Noninvasive Sonic Tomography for the Detection of Internal Defects in Relict Woodlands of Polylepis in Peru
Abstract
1. Introduction
2. Materials and Methods
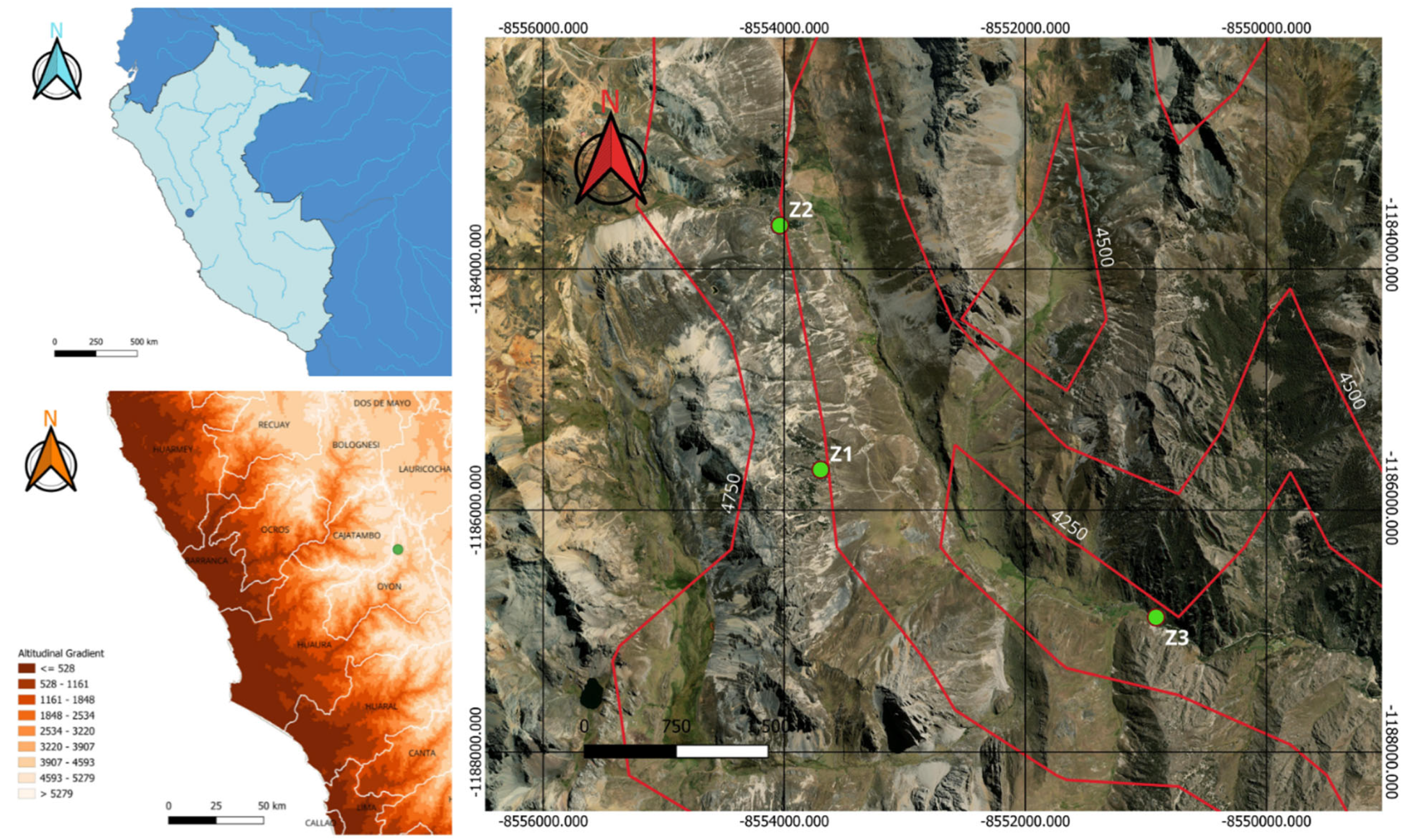
2.1. Study Sites
2.2. Sonic Tomography
2.3. Data Analysis
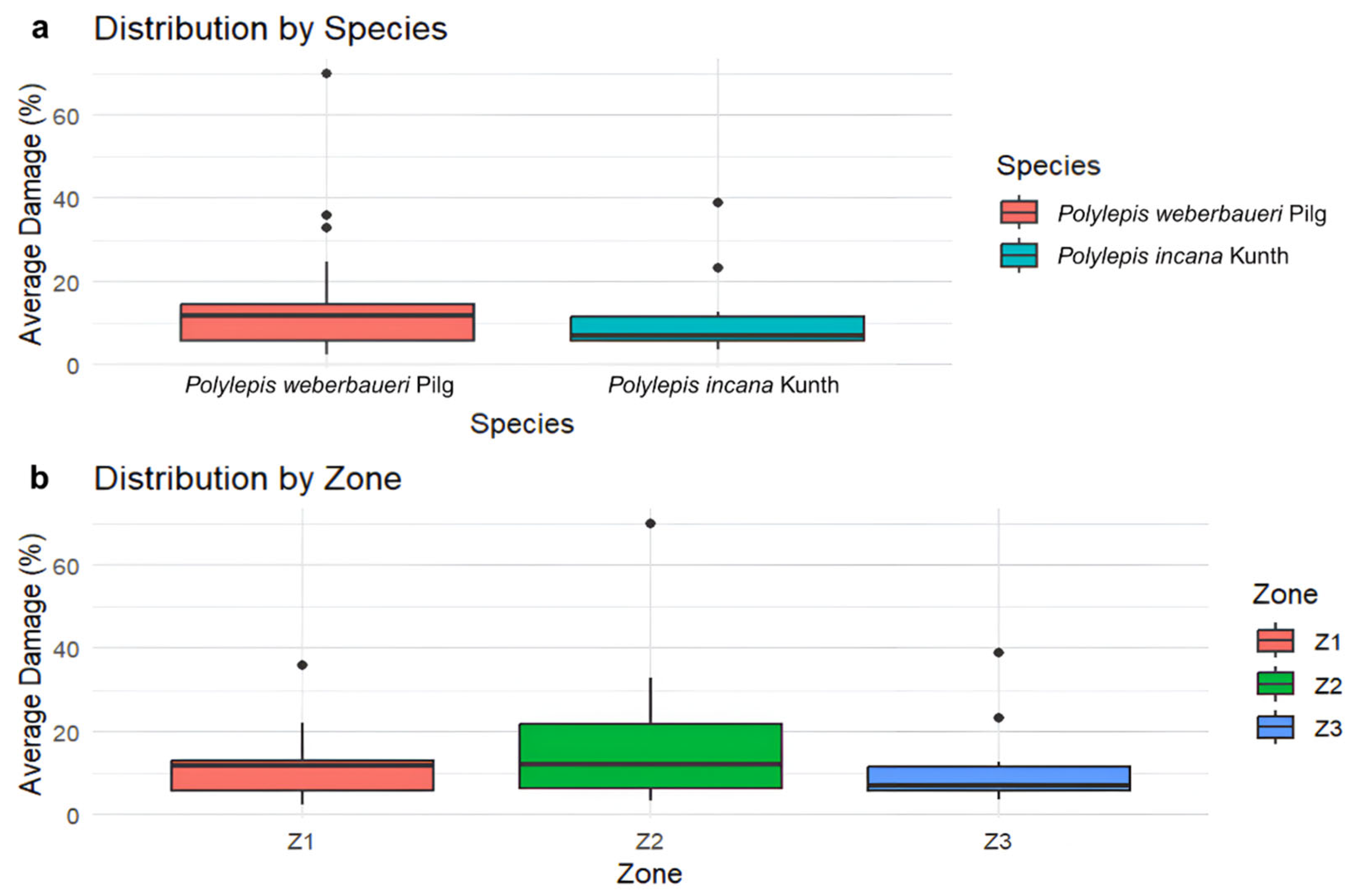
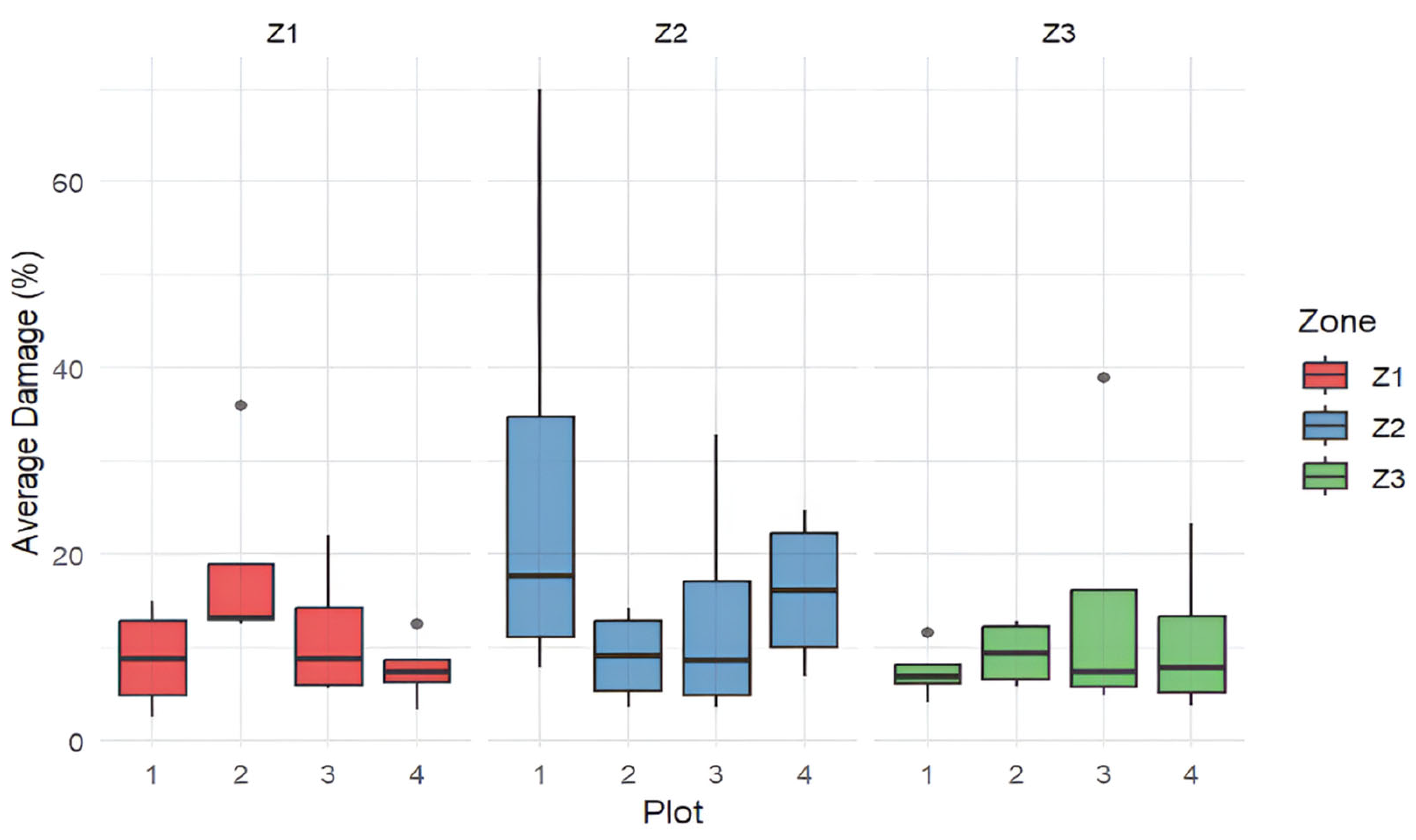
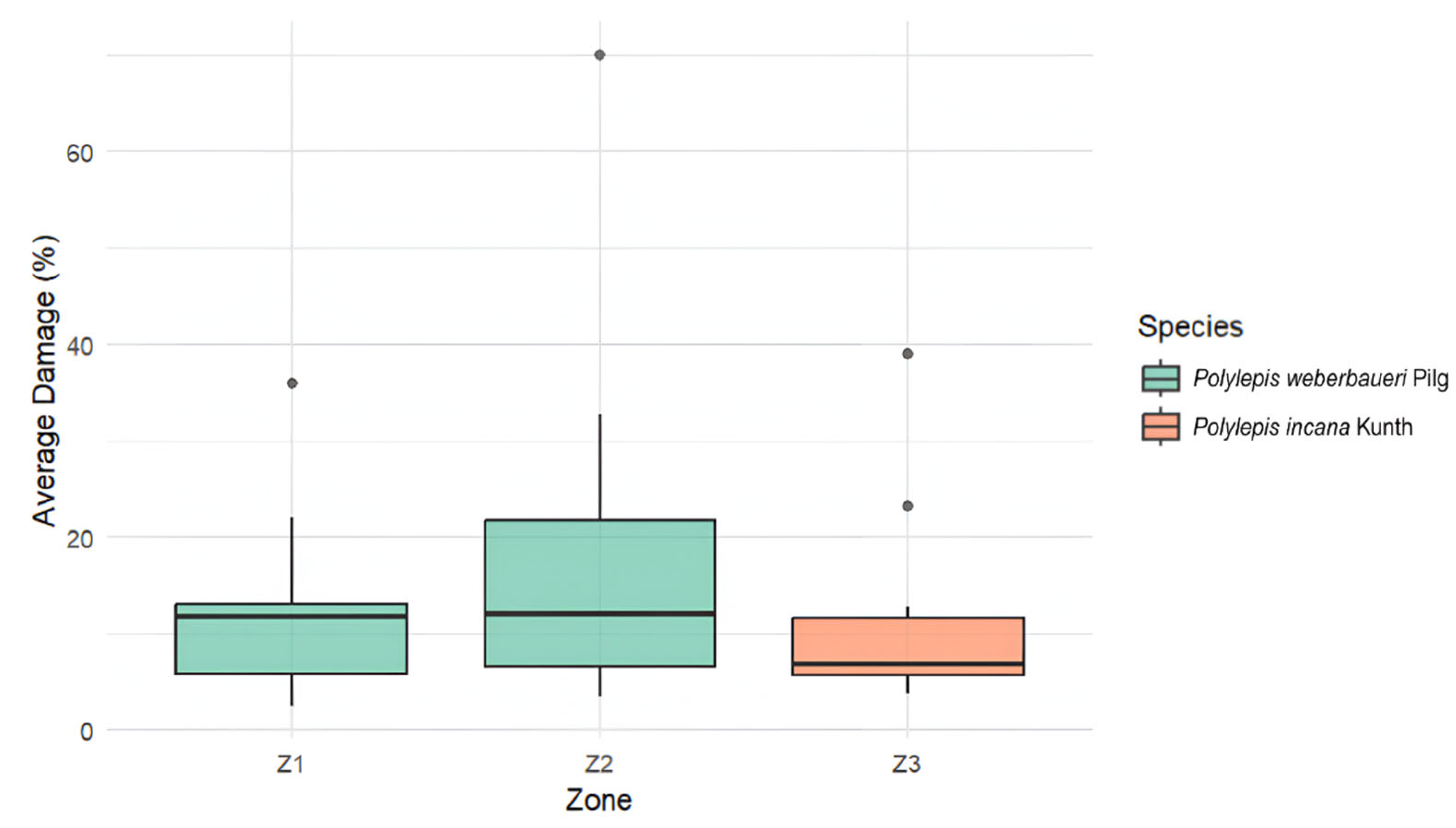
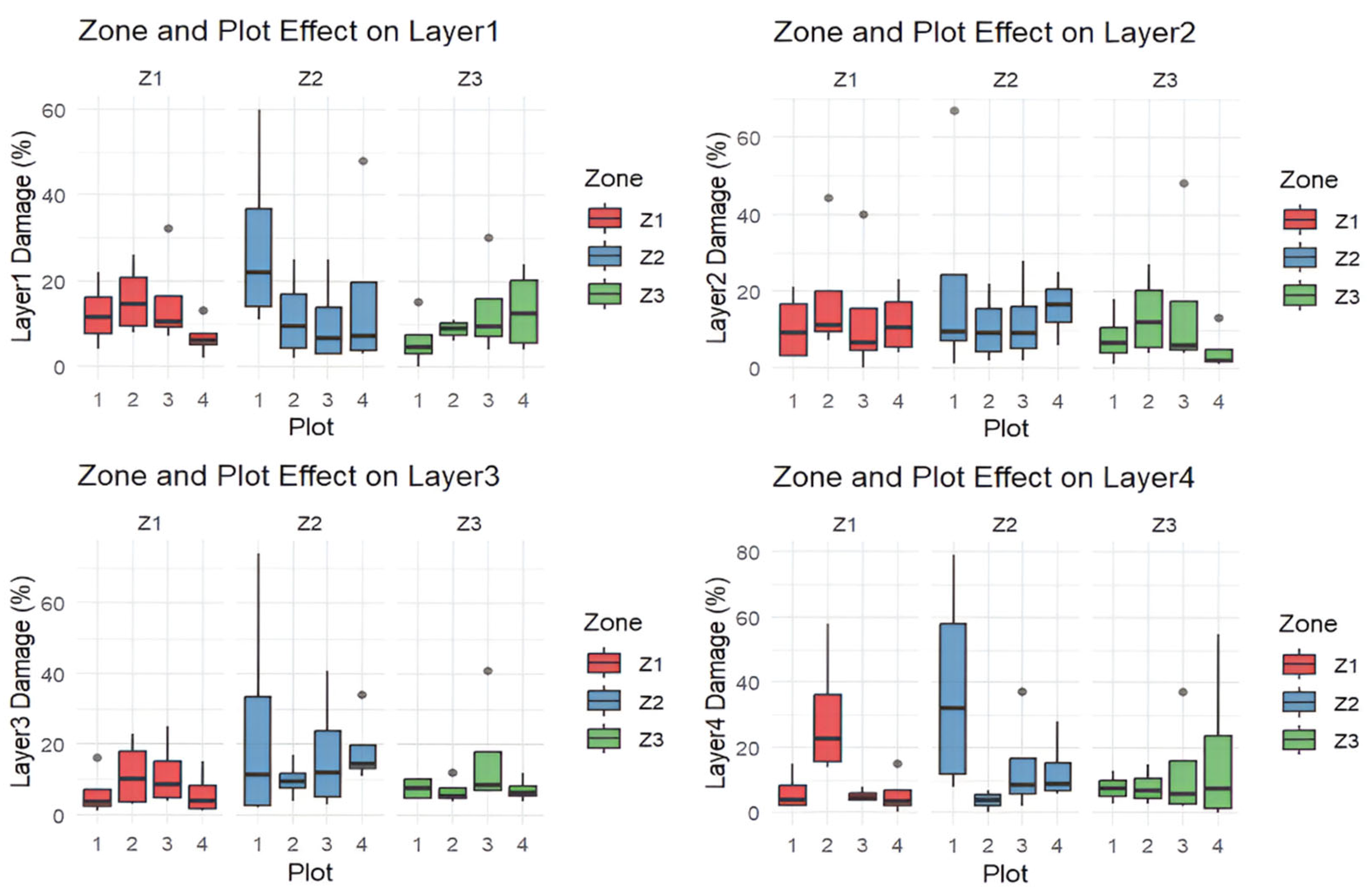
3. Results
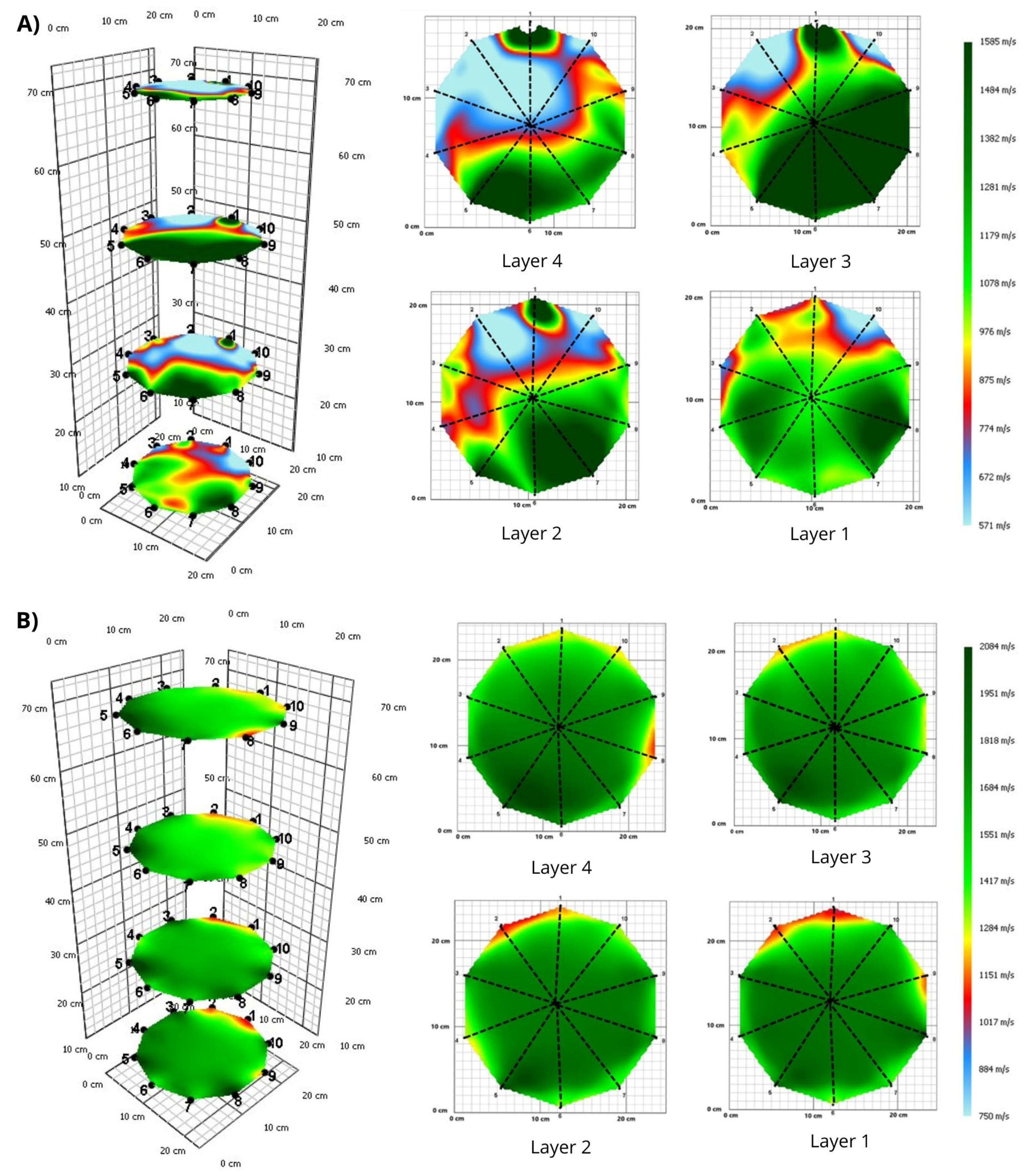
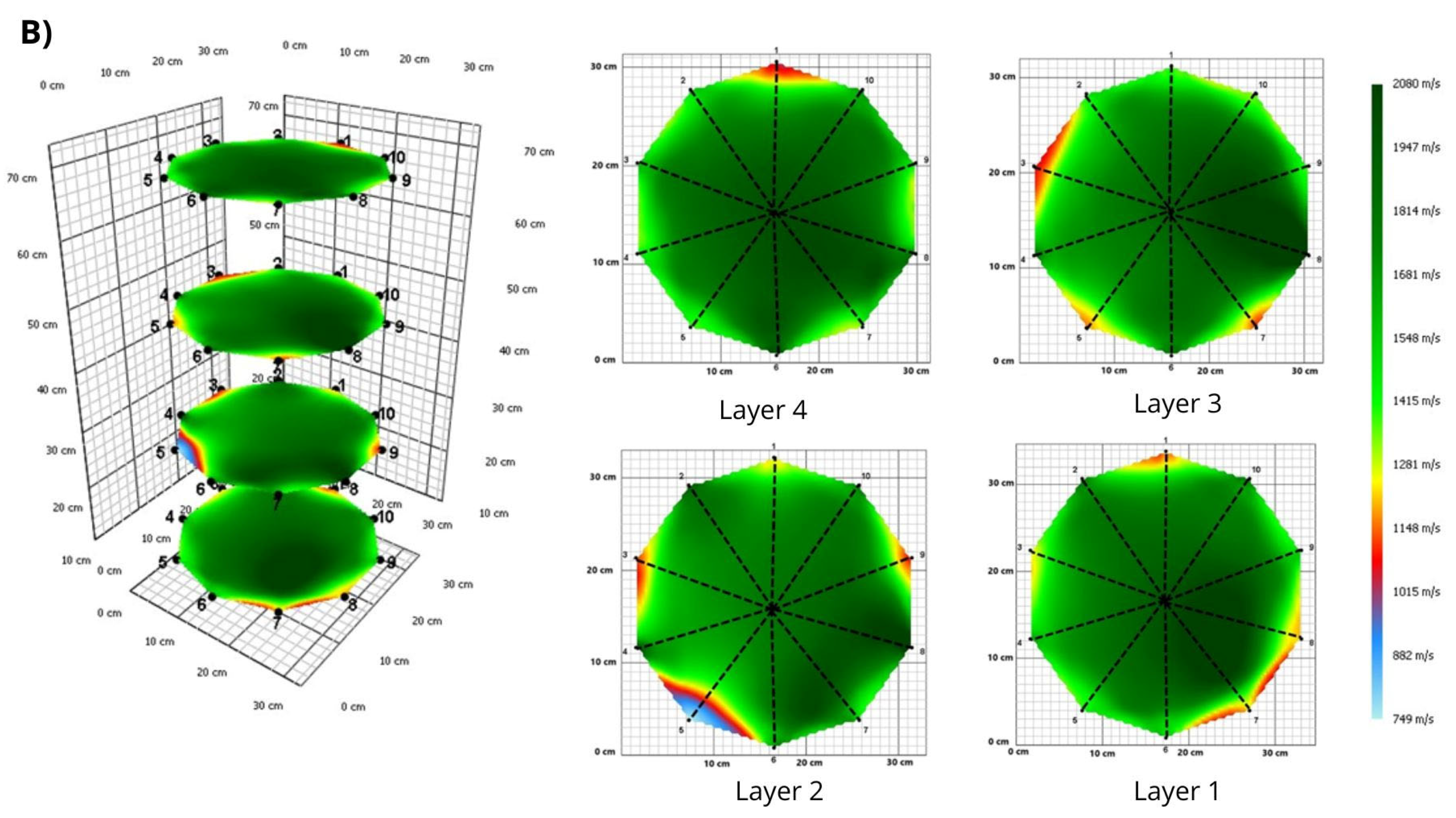
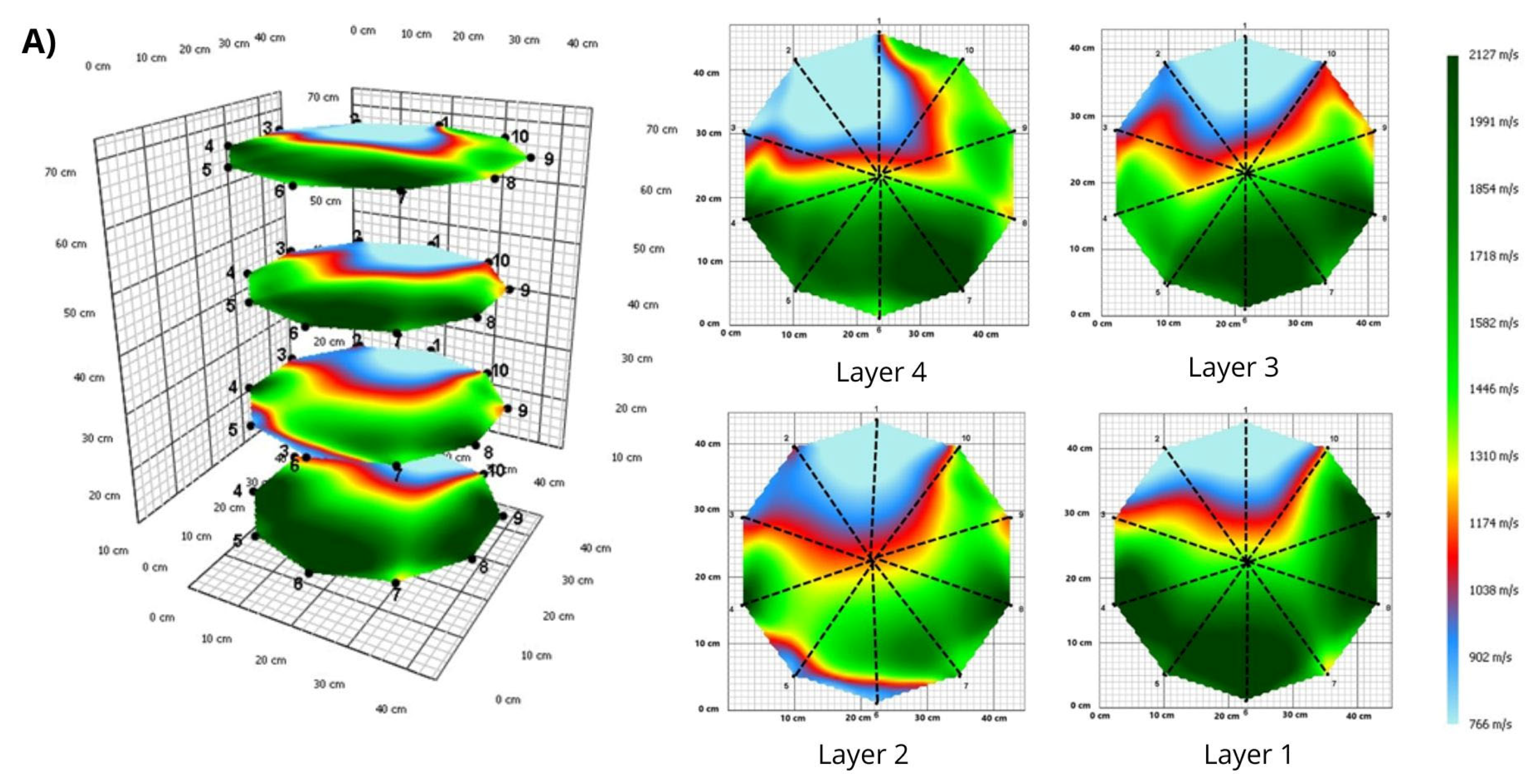
3.1. Tomograms and Level of Wood Decay
3.2. Data Analysis Results
3.3. Conservation Status
4. Discussion
4.1. Sonic Tomography
4.2. Wood Decay in Study Sites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Catchpole, S.; Rojas, V.; Medina, M.; Ruiz, V.H. Barreras geográficas: ¿Intervienen en la composición y distribución longitudinal en la comunidad íctica de un río? Gayana 2020, 84, 129–143. [Google Scholar] [CrossRef]
