Restoration of Understory Plant Species and Functional Diversity in Temperate Plantations Along Successional Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region and Plot Data Collection
2.1.1. Site Description
2.1.2. Sampling Design
2.1.3. Vegetation Data
2.2. Environmental Factors
2.2.1. Environmental Variables at the Landscape Scale
2.2.2. Environmental Variables at Local Scale
2.2.3. Selection of Plant Traits
2.3. Data Analysis
3. Results
4. Discussion
4.1. Accumulation of Species Richness, Diversity, and Functional Diversity
4.2. Divergence of Species Composition
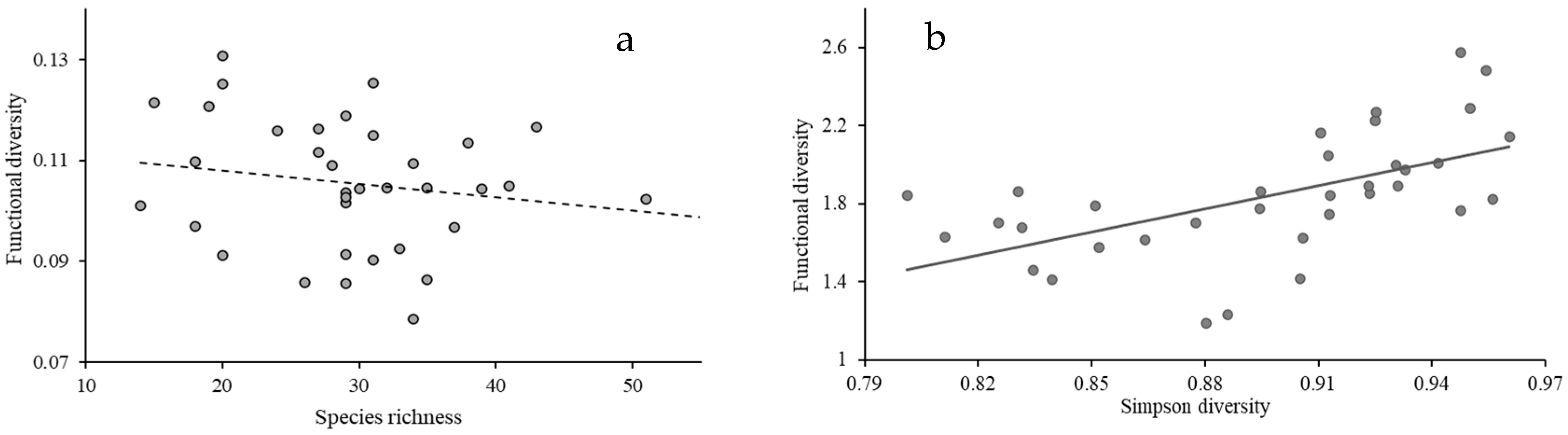
4.3. Relationship Between Functional Diversity and Species Diversity
4.4. Response of Functional Diversity to the Environment
4.5. Soil Biogeochemical Drivers of Biodiversity Dynamics
4.6. Limitations in Experimental Design
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Species Name | Generic Name | Family Name |
|---|---|---|
| Artemisia argyi | Artemisia | Asteraceae |
| Rumex patientia | Rumex | Polygonaceae |
| Cyperus nipponicus | Cyperus | Cyperaceae |
| Imperata cylindrica | Imperata | Poaceae |
| Thymus mongolicus | Thymus | Labiatae |
| Patrinia scabiosaefolia | Patrinia | Valerianaceae |
| Echinochloa crusgalli | Echinochloa | Poaceae |
| Viola variegata | Viola | Violaceae |
| Ixeris sonchifolia | Ixeris | Asteraceae |
| Bupleurum chinense | Bupleurum | Umbellifera |
| Agropyron cristatum | Agropyron | Poaceae |
| Vicia faba | Vicia | Leguminosae |
| Xanthium sibiricum | Xanthium | Asteraceae |
| Melilotus suaveolens | Melilotus | Leguminosae |
| Stellaria dichotoma | Stellaria | Caryophyllaceae |
| Potentilla supina | Potentilla | Rosaceae |
| Plantago asiatica | Plantago | Plantaginaceae |
| Arabis pendula | Arabis | Brassicaceae |
| Salix babylonica | Salix | Salicaceae |
| Cirsium selosum | Cirsium | Asteraceae |
| Allium fistulosum | Allium | Liliaceae |
| Cleistogenes caespitosa | Cleistogenes | Poaceae |
| Geranium dahuricum | Geranium | Geraniaceae |
| Saussurea davurica | Saussurea | Asteraceae |
| Astragalus dahuricus | Astragalus | Leguminosae |
| Calystegia hederacea | Calystegia | Convolvulaceae |
| Cirsium japonicum | Cirsium | Asteraceae |
| Artemisia sieversiana | Artemisia | Asteraceae |
| Euphorbia humifusa | Euphorbia | Euphorbiaceae |
| Cynanchum thesioides | Cynanchum | Asclepiadaceae |
| Sanguisorba officinalis | Sanguisorba | Rosaceae |
| Androsace umbellata | Androsace | Primulaceae |
| Malva crispa | Malva | Malvaceae |
| Lepidium apetalum | Lepidium | Brassicaceae |
| Heracleum hemsleyanum | Heracleum | Umbellifera |
| Clematis brevicaudata | Clematis | Ranunculaceae |
| Potentilla multicaulis | Potentilla | Rosaceae |
| Schizonepeta multifida | Schizonepeta | Labiatae |
| Roegneria kamoji | Roegneria | Poaceae |
| Cynanchum chinense | Cynanchum | Asclepiadaceae |
| Potentilla anserina | Potentilla | Rosaceae |
| Potentilla bifurca | Potentilla | Rosaceae |
| Potentilla discolor | Potentilla | Rosaceae |
| Amaranthus retroflexus | Amaranthus | Amaranthaceae |
| Saposhnikovia divaricata | Pastinaca | Umbellifera |
| Clinopodium chinense | Clinopodium | Labiatae |
| Saussurea runcinata | Saussurea | Asteraceae |
| Chrysanthemum lavandulaefolium | Chrysanthemum | Asteraceae |
| Brassica oleracea | Brassica | Brassicaceae |
