Tree Diversity and Identity Effects on Aboveground Biomass Are Stronger than Those of Abiotic Drivers in Coniferous and Broadleaved Forest Restoration Sites of South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Data Collection, and Calculation of AGB
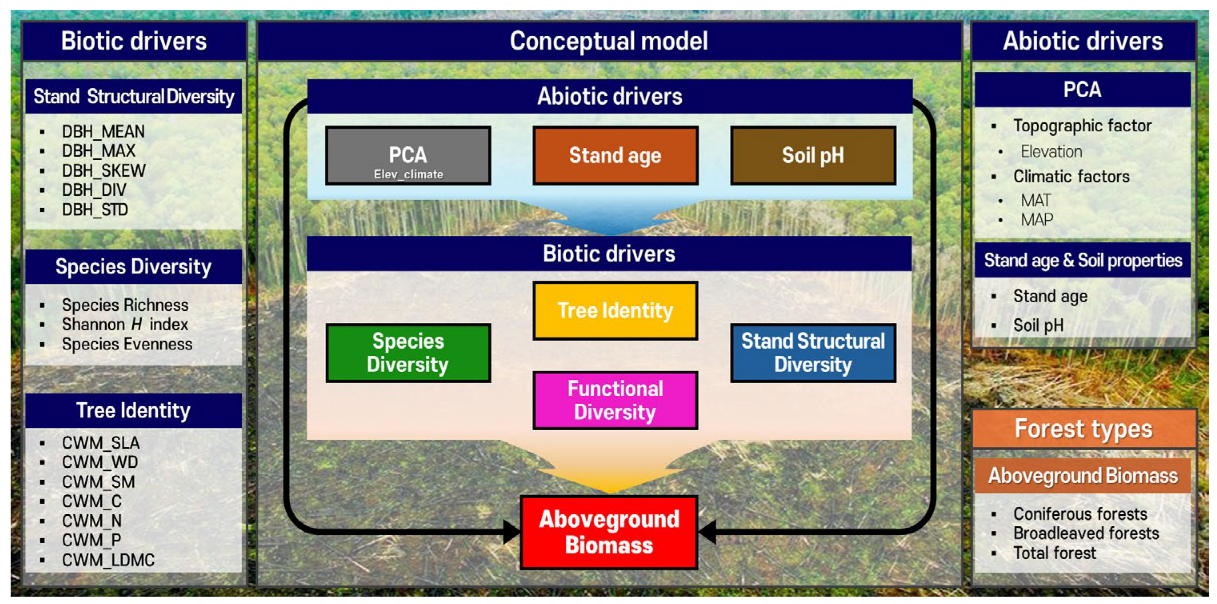
2.2. Quantification of Biotic Drivers
2.3. Quantification of Abiotic Drivers
2.4. Statistical Analysis
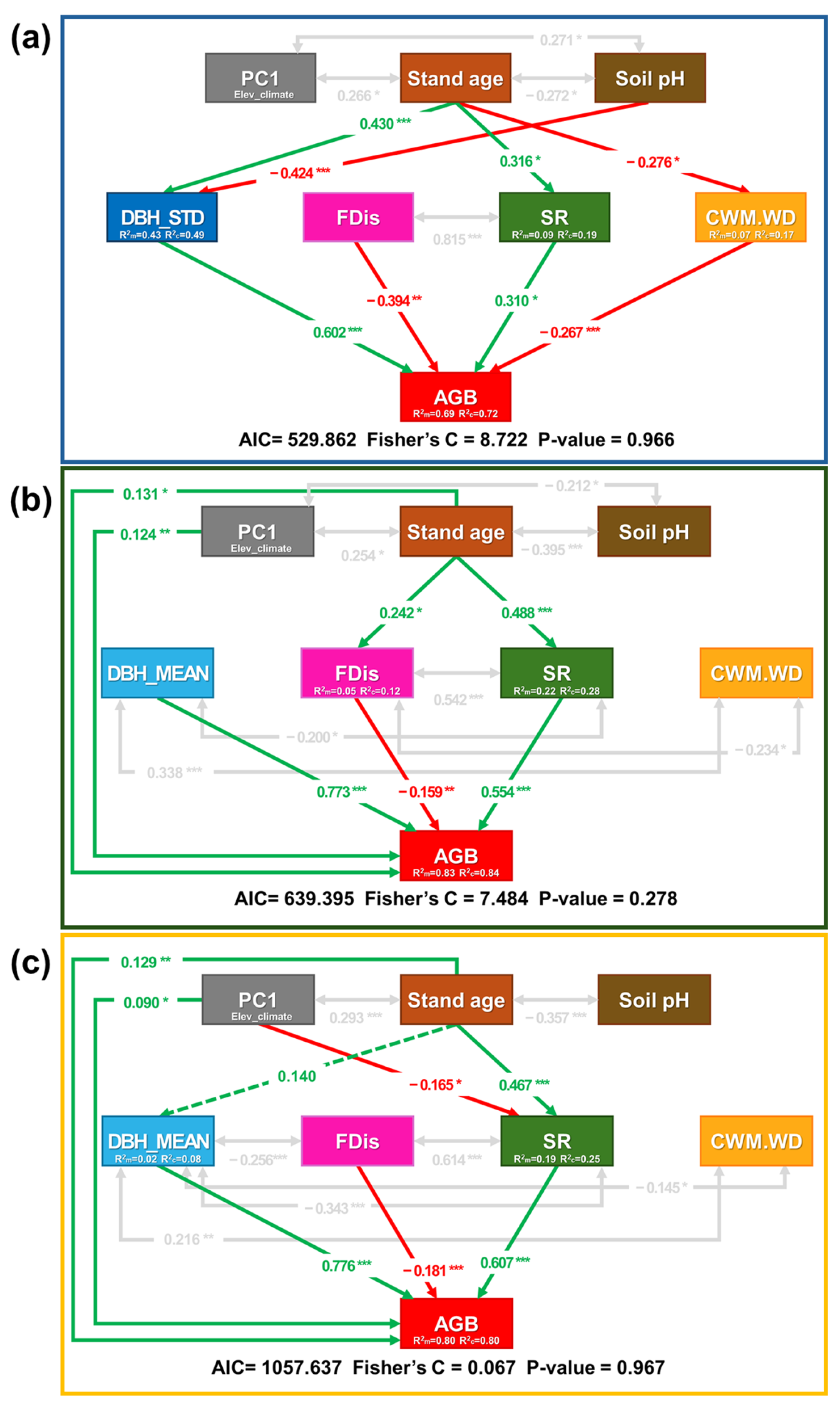
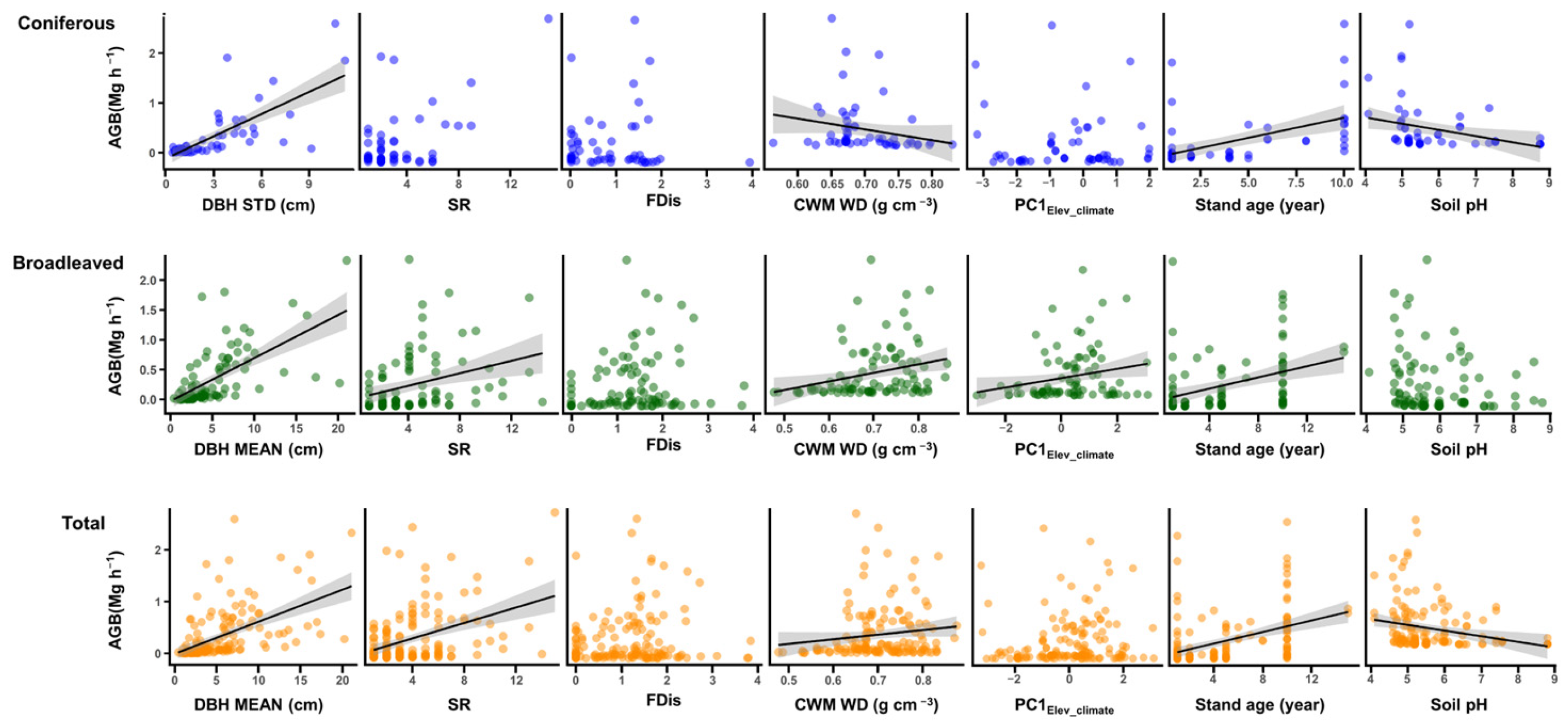
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- King, A.W.; Hayes, D.J.; Huntzinger, D.N.; West, T.O.; Post, W.M. North American carbon dioxide sources and sinks: Magnitude, attribution, and uncertainty. Front. Ecol. Environ. 2012, 10, 512–519. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing forest degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IGES: Hayama, Japan, 2006.
- GOFC-GOLD. GOFC-GOLD REDD Sourcebook Side Event, UNFCCC COP; GOFC-GOLD: Washington, DC, USA, 2008. [Google Scholar]
- de Jong, W.; Börner, J.; Pacheco, P.; Pokorny, B.; Sabogal, C.; Benneker, C.; Cano, W.; Cornejo, C.; Evans, K.; Ruiz, S.; et al. Amazon Forests at the Crossroads: Pressures, Responses and Challenges; International Union of Forest Research Organizations (IUFRO): Vienna, Austria, 2010; Volume 25, pp. 283–298. [Google Scholar]
- Duffy, J.E. Why biodiversity is important to the functioning of real—World ecosystems. Front. Ecol. Environ. 2009, 7, 437–444. [Google Scholar] [CrossRef]
- Jong, W.D.; Liu, J.; Long, H. The forest restoration frontier. Ambio 2021, 50, 2224–2237. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Minnemeyer, S.; Laestadius, L.; Sizer, N.; Saint-Laurent, C.; Potapov, P. A World of Opportunity: The Global Restoration Opportunity Map; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Brudvig, L.A. The restoration of biodiversity: Where has research been and where does it need to go? Am. J. Bot. 2011, 98, 549–558. [Google Scholar] [CrossRef]
