Using Douglas Fir and European Larch Needles for the Assessment of Their Retention Capacity for Atmospheric Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Statistical Analysis
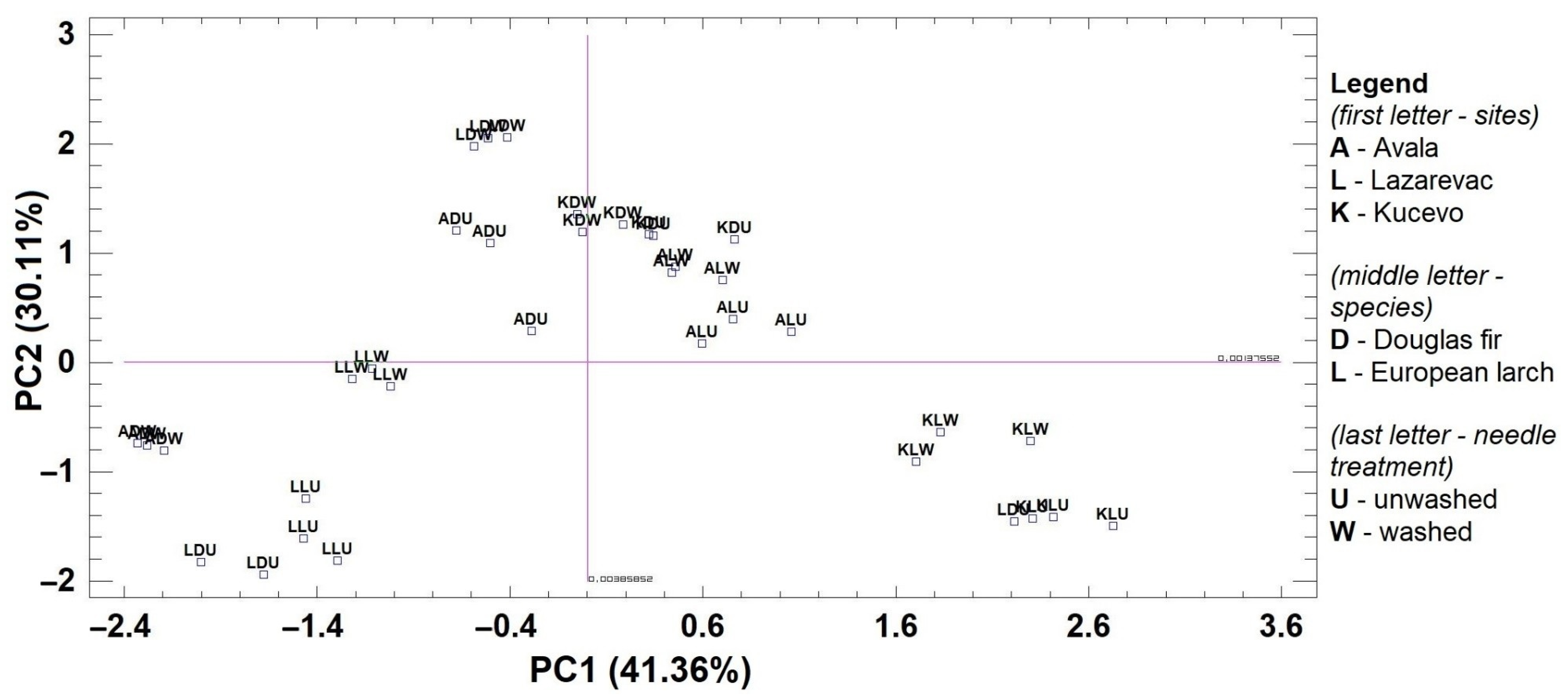
3. Results
4. Discussion
5. Conclusions
- −
- Considering the content of almost all the analyzed heavy metals, Lazarevac was the most polluted site for both species studied, confirming the main hypothesis of this study;
- −
- The concentrations of all the heavy metals analyzed at the researched sites for both species were within the maximum allowable limits, apart from nickel (Ni), the concentration of which at the very polluted area of Lazarevac was slightly over the usual limit values;
- −
- Higher contents of almost all the heavy metals analyzed, apart from (Ni), were found in the unwashed compared to the washed needles;
- −
- The content of cadmium (Cd) was higher in the unwashed EL needles than in the unwashed DF needles;
- −
- The content of copper (Cu) and lead (Pb) was higher in the unwashed compared to the washed DF needles, and the same applied to the Pb content in the EL needles;
- −
- EL has a greater potential to accumulate cobalt (Co) than DF on polluted sites;
- −
- Considering the calculated values of the predictive foliar metal accumulation index (MAI), DF could be a good planting choice in urban areas where heavy metals contamination is significant.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Urošević, J.; Stanković, D.; Jokanović, D.; Trivan, G.; Rodzkin, A.; Jović, Đ.; Jovanović, F. Phytoremediation Potential of Different Genotypes of Salix alba and S. viminalis. Plants 2024, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Arsenov, D.; Župunski, M.; Borišev, M.; Nikolič, N.; Orlović, S.; Pilipović, A.; Pajevič, S. Exogenously Applied Citric Acid Enhances Antioxidant Defense and Phytoextraction of Cadmium by Willows (Salix Spp.). Water Air Soil Pollut. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Voutsa, D.; Samara, C. Labile and bioaccessible fractions of heavy metals in the airborne particulate matter from urban and industrial areas. Atmos. Environ. 2002, 36, 3583–3590. [Google Scholar] [CrossRef]
- Prasad, M.N.V. Phytoremediation of Metal-Polluted Ecosystems: Hype for Commercialization. Russ. J. Plant Physiol. 2003, 50, 686–701. [Google Scholar] [CrossRef]