- Escobar Vásquez, S.P. Estructura Genética de Poblaciones Transandinas de Prestoea acuminata (Willd.) H. E. Moore en los Andes Septentrionales de Ecuador. Bachelor’s Thesis, Pontificia Universidad Católica del Ecuador, Quito, Ecuador, 2011. [Google Scholar]
- Beltrán, H.; Galán de Mera, A. Patrones de distribución de las especies de Senecio L. (Asteraceae) en el Perú. Rev. Peru. Biol. 2022, 29, e21463. [Google Scholar] [CrossRef]
- Alberdi Nieves, V. Modelado de distribución de especies en los bosques de los andes meridionales. Pap. Geogr. 2021, 66, 195–207. [Google Scholar] [CrossRef]
- Ceroni Stuva, A.; Castro Cepero, V. Diversidad vegetal del sistema agrario del distrito de Cajatambo, Lima: Ecosistemas agricultura y matorral. Arnaldoa 2022, 29, 31–48. [Google Scholar]
- Vallejos-Garrido, P.; Pino, K.; Espinoza-Aravena, N.; Pari, A.; Inostroza-Michael, O.; Toledo-Muñoz, M.; Castillo-Ravanal, B.; Romero-Alarcón, V.; Hernández, C.E.; Palma, R.E.; et al. The importance of the Andes in the evolutionary radiation of Sigmodontinae (Rodentia, Cricetidae), the most diverse group of mammals in the Neotropics. Sci. Rep. 2023, 13, 2207. [Google Scholar] [CrossRef]
- Avalos, G. Periodos secos y húmedos en la vertiente occidental de los Andes peruanos. In Informe Técnico N°006; Servicio Nacional de Meteorología e Hidrología del Perú: Lima, Peru, 2016; Available online: https://repositorio.senamhi.gob.pe/bitstream/handle/20.500.12542/832/Periodos-secos-y-h%c3%bamedos-en-la-vertiente-occidental-de-los-Andes-peruanos.pdf?sequence=1&is-Allowed=y (accessed on 30 October 2024).