- Harris, J. Soil microbial communities and restoration ecology: Facilitators or followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef]
- Paul, K.I.; Cunningham, S.C.; England, J.R.; Roxburgh, S.H.; Preece, N.D.; Lewis, T.; Brooksbank, K.; Crawford, D.F.; Polglase, P.J. Managing reforestation to sequester carbon, increase biodiversity potential and minimize loss of agricultural land. Land Use Policy 2016, 51, 135–149. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Carwardine, J.; Hawkins, C.; Polglase, P.; Possingham, H.P.; Reeson, A.; Renwick, A.R.; Watts, M.; Martin, T.G. Spatial priorities for restoring biodiverse carbon forests. BioScience 2015, 65, 372–382. [Google Scholar] [CrossRef]
- Hagger, V.; Wilson, K.; England, J.R.; Dwyer, J.M. Water availability drives aboveground biomass and bird richness in forest restoration plantings to achieve carbon and biodiversity cobenefits. Ecol. Evol. 2019, 9, 14379–14393. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef]
- Hanif, M.A.; Yu, Q.; Rao, X.; Shen, W. Disentangling the contributions of plant taxonomic and functional diversities in shaping aboveground biomass of a restored forest landscape in Southern China. Plants 2019, 8, 612. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, C.B.; Lee, M.K. Tree size variation induced by stand age mainly regulates aboveground biomass across three major stands of temperate forests in South Korea. Front. For. Glob. Change 2023, 6, 1229661. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ali, A. Functional identity regulates aboveground biomass better than trait diversity along abiotic conditions in global forest metacommunities. Ecography 2022, 2022, e05854. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Gao, W.; Liang, M.; Xiang, W.; Fu, L.; Guo, H.; He, X.; Sharma, R.P.; Chen, Z.; Li, Y.; Zhou, M.; et al. Development stage—Dependent effects of biodiversity on aboveground biomass of temperate forests. Ecography 2025, 2025, e07414. [Google Scholar] [CrossRef]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bongalov, B.; Burslem, D.F.