- Moudouma, C.F.M.; Riou, C.; Gloaguen, V.; Saladin, G. Hybrid larch (Larix × eurolepis Henry): A good candidate for cadmium phytoremediation? Environ. Sci. Pollut. Res. 2013, 20, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H. Metal Hyperaccumulation in Plants: A Review Focusing on Phytoremediation Technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- A Preliminary Investigation in Using Pohlia nutans and Larix decidua as Biomonitors of Air Pollution by the Coke Industry in Wałbrzych (SW Poland). Pol. J. Environ. Stud. 2008, 17, 121–128.
- Kuroda, K.; Kagawa, A.; Tonosaki, M. Radiocesium concentrations in the bark, sapwood and heartwood of three tree species collected at Fukushima forests half a year after the Fukushima Dai-ichi nuclear accident. J. Environ. Radioact. 2013, 122, 37–42. [Google Scholar] [CrossRef]
- Chojnacka-Ożga, L.; Ożga, W. The Impact of Air Pollution on the Growth of Scots Pine Stands in Poland on the Basis of Dendrochronological Analyses. Forests 2021, 12, 1421. [Google Scholar] [CrossRef]
- Hana, A.; Leila Sahli, a. Using tree leaves and barks collected from contaminated and uncontaminated areas as indicators of air metallic pollution. Int. J. Phytoremediation 2019, 21, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Mingorance, M.D.; Oliva, S.R. Heavy metals content in N. oleander leaves as urban pollution assessment. Environ. Monit. Assess 2006, 119, 57–68. [Google Scholar] [CrossRef]
- Safari, M.; Ramavandi, B.; Sanati, A.M.; Sorial, G.A.; Hashemi, S.; Tahmasebi, S. Potential of trees leaf/bark to control atmospheric metals in a gas and petrochemical zone. J. Environ. Manag. 2018, 222, 12–20. [Google Scholar] [CrossRef]
- Fox, T.C.; Guerinot, M.L. Molecular Biology of Cation Transport in Plants. Annu. Rev. Plant Biol. 1998, 49, 669–696. [Google Scholar] [CrossRef]
- Marco, O.; Manuela, P.; Manuela, P.; Maurizio, S.; Maria Perla, C. Heavy metals variations in some conifers in Valle d’Aosta (Western Italian Alps) from 1930 to 2000. Microchem. J. 2002, 73, 237–244. [Google Scholar] [CrossRef]
- Hamanishi, E.T.; Campbell, M.M. Genome-wide responses to drought in forest trees. For. Int. J. For. Res. 2011, 84, 273–283. [Google Scholar] [CrossRef]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Penuelas, J. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 2013, 4, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y. Study on adsorption and remediation of heavy metals by poplar and larch in contaminated soil. Environ. Sci. Pollut. Res. Int. 2010, 17, 1331–1338. [Google Scholar] [CrossRef]