- White-Nockleby, C.; Prieto, M.; Yager, K.; Meneses, R.I. Understanding bofedales as cultural landscapes in the central Andes. Wetlands 2021, 41, 102. [Google Scholar] [CrossRef]
- Flores, G.S. Biogeografía de un Bosque alto Andino: Historia e Impactos del Cambio Climático en los Queñuales Peruanos. Bachelor’s Thesis, Pontificia Universidad Católica del Perú, Lima, Peru, 2017. [Google Scholar]
- MINAM. Mapa Nacional de Cobertura Vegetal, 1st ed.; Dirección General de Evaluación, Valoración y Financiamiento del Patrimonio Natural, Ministerio del Ambiente: Lima, Peru, 2015; pp. 73–79. [Google Scholar]
- Franco, P.; Cáceres, C.; Navarro, M.; Jove, C.; Ignacio, J.; Oyague, E. Bosques de Polylepis tarapacana en la cuenca Maure, extremo sur del Perú. Oportunidades para su conservación. Estud. Geogr. 2021, 82, e059. [Google Scholar] [CrossRef]
- Calizaya, A.C.M. Dasonomía del bosque de queñua (Polylepis spp.) de la comunidad Quello Quello en el distrito de Lampa, Puno-Perú. Rev. Investig. 2022, 11, 142–154. [Google Scholar] [CrossRef]
- Boza Espinoza, T.E.; Kessler, M. A monograph of the genus Polylepis (Rosaceae). PhytoKeys 2022, 203, 1–274. [Google Scholar] [CrossRef]
- Morales-Aranibar, L.; Morales-Aranibar, C. Maps of the distribution of Polylepis forests in southern Peru. In Global Precision Ag Innovation 2022, Proceedings of the XV International Scientific Conference “INTERAGROMASH 2022”, Rostov-on-Don, Russia, 25–27 May 2022, 2nd ed.; Beskopylny, A., Shamtsyan, M., Artiuk, V., Eds.; Springer: New York, NY, USA, 2022; Volume 1, pp. 3009–3018. [Google Scholar]
- Gosling, W.D.; Hanselman, J.A.; Knox, C.; Valencia, B.G.; Bush, M.B. Long term drivers of change in Polylepis woodland distribution in the central Andes. J. Veg. Sci. 2009, 20, 1041–1052. [Google Scholar] [CrossRef]
- Gareca, E.E.; Fernández, M.; Stanton, S. Dendrochronological investigation of the high Andean tree species Polylepis besseri and implications for management and conservation. Biodivers. Conserv. 2010, 19, 1839–1851. [Google Scholar] [CrossRef]
- Requena-Rojas, E.J.; Crispín-DelaCruz, D.B.; Ticse-Otarola, G.; Quispe-Melgar, H.R.; Inga Guillen, J.G.; Camel Paucar, V.; Anthony, G.; Ames Martinez, F.N.; Morales, M.S. Temporal growth variation in high-elevation forests: Case study of Polylepis forests in central Andes. In Latin American Dendroecology: Combining Tree-Ring Sciences and Ecology in a Megadiverse Territory, 1st ed.; Pompa-García, M., Camarero, J.J., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 263–279. [Google Scholar]
- Renison, D.; Cuyckens, G.A.E.; Pacheco, S.; Guzmán, G.F.; Grau, H.R.; Marcora, P.; Robledo, G.; Cingolani, A.M.; Dominguez, J.; Landi, M.; et al. Distribución y estado de conservación de las poblaciones de árboles y arbustos del género Polylepis (Rosaceae) en las montañas de Argentina. Ecol. Austral 2013, 23, 27–36. [Google Scholar] [CrossRef]
- Renison, D.; Morales, L.; Cuyckens, G.A.; Sevillano, C.S.; Cabrera Amaya, D.M. Ecología y conservación de los bosques y arbustales de Polylepis: ¿qué sabemos y qué ignoramos? Ecol. Austral 2018, 28, 163–174. [Google Scholar] [CrossRef]
- Mendoza, W.; Cano, A. Diversidad del género Polylepis (Rosaceae, Sanguisorbeae) en los Andes peruanos. Rev. Peru. Biol. 2011, 18, 197–200. [Google Scholar] [CrossRef]
- Mendoza, W.; León, B. Rosaceae endémicas del Perú. Rev. Peru Biol. 2006, 13, 583–585. [Google Scholar] [CrossRef]
- Díaz, R.F.D. Distribución Altitudinal de Psilidos (Hemiptera) en Relictos de Polylepis (Rosales) en la Microcuenca de Pumamarka. Bachelor’s Thesis, Universidad Nacional San Antonio de Abad del Cusco, Cusco, Peru, 2011. [Google Scholar]
- López, V.L.; Cellini, J.M.; Cuyckens, G.A.E. Influencia del micrositio y el ambiente en la instalación de Polylepis tarapacana en los Altos Andes. Neotrop. Biodivers. 2021, 7, 135–145. [Google Scholar] [CrossRef]
- Zutta, B.; Rundel, P. Modeled shifts in Polylepis species ranges in the Andes from the Last Glacial Maximum to the present. Forests 2017, 8, 232. [Google Scholar] [CrossRef]
- Dourojeanni, P. Distribución y Conectividad de Bosques Altoandinos (Polylepis) en la Cuenca alta del río Pativilca. Bachelor’s Thesis, Pontificia Universidad Católica del Perú, Lima, Peru, 2008. [Google Scholar]
- Soteras, F.; Renison, D.; Becerra, A.G. Growth response, phosphorus content and root colonization of Polylepis australis Bitt. seedlings inoculated with different soil types. New For. 2013, 44, 577–589. [Google Scholar] [CrossRef]
- Cuyckens, G.A.; Renison, D. Ecology and conservation of Polylepis montane forests: An introduction to the special issue. Ecol. Austral 2018, 28, 157–162. [Google Scholar] [CrossRef]
- Giorgis, M.A.; Cingolani, A.M.; Teich, I.; Renison, D.; Hensen, I. Do Polylepis australis trees tolerate herbivory? Seasonal patterns of shoot growth and its consumption by livestock. Plant Ecol. 2010, 207, 307–319. [Google Scholar] [CrossRef]
- Aguilar, L.C.; Piepenstock, A.; Burgoa, W. Especies nativas kewiña (Polylepis sp.) y kiswara (Buddleja sp.) en barreras vivas: Una alternativa para reducir la degradación de suelos y mejorar las condiciones de vida en la zona altoandina de Bolivia. Acta Nova 2009, 4, 425–438. [Google Scholar]
- Gálvez Cárdenas, G. Evaluación de los Bosques de Polylepis y Plan de Restauración Ecológica en la Microcuenca de Cancha Cancha-Calca. Bachelor’s Thesis, Universidad Nacional San Antonio de Abad del Cusco, Cusco, Peru, 2013. [Google Scholar]
- Montalvo, J.; Minga, D.; Verdugo, A.; López, J.; Guazhambo, D.; Pacheco, D.; Siddons, D.; Crespo, A.; Zárate, E. Características morfológico-funcionales, diversidad arbórea, tasa de crecimiento y de secuestro de carbono en especies y ecosistemas de Polylepis del sur de Ecuador. Ecol. Austral 2018, 28, 249–261. [Google Scholar] [CrossRef]
- Crispin De La Cruz, D.B. Influencia de la Variabilidad Climática en el Crecimiento Radial de Polylepis Tarapacana Phill. En Chiluyo—Tacna. Bachelor’s Thesis, Universidad Continental, Huancayo, Peru, 2021. [Google Scholar]
- Cervantes, R.; Sánchez, J.M.; Alegre, J.; Rendón, E.; Baiker, J.R.; Locatelli, B.; Bonnesoeur, V. Contribución de los ecosistemas altoandinos en la provisión del servicio ecosistémico de regulación hídrica. Ecol. Apl. 2021, 20, 137–146. [Google Scholar] [CrossRef]