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Chen, K.; Li, T.; Yang, M.; Zhou, X.; Peng, C. The effects of environmental factors and plant diversity on forest carbon sequestration vary between eastern and western regions of China. J. Clean. Prod. 2024, 437, 140371. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zhang, J.; Liu, Y.; Li, H.; Zhou, B.; Shen, B.; Hou, L.; Xu, D.; Ding, L.; et al. Spatial and temporal patterns of above-and below-ground biomass over the Tibet Plateau grasslands and their sensitivity to climate change. Sci. Total Environ. 2024, 919, 170900. [Google Scholar] [CrossRef]
- Coradini, K.; Krejčová, J.; Frouz, J. Potential of vegetation and woodland cover recovery during primary and secondary succession, a global quantitative review. Land Degrad. Dev. 2022, 33, 512–526. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Villa, P.M.; Silla, F.; Gomes, L.P.; Meira-Neto, J.A.A.; Torres, C.M.M.; Neri, A.V. Functional composition enhances aboveground carbon stock during tropical late-secondary forest succession. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 1–11. [Google Scholar] [CrossRef]
- Mensah, S.; Noulèkoun, F.; Salako, V.K.; Lokossou, C.S.; Akouété, P.; Seifert, T.; Glèlè Kakaï, R. Structural and taxonomic diversity predict above—Ground biomass better than functional measures of maximum height in mixed—Species forests. Appl. Veg. Sci. 2023, 26, e12732. [Google Scholar] [CrossRef]
- Ali, A. Forest stand structure and functioning: Current knowledge and future challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Su, L.; Heydari, M.; Omidipour, R.; Soheili, F.; Cheraghi, J.; Villa, P.M.; Prévosto, B. Stand structural diversity and elevation rather than functional diversity drive aboveground biomass in historically disturbed semiarid oak forests. For. Ecol. Manag. 