Soil Biogeochemical Feedback to Fire in the Tropics: Increased Nitrification and Denitrification Rates and N2O Emissions Linked to Labile Carbon and Nitrogen Fractions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Soil Properties
2.3. DNA Extraction and Quantitative PCR Analyses
2.4. Soil N2O Measurements
2.5. Statistical Analyses
3. Results
3.1. Soil Physicochemical Properties
3.2. N-Cycling Functional Gene Abundances
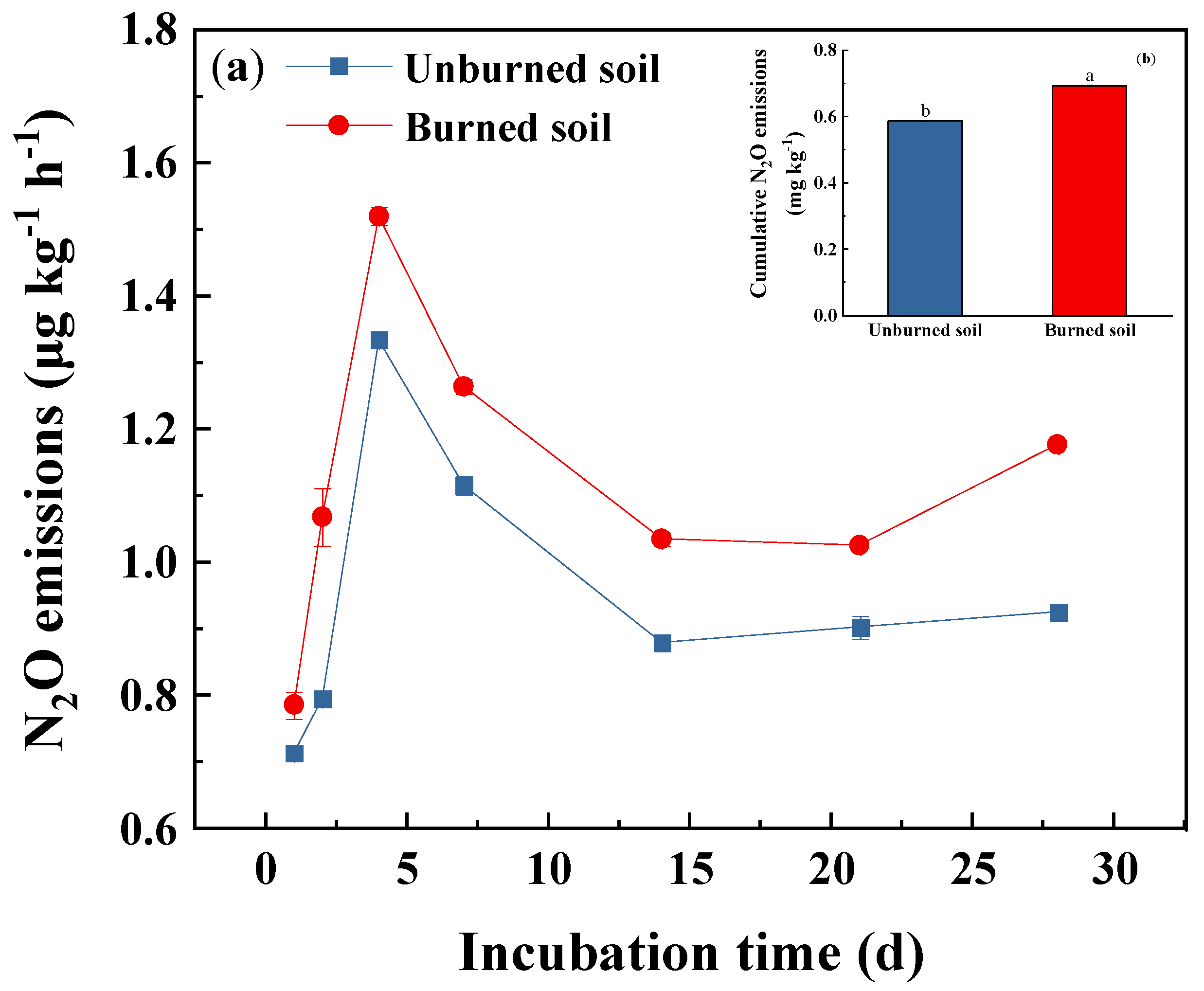
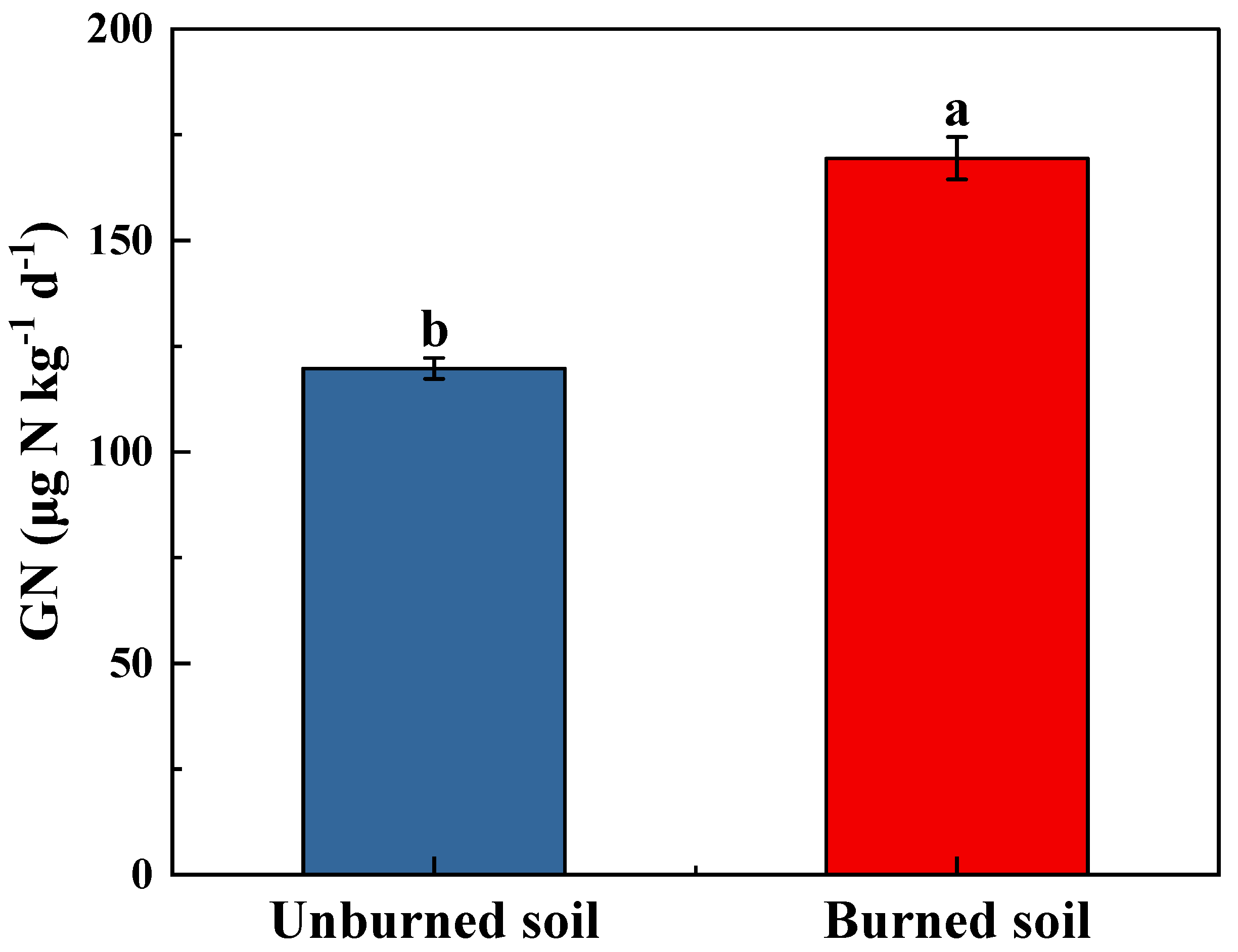
3.3. Soil N2O Fluxes and Gross Nitrification Rate
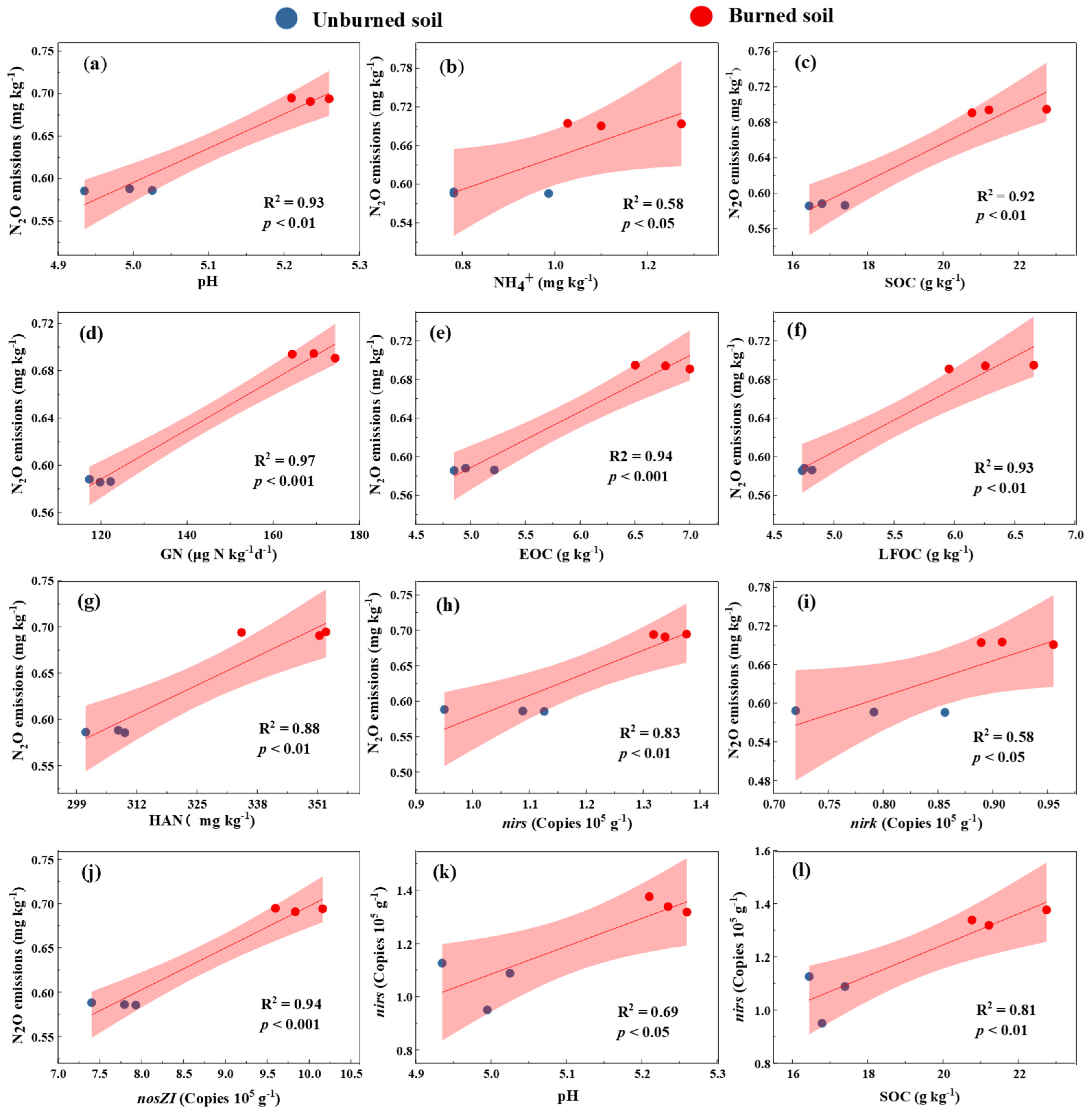
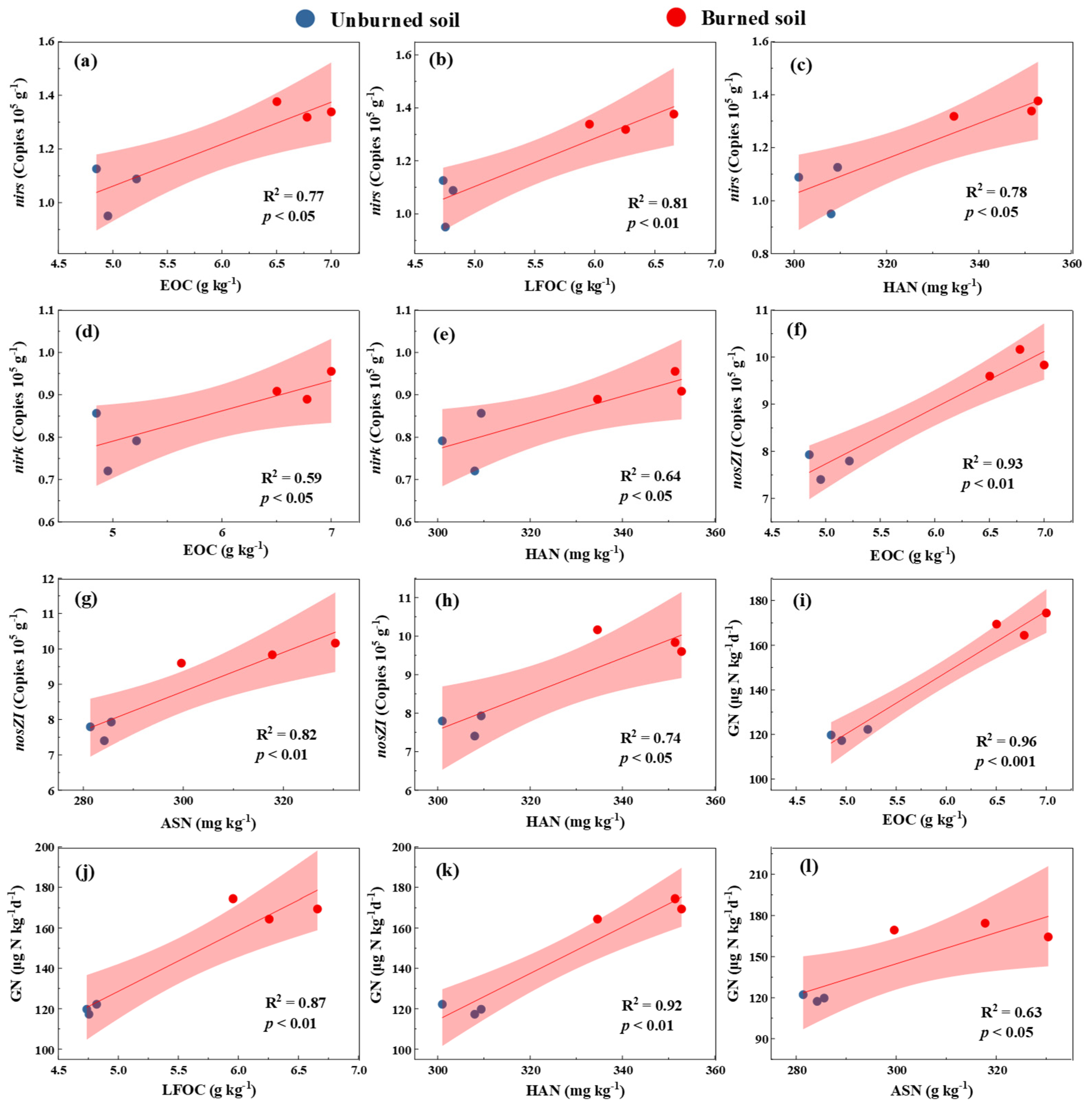
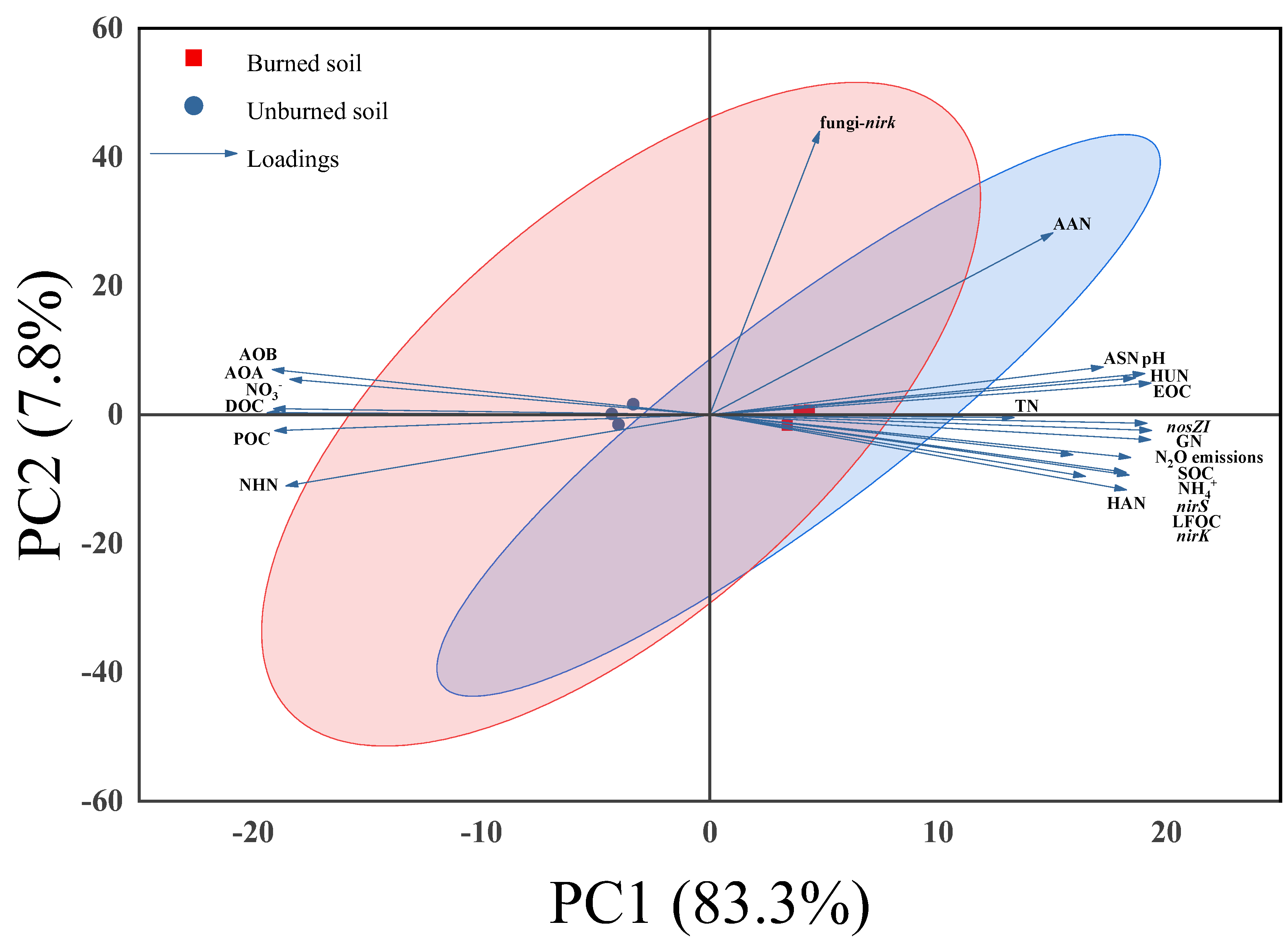
3.4. Relationships Among Studied Variables
4. Discussion
4.1. Fire Increased Soil NO3− Production
4.2. Fire Stimulated N2O Emissions by Increasing GN and Affecting C and N Fractions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lizundia-Loiola, J.; Oton, G.; Ramo, R.; Chuvieco, E. A spatio-temporal active-fire clustering approach for global burned area mapping at 250 m from MODIS data. Remote Sens. Environ. 2020, 236, 111493. [Google Scholar] [CrossRef]
- Butler, O.M.; Elser, J.J.; Lewis, T.; Mackey, B.; Chen, C. The phosphorus-rich signature of fire in the soil-plant system: A global meta-analysis. Ecol. Lett. 2018, 21, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.F.A.; Ahlstrom, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Alonso-Canas, I.; Chuvieco, E. Global burned area mapping from ENVISAT-MERIS and MODIS active fire data. Remote Sens. Environ. 2015, 163, 140–152. [Google Scholar] [CrossRef]
- Blunden, M.H.; Florence, L.; Bryceson, S.R.; Clark, G.J.; Morgan, J.W.; Wood, J.L. Fire regime impacts on soil microbes, soil organic carbon and ground cover in an Australian tropical savanna. Int. J. Wildland Fire 2024, 33, 12. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Marfella, L.; Mairota, P.; Marzaioli, R.; Glanville, H.C.; Pazienza, G.; Rutigliano, F.A. Long-term impact of wildfire on soil physical, chemical and biological properties within a pine forest. Eur. J. For. Res. 2024, 143, 1379–1399. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, C.; Li, Q.; Lin, W. Isotope signatures of N2O emitted from vegetable soil: Ammonia oxidation drives N2O production in NH4+-fertilized soil of North China. Sci. Rep. 2016, 6, 29257. [Google Scholar] [CrossRef]
