway spruce: Anatomically based defense responses and increased resistance against fungal infection. Tree Physiol. 2008, 28, 29–35. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.E.; Franceschi, V.R.; Solheim, H.; Krekling, T.; Christiansen, E. Wound-induced traumatic resin duct development in stems of Norway spruce (Pinaceae): Anatomy and cytochemical traits. Am. J. Bot. 2000, 87, 302–313. [Google Scholar] [CrossRef] [PubMed]
- La Manna, L.; Carabelli, F.; Gómez, M.; Matteucci, S.D. Disposición espacial de parches de Austrocedrus chilensis con síntomas de defoliación y mortalidad en el Valle 16 de Octubre. Bosque 2008, 29, 23–32. [Google Scholar] [CrossRef]
- Tardif, J.C.; Conciatori, F. Microscopic examination of wood: Sample preparation and techniques for light microscopy. In Plant Microtechniques and Protocols; Yeung, E., Stasolla, C., Sumner, M., Huang, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 373–415. [Google Scholar] [CrossRef]
- D’Ambrosio de Argüeso, A. Manual de Técnicas en Histología Vegetal, 1st ed.; Ed Hemisferio Sur S.A: Buenos Aires, Argentina, 1986; 83p. [Google Scholar]
- Nava, L.R.; Isaías, A.Q.; Olvera, C.D.L.P.P. Comparación histoquímica de albura y duramen de tres especies de Quercus. Madera y Bosques 1999, 5, 27–41. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Slusarenko, A.J. Production of Salicylic Acid Precursors Is a Major Function of Phenylalanine Ammonia-Lyase in the Resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef]
- De Magistris, A.A.; Castro, M.A. Bark anatomy of southern South American Cupressaceae. IAWA J. 2001, 22, 367–383. [Google Scholar] [CrossRef]
- Castro, M.A. Cortezas. Especies Leñosas de los Bosques Andinos Patagónicos Argentina; LOLA: Buenos Aires, Argentina, 2009; 280p. [Google Scholar]
- Troncoso, O.; Greslebin, A. Trabeculae in Patagonian mountain cypress (Austrocedrus chilensis) associated with Phytophthora austrocedri infection. IAWA J. 2018, 39, 209–220. [Google Scholar] [CrossRef]
- Tippett, J.T.; Shea, S.R.; Hill, T.C.; Shearer, B.L. Development of Lesions Caused by Phytophthora cinnamomi in the Secondary Phloem of Eucalyptus marginata. Aust. J. Bot. 1983, 31, 197–210. [Google Scholar] [CrossRef]
- Davison, E.M.; Stukely, M.J.; Crane, C.E.; Tay, F.C.S. Invasion of phloem and xylem of woody stems and roots of Eucalyptus marginata and Pinus radiata by Phythophtora cinnamomi. Phytopathology 1994, 84, 335–340. [Google Scholar] [CrossRef]
- Oh, E.; Hansen, E.M. Histopathology of Infection and Colonization of Susceptible and Resistant Port-Orford-Cedar by Phytophthora lateralis. Phytopathology 2007, 97, 684–693. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Christiansen, E.; Franceschi, V.R. Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: A phylogenetic perspective. Tree Physiol. 2004, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, F.; Werres, S. Histological studies of Phytophthora ramorum in Rhododendron twigs. Can. J. Bot. 2004, 82, 1481–1489. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krekling, T.; Berryman, A.A.; Christiansen, E. Specialized phloem parenchyma cells in Norway spruce (Pinaceae) bark are an important site of defense reactions. Am. J. Bot. 1998, 85, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Ockels, F.S.; Eyles, A.; McPherson, B.A.; Wood, D.L.; Bonello, P. Phenolic Chemistry of Coast Live Oak Response to Phytophthora ramorum Infection. J. Chem. Ecol. 2007, 33, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.M.; Mcpherson, B.A.; Wood, D.L.; Garbelotto, M.; Bonello, P. Relationship between field resistance to Phytophthora ramorum and constitutive phenolic chemistry of coast live oak. For. Pathol. 2011, 41, 464–469. [Google Scholar] [CrossRef]
- Philips, D.; Grant, B.R.; Weste, G. Histological changes in the roots of an avocado cultivar, Duke 7, infected with Phytophthora cinnamomi. Phytopathology 1986, 77, 691–698. [Google Scholar] [CrossRef]
- Repka, V.; Fischerova, I.; Silharova, K. Methyl jasmonate induces a hypersensitive-like response of grapevine in the absence of avirulent pathogens. Vitis-Geilweilerhof 2001, 40, 5–10. [Google Scholar]
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl Jasmonate is a Potent Elicitor of Multiple Defense Responses in Grapevine Leaves and Cell-Suspension Cultures. Biol. Plant. 2004, 48, 273–283. [Google Scholar] [CrossRef]
- Rao, M.V.; Lee, H.-I.; Creelman, R.A.; Mullet, J.E.; Davis, K.R. Jasmonic Acid Signaling Modulates Ozone-Induced Hypersensitive Cell Death. Plant Cell 2000, 12, 1633–1646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andi, S.; Taguchi, F.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. Effect of Methyl Jasmonate on Harpin-Induced Hypersensitive Cell Death, Generation of Hydrogen Peroxide and Expression of PAL mRNA in Tobacco Suspension Cultured BY-2 Cells. Plant Cell Physiol. 2001, 42, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, A.E.; Patiño, L.F.; Morales, J.G. Histological changes induced during the biotrophic phase of infection of three potato varieties by Phytophthora infestans (Mont.) de Bary. J. Plant Prot. Res. 2019, 59, 465–478. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troncoso, O.; Greslebin, A.G. Histological and Histochemical Analysis of Austrocedrus chilensis Trees Healthy and Infected with Phytophthora austrocedri. Forests 2025, 16, 1073. https://doi.org/10.3390/f16071073
Troncoso O, Greslebin AG. Histological and Histochemical Analysis of Austrocedrus chilensis Trees Healthy and Infected with Phytophthora austrocedri. Forests. 2025; 16(7):1073. https://doi.org/10.3390/f16071073
Chicago/Turabian StyleTroncoso, Oscar, and Alina G. Greslebin. 2025. "Histological and Histochemical Analysis of Austrocedrus chilensis Trees Healthy and Infected with Phytophthora austrocedri" Forests 16, no. 7: 1073. https://doi.org/10.3390/f16071073
APA StyleTroncoso, O., & Greslebin, A. G. (2025). Histological and Histochemical Analysis of Austrocedrus chilensis Trees Healthy and Infected with Phytophthora austrocedri. Forests, 16(7), 1073. https://doi.org/10.3390/f16071073