| Polygonum alpinum | Polygonum | Polygonaceae |
| Setaria viridis | Setaria | Poaceae |
| Cynodon dactylon | Cynodon | Poaceae |
| Lycium chinense | Lycium | Solanaceae |
| Vicia cracca | Vicia | Leguminosae |
| Bidens pilosa | Bidens | Asteraceae |
| Rorippa indica | Rorippa | Brassicaceae |
| Campylotropis macrocarpa | Campylotropis | Leguminosae |
| Salix chaenomeloides | Salix | Salicaceae |
| Pinus thunbergii | Pinus | Pinaceae |
| Caragana rosea | Caragana | Leguminosae |
| Ostryopsis davidiana | Ostryopsis | Betulaceae |
| Halenia corniculata | Genthianaceae | Halenia |
| Medicago ruthenica | Medicago | Leguminosae |
| Scabiosa tschiliensis | Scabiosa | Dipsacaceae |
| Larix principis-rupprechtii | Larix | Pinaceae |
| Cucumis sativus | Cucumis | Cucurbitaceae |
| Artemisia annua | Artemisia | Asteraceae |
| Polygonatum sibiricum | Polygonatum | Liliaceae |
| Chenopodium glaucum | Chenopodium | Chenopodiaceae |
| Leontopodium leontopodioides | Leontopodium | Asteraceae |
| Agastache rugosa | Labiatae | Agastache |
| Kummerowia striata | Kummerowia | Leguminosae |
| Panicum miliaceum | Panicum | Poaceae |
| Populus × canadensis | Populus | Salicaceae |
| Chenopodium acuminatum | Chenopodium | Chenopodiaceae |
| Equisetum ramosissimum | Equisetum | Equisetaceae |
| Arthraxon hispidus | Arthraxon | Poaceae |
| Vicia sativa | Vicia | Leguminosae |
| Sonchus arvensis | Sonchus | Asteraceae |
| Diospyros lotus | Diospyros | Ebenaceae |
| Mulgedium tataricum | Mulgedium | Asteraceae |
| Sonchus oleraceus | Sonchus | Asteraceae |
| Ixeris polycephala | Ixeris | Asteraceae |
| latifolius Tausch | Echinops | Asteraceae |
| Oxytropis caerulea | Oxytropis | Leguminosae |
| Stellera chamaejasme | Stellera | Euphorbiaceaec |
| Geranium wilfordii | Geranium | Geraniaceae |
| Lomatogonium carinthiacum | Lomatogonium | Gentianaceae |
| Salix chaenomeloides | Salix | Salicaceae |
| Asparagus schoberioides | Aspargus | Aspargus |
| Agrimonia pilosa | Agrimonia | Rosaceae |
| Phragmites australis | Phragmites | Poaceae |
| Rhamnus bungeana | Rhamnus | Rhamnaceae |
| Vigna radiata | Vigna | Leguminosae |
| Ampelopsis humulifolia | Ampelopsis | Vitaceae |
| Portulaca oleracea | Portulaca | Portulacaceae |
| Iris lectea | Iris | Iridaceae |
| Solanum tuberosum | Solanum | Solanaceae |
| Digitaria sanguinalis | Digitaria | Poaceae |
| Datura stramonium | Datura | Solanaceae |
| Erodium stephanianum | Erodium | Geraniaceae |
| Rosa bella | Rosa | Rosaceae |
| Gueldenstaedtia verna | Gueldenstaedtia | Leguminosae |
| Clematis hexapetala | Clematis | Ranunculaceae |
| Unknown | Astragalus | Leguminosae |
| Unknown | Polygonum | Polygonaceae |
| Medicago sativa | Medicago | Leguminosae |
| Hemistepta lyrata | Hemistepta | Asteraceae |
| Caragana korshinskii | Caragana | Leguminosae |
| Caragana korshinskii | Caragana | Leguminosae |
| Aconitum barbatum | Aconitum | Ranunculaceae |
| Myosoton aquaticum | Malachium | Caryophyllaceae |
| Eleusine indica | Eleusine | Poaceae |
| Galium verum | Galium | Rubiaceae |
| Elymus dahuricus | Elymus | Poaceae |
| Thermopsis lanceolata | Thermopsis | Betulaceae |
| Plantago depressa | Plantago | Plantaginaceae |
| Potentilla flagellaris | Potentilla | Rosaceae |
| Taraxacum mongolium | Taraxacum | Asteraceae |