- Tarifa, T.; Yensen, E. Mamíferos de los bosques de Polylepis de Bolivia. Rev. Boliv. Ecol. Conserv. Ambient. 2001, 9, 29–43. [Google Scholar]
- Servat, G.P.; Mendoza, W.; Ochoa, J.A. Flora y fauna de cuatro bosques de Polylepis (Rosaceae) en la Cordillera del Vilcanota (Cusco, Peru). Ecol. Apl. 2002, 1, 25–35. [Google Scholar] [CrossRef]
- Canales, Á.; Huarasa, Y. Poder germinativo de Polylepis incana con aplicación de diferentes tratamientos de agua. Rev. Cuba. Cienc. For. 2020, 8, 495–506. [Google Scholar]
- Jameson, J.S.; Ramsay, P.M. Changes in high-altitude Polylepis forest cover and quality in the Cordillera de Vilcanota, Peru, 1956–2005. Biol. Conserv. 2007, 138, 38–46. [Google Scholar] [CrossRef]
- Cierjacks, A.; Salgado, S.; Wesche, K.; Hensen, I. Post-fire population dynamics of two tree species in high-altitude Polylepis forests of central Ecuador. Biotropica 2008, 40, 176–182. [Google Scholar] [CrossRef]
- Teich, I.; Cingolani, A.M.; Renison, D.; Hensen, I.; Giorgis, M.A. Do domestic herbivores retard Polylepis australis Bitt. woodland recovery in the mountains of Córdoba, Argentina? For. Ecol. Manag. 2005, 219, 229–241. [Google Scholar] [CrossRef]
- Purcell, J.; Brelsford, A. Reassessing the causes of decline of Polylepis, a tropical subalpine forest. Ecotropica 2004, 10, 155–158. [Google Scholar]
- Kessler, M. Bosques de Polylepis. Bot. Econ. Andes Cent. 2006, 11, 110–120. [Google Scholar]
- Zutta, B.R.; Rundel, P.W.; Saatchi, S.; Casana, J.D.; Gauthier, P.; Soto, A.; Velazco, Y.; Buermann, W. Prediciendo la distribución de Polylepis: Bosques Andinos vulnerables y cada vez más importantes. Rev. Peru. Biol. 2012, 19, 205–212. [Google Scholar] [CrossRef]
- Segovia-Salcedo, M.C.; Domic, A.; Boza, T.E.; Kessler, M. Situación taxonómica de las especies del género Polylepis: Implicancias para los estudios ecológicos, la conservación y la restauración de sus bosques. Ecol. Austral 2018, 28, 188–201. [Google Scholar] [CrossRef]
- Boa, E. An illustrated guide to the state of health of trees. In Recognition and Interpretation of Symptoms and Damage, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 1–3. [Google Scholar]
- Díaz-Rivera, J.C.; Aguirre-Salado, C.A.; Loredo-Osti, C.; Escoto-Rodríguez, M. Identificación del estado fitosanitario de árboles mediante aprendizaje automático e imágenes de muy alta resolución espacial. Sci. Agropecu. 2024, 15, 177–189. [Google Scholar] [CrossRef]
- Macías-Muro, A.; Martínez-Trinidad, T.; Valdez-Lazalde, J.R.; Romero-Sánchez, M.E.; Vaquera-Huerta, H. Evaluación de la salud del arbolado urbano a través de imágenes satelitales en Guadalajara, México. Entreciencias 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Qiu, Q.; Lau, D. Assessment of Trees’ Structural Defects via Hybrid Deep Learning Methods Used in Unmanned Aerial Vehicle (UAV) Observations. Forests 2024, 15, 1374. [Google Scholar] [CrossRef]
- Hu, G.; Yin, C.; Wan, M.; Zhang, Y.; Fang, Y. Recognition of diseased Pinus trees in UAV images using deep learning and AdaBoost classifier. Biosyst. Eng. 2020, 194, 138–151. [Google Scholar] [CrossRef]
- Krtalić, A.; Linardić, D.; Pernar, R. Framework for spatial and temporal monitoring of urban forest and vegetation conditions: Case study Zagreb, Croatia. Sustainability 2021, 13, 6055. [Google Scholar] [CrossRef]
- Bernabei, M.; Macchioni, N. La datación dendrocronológica en el estudio de los edificios históricos. Loggia Arquit. Restaur. 2012, 24–25, 104–111. [Google Scholar] [CrossRef]
- Musule, R.; Bárcenas-Pazos, G.M.; Pineda-López, M.R.; Houbron, E.P.; Sánchez-Velásquez, L.R. Desarrollo y evaluación de un método racional y no destructivo para la toma de muestras de maderas blandas utilizadas en análisis químicos. Madera Bosques 2018, 24, e2411427. [Google Scholar] [CrossRef]
- Mendoza, L.; Castro, F.F.; Godoy, V.M. Evaluación visual del estado fito-sanitario de los árboles en 3 paseos (parques) del casco antiguo de la ciudad de colón y su riesgo potencial para la ciudadanía. Rev. Semilla Este 2022, 2, 29–47. [Google Scholar]
- Mojiol, A.R. Tree health assessment for roadside tree in Kota Kinabalu City Centre, Sabah. Borneo Sci. 2023, 39, 104–113. [Google Scholar] [CrossRef]
- Kozlowski, T. Tree Physiology and Forest Pests. Forestry 1969, 67, 118–123. [Google Scholar]
- Del Pilar Colán de la Vega, X.; Rodriguez, J.A.C.; Yanavilca, A.E.M.; Reyes, J.J.A.; Rodríguez, M.J.M. ¿Existe riesgo de caída d e árboles de Schinus molle por la presencia de tumores en la ciudad de Lima? Espac. Desarro. 2019, 34, 175–200. [Google Scholar] [CrossRef]
- Du, X.; Zheng, Y.; Feng, H. Optimizing sensor positions in the stress wave tomography of internal defects in hardwood. Forests 2024, 15, 465. [Google Scholar] [CrossRef]
- Zhang, J.; Khoshelham, K. 3D reconstruction of internal wood decay using photogrammetry and sonic tomography. Photogramm. Rec. 2020, 35, 357–374. [Google Scholar] [CrossRef]
- Socco, L.; Sambuelli, L.; Martinis, R.; Comino, E.; Nicolotti, G. Feasibility of ultrasonic tomography for nondestructive testing of decay on living trees. Res. Nondestruct. Eval. 2004, 15, 31–54. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Hawwa, M.A. IC Tomography and Infrared Tomography Techniques to Monitor Defect Sites in Palm Tree Trunks. Adv. Mater. Res. 2012, 445, 1053–1057. [Google Scholar] [CrossRef]
- Johnstone, D.; Moore, G.; Tausz, M.; Nicolas, M. The Measurement of Wood Decay in Landscape Trees. Arboric. Urban For. 2010, 36, 121–127. [Google Scholar] [CrossRef]
- Duarte Coelho, A.P. Urban Tree Risk Assessment: Proposal of a Protocol for Montevideo, Uruguay. Ph.D. Thesis, Universidad de la República, Montevideo, Uruguay, 2021. [Google Scholar]
- Suchocka, M.; Jelonek, T.; Błaszczyk, M.; Wińska-Krysiak, M.; Kubus, M.; Ziemiański, M.; Kalaji, H.M. Risk assessment of hollow-bearing trees in urban forests. Sci. Rep. 2023, 13, 22214. [Google Scholar] [CrossRef]
- Ostrovsky, R.; Kobza, M.; Gažo, J. Extensively damaged trees tested with acoustic tomography considering tree stability in urban greenery. Trees 2017, 31, 1015–1023. [Google Scholar] [CrossRef]