- Kastori, R. Fiziologija Biljaka; Nauka: Beograd, Serbia, 1993. (In Serbian) [Google Scholar]
- Bidwell, R.G.S. Plant Physiology; Macmillan: New York, NY, USA, 1974. [Google Scholar]
- Adriano, D. Trace Elements in the Terrestrial Environment; Springer: New York, NY, USA, 1986. [Google Scholar]
- Aničić, M. Aktivni Biomonitoring Atmosferske Depozicije Elemenata u Tragovima u Urbanoj Sredini Korišćenjem Mahovine Sphagnum girgensohnii Russow. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2010. (In Serbian). [Google Scholar]
- Dragica, S.; Ružica, I.; Mirjana, Š.-N.; Dragica, V.; Slobodanka, P. Contents of the heavy metals nickel and lead in leaves of Paulownia elongata S.Y. Hu and Paulownia fortunei Hems. in Serbia. Arch. Biol. Sci. 2009, 61, 827–834. [Google Scholar] [CrossRef]
- Pais, I. Criteria of essentiality, beneficiality and toxicity of chemical elements. Acta Aliment. 1992, 21, 145–152. [Google Scholar]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- De Nicola, F.; Maisto, G.; Prati, M.; Alfani, A. Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environ. Pollut. 2008, 153, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Sabina Rossini, O.; Benito Valdés, a. Influence of Washing on Metal Concentrations in Leaf Tissue. Commun. Soil Sci. Plant Anal. 2004, 35, 1543–1552. [Google Scholar] [CrossRef]
- Antonio, J.; Miguel, T.; Francisco, J.; Juan, C. A chemical speciation of trace metals for fine urban particles. Atmos. Environ. 2002, 36, 773–780. [Google Scholar] [CrossRef]
- Milošević, I.R.; Zivkovic, S.; Momcilovic, M.; Visnjic-Jeftic, Z.; Veselinovic, M.; Marković, I.D.; Marković, D.M. Field experiment on the uptake of lead, strontium, cobalt, and nickel in the wood and bark of spruce (Picea abies L.) and Douglas-fir (Pseudotsuga menziesii Mirb.). J. Serbian Chem. Soc. 2025, 71, 9. [Google Scholar] [CrossRef]
- Rautio, P.F.A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. Version 2020-3. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 16. Available online: http://www.icp-forests.org/Manual.htm (accessed on 14 April 2025).