- Arai, H.; Hadi, A.; Darung, U.; Limin, S.H.; Takahashi, H.; Hatano, R.; Inubushi, K. Land use change affects microbial biomass and fluxes of carbon dioxide and nitrous oxide in tropical peatlands. Soil Sci. Plant Nutr. 2014, 60, 423–434. [Google Scholar] [CrossRef]
- Guetlein, A.; Gerschlauer, F.; Kikoti, I.; Kiese, R. Impacts of climate and land use on N2O and CH4 fluxes from tropical ecosystems in the Mt. Kilimanjaro region, Tanzania. Glob. Change Biol. 2018, 24, 1239–1255. [Google Scholar] [CrossRef]
- Krishna, M.; AchutaRao, B.A.N.; Aldrian, E.; Armour, K. Climate Change 2021: The Physical Science Basis. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 8 June 2025).
- Metcalfe, D.B.; Rocha, W.; Balch, J.K.; Brando, P.M.; Doughty, C.E.; Malhi, Y. Impacts of fire on sources of soil CO2 efflux in a dry Amazon rain forest. Glob. Change Biol. 2018, 24, 3629–3641. [Google Scholar] [CrossRef]
- Hu, M.; Song, J.; Li, S.; Li, Z.; Hao, Y.; Di, M.; Wan, S. Understanding the effects of fire and nitrogen addition on soil respiration of a field study by combining observations with a meta-analysis. Agric. For. Meteorol. 2020, 292, 108106. [Google Scholar] [CrossRef]
- Akande, O.J.; Ma, Z.; Huang, C.; He, F.; Chang, S.X. Meta-analysis shows forest soil CO2 effluxes are dependent on the disturbance regime and biome type. Ecol. Lett. 2023, 26, 765–777. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, J.; Liu, L.; El-Tarabily, K.A.; Uwiragiye, Y.; Dan, X.; Tang, S.; Wu, Y.; Zhu, T.; Meng, L.; et al. Fire Reduces Soil Nitrate Retention While Increasing Soil Nitrogen Production and Loss Globally. Environ. Sci. Technol. 2024, 58, 23004–23017. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Feng, H.; Peng, C.; Du, J.; Wang, W.; Kneeshaw, D.; Pan, C.; Roberge, G.; Feng, L.; Chen, A. Fire effects on soil CH4 and N2O fluxes across terrestrial ecosystems. Sci. Total Environ. 2024, 948, 174708. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.Z.; Homyak, P.M. Post-fire soil emissions of nitric oxide (NO) and nitrous oxide (N2O) across global ecosystems: A review. Biogeochemistry 2023, 165, 291–309. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, L.; Wang, L.; Qian, H.; Hou, C.; Wen, S.; Wang, B.; Huang, Q.; Chen, W. Abundance for subgroups of denitrifiers in soil aggregates asscociates with denitrifying enzyme activities under different fertilization regimes. Appl. Soil Ecol. 2021, 166, 103983. [Google Scholar] [CrossRef]