- Karlinasari, L.; Lestari, A.T.; Nababan, M.Y.S.; Siregar, I.Z.; Nandika, D. Assessment of urban tree condition using sonic tomography technology. IOP Conf. Ser. Earth Environ. Sci. 2018, 203, 012030. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Ballesteros, J.O.; Barrios-Rodriguez, C.A.; Bonadies, E.F.; Cedeño-Sánchez, M.L.; Fossatti-Caballero, N.J.; Trejos- Rodríguez, M.M.; Pérez-Suñiga, J.M.; Holub-Young, K.S.; Henn, L.A.W.; et al. Use of Sonic Tomography to Detect and Quantify Wood Decay in Living Trees. Appl. Plant Sci. 2016, 4, 1600060. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lee, G.; Shin, J. Reliability of Noninvasive Sonic Tomography for the Detection of Internal Defects in Old, Large Trees of Abies holophylla Maxim. Forests 2021, 12, 1131. [Google Scholar] [CrossRef]
- Cardenas-Rengifo, G.P.; Baselly-Villanueva, J.R.; Chumbimune-Vivanco, S.Y.; Macedo-Ramírez, A.T.; Salazar, E.; Minaya, B.; Quintana, S.; Cabudivo, A.; Palma, S.S.A.; Álvarez-Álvarez, P.; et al. Using Acoustic Tomography to Model Wood Deterioration in Cedrelinga cateniformis Ducke in the Peruvian Amazon. Forests 2024, 15, 778. [Google Scholar] [CrossRef]
- León, B.; Roque, J.; Ulloa Ulloa, C.; Pitman, N.; Jørgensen, P.M.; Cano, A. (Eds.) El Libro Rojo de las Plantas Endémicas del Perú [The Red Book of Endemic Plants of Peru]; Universidad Nacional Mayor de San Marcos: Lima, Peru, 2006. [Google Scholar]
- Bax, V.; Castro-Núñez, A.; Francesconi, W. Assessment of potential climate change impacts on montane forests in the Peruvian Andes: Implications for conservation prioritization. Forests 2021, 12, 375. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest Health and Global Change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Paulino, E.; La Torre, M.I.; Huamán, L. Altitudinal distribution of the phanerogamic flora in Oyón, Lima, Peru. Biologist 2013, 13, 21–33. [Google Scholar]
- SENAMHI. Available online: https://www.senamhi.gob.pe/site/descarga-datos/ (accessed on 23 October 2024).
- Angulo-Ruiz, W.E.; Fasabi-Pashanasí, H.; Rengifo-Pérez, C.P.; Valdivia-Márquez, L.N. Non-Destructive Technique Based on Acoustic Tomography for the Identification of Internal Defects in Trees. Sci. Agropecu. 2021, 12, 65–71. [Google Scholar] [CrossRef]
- Chumbimune, S.Y.; Cardenas, G.P.; Saravia, D.; Valqui, L.; Salazar, W.; Arbizu, C.I. Methodology for avocado (Persea americana Mill.) orchard evaluation using different measurement technologies. Chil. J. Agric. Anim. Sci. 2022, 38, 259–273. [Google Scholar] [CrossRef]
- Angulo Ruíz, W.E. Estudio de Sanidad Forestal Mediante Técnicas Acústicas No Destructivas de una Plantación Forestal “Tornillo” Proveniente de la Región Loreto, 1st ed.; Ministerio de Agricultura y Riego (MINAGRI): Lima, Peru, 2018; pp. 1–8. [Google Scholar]
- Helmanto, H.; Kristiati, E.; Wardhani, F.F.; Zulkarnaen, R.N.; Sahromi; Mujahidin; Rachmadiyanto, A.N.; Abdurachman. Tree health assessment of Agathis borneensis Warb. in Bogor Botanical Garden using arborsonic. IOP Conf. Ser. Earth Environ. Sci. 2018, 203, 012032. [Google Scholar] [CrossRef]
- Simpson, B.B. A Revision of the Genus Polylepis (Rosaceae: Sanguisorbeae), 43rd ed.; Smithsonian Contributions to Botany: Washington, DC, USA, 1979; pp. 15–56. [Google Scholar] [CrossRef]
- Kessler, M.; Schmidt-Lebuhn, A.N. Taxonomical and distributional notes on Polylepis (Rosaceae). Org. Divers. Evol. 2006, 6, 67–70. [Google Scholar] [CrossRef]
- Brooks, M.; Kristensen, K.; van Benthem, K.; Magnusson, Á.; Berg, C.; Nielsen, A.; Skaug, H.; Mächler, M.; Bolker, B. glmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Brashaw, B.K.; Bucur, V.; Divos, F.; Gonçalves, R. Nondestructive Testing and Evaluation of Wood: A Worldwide Research Update. For. Prod. J. 2009, 59, 7–14. [Google Scholar]
- Baláš, M.; Gallo, J.; Kuneš, I. Work sampling and work process optimization in sonic and electrical resistance tree tomography. J. For. Sci. 2020, 66, 9–21. [Google Scholar] [CrossRef]
- Toro, M.E.; Velásquez, J.E. Aplicaciones del ultrasonido en la evaluación no destructiva de la madera. Rev. Copérnic. 2005, 2, 273–282. [Google Scholar]
- Lin, C.J.; Huang, Y.H.; Huang, G.S.; Wu, M.L. Detection of Decay Damage in Iron-Wood Living Trees by Nondestructive Techniques. J. Wood Sci. 2016, 62, 42–51. [Google Scholar] [CrossRef]
- Morales, M.J.; Rodríguez, C.; Rubio de Hita, P. Use of Ultrasound as a Nondestructive Evaluation Technique for Sustainable Interventions on Wooden Structures. Build. Environ. 2014, 82, 247–257. [Google Scholar] [CrossRef]
- Oliveira, F.G.R.; Campos, J.A.O.; Pletz, E.; Sales, A. Nondestructive Evaluation of Wood Using Ultrasonic Technique. Maderas Cienc. Technol. 2002, 4, 133–139. [Google Scholar] [CrossRef]
- Fathi, H.; Nasir, V.; Kazemirad, S. Prediction of the Mechanical Properties of Wood Using Guided Wave Propagation and Machine Learning. Constr. Build. Mater. 2020, 262, 120848. [Google Scholar] [CrossRef]
- Vergara, P.M.; Fierro, A.; Carvajal, M.A.; Alaniz, A.J. Using sonic tomography to assess the relationship between internal wood decay and saproxylic beetle communities. Environ. Technol. Innov. 2022, 28, 102677. [Google Scholar] [CrossRef]
- Coelho-Duarte, A.P.; Vallejos-Barra, Ó.; Ponce-Donoso, M. Evaluación de la probabilidad de falla de árboles urbanos usando tecnologías no destructivas. Mem. Investig. Ing. 2023, 24, 172–187. [Google Scholar] [CrossRef]
- Simon, J.; Machar, I.; Brus, J.; Pechanec, V. Combining a growth-simulation model with acoustic-wood tomography as a decision-support tool for adaptive management and conservation of forest ecosystems. Ecol. Inform. 2015, 30, 309–312. [Google Scholar] [CrossRef]
- Son, J.; Kim, S.; Shin, J.; Lee, G.; Kim, H. Reliability of non-destructive sonic tomography for detection of defects in old Zelkova serrata (Thunb.) Makino trees. For. Sci. Technol. 2021, 17, 110–118. [Google Scholar] [CrossRef]
- Marra, R.E.; Brazee, N.J.; Fraver, S. Estimating carbon loss due to internal decay in living trees using tomography: Implications for forest carbon budgets. Environ. Res. Lett. 2018, 13, 105004. [Google Scholar] [CrossRef]
- Rachmadiyanto, A.N.; Hariri, M.R.; Primananda, E.; Suhatman, A.; Kuswara, U. Evaluación de la Salud de 12 Árboles Emblemáticos Patrimoniales en los Jardines Botánicos de Bogor. Bol. Kebun Raya 2021, 24, 104–116. [Google Scholar] [CrossRef]
- Swari, K.K.I.; Sari, D.A.I.T.; Hanum, S.F.; Rahayu, A. Evaluation on Fallen Trees of Hesperocyparis guadalupensis (S. Watson) Bartel and Pavetta sp. in Bali Botanic Garden Based on Visual Assessment and Acoustic Tomography. J. Ilmu Kehutan. 2022, 16, 108–114. [Google Scholar] [CrossRef]