| Stemmacantha uniflora | Stemmacantha | Asteraceae |
| Capsella bursa-pastoris | Capsella | Brassicaceae |
| Rubia cordifolia | Rubia | Rubiaceae |
| Clematis aethusifolia | Clematis | Ranunculaceae |
| Gentiana macrophylla | Gentiana | Gentianaceae |
| Corydalis repens | Corydalis | Papaveraceae |
| Euphorbia esula | Euphorbia | Euphorbiaceae |
| Hippophae rhamnoides | Hippophae | Elaeagnaceae |
| Oxytropis psamocharis | Oxytropis | Leguminosae |
| Allium senescens | Allium | Liliaceae |
| Kalimeris lautureana | Kalimeris | Asteraceae |
| Armeniaca sibirica | Armeniaca | Rosaceae |
| Potentilla kleiniana | Potentilla | Rosaceae |
| Duchesnea indica | Duchesnea | Rosaceae |
| Adenophora polyantha | Adenophora | Campanulaceae |
| Geranium sibiricum | Geranium | Geraniaceae |
| Amethystea caerulea | Amethystea | Labiatae |
| Ammannia baccifera | Ammannia | Lythraceae |
| Polygonum lapathifolium | Polygonum | Polygonaceae |
| Medicago lupluina | Medicago | Leguminosae |
| Asparagus cochinchinensis | Asparagus | Liliaceae |
| Hyoscyamus niger | Hyoscyamus | Solanaceae |
| Convolvulus arvensis | Convolvulus | Convolvulaceae |
| Beta vulgaris | Beta | Chenopodiaceae |
| Acalypha australis | Acalypha | Euphorbiaceaec |
| Mazus japonicus | Mazus | Ranunculaceae |
| Spiraea pubescens | Spiraea | Rosaceae |
| Orostachys fimbriatus | Orostachys | Crassulaceae |
| Vicia unijuga | Vicia | Leguminosae |
| Potentilla chinensis | Potentilla | Rosaceae |
| Equisetum arvense | Equisetum | Equisetaceae |
| Saussurea ussuriensis | Saussurea | Asteraceae |
| Myosotis silvatica | Myosotis | Boraginaceae |
| Carex rigescens | Carex | Cyperaceae |
| Berberis poiretii | Berberis | Berberidaceae |
| Leonurus sibiricus | Leonurus | Labiatae |
| Polygala tenuifolia | Polygala | Polygalaceae |
| Unknown | Sedum | Crassulaceae |
| Dracocephalum moldavica | Dracocephalum | Labiatae |
| Elsholtzia ciliata | Elsholtzia | Labiatae |
| Chrysanthemum chanetii | Chrysanthemum | Asteraceae |
| Bidens parviflora | Bidens | Asteraceae |
| Oxytropis glabra | Oxytropis | Leguminosae |
| Rhamnus parvifolia | Rhamnus | Rhamnaceae |
| Populus simonii | Populus | Salicaceae |
| Inula japonica | Inula | Asteraceae |
| Scorzonera austriaca | Scorzonera | Asteraceae |
| Linum usitatissimum | Linum | Linaceae |
| Corydalis yanhusuo | Corydalis | Papaveraceae |
| Leymus chinense | Leymus | Poaceae |
| Glycine soja | Glycine | Leguminosae |
| Arundinella hirta | Arundinella | Poaceae |
| Deyeuxia arundinacea | Deyeuxia | Poaceae |
| Avena fatua | Avena | Poaceae |
| Papaver nudicaule | Papaver | Papaveraceae |
| Iris dichotoma | Iris | Iridaceae |
| Leonurus artemisia | Leonurus | Labiatae |
| Artemisia capillaris | Artemisia | Asteraceae |
| Poa sphondylodes | Poa | Poaceae |
| Pinus tabulaeformis | Pinus | Pinaceae |
| Ulmus pumila | Ulmus | Ulmaceae |
| Ulmus pumila | Ulmus | Ulmaceae |
| Achnatherum sibiricum | Achnatherum | Poaceae |
| Zea mays | Zea | Poaceae |
| polygonatum odratum | Polygonatum | Liliaceae |
| Pharbitis purpurea | Pharbitis | Convolvulaceae |
| Polygala tenuifolia | Polygala | Polygalaceae |
| Viola prioantha | Viola | Violaceae |
| Poa annua | Poa | Poaceae |
| Thalictrum squarrosum | Thalictrum | Ranunculaceae |
| Potentilla bifurca | Potentilla | Rosaceae |
| Halerpestes ruthenica | Halerpestes | Ranunculaceae |