- Bahram, M.; Espenberg, M.; Parn, J.; Lehtovirta-Morley, L.; Anslan, S.; Kasak, K.; Koljalg, U.; Liira, J.; Maddison, M.; Moora, M.; et al. Structure and function of the soil microbiome underlying N2O emissions from global wetlands. Nat. Commun. 2022, 13, 1430. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, K.T. Wildfires May Alter the Nitrogen Cycle—And Air Pollution. Eos 2021, 102. [Google Scholar] [CrossRef]
- Srikanthasamy, T.; Barot, S.; Koffi, F.K.; Tambosco, K.; Marcangeli, Y.; Carmignac, D.; N’Dri, A.B.; Gervaix, J.; Le Roux, X.; Lata, J.-C. Short-term impact of fire on the total soil microbial and nitrifier communities in a wet savanna. Ecol. Evol. 2021, 11, 9958–9969. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Fu, B.; Wang, Q.; Xu, M.; Wang, H.; Yang, F.; Liu, G. Effects of land-use change and fertilization on N2O and NO fluxes, the abundance of nitrifying and denitrifying microbial communities in a hilly red soil region of southern China. Appl. Soil Ecol. 2017, 120, 111–120. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Q.; Yang, Y.; Yuan, M.; Ma, X.; Chiariello, N.R.; Docherty, K.M.; Field, C.B.; Gutknecht, J.L.; Hungate, B.A. Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning. Glob. Change Biol. 2020, 26, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhan, X.; Tigabu, M.; He, Y.; Li, Z.; Wang, G.; Guo, F. Rhizosphere soil microbial communities and nitrogen transformation response to forest fire smoke. Appl. Soil Ecol. 2025, 208, 105990. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Xu, X.; Luo, Y.; Chen, C.; Ju, C.; Chen, H.Y.H.; Shi, Z.; Ruan, H. Carbon and nitrogen dynamics in tropical ecosystems following fire. Glob. Ecol. Biogeogr. 2022, 31, 378–391. [Google Scholar] [CrossRef]
- Duan, P.P.; Zhou, J.; Feng, L.; Jansen-Willems, A.B.; Xiong, Z.Q. Pathways and controls of N2O production in greenhouse vegetable production soils. Biol. Fertil. Soils 2019, 55, 285–297. [Google Scholar] [CrossRef]