- Nowak, T.P.; Jasieńko, J.; Hamrol-Bielecka, K. In Situ Assessment of Structural Timber Using the Resistance Drilling Method–Evaluation of Usefulness. Constr. Build. Mater. 2016, 102, 403–415. [Google Scholar] [CrossRef]
- Goh, C.L.; Rahim, R.A.; Rahiman, M.H.F.; Talib, M.T.M.; Tee, Z.C. Sensing Wood Decay in Standing Trees: A Review. Sens. Actuators Phys. 2017, 269, 276–282. [Google Scholar] [CrossRef]
- Soge, A.O.; Popoola, O.I.; Adetoyinbo, A.A. Detection of Wood Decay and Cavities in Living Trees: A Review. Can. J. For. Res. 2020, 51, 937–947. [Google Scholar] [CrossRef]
- Bleive, A.; Liepins, J.; Liepins, K. Internal Decay Assessment Using Drilling Resistance in Mature Common Alder (Alnus glutinosa (L.) Gaertn.) Stands. For. Wood Process. 2022, 37, 37–43. [Google Scholar] [CrossRef]
- Brancheriau, L.; Ghodrati, A.; Gallet, P.; Thaunay, P.; Lasaygues, P. Application of ultrasonic tomography to characterize the mechanical state of standing trees (Picea abies). J. Phys. Conf. Ser. 2012, 353, 012007. [Google Scholar] [CrossRef]
- Wang, X.; Allison, R. Decay Detection in Red Oak Trees Using a Combination of Visual Inspection, Acoustic Testing, and Resistance Microdrilling. Arboric. Urb. For. 2008, 34, 1–4. [Google Scholar] [CrossRef]
- Echavarría-Cháirez, F.G.; Medina-García, G.; Ruiz-Corral, J.A. Efecto en la Erosión Hídrica del Suelo en Pastizales y Otros Tipos de Vegetación por Cambios en el Patrón de Lluvias por el Calentamiento Global en Zacatecas, México. Rev. Mex. Cienc. Pecu. 2020, 11, 63–74. [Google Scholar] [CrossRef]
- Avon, C.; Dumas, Y.; Bergès, L. Management practices increase the impact of roads on plant communities in forests. Biol. Conserv. 2013, 159, 24–31. [Google Scholar] [CrossRef]
- Rangel-Churio, O.; Arellano-Peña, H. Bosques de Polylepis: Un tipo de vegetación condenado a la extinción. In Colombia Diversidad Biotica X: Cambio Global (Natural) y Climático (Antrópico) en el Páramo Colombiano; Rangel-Churio, O., Ed.; Instituto de Ciencias Naturales: Bogotá, Colombia, 2010; pp. 443–478. [Google Scholar]
- Johnson, D.J.; Magee, L.; Pandit, K.; Bourdon, J.; Broadbent, E.N.; Glenn, K.; Kaddoura, Y.; Machado, S.; Nieves, J.; Wilkinson, B.E.; et al. Canopy Tree Density and Species Influence Tree Regeneration Patterns and Woody Species Diversity in a Longleaf Pine Forest. For. Ecol. Manag. 2021, 490, 119082. [Google Scholar] [CrossRef]
- Vistín, D.A.; Salas, E.M.; Balseca, J.E.; Lara, N.X. Distribución potencial de Polylepis incana en los Andes ecuatorianos para estudios de fisiología vegetal y planes de rehabilitación forestal. Ecol. Austral 2022, 33, 001–012. [Google Scholar] [CrossRef]
- Fajardo-Gutiérrez, F.; Infante-Betancour, J.; Cabrera-Amaya, D.M. Modelización de la distribución potencial del género Polylepis en Colombia y consideraciones para su conservación. Ecol. Austral 2018, 28 (Suppl. 1), 202–215. [Google Scholar] [CrossRef]
- Sevillano-Ríos, C.S.; Rodewald, A.D. Avian community structure and habitat use of Polylepis forests along an elevation gradient. PeerJ 2017, 5, e3220. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Kessler, M.; Ruokolainen, K.; Hertel, D. Accessibility predicts structural variation of Andean Polylepis forests. Biodivers. Conserv. 2011, 20, 1789–1802. [Google Scholar] [CrossRef]
- Kosmala, M.; Rosłon-Szeryńska, E.; Suchocka, M. Influence of mechanical damage on the condition of trees. Ann. Wars. Univ. Life Sci.—SGGW Hortic. Landsc. Archit. 2008, 29, 137–144. [Google Scholar]
- Kacprzyk, M.; Matsiakh, I.; Musolin, D.L.; Selikhovkin, A.V.; Baranchikov, Y.N.; Burokiene, D.; Cech, T.; Talgø, V.; Vettraino, A.M.; Vannini, A.; et al. Damage to stems, branches and twigs of broadleaf woody plants. In Field Guide for the Identification of Damage on Woody Sentinel Plants, 1st ed.; Roques, A., Cleary, M., Matsiakh, I., Eschen, R., Eds.; CABI: Wallingford, UK, 2017; pp. 104–134. [Google Scholar]
- Purcell, L. Lo Esencial para la Poda de Árboles. For. Nat. Resour. 2015, 506, 1–18. [Google Scholar]
- Downer, A.J.; Perry, E.J. Wood Decay Fungi in Landscape Trees. Pest Notes 2019, 74109, 1–6. [Google Scholar]
- Sosa, D.F. Aislamiento y Evaluación de la Actividad Enzimática de Hongos Descomponedores de Madera en la Reserva Natural la Montaña del Ocaso, Quimbaya-Quindío. Bachelor’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2006. [Google Scholar]
- González, V.; Tuset, J.J.; Hinarejos, R. Hongos asociados a la podredumbre del leño (caries) de los cítricos. Levante Agric. 2007, 384, 60–65. [Google Scholar]
- Prieto, D.; Devesa, J.; Martínez, E.L.; Alvarez, C. Ceratocystis fimbriata como Causa de la Llaga Macana del Cafeto. Rev. Prot. Veg. 1987, 2, 16–21. [Google Scholar]
- Arguedas-Gamboa, M. Clasificación de Tipos de Daños Producidos por Insectos Forestales. Segunda Parte. Rev. For. Mesoam. Kurú 2006, 3, 64. [Google Scholar]
- Magne Flores, A.M. Valoración Fitosanitaria de la Kiswara (Buddleja coriácea Remy) y la Queñua (Polylepis incana Kunth) Especies Ornamentales en la Ciudad de El Alto-La Paz. Bachelor’s Thesis, Universidad Mayor de San Andrés, La Paz, Bolivia, 2015. [Google Scholar]
- Delgado, L.; Pedraza Pérez, R.A. La Madera Muerta de los Ecosistemas Forestales. For. Veracruzana 2002, 4, 59–66. [Google Scholar]
- García Guzmán, S.M.; Moreta Reyes, D.M. Identificación y Control de Plagas y Enfermedades en Polylepis racemosa en la Zona de Toacazo, Tanicuchi y Pastocalle. Bachelor’s Thesis, Universidad Politécnica Salesiana, Quito, Ecuador, 2019. [Google Scholar]
- Cuyckens, G.A.E.; Christie, D.A.; Domic, A.I.; Malizia, L.R.; Renison, D. Climate change and the distribution and conservation of the world’s highest elevation woodlands in the South American Altiplano. Glob. Planet Change 2016, 137, 79–87. [Google Scholar] [CrossRef]
- Franco-León, P.; Navarro Guzman, M.A.; Oyague Passuni, E.; Ignacio Apaza, J.; Jove Chipana, C. Fragmentation, birds, and conservation of the Polylepis Forest in Southern Peru. Cuad. Investig. Geogr. 2024, 50, 179–199. [Google Scholar] [CrossRef]
- Quinteros-Casaverde, N.; Flores-Negrón, C.F.; Williams, D.A. Low Genetic Diversity and Fragmentation Effects in a Wind- Pollinated Tree, Polylepis multijuga Plige (Rosaceae) in the High Andes. Conserv. Genet. 2012, 13, 593–603. [Google Scholar] [CrossRef]