- Michel, H. Preparation steps in environmental trace element analysis—Facts and traps. Talanta 2001, 54, 1021–1038. [Google Scholar] [CrossRef]
- Dean, J.R. Methods for Environmental Trace Analysis; John Wiley and Sons: New York, NY, USA, 2003; Volume 12. [Google Scholar]
- Zeiner, M.; Juranović Cindrić, I. Accumulation of Major, Minor and Trace Elements in Pine Needles (Pinus nigra) in Vienna (Austria). Molecules 2021, 26, 3318. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Wang, Y.; Tang, L.; Wang, K.; Shi, F. The Effect of Different Cleaning Methods on Needles for Assessing the Atmospheric Heavy Metal Retention Capacity of Three Coniferous Trees. Appl. Sci. 2021, 11, 1668. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, E.; Han, Y.; Wang, R. Variation of Heavy Metal Enrichment Efficiency in Roadside Trees of Sophora japonica L. with Different Diameters at Breast Height. Acta Ecol. Sin. 2015, 35, 5353–5363. [Google Scholar]
- Xu, H.; Wang, W.; Wang, H.; Sun, Y.; Zhong, Z.; Wang, S. Differences in quantity and composition of leaf particulate matter and morphological structures in three evergreen trees and their association in Harbin, China. Environ. Pollut. 2019, 252, 1772–1790. [Google Scholar] [CrossRef]
- Murphy, A.P.; Coudert, M.; Barker, J. Plants as biomarkers for monitoring heavy metal contaminants on landfill sites using sequential extraction and inductively coupled plasma atomic emission spectrophotometry (ICP-AES). J. Environ. Monit. 2000, 2, 621–627. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, G.R.; Koller, C.E.; Blomberg, S.P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 2007, 69, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Alhashemi, A.S.; Karbassi, A.R.; Kiabi, B.H.; Monavari, S.M.; Nabavi, S.M.; Sekhavatjou, M.S. Bioaccumulation of trace elements in trophic levels of wetland plants and waterfowl birds. Biol. Trace Elem. Res. 2011, 142, 500–516. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, J.Q.; Bi, X.H.; Feng, Y.C.; Wu, J.H. Pollution Characteristics and Sources of Heavy Metals in PM10 and PM2.5 in Tianjin City. Acta Sci. Nat. Univ. Nankaiensis 2013, 46, 1–7. [Google Scholar]
- Li, Y.Q.L.; Yang, M.; Liang, M.; Chen, Q.B.; Deng, Z.H. Study on Dust-retention and Ability to Absorb Heavy Metals of Greening Tree Species. Mod. Agric. Sci. Technol. 2016, 5, 212–217. [Google Scholar]
- Xie, C.; Yan, L.; Liang, A.; Che, S. Understanding the washoff processes of PM2.5 from leaf surfaces during rainfall events. Atmos. Environ. 2019, 214, 116844. [Google Scholar] [CrossRef]
- Chen, P.; Bi, X.; Zhang, J.; Wu, J.; Feng, Y. Assessment of heavy metal pollution characteristics and human health risk of exposure to ambient PM2.5 in Tianjin, China. Particuology 2015, 20, 104–109. [Google Scholar] [CrossRef]
- Mei, F.; Xu, C.; Zhou, L. Chemical species and bioavailbility of Cu, Pb Zn, Ni and Cd of dustfall from Xi’an parks in China. Environ. Chem. 2011, 30, 1284–1290. [Google Scholar]
- Jean Damascene, H.; Zhou, J.; Yang, W.; Gu, Q.; Yu, X. Biochar-bacteria-plant partnerships: Eco-solutions for tackling heavy metal pollution. Ecotoxicol. Environ. Saf. 2020, 204, 111020. [Google Scholar] [CrossRef]
- Chen, R.; Kan, H.; Chen, B.; Huang, W.; Bai, Z.; Song, G.; Pan, G. Association of Particulate Air Pollution with Daily Mortality: The China Air Pollution and Health Effects Study. Am. J. Epidemiol. 2012, 175, 1173–1181. [Google Scholar] [CrossRef]
- Kamal Jyoti, M.; Anil Kumar, D.; Mohit, A.; Ashok, D. Estimating premature mortality attributable to PM2.5 exposure and benefit of air pollution control policies in China for 2020. Sci. Total Environ. 2018, 612, 683–693. [Google Scholar] [CrossRef]