- Malghani, S.; Yoo, G.Y.; Giesemann, A.; Well, R.; Kang, H. Combined application of organic manure with urea does not alter the dominant biochemical pathway producing N2O from urea treated soil. Biol. Fertil. Soils 2020, 56, 331–343. [Google Scholar] [CrossRef]
- Wang, N.; Luo, J.-L.; Juhasz, A.L.; Li, H.-B.; Yu, J.-G. Straw decreased N2O emissions from flooded paddy soils via altering denitrifying bacterial community compositions and soil organic carbon fractions. Fems Microbiol. Ecol. 2020, 96, fiaa046. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Wang, C.; Wan, Y.; Yang, R.; Mou, J.; Liu, J.; Wu, Y.; Tang, S.; Zhu, T.; et al. Carbon and nitrogen fractions control soil N2O emissions and related functional genes under land-use change in the tropics. Environ. Pollut. 2023, 335, 122370. [Google Scholar] [CrossRef]
- Lan, J. Responses of soil organic carbon components and their sensitivity to karst rocky desertification control measures in Southwest China. J. Soils Sediments 2021, 21, 978–989. [Google Scholar] [CrossRef]
- Han, X.; Liu, X.; Li, Z.; Li, J.; Yuan, Y.; Li, H.; Zhang, L.; Liu, S.; Wang, L.; You, C.; et al. Characteristics of Soil Organic Carbon Fractions and Stability along a Chronosequence of Cryptomeria japonica var. sinensis Plantation in the Rainy Area of Western China. Forests 2022, 13, 1663. [Google Scholar] [CrossRef]
- Lu, H.; He, H.; Zhao, J.; Zhang, W.; Xie, H.; Hu, G.; Liu, X.; Wu, Y.; Zhang, X. Dynamics of fertilizer-derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil 2013, 373, 595–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhou, C.; Lei, P.; Sootahar, M.K.; Ni, J. Organic and Inorganic Nitrogen forms as Affected by Phosphorus Additions in a Maize-Mustard Cropping System. Clean-Soil Air Water 2022, 50, 2100240. [Google Scholar] [CrossRef]
- Taikui, L.; Zhanling, G.; Changlin, K.; Jinling, L.; Xiangning, Z.; Xiaolin, Y. Effects of Extraction Conditions on the Test Results of Soil Dissolved Organic Carbon. J. Ecol. Environ. Sci. 2017, 26, 1878. [Google Scholar] [CrossRef]
- Xuesong, G.; Peng, H.; Liangji, D.; Shirong, Z.; Chun, H. Distribution characteristics of soil total organic carbon and particle organic carbon on the purple hilly region slopes. J. Ecol. Environ. Sci. 2009, 18, 337. [Google Scholar] [CrossRef]