2023, 543, 121139. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Zhang, X.; Wang, H. Effects of Tree Diversity, Functional Composition, and Large Trees on the Aboveground Biomass of an Old-Growth Subtropical Forest in Southern China. Forests 2023, 14, 994. [Google Scholar] [CrossRef]
- Granda, E.; Gazol, A.; Camarero, J.J. Functional diversity differently shapes growth resilience to drought for co-existing pine species. J. Veg. Sci. 2018, 29, 265–275. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Zhou, S.; Song, J.; Fu, B. Soil moisture determines the recovery time of ecosystems from drought. Glob. Change Biol. 2023, 29, 3562–3574. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chen, H.Y.; Chang, S.X.; Zhao, Y.T.; Yang, X.D.; Xu, M.S. Stand structural diversity rather than species diversity enhances aboveground carbon storage in secondary subtropical forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.; Rey Benayas, J.M. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef]
- Suwanto, A.; Takarina, N.D.; Koestoer, R.H.; Frimawaty, E. Diversity, biomass, covers, and NDVI of restored mangrove forests in Karawang and Subang Coasts, West Java, Indonesia. Biodiversitas 2021, 22, 4115–4122. [Google Scholar] [CrossRef]
- Holl, K.D.; Zahawi, R.A. Factors explaining variability in woody above-ground biomass accumulation in restored tropical forest. For. Ecol. Manag. 2014, 319, 36–43. [Google Scholar] [CrossRef]
- Müller, J.; Mitesser, O.; Cadotte, M.W.; van der Plas, F.; Mori, A.S.; Ammer, C.; Chao, A.; Scherer-Lorenzen, M.; Baldrian, P.; Bässler, C.; et al. Enhancing the structural diversity between forest patches—A concept and real—World experiment to study biodiversity, multifunctionality and forest resilience across spatial scales. Glob. Change Biol. 2023, 29, 1437–1450. [Google Scholar] [CrossRef]