- Vilotić, D. Uporedna Anatomija Drveta; Univerzitet u Beogradu: Belgrade, Serbia, 2020; pp. 1–176, (In English: Comparative wood anatomy). [Google Scholar]
- Li, Z.; Zou, R.L.; Li, J.; Huang, J.; Zhu, B.H. Source Apportionment of Heavy Metals (Pb, Cd, As, Hg) in PM2.5 and PM10 in a City in China. Adv. Mater. Res. 2014, 908, 14–17. [Google Scholar]
- Snezana, M.S.; Dusanka Dj, M.; Renata, M.K.; Ana, A.I. Assessment of airborne heavy metal pollution using plant parts and topsoil. Ecotoxicol. Environ. Saf. 2012, 76, 209–214. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Piotrowska, M. Zanieczyszczenie Glebi Roslin Uprawnych Pierwiastkami Sladowymi; CBR-opracowanie problemowe: Warszawa, Poland, 1984. (In Polish) [Google Scholar]
- Celik, A.; Kartal, A.A.; Akdoğan, A.; Kaska, Y. Determining the heavy metal pollution in Denizli (Turkey) by using Robinio pseudo-acacia L. Environ. Int. 2005, 31, 105–112. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Hu, Y.; Wang, D.; Wei, L.; Zhang, X.; Song, B. Bioaccumulation of heavy metals in plant leaves from Yan’an city of the Loess Plateau, China. Ecotoxicol. Environ. Saf. 2014, 110, 82–88. [Google Scholar] [CrossRef]
- Sevgi, Y.; Murat, Z. Monitoring environmental pollution in Erzurum by chemical analysis of Scots pine (Pinus sylvestris L.) needles. Environ. Int. 2004, 29, 1041–1047. [Google Scholar] [CrossRef]
- Bénédicte, V.; François, P.; Sandrine, P.; Jean-Claude, P. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: Bioaccumulation in soil, Graminaceae and land snails. Chemosphere 2004, 55, 1349–1359. [Google Scholar] [CrossRef]
- Alfani, A.; Baldantoni, D.; Maisto, G.; Bartoli, G. Temporal and spatial variation in C, N, S and trace element contents in the leaves of Quercus ilex within the urban area of Naples. Environ. Pollut. 2000, 109, 119–129. [Google Scholar] [CrossRef]
- M’hamed, M.; Benchaaben, H.; Abdelkader, D.; Nadira, A. Détection de la pollution de lʼair dʼorigine routière par certaines espèces végétales bioaccumulatrices de quelques métaux lourds (Pb, Zn, Cu). Pollut. Atmos. 2007, 196, 385–394. [Google Scholar]
- Ejidike, I.P.; Onianwa, P.C. Assessment of Trace Metals Concentration in Tree Barks as Indicator of Atmospheric Pollution within Ibadan City, South-West, Nigeria. J. Anal. Methods Chem. 2015, 2015, 243601. [Google Scholar] [CrossRef]
- Cédric, A.; Vincent, G.; Céline Faugeron, a. Phytoremediation of Cadmium-Contaminated Soils by Young Douglas Fir Trees: Effects of Cadmium Exposure on Cell Wall Composition. Int. J. Phytoremediation 2014, 16, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Arsenov, D. Physiological Aspects of the Potential of Willows in Assisted Phytoremediation of Cadmium Using Citric Acid. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2018. [Google Scholar]
- Macnicol, R.D.; Beckett, P.H.T. Critical tissue concentrations of potentially toxic elements. Plant Soil 1985, 85, 107–129. [Google Scholar] [CrossRef]
- Babar, S.; Mohsin, T.; Abdul, R.; Sardar Alam, C.; Shah, F.; Shamsur, R.; Anket, S. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Hussain, M.B.; Ali, S.; Azam, A.; Hina, S.; Farooq, M.A.; Ali, B.; Bharwana, S.A.; Gill, M.B. Morphological, physiological and biochemical responses of plants to nickel stress: A review. Afr. J. Agric. Res. 2013, 8, 1596–1602. [Google Scholar]
- Gough, L.P.; Shacklette, H.T.; Case, A.A. Element Concentrations Toxic to Plants, Animals, and Man; United States Government Publishing Office: Washington, DC, USA, 1979.