- Bingyan, L.; Haiyan, X.; Xiaodong, W.; Biying, C.; Zipeng, G.; Shouye, X. Differences of Soil Organic Carbon Components in Different Grassland Types of Qilian Mountain. J. Earth Sci. 2024, 49, 1487–1497. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Y.; Liu, D.; Zhang, S.; Lan, B.; He, L.; Yu, Z.; Zhou, S.; Chen, X.; Qu, Y. Characteristics of organic nitrogen fractions in sediments of the water level fluctuation zone in the tributary of the Yangtze River. Sci. Total Environ. 2019, 653, 327–333. [Google Scholar] [CrossRef]
- Wang, D.; Sheng, K.; Zhao, W.; Li, L.; Zhang, Q.; Wang, Y. Dominance of archaeal ammonia-oxidizers in soil nitrification across different soil types and fertilities in North China plain. Eur. J. Soil Biol. 2021, 106, 103354. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Taylor, A.E.; Bakken, L.R.; Bottomley, P.J.; Myrold, D.D.; Dorsch, P. Relative activity of ammonia oxidizing archaea and bacteria determine nitrification-dependent N2O emissions in Oregon forest soils. Soil Biol. Biochem. 2019, 139, 107612. [Google Scholar] [CrossRef]
- Elrys, A.S.; Ali, A.; Zhang, H.M.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Müller, C.; Chang, S.X. Patterns and drivers of global gross nitrogen mineralization in soils. Glob. Change Biol. 2021, 27, 5950–5962. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Liu, J.; Wu, J.; Li, B.; Fang, C.; Nie, M. Spatiotemporal variability of fire effects on soil carbon and nitrogen: A global meta-analysis. Glob. Change Biol. 2021, 27, 4196–4206. [Google Scholar] [CrossRef]
- Meng, C.; Liu, Y.; Su, W.; Zhang, J.; Luo, Y.; Zhang, Q.; Di, H.; Tang, C.; Xu, J.; Li, Y. Nutrient availability and microbial traits constrained by soil texture modulate the impact of forest fire on gross nitrogen mineralization. For. Ecol. Manag. 2023, 541, 121067. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, H.; Afzal, M.R.; Gao, X.; Li, Y.; Subbarao, G.V.; Zhu, Y. BNI-release mechanisms in plant root systems: Current status of understanding. Biol. Fertil. Soils 2022, 58, 225–233. [Google Scholar] [CrossRef]