- Simpson, B.B. Speciation and Specialization of Polylepis in the Andes. High Altitude Tropical Biogeography; Vulllemeire, F., Monasterio, M., Eds.; Oxford University Press: Oxford, UK, 1986; pp. 304–316. [Google Scholar]
- Gareca, E.E.; Breyne, P.; Vandepitte, K.; Cahill, J.R.A.; Fernandez, M.; Honnay, O. Genetic Diversity of Andean Polylepis Woodlands and Inferences Regarding Their Fragmentation History. Bot. J. Linn. Soc. 2013, 172, 544–554+653. [Google Scholar] [CrossRef]
- Useros Fernández, J.L. El Cambio Climático: Sus Causas y Efectos Medioambientales. An. R. Acad. Med. Cir. Val. 2013, 50, 71–98. [Google Scholar]
- Segovia-Salcedo, M.C.; Caiza Guamba, J.C.; Kessler, M.; Ramsay, P.M.; Espinoza Boza, T.; Renison, D.; Quispe-Melgar, H.R.; Urquiaga-Flores, E.; Rodriguez-Caton, M.; Ames-Martínez, F.N.; et al. ¿Cómo Avanzar en la Conservación de los Bosques de Polylepis y su Diversidad Biológica? Neotrop. Biodivers. 2021, 7, 318–326. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Argibay, D.S.; Renison, D. Efecto del fuego y la ganadería en bosques de Polylepis australis (Rosaceae) a lo largo de un gradiente altitudinal en las montañas del centro de la Argentina. Bosque 2018, 39, 145–150. [Google Scholar] [CrossRef]
- Campomanes Principe, Y.T. Escenario de Distribución de los Bosques de Polylepis al 2030 Frente a los Elementos Climatológicos de Temperatura y Precipitación, en el Distrito de Pomabamba-Ancash, Utilizando Maxent y GIS, 2017. Bachelor’s Thesis, Universidad César Vallejo, Lima, Peru, 2017. [Google Scholar]
- Andrade, C. Sample Size and Its Importance in Research. Indian J. Psychol. Med. 2020, 42, 102–103. [Google Scholar] [CrossRef]
- Sevillano-Ríos, C.S.; Rodewald, A.D.; Morales, L.V. Ecología y conservación de las aves asociadas con Polylepis:¿qué sabemos de esta comunidad cada vez más vulnerable? Ecol. Austral 2018, 28, 216–222. [Google Scholar] [CrossRef]




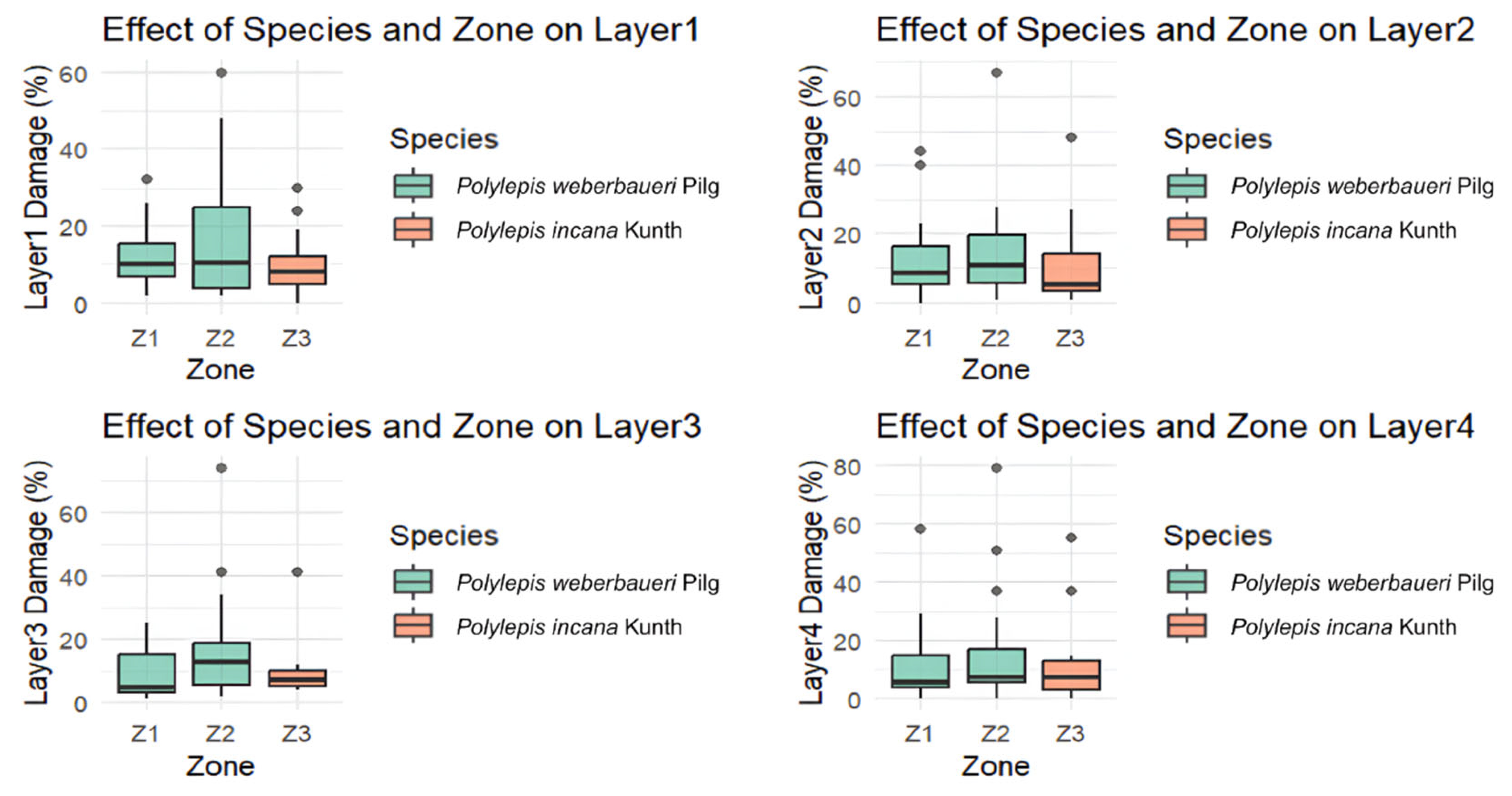
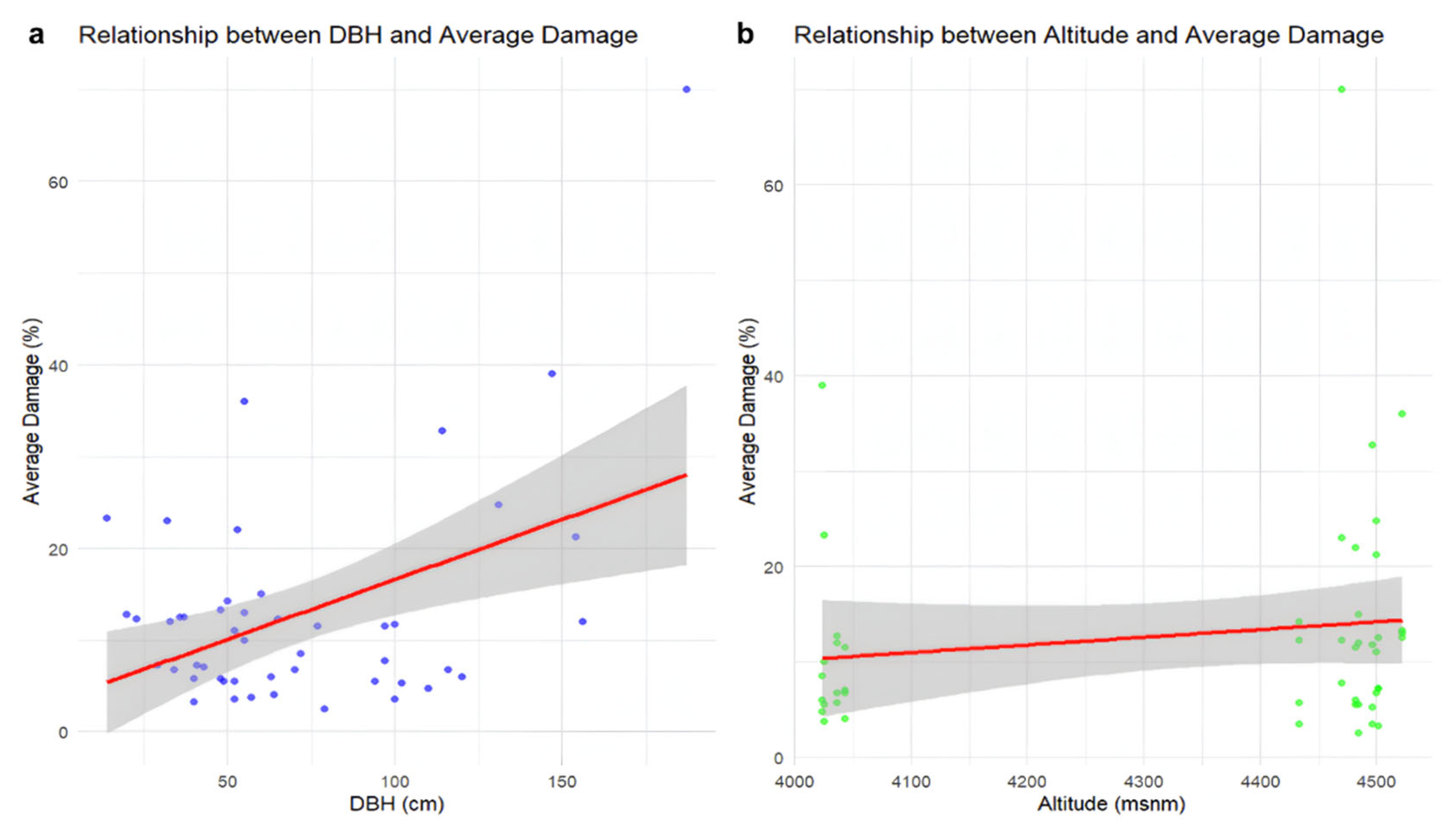
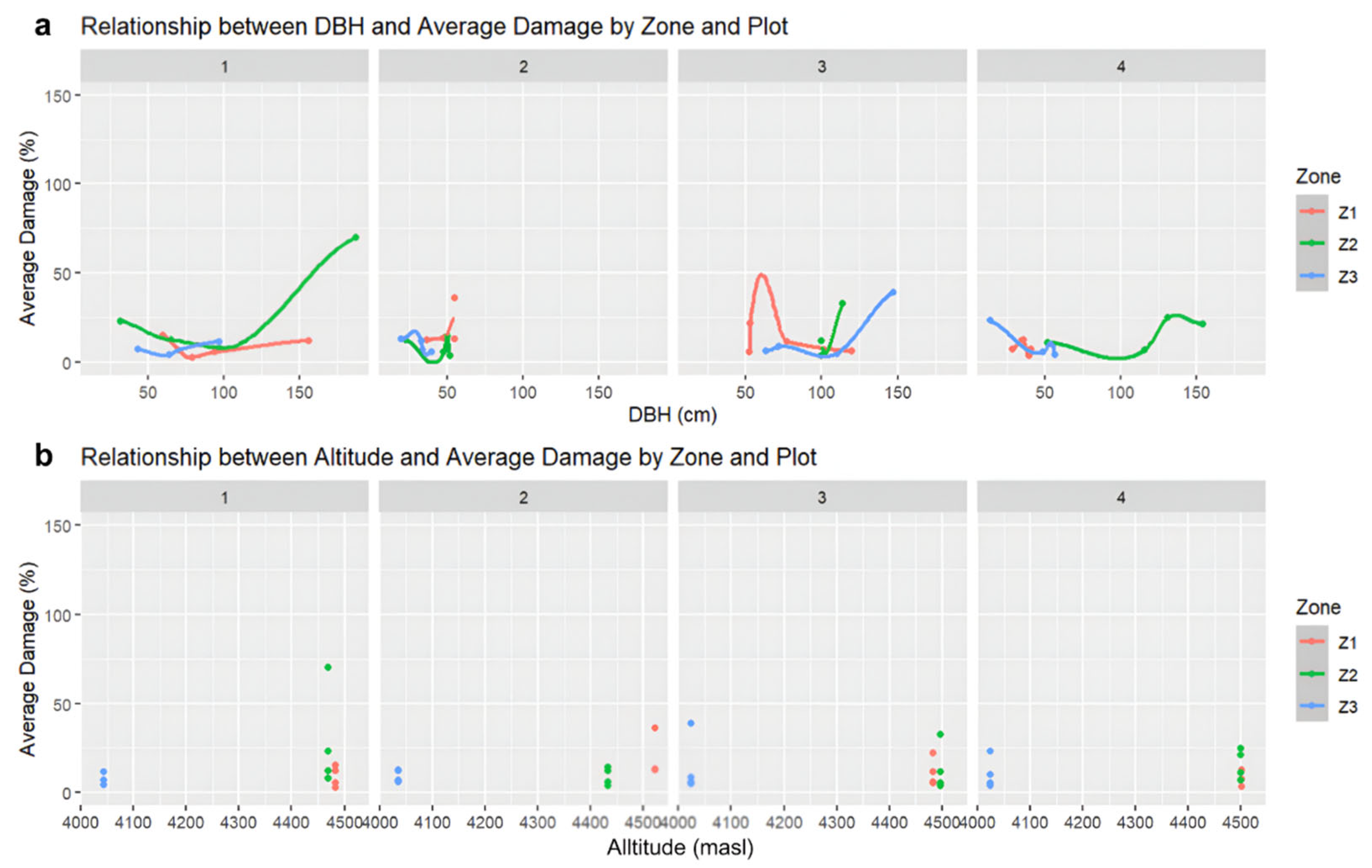
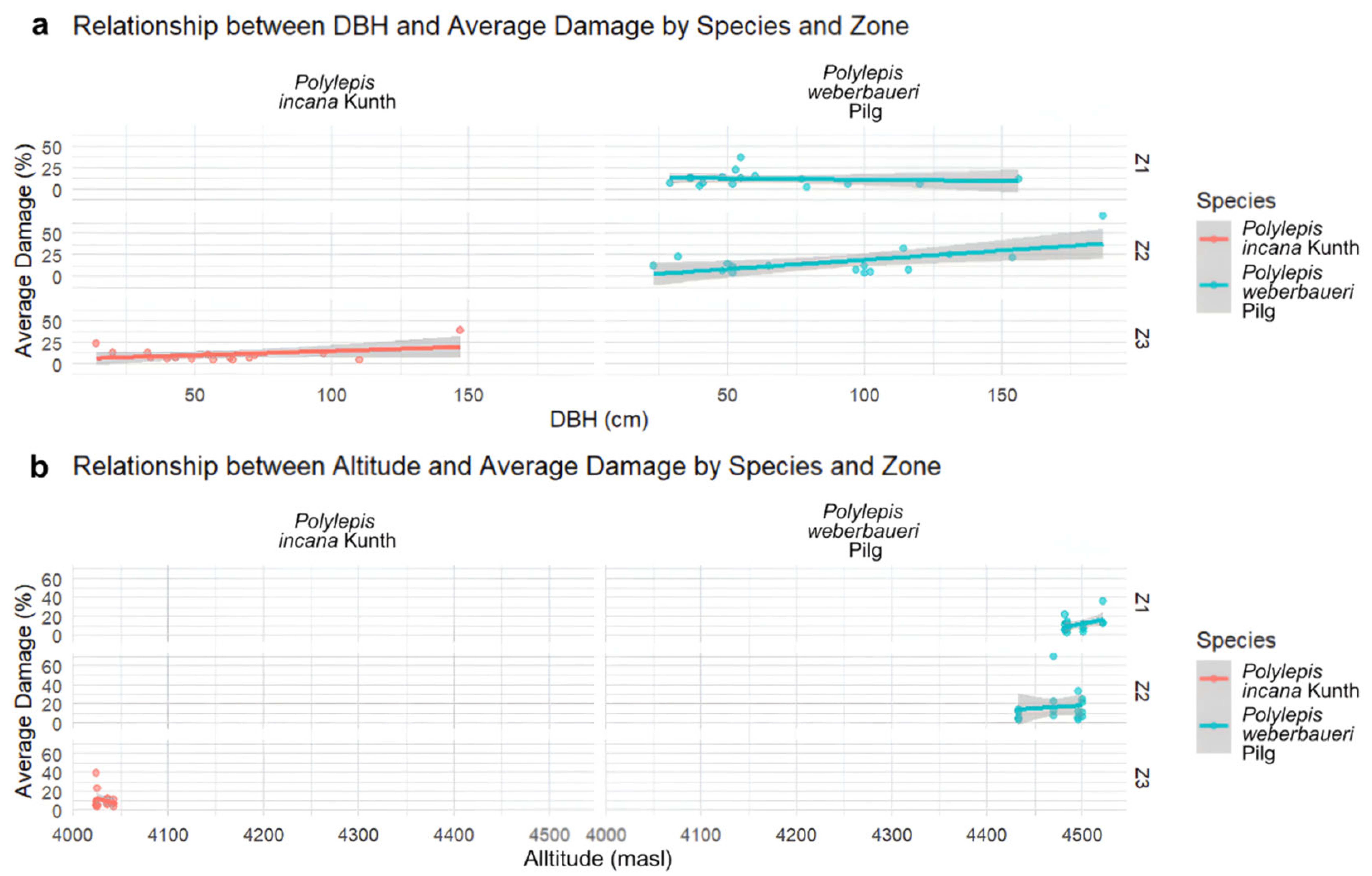
| Study Area | Plot | Tree |
DBH (cm) |
Altitude m a.s.l. | Layer 1 | Layer 2 | Layer 3 | Layer 4 |
Average Damage. % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 94 | 4484 | 9 | 3 | 4 | 6 | 5.5 |
| 1 | 1 | 2 | 79 | 4484 | 4 | 3 | 1 | 2 | 2.5 |
| 1 | 1 | 3 | 60 | 4484 | 14 | 15 | 16 | 15 | 15 |
| 1 | 1 | 4 | 156 | 4484 | 22 | 21 | 3 | 2 | 12 |
| 1 | 2 | 1 | 55 | 4522 | 10 | 10 | 3 | 29 | 13 |
| 1 | 2 | 2 | 37 | 4522 | 8 | 12 | 16 | 14 | 12.5 |
| 1 | 2 | 3 | 48 | 4522 | 26 | 7 | 4 | 16 | 13.25 |
| 1 | 2 | 4 | 55 | 4522 | 19 | 44 | 23 | 58 | 36 |
| 1 | 3 | 1 | 120 | 4482 | 11 | 0 | 5 | 8 | 6 |
| 1 | 3 | 2 | 52 | 4482 | 7 | 6 | 4 | 5 | 5.5 |
| 1 | 3 | 3 | 77 | 4482 | 10 | 7 | 25 | 4 | 11.5 |
| 1 | 3 | 4 | 53 | 4482 | 32 | 40 | 12 | 4 | 22 |
| 1 | 4 | 1 | 36 | 4501 | 6 | 23 | 6 | 15 | 12.5 |
| 1 | 4 | 2 | 29 | 4501 | 6 | 4 | 15 | 4 | 7.25 |
| 1 | 4 | 3 | 40 | 4501 | 2 | 6 | 2 | 3 | 3.25 |
| 1 | 4 | 4 | 41 | 4501 | 13 | 15 | 1 | 0 | 7.25 |
| 2 | 1 | 1 | 97 | 4470 | 11 | 9 | 3 | 8 | 7.75 |
| 2 | 1 | 2 | 32 | 4470 | 29 | 10 | 2 | 51 | 23 |
| 2 | 1 | 3 | 65 | 4470 | 15 | 1 | 20 | 13 | 12.25 |
| 2 | 1 | 4 | 187 | 4470 | 60 | 67 | 74 | 79 | 70 |
| 2 | 2 | 1 | 52 | 4433 | 5 | 2 | 4 | 3 | 3.5 |
| 2 | 2 | 2 | 23 | 4433 | 14 | 13 | 17 | 5 | 12.25 |
| 2 | 2 | 3 | 48 | 4433 | 2 | 5 | 9 | 7 | 5.75 |
| 2 | 2 | 4 | 50 | 4433 | 25 | 22 | 10 | 0 | 14.25 |
| 2 | 3 | 1 | 100 | 4496 | 10 | 12 | 18 | 7 | 11.75 |
| 2 | 3 | 2 | 100 | 4496 | 3 | 6 | 3 | 2 | 3.5 |
| 2 | 3 | 3 | 114 | 4496 | 25 | 28 | 41 | 37 | 32.75 |
| 2 | 3 | 4 | 102 | 4496 | 3 | 2 | 6 | 10 | 5.25 |
| 2 | 4 | 1 | 52 | 4500 | 10 | 14 | 14 | 6 | 11 |
| 2 | 4 | 2 | 154 | 4500 | 4 | 19 | 34 | 28 | 21.25 |
| 2 | 4 | 3 | 116 | 4500 | 3 | 6 | 11 | 7 | 6.75 |
| 2 | 4 | 4 | 131 | 4500 | 48 | 25 | 15 | 11 | 24.75 |
| 3 | 1 | 1 | 64 | 4043 | 4 | 1 | 5 | 6 | 4 |
| 3 | 1 | 2 | 70 | 4043 | 0 | 8 | 10 | 9 | 6.75 |
| 3 | 1 | 3 | 97 | 4043 | 15 | 18 | 10 | 3 | 11.5 |
| 3 | 1 | 4 | 43 | 4043 | 5 | 5 | 5 | 13 | 7 |
| 3 | 2 | 1 | 40 | 4036 | 10 | 4 | 6 | 3 | 5.75 |
| 3 | 2 | 2 | 33 | 4036 | 11 | 27 | 5 | 5 | 12 |
| 3 | 2 | 3 | 20 | 4036 | 6 | 18 | 12 | 15 | 12.75 |
| 3 | 2 | 4 | 34 | 4036 | 8 | 6 | 4 | 9 | 6.75 |
| 3 | 3 | 1 | 72 | 4024 | 11 | 7 | 7 | 9 | 8.5 |
| 3 | 3 | 2 | 147 | 4024 | 30 | 48 | 41 | 37 | 39 |
| 3 | 3 | 3 | 110 | 4024 | 4 | 5 | 7 | 3 | 4.75 |
| 3 | 3 | 4 | 63 | 4024 | 8 | 4 | 10 | 2 | 6 |
| 3 | 4 | 1 | 57 | 4025 | 6 | 2 | 7 | 0 | 3.75 |
| 3 | 4 | 2 | 55 | 4025 | 19 | 13 | 6 | 2 | 10 |
| 3 | 4 | 3 | 14 | 4025 | 24 | 2 | 12 | 55 | 23.25 |
| 3 | 4 | 4 | 49 | 4025 | 4 | 1 | 4 | 13 | 5.5 |
| Z1 | Z2 | Z3 | Conservation Status | |
|---|---|---|---|---|
| Polylepis weberbaueri Pilg. | ✓ | ✓ | ✗ | aNT, cVu |
| Polylepis incana Kunth | ✗ | ✗ | ✓ | bLC, dCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinteros-Gómez, Y.; Salinas-Inga, A.; Macedo-Bedoya, J.; Peralta-Alcantara, E.; La Rosa-Sánchez, M.; Gonzales, F.C.; Yamunaque, A.; Angeles-Alvarez, F.; Gómez-Ticerán, D.; Dávila, O.L.S. Noninvasive Sonic Tomography for the Detection of Internal Defects in Relict Woodlands of Polylepis in Peru. Forests 2025, 16, 957. https://doi.org/10.3390/f16060957
Quinteros-Gómez Y, Salinas-Inga A, Macedo-Bedoya J, Peralta-Alcantara E, La Rosa-Sánchez M, Gonzales FC, Yamunaque A, Angeles-Alvarez F, Gómez-Ticerán D, Dávila OLS. Noninvasive Sonic Tomography for the Detection of Internal Defects in Relict Woodlands of Polylepis in Peru. Forests. 2025; 16(6):957. https://doi.org/10.3390/f16060957
Chicago/Turabian StyleQuinteros-Gómez, Yakov, Abel Salinas-Inga, Jehoshua Macedo-Bedoya, Enzo Peralta-Alcantara, Marcel La Rosa-Sánchez, Fernando Camones Gonzales, Alexandra Yamunaque, Franco Angeles-Alvarez, Doris Gómez-Ticerán, and Olga Lidia Solano Dávila. 2025. "Noninvasive Sonic Tomography for the Detection of Internal Defects in Relict Woodlands of Polylepis in Peru" Forests 16, no. 6: 957. https://doi.org/10.3390/f16060957
APA StyleQuinteros-Gómez, Y., Salinas-Inga, A., Macedo-Bedoya, J., Peralta-Alcantara, E., La Rosa-Sánchez, M., Gonzales, F. C., Yamunaque, A., Angeles-Alvarez, F., Gómez-Ticerán, D., & Dávila, O. L. S. (2025). Noninvasive Sonic Tomography for the Detection of Internal Defects in Relict Woodlands of Polylepis in Peru. Forests, 16(6), 957. https://doi.org/10.3390/f16060957