- Morrissey, J.; Baxter, I.R.; Lee, J.; Li, L.; Lahner, B.; Grotz, N.; Kaplan, J.; Salt, D.E.; Guerinot, M.L. The Ferroportin Metal Efflux Proteins Function in Iron and Cobalt Homeostasis in Arabidopsis. Plant Cell 2009, 21, 3326–3338. [Google Scholar] [CrossRef]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance†. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef]
- Popović, V.; Šešlija Jovanović, D.; Miletić, Z.; Milovanović, J.; Lučić, A.; Rakonjac, L.; Miljković, D. The evaluation of hazardous element content in the needles of the Norway spruce (Picea abies L.) that originated from anthropogenic activities in the vicinity of the native habitats. Environ. Monit. Assess 2022, 195, 109. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. Accumulation of heavy metals and antioxidant responses in Pinus sylvestris L. needles in polluted and non-polluted sites. Ecotoxicology 2016, 25, 970–981. [Google Scholar] [CrossRef]
- Golnar, M.; Navid, K.; Hasan Baha, B. Bio-monitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecol. Eng. 2016, 90, 331–335. [Google Scholar] [CrossRef]
- De La Cruz, A.R.; Ferreira, L.; Andrade, V.P.; Gioda, A. Biomonitoring of toxic elements in plants collected near leather tanning industry. J. Braz. Chem. Soc. 2019, 30, 256–264. [Google Scholar] [CrossRef]


| Site | Douglas Fir | European Larch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cu | Co | Cd | Pb | Ni | Cu | Co | Cd | Pb | ||
| Lazarevac | Mean 1 | 14.11 a | 3.33 a | 0.67 a | 0.19 a | 0.46 a | 17.50 a | 3.13 b | 0.56 b | 0.16 c | 0.50 a |
| SD | 5.98 | 0.42 | 0.08 | 0.07 | 0.40 | 0.95 | 0.06 | 0.06 | 0.01 | 0.12 | |
| CV (%) | 42.39 | 12.49 | 11.25 | 38.75 | 87.16 | 5.45 | 1.84 | 10.40 | 6.25 | 23.70 | |
| Avala | Mean | 6.09 b | 3.43 a | 0.16 b | 0.13 a | 0.16 a | 13.70 b | 4.07 a | 0.19 c | 0.19 b | 0.04 b |
| SD | 0.42 | 0.06 | 0.04 | 0.03 | 0.24 | 0.15 | 0.12 | 0.01 | 0.01 | 0.07 | |
| CV (%) | 6.89 | 1.68 | 25.80 | 20.35 | 153.24 | 1.06 | 2.84 | 5.26 | 2.99 | 173.21 | |
| Kučevo | Mean | 8.43 ab | 3.67 a | 0.14 b | 0.16 a | 0.00 a | 7.36 c | 4.07 a | 0.76 a | 0.27 a | 0.00 b |
| SD | 0.11 | 0.29 | 0.03 | 0.01 | 0.00 | 0.11 | 0.12 | 0.02 | 0.01 | 0.00 | |
| CV (%) | 1.35 | 7.87 | 22.43 | 3.69 | 0.00 | 1.46 | 2.84 | 2.28 | 3.70 | 0.00 | |
| ANOVA | F-ratio | 4.26 | 1.01 | 97.86 | 1.21 | 2.50 | 250.10 | 87.11 | 196.81 | 122.71 | 37.01 |
| p-value 2 | 0.0706 | 0.4178 | 0.0000 | 0.3613 | 0.1234 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0004 | |
| Species | Ni 1 | Cu | Co | Cd | Pb | MAI | |
|---|---|---|---|---|---|---|---|
| Douglas fir | 9.54 ± 4.40 a | 3.48 ± 0.28 a | 0.32 ± 0.25 a | 0.16 ± 0.04 b | 0.31 ± 0.29 a | 4.14 | |