- Subbarao, G.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.; Hash, C.; George, T.; Rao, P.S.; Nardi, P. Biological nitrification inhibition—A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.a.-B.; Alef, K. Amino sugars and muramic acid—Biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Hassan, W.; Bashir, S.; Ahmed, N.; Tanveer, M.; Shah, A.N.; Bano, R.; David, J. Labile Organic Carbon Fractions, Regulator of CO2 Emission: Effect of Plant Residues and Water Regimes. Clean-Soil Air Water 2016, 44, 1358–1367. [Google Scholar] [CrossRef]
- Inatomi, M.; Hajima, T.; Ito, A. Fraction of nitrous oxide production in nitrification and its effect on total soil emission: A meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 2019, 14, e0219159. [Google Scholar] [CrossRef]
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Change Biol. 2020, 26, 103–118. [Google Scholar] [CrossRef]
- Verhamme, D.T.; Prosser, J.I.; Nicol, G.W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef]
- Rutting, T.; Schleusner, P.; Hink, L.; Prosser, J.I. The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biol. Biochem. 2021, 160, 108353. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, G.; Zhang, J.; Chen, Y.; Yu, M.; Lu, L.; Li, H.; Zhu, Y.; Yuan, Y.; Huang, A.; et al. Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl. Microbiol. Biotechnol. 2015, 99, 4059–4070. [Google Scholar] [CrossRef]
- Li, X.; Qiao, J.; Li, S.; Haggblom, M.M.; Li, F.; Hu, M. Bacterial Communities and Functional Genes Stimulated During Anaerobic Arsenite Oxidation and Nitrate Reduction in a Paddy Soil. Environ. Sci. Technol. 2020, 54, 2172–2181. [Google Scholar] [CrossRef]
- van Lent, J.; Hergoualc’h, K.; Verchot, L.V. Reviews and syntheses: Soil N2O and NO emissions from land use and land-use change in the tropics and subtropics: A meta-analysis. Biogeosciences 2015, 12, 7299–7313. [Google Scholar] [CrossRef]
- Long, X.-E.; Huang, Y.; Chi, H.; Li, Y.; Ahmad, N.; Yao, H. Nitrous oxide flux, ammonia oxidizer and denitrifier abundance and activity across three different landfill cover soils in Ningbo, China. J. Clean. Prod. 2018, 170, 288–297. [Google Scholar] [CrossRef]
- Gao, W.; Yao, Y.; Gao, D.; Wang, H.; Song, L.; Sheng, H.; Cai, T.; Liang, H. Responses of N2O emissions to spring thaw period in a typical continuous permafrost region of the Daxing’an Mountains, northeast China. Atmos. Environ. 2019, 214, 116822. [Google Scholar] [CrossRef]