| Stipa capillata | Stipa | Poaceae |
| Astragalus adsurgens | Astragalus | Leguminosae |
| Axyris amaranthoides | Axyris | Chenopodiaceae |
| Salsola collina | Salsola | Chenopodiaceae |
| Artemisia scoparia | Artemisia | Asteraceae |
| Oplismenus compositus | Oplismenus | Poaceae |
| Aster tataricus | Aster | Asteraceae |
| Oxalis corniculata | Oxalis | Oxalidaceae |
References
- Abbas, S.; Nichol, J.E.; Zhang, J.; Fischer, G.A. The accumulation of species and recovery of species composition along a 70 year succession in a tropical secondary forest. Ecol. Indic. 2019, 106, 105524. [Google Scholar] [CrossRef]
- Le, H.D.; Smith, C.; Herbohn, J.; Harrison, S. More than just trees: Assessing reforestation success in tropical developing countries. J. Rural Stud. 2012, 28, 5–19. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Ferraz, S.F.B. Role of eucalypt and other planted forests in biodiversity conservation and the provision of biodiversity-related ecosystem services. For. Ecol. Manag. 2013, 301, 43–50. [Google Scholar] [CrossRef]
- Hazarika, R.; Bolte, A.; Bednarova, D.; Chakraborty, D.; Gaviria, J.; Kanzian, M.; Kowalczyk, J.; Lackner, M.; Lstibůrek, M.; Longauer, R.; et al. Multi-actor perspectives on afforestation and reforestation strategies in Central Europe under climate change. Ann. For. Sci. 2021, 78, 60. [Google Scholar] [CrossRef]
- MacDonald, H. Envisioning better forest transitions: A review of recent forest transition scholarship. Heliyon 2023, 9, e20429. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Nunes, S.; Gastauer, M.; Cavalcante, R.B.L.; Ramos, S.J.; Caldeira, C.F.; Silva, D.; Rodrigues, R.R.; Salomão, R.; Oliveira, M.; Souza-Filho, P.W.M.; et al. Challenges and opportunities for large-scale reforestation in the Eastern Amazon using native species. For. Ecol. Manag. 2020, 466, 118120. [Google Scholar] [CrossRef]
- Kremer, K.; Jonsson, B.G.; Dutta, T.; Tavares, M.F.; Bauhus, J. Single-vs mixed-species plantations: A systematic review on the effects on biodiversity. Biol. Conserv. 2025, 307, 111182. [Google Scholar] [CrossRef]
- Hua, F.; Wang, X.; Zheng, X.; Brendan, F.; Wang, L.; Zhu, J.; Tang, Y.; Yu, D.W.; Wilcove, D.S. Opportunities for biodiversity gains under the world’s largest reforestation programme. Nat. Commun. 2016, 7, 12717. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Lamb, D. Undertaking large-scale forest restoration to generate ecosystem services. Restor. Ecol. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Mammola, S.; Carmona, C.P.; Guillerme, T.; Cardoso, P. Concepts and applications in functional diversity. Funct. Ecol. 2021, 35, 1869–1885. [Google Scholar] [CrossRef]
- Chelli, S.; Bricca, A.; Tsakalos, J.L.; Andreetta, A.; Bonari, G.; Campetella, G.; Carnicelli, S.; Cervellini, M.; Puletti, N.; Wellstein, C.; et al. Multiple drivers of functional diversity in temperate forest understories: Climate, soil, and forest structure effects. Sci. Total Environ. 2024, 916, 170258. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, A.; Van Daele, T.; Van Landuyt, W.; Van Reeth, W. Biodiversity and ecosystem services: Complementary approaches for ecosystem management? Ecol. Indic. 2012, 21, 123–133. [Google Scholar] [CrossRef]