| European larch | 12.85 ± 4.21 a | 3.76 ± 0.45 a | 0.50 ± 0.24 a | 0.21 ± 0.05 a | 0.18 ± 0.24 a | 3.76 | |
| ANOVA | F-ratio | 2.37 | 2.22 | 2.17 | 4.46 | 0.93 | -- |
| p-value 2 | 0.1434 | 0.1553 | 0.1602 | 0.0407 | 0.3493 | -- | |
| Needle Treatment | Douglas Fir | European Larch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cu | Co | Cd | Pb | Ni | Cu | Co | Cd | Pb | ||
| Unwashed | Mean 1 | 9.54 a | 3.47 a | 0.32 a | 0.16 a | 0.31 a | 12.85 a | 3.76 a | 0.50 a | 0.21 a | 0.18 a |
| SD | 4.66 | 0.29 | 0.27 | 0.05 | 0.31 | 4.46 | 0.47 | 0.25 | 0.05 | 0.25 | |
| CV (%) | 48.88 | 8.48 | 81.90 | 29.01 | 100.66 | 34.70 | 12.64 | 50.08 | 23.80 | 139.09 | |
| Washed with water | Mean | 10.84 a | 2.91 b | 0.26 a | 0.12 a | 0.00 b | 12.11 a | 3.74 a | 0.41 a | 0.17 a | 0.00 b |
| SD | 6.37 | 0.39 | 0.26 | 0.03 | 0.00 | 4.20 | 0.61 | 0.22 | 0.05 | 0.00 | |
| CV (%) | 58.76 | 0.39 | 100.49 | 22.40 | 0.00 | 34.71 | 16.37 | 54.98 | 26.96 | 0.00 | |
| ANOVA | F-ratio | 0.24 | 12.07 | 0.31 | 4.27 | 8.88 | 0.13 | 0.01 | 0.74 | 2.83 | 4.65 |
| p-value 2 | 0.6284 | 0.0031 | 0.5837 | 0.0553 | 0.0088 | 0.7211 | 0.9403 | 0.4033 | 0.1121 | 0.0466 | |
| Component | Eigenvalue | Percentage | Ni | Cu 1 | Co | Cd | Pb. |
|---|---|---|---|---|---|---|---|
| PC1 | 2.07 | 41.36 | −0.64 | 0.85 | 0.12 | 0.85 | −0.45 |
| PC2 | 1.51 | 30.11 | −0.49 | 0.06 | −0.94 | −0.50 | −0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokanović, D.; Stojiljković, I.; Nikolić Jokanović, V.; Živanović, K.; Marinković, M.; Tubić, B.; Jovanović, F. Using Douglas Fir and European Larch Needles for the Assessment of Their Retention Capacity for Atmospheric Heavy Metals. Forests 2025, 16, 980. https://doi.org/10.3390/f16060980
Jokanović D, Stojiljković I, Nikolić Jokanović V, Živanović K, Marinković M, Tubić B, Jovanović F. Using Douglas Fir and European Larch Needles for the Assessment of Their Retention Capacity for Atmospheric Heavy Metals. Forests. 2025; 16(6):980. https://doi.org/10.3390/f16060980
Chicago/Turabian StyleJokanović, Dušan, Ivana Stojiljković, Vesna Nikolić Jokanović, Kristina Živanović, Marko Marinković, Bojan Tubić, and Filip Jovanović. 2025. "Using Douglas Fir and European Larch Needles for the Assessment of Their Retention Capacity for Atmospheric Heavy Metals" Forests 16, no. 6: 980. https://doi.org/10.3390/f16060980
APA StyleJokanović, D., Stojiljković, I., Nikolić Jokanović, V., Živanović, K., Marinković, M., Tubić, B., & Jovanović, F. (2025). Using Douglas Fir and European Larch Needles for the Assessment of Their Retention Capacity for Atmospheric Heavy Metals. Forests, 16(6), 980. https://doi.org/10.3390/f16060980