- Garcia-Ruiz, R.; Gomez-Munoz, B.; Hatch, D.J.; Bol, R.; Baggs, E.M. Soil mineral N retention and N2O emissions following combined application of 15N-labelled fertiliser and weed residues. Rapid Commun. Mass Spectrom. 2012, 26, 2379–2385. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, Y.; Guo, D.; Wang, Q.; Bao, Y.; Li, L. Effects of Multi-nutrient Additions on GHG Fluxes in a Temperate Grassland of Northern China. Ecosystems 2014, 17, 657–672. [Google Scholar] [CrossRef]
- Adkins, J.; Miesel, J.R. Post-fire effects of soil heating intensity and pyrogenic organic matter on microbial anabolism. Biogeochemistry 2021, 154, 555–571. [Google Scholar] [CrossRef]
- Liu, F.; Qin, S.; Fang, K.; Chen, L.; Peng, Y.; Smith, P.; Yang, Y. Divergent changes in particulate and mineral-associated organic carbon upon permafrost thaw. Nat. Commun. 2022, 13, 5073. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wu, Z.; Shen, Y.; Cai, Y. Effect of annual prescribed burning of wetlands on soil organic carbon fractions: A 5-year study in Poyang, China. Ecol. Eng. 2019, 138, 219–226. [Google Scholar] [CrossRef]
- Timothy, D.A.; Moses, I.; Johnstone, K.; Nekesa, O.A. Effects of wildland fire on the tropical alpine moorlands of Mount Kenya. Catena 2017, 149, 300–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Zheng, X.; Ren, X.; Cardenas, L.; Carswell, A.; Misselbrook, T. Land-use type affects N2O production pathways in subtropical acidic soils. Environ. Pollut. 2018, 237, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Koegel-Knabner, I.; Schulze, E.D. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef]
- Trotta, G.; Cadez, L.; Boscutti, F.; Vuerich, M.; Asquini, E.; Boscarol, G.; Cingano, P.; Azzani, G.; Pischedda, S.; Tomao, A.; et al. Interpreting the shifts in forest structure, plant community composition, diversity, and functional identity by using remote sensing-derived wildfire severity. Fire Ecol. 2024, 20, 94. [Google Scholar] [CrossRef]
- Penman, T.D.; Towerton, A. Soil temperatures during autumn prescribed burning: Implications for the germination of fire responsive species? Int. J. Wildland Fire 2008, 17, 572–578. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Stocks, B.J. Fire, Climate Change, and Carbon Cycling in the Boreal Forest; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 138. [Google Scholar]