- Son, Y.M.; Kim, R.H.; Lee, K.H.; Pyo, J.K.; Kim, S.W.; Hwang, J.S.; Lee, S.J. Carbon Emission Factors and Biomass Allometric Equations by Species in Korea; Korea Forest Research Institute Report; National Institute of Forest Science: Seoul, Republic of Korea, 2014; pp. 14–18. [Google Scholar]
- Heiskanen, J.; Pellikka, P.; Betemariam, E.A.; Packalen, P. Field Measurement Guidelines for Aboveground Biomass and Fuel Wood Stocks; Building Biocarbon and Rural Development in West Africa (BIODEV); World Agroforestry Centre: Nairobi, Kenya, 2013. [Google Scholar]
- Korea Forest Service. 2022 Forest Restoration Implementation Plan. Available online: https://www.forest.go.kr/ (accessed on 10 June 2024).
- Gamboa-Blanco, E.A.; Dupuy, J.M.; Portillo-Quintero, C.A.; Caughlin, T.; Hernández-Stefanoni, J.L. Effects of successional age, plot size, and tree size on the relationship between diversity and aboveground biomass in tropical dry forests. Plant Ecol. 2024, 225, 803–817. [Google Scholar] [CrossRef]
- Memiaghe, H.R.; Lutz, J.A.; Korte, L.; Alonso, A.; Kenfack, D. Ecological importance of small-diameter trees to the structure, diversity and biomass of a tropical evergreen forest at Rabi, Gabon. PLoS ONE 2016, 11, e0154988. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Chen, H.Y.; You, W.H.; Yan, E.R. Multiple abiotic and biotic drivers of aboveground biomass shift with forest stratum. For. Ecol. Manag. 2019, 436, 1–10. [Google Scholar] [CrossRef]
- Martin-Guay, M.O.; Belluau, M.; Côté, B.; Handa, I.T.; Jewell, M.D.; Khlifa, R.; Munson, A.D.; Rivest, M.; Whalen, J.K.; Rivest, D. Tree identity and diversity directly affect soil moisture and temperature but not soil carbon ten years after planting. Ecol. Evol. 2022, 12, e8509. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0-12; 2014. Available online: https://www.researchgate.net/publication/312463190_FD_Measuring_functional_diversity_from_multiple_traits_and_other_tools_for_functional_ecology (accessed on 10 April 2025).