| Parameter | Unburned Soil | Burned Soil |
|---|---|---|
| pH | 4.99 ± 0.05 b | 5.24 ± 0.03 a |
| TN (g kg−1) | 1.26 ± 0.00 a | 1.29 ± 0.03 a |
| NH4+ (mg kg−1) | 0.85 ± 0.12 b | 1.13 ± 0.13 a |
| NO3− (mg kg−1) | 6.87 ± 0.07 a | 6.36 ± 0.05 b |
| SOC (g kg−1) | 16.9 ± 0.48 b | 21.6 ± 1.04 a |
| EOC (g kg−1) | 5.01 ± 0.19 b | 6.76 ± 0.25 a |
| DOC (g kg−1) | 0.15 ± 0.00 a | 0.14 ± 0.00 b |
| LFOC (g kg−1) | 4.77 ± 0.04 b | 6.29 ± 0.35 a |
| POC (g kg−1) | 1.42 ± 0.04 a | 0.93 ± 0.09 b |
| ASN (mg kg−1) | 284 ± 2.14 b | 316 ± 15.5 a |
| HUN (mg kg−1) | 63.5 ± 16.7 b | 117 ± 4.92 a |
| AAN (mg kg−1) | 182 ± 21.0 a | 215 ± 17.6 a |
| HAN (mg kg−1) | 306 ± 4.50 b | 346 ± 10.1 a |
| NHN (mg kg−1) | 425 ± 33.1 a | 297 ± 25.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, M.; Yatoo, A.M.; Zhang, R.; Feng, J.; Sun, X.; Wan, Y.; Wen, Y.; Wu, Y.; He, Q.; Meng, L.; et al. Soil Biogeochemical Feedback to Fire in the Tropics: Increased Nitrification and Denitrification Rates and N2O Emissions Linked to Labile Carbon and Nitrogen Fractions. Forests 2025, 16, 983. https://doi.org/10.3390/f16060983
Kong M, Yatoo AM, Zhang R, Feng J, Sun X, Wan Y, Wen Y, Wu Y, He Q, Meng L, et al. Soil Biogeochemical Feedback to Fire in the Tropics: Increased Nitrification and Denitrification Rates and N2O Emissions Linked to Labile Carbon and Nitrogen Fractions. Forests. 2025; 16(6):983. https://doi.org/10.3390/f16060983
Chicago/Turabian StyleKong, Mengru, Ali Mohd Yatoo, Rui Zhang, Junjie Feng, Xiaomeng Sun, Yunxing Wan, Yuhong Wen, Yanzheng Wu, Qiuxiang He, Lei Meng, and et al. 2025. "Soil Biogeochemical Feedback to Fire in the Tropics: Increased Nitrification and Denitrification Rates and N2O Emissions Linked to Labile Carbon and Nitrogen Fractions" Forests 16, no. 6: 983. https://doi.org/10.3390/f16060983
APA StyleKong, M., Yatoo, A. M., Zhang, R., Feng, J., Sun, X., Wan, Y., Wen, Y., Wu, Y., He, Q., Meng, L., Zhang, J., & Elrys, A. S. (2025). Soil Biogeochemical Feedback to Fire in the Tropics: Increased Nitrification and Denitrification Rates and N2O Emissions Linked to Labile Carbon and Nitrogen Fractions. Forests, 16(6), 983. https://doi.org/10.3390/f16060983