- R Core Team. R: A Language and Environment for Statistical Computing, R version 3.5. 0; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Finegan, B.; Peña-Claros, M.; de Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Eguiguren Velepucha, P.; Fernandez, F.; et al. Does functional trait diversity predict above—Ground biomass and productivity of tropical forests? Testing three alternative hypotheses. J. Ecol. 2015, 103, 191–201. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E. New handbook for standardised measurement of plant functional traits worldwide. Aust. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, Y.J.; Lee, C.B. Ecosystem multifunctionality in temperate forests of South Korea is primarily controlled by structural diversity and potential moisture availability with synergy effects between ecosystem functions. J. Environ. Manag. 2025, 382, 125449. [Google Scholar] [CrossRef]
- Munnaf, M.A.; Haesaert, G.; Van Meirvenne, M.; Mouazen, A.M. Site-specific seeding using multi-sensor and data fusion techniques: A review. Adv. Agron. 2020, 161, 241–323. [Google Scholar]
- Toledo, R.M.; Santos, R.F.; Baeten, L.; Perring, M.P.; Verheyen, K. Soil properties and neighbouring forest cover affect above—Ground biomass and functional composition during tropical forest restoration. Appl. Veg. Sci. 2018, 21, 179–189. [Google Scholar] [CrossRef]
- Chun, J.H.; Lee, C.B. Partitioning the regional and local drivers of phylogenetic and functional diversity along temperate elevational gradients on an East Asian peninsula. Sci. Rep. 2018, 8, 2853. [Google Scholar] [CrossRef]
- Gao, M.; Piao, S.; Chen, A.; Yang, H.; Liu, Q.; Fu, Y.H.; Janssens, I.A. Divergent changes in the elevational gradient of vegetation activities over the last 30 years. Nat. Commun. 2019, 10, 2970. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, A.M.; De Kauwe, M.G.; Roderick, M.L.; Burrell, A.; Lehmann, P.; Pitman, A.J. Annual precipitation explains variability in dryland vegetation greenness globally but not locally. Glob. Change Biol. 2021, 27, 4367–4380. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Shi, P.; Li, W.; He, Y.; Zhang, X.; Shen, Z.; Chai, S. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan Plateau. Sci. China Life Sci. 2010, 53, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.H.; Ali, A.; Lee, C.B. Topography and forest diversity facets regulate overstory and understory aboveground biomass in a temperate forest of South Korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef]
- Ali, A.; Wang, L.Q. Big-sized trees and forest functioning: Current knowledge and future perspectives. Ecol. Indic. 2021, 127, 107760. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Yi, S.; Wu, P.; Peng, X.; Tang, Z.; Bai, F.; Sun, X.; Gao, Y.; Qin, H.; Yu, X.; Wang, R.; et al. Biodiversity, environmental context and structural attributes as drivers of aboveground biomass in shrublands at the middle and lower reaches of the Yellow River basin. Sci. Total Environ. 2021, 774, 145198. [Google Scholar] [CrossRef]
- Forrester, D.I. Does individual-tree biomass growth increase continuously with tree size? For. Ecol. Manag. 2021, 481, 118717. [Google Scholar] [CrossRef]
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large trees as key elements of carbon storage and dynamics after selective logging in the Eastern Amazon. For. Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- Yang, Y.; Jing, L.; Li, Q.; Liang, C.; Dong, Q.; Zhao, S.; Chen, Y.; She, D.; Zhang, X.; Wang, L.; et al. Big-sized trees and higher species diversity improve water holding capacities of forests in northeast China. Sci. Total Environ. 2023, 880, 163263. [Google Scholar] [CrossRef]
- Ullah, F.; Gilani, H.; Sanaei, A.; Hussain, K.; Ali, A. Stand structure determines aboveground biomass across temperate forest types and species mixture along a local-scale elevational gradient. For. Ecol. Manag. 2021, 486, 118984. [Google Scholar] [CrossRef]
- Rozendaal, D.M.; Chazdon, R.L. Demographic drivers of tree biomass change during secondary succession in northeastern Costa Rica. Ecol. Appl. 2015, 25, 506–516. [Google Scholar] [CrossRef]
- Burgess-Conforti, J.R.; Moore, P.A.; Owens, P.R.; Miller, D.M.; Ashworth, A.J.; Hays, P.D.; Evans-White, M.A.; Anderson, K.R. Are soils beneath coniferous tree stands more acidic than soils beneath deciduous tree stands? Environ. Sci. Pollut. Res. 2019, 26, 14920–14929. [Google Scholar] [CrossRef]
- Raza, S.; Zamanian, K.; Ullah, S.; Kuzyakov, Y.; Virto, I.; Zhou, J. Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod. 2021, 315, 128036. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, R.; Mulder, J.; Zhang, T.; Duan, L. Nitrogen saturation, soil acidification, and ecological effects in a subtropical pine forest on acid soil in southwest China. J. Geophys. Res. Biogeosciences 2015, 120, 2457–2472. [Google Scholar] [CrossRef]
- Tang, C.Q.; Tang, C.Q. Evergreen Broad-Leaved Forests. The Subtropical Vegetation of Southwestern China: Plant Distribution, Diversity and Ecology; Springer: Dordrecht, The Netherlands, 2015; pp. 49–112. [Google Scholar]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Lee, H.; Kim, T.K.; Cho, S.; Yoon, J.; Kim, H.S. Changes of tree species composition and distribution patterns in mts. Jiri and baegun, Republic of Korea over 15 years. Forests 2020, 11, 186. [Google Scholar] [CrossRef]
- Petrokas, R.; Baliuckas, V.; Manton, M. Successional categorization of European hemi-boreal forest tree species. Plants 2020, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Wang, L.Q.; Kazempour Larsary, M.; Chaudhary, R.; Joshi, P.R.; Ali, A. Functional composition of tall-statured trees underpins aboveground biomass in tropical forests. J. For. Res. 2023, 34, 333–344. [Google Scholar] [CrossRef]
- Yazaki, T.; Hirano, T.; Sano, T. Biomass accumulation and net primary production during the early stage of secondary succession after a severe forest disturbance in northern Japan. Forests 2016, 7, 287. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Hao, G.; Ma, K.; Bongers, F.; Sterck, F.J. Conifer and broadleaved trees differ in branch allometry but maintain similar functional balances. Tree Physiol. 2020, 40, 511–519. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Q.; Ma, S.; Feng, Y.; Fang, W.; Ji, C.; Zhu, J.; Wang, Z.; Wang, S.; Tang, Z.; et al. Climate and forest attributes influence above—Ground biomass of deciduous broadleaf forests in China. J. Ecol. 2023, 111, 495–508. [Google Scholar] [CrossRef]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Above—Ground biomass is driven by mass—Ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2018, 106, 561–570. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Ma, Y.; Eziz, A.; Halik, Ü.; Abliz, A.; Kurban, A. Precipitation and Temperature Influence the Relationship between Stand Structural Characteristics and Aboveground Biomass of Forests—A Meta-Analysis. Forests 2023, 14, 896. [Google Scholar] [CrossRef]
- Sun, Z.; Sonsuthi, A.; Jucker, T.; Ali, A.; Cao, M.; Liu, F.; Cao, G.; Hu, T.; Ma, Q.; Guo, Q.; et al. Top canopy height and stem size variation enhance aboveground biomass across spatial scales in seasonal tropical forests. Plants 2023, 12, 1343. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; García-Valdés, R.; Bugmann, H. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, X.; Jiang, J.; DeAngelis, D.L.; Fu, Z.; Zhang, J. Similarity of plant functional traits and aggregation pattern in a subtropical forest. Ecol. Evol. 2017, 7, 4086–4098. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, J.-S.; Park, J.; Lee, Y.-J.; Lee, M.-K.; Lim, C.-Y.; Lee, C.-B. Tree Diversity and Identity Effects on Aboveground Biomass Are Stronger than Those of Abiotic Drivers in Coniferous and Broadleaved Forest Restoration Sites of South Korea. Forests 2025, 16, 979. https://doi.org/10.3390/f16060979
Kwak J-S, Park J, Lee Y-J, Lee M-K, Lim C-Y, Lee C-B. Tree Diversity and Identity Effects on Aboveground Biomass Are Stronger than Those of Abiotic Drivers in Coniferous and Broadleaved Forest Restoration Sites of South Korea. Forests. 2025; 16(6):979. https://doi.org/10.3390/f16060979
Chicago/Turabian StyleKwak, Ji-Soo, Joonhyung Park, Yong-Ju Lee, Min-Ki Lee, Chae-Young Lim, and Chang-Bae Lee. 2025. "Tree Diversity and Identity Effects on Aboveground Biomass Are Stronger than Those of Abiotic Drivers in Coniferous and Broadleaved Forest Restoration Sites of South Korea" Forests 16, no. 6: 979. https://doi.org/10.3390/f16060979
APA StyleKwak, J.-S., Park, J., Lee, Y.-J., Lee, M.-K., Lim, C.-Y., & Lee, C.-B. (2025). Tree Diversity and Identity Effects on Aboveground Biomass Are Stronger than Those of Abiotic Drivers in Coniferous and Broadleaved Forest Restoration Sites of South Korea. Forests, 16(6), 979. https://doi.org/10.3390/f16060979