- Berisha, N.; Geci, D. The analysis of the influence of grazing intensity on the diversity and abundance of plants and spiders (Arachnida: Araneae). Eur. J. Environ. Sci. 2023, 13, 31–38. [Google Scholar] [CrossRef]
- Genung, M.A.; Fox, J.; Winfree, R. Species loss drives ecosystem function in experiments, but in nature the importance of species loss depends on dominance. Glob. Ecol. Biogeogr. 2020, 29, 1531–1541. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Luzuriaga, A.L.; Sánchez, A.M.; López-Angulo, J.; Escudero, A. Habitat fragmentation determines diversity of annual plant communities at landscape and fine spatial scales. Basic Appl. Ecol. 2018, 29, 12–19. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Q.; Luo, P.; Zhang, Y.; Gu, X.; Lin, B. Direct and indirect effects of environmental factors, spatial constraints, and functional traits on shaping the plant diversity of montane forests. Ecol. Evol. 2020, 10, 557–568. [Google Scholar] [CrossRef]
- Onandia, G.; Schittko, C.; Ryo, M.; Bernard-Verdier, M.; Heger, T.; Joshi, J.; Kowarik, I.; Gessler, A. Ecosystem functioning in urban grasslands: The role of biodiversity, plant invasions and urbanization. PLoS ONE 2019, 14, e0225438. [Google Scholar] [CrossRef]
- Nichol, J.E.; Abbas, S. Evaluating Plantation Forest vs. Natural Forest Regeneration for Biodiversity Enhancement in Hong Kong. Forests 2021, 12, 593. [Google Scholar] [CrossRef]
- Matzek, V.; Wilson, K.A. Public support for restoration: Does including ecosystem services as a goal engage a different set of values and attitudes than biodiversity protection alone? PLoS ONE 2021, 16, e0245074. [Google Scholar] [CrossRef] [PubMed]
- Casas-Pinilla, L.C.; Iserhard, C.A.; Richter, A.; Gawlinski, K.; Cavalheiro, L.B.D.; Romanowski, H.P.; Kaminski, L.A. Different-aged Pinus afforestation does not support typical Atlantic Forest fruit-feeding butterfly assemblages. For. Ecol. Manag. 2022, 518, 120279. [Google Scholar] [CrossRef]
- Pandey, B.; Agrawal, M.; Singh, S. Coal mining activities change plant community structure due to air pollution and soil degradation. Ecotoxicology 2014, 23, 1474–1483. [Google Scholar] [CrossRef]
- Chen, B.; Iannone, B.V., III. FRAGSTATS: A Free Tool for Quantifying and Evaluating Spatial Patterns: FOR362/FR431, 6/2020. EDIS 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Hu, W.; Yu, Z. Importance of Soil Organic Matter and the Species Pool for Local Species Richness in Montane Ecosystems. Sustainability 2021, 13, 10634. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Chambers, J.C.; Board, D.I.; Roundy, B.A.; Weisberg, P.J. Removal of perennial herbaceous species affects response of Cold Desert shrublands to fire. J. Veg. Sci. 2017, 28, 975–984. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Ollenburger, M.; Basso, B.; DeHaan, L.R. Soil and Water Quality Rapidly Responds to the Perennial Grain Kernza Wheatgrass. Agron. J. 2013, 105, 735–744. [Google Scholar] [CrossRef]
- Lindemann-Matthies, P.; Junge, X.; Matthies, D. The influence of plant diversity on people’s perception and aesthetic appreciation of grassland vegetation. Biol. Conserv. 2010, 143, 195–202. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Package ‘fd’. Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. 2014. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 19 February 2025).
- Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Biometris: Ithaca, NY, USA, 2012. [Google Scholar]
- Pinheiro, H.T.; Rocha, L.A.; Macieira, R.M.; Carvalho-Filho, A.; Anderson, A.B.; Bender, M.G.; Di Dario, F.; Ferreira, C.E.L.; Figueiredo-Filho, J.; Francini-Filho, R.; et al. South-western Atlantic reef fishes: Zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers. Distrib. 2018, 24, 951–965. [Google Scholar] [CrossRef]
- Bennett, J.A.; Riibak, K.; Kook, E.; Reier, U.; Tamme, R.; Bueno, C.G.; Partel, M. Species pools, community completeness and invasion: Disentangling diversity effects on the establishment of native and alien species. Ecol. Lett. 2016, 19, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, F.; Honnay, O.; De Kort, H. The role of dispersal limitation and reforestation in shaping the distributional shift of a forest herb under climate change. Divers. Distrib. 2021, 27, 1775–1791. [Google Scholar] [CrossRef]
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Can hybrid poplar plantations accelerate the restoration of forest understory attributes on abandoned fields? For. Ecol. Manag. 2013, 287, 77–89. [Google Scholar] [CrossRef]
- Yang, H.; Pan, C.; Wu, Y.; Qing, S.; Wang, Z.; Wang, D. Response of understory plant species richness and tree regeneration to thinning in Pinus tabuliformis plantations in northern China. For. Ecosyst. 2023, 10, 100105. [Google Scholar] [CrossRef]
- Prach, K.; Walker, L.R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 2011, 26, 119–123. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsitsoni, T.; Tsakaldimi, M.; Zagas, T. Reforestation of degraded Kermes oak shrublands with planted pines: Effects on vegetation cover, species diversity and community structure. New For. 2012, 43, 1–11. [Google Scholar] [CrossRef]
- Ma, J.; Liu, S.; Shi, Z.; Zhang, Y.; Kang, B.; Chen, B. Changes in species composition and diversity in the restoration process of sub-alpine dark brown coniferous forests in western Sichuan Province, China. Front. For. China 2008, 3, 300–307. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Mitchell Aide, T.; Alvarez-Davila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Mestre, L.; Toro-Manríquez, M.; Soler, R.; Huertas-Herrera, A.; Martínez-Pastur, G.; Lencinas, M.V. The influence of canopy-layer composition on understory plant diversity in southern temperate forests. For. Ecosyst. 2017, 4, 6. [Google Scholar] [CrossRef]
- Ricotta, C.; Szeidl, L.; Pavoine, S. Towards a unifying framework for diversity and dissimilarity coefficients. Ecol. Indic. 2021, 129, 107971. [Google Scholar] [CrossRef]
- Lazarina, M.; Michailidou, D.E.; Tsianou, M.; Kallimanis, A.S. How Biodiversity, Climate and Landscape Drive Functional Redundancy of British Butterflies. Insects 2023, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Siwicka, E.; Thrush, S.F.; Hewitt, J.E. Linking changes in species-trait relationships and ecosystem function using a network analysis of traits. Ecol. Appl. 2020, 30, e02010. [Google Scholar] [CrossRef] [PubMed]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Hines, J.; Isbell, F.; van der Plas, F.; Hobbie, S.E.; Kazanski, C.E.; Lehmann, A.; Liu, M.; Lochner, A.; Rillig, M.C.; et al. Plant diversity maintains multiple soil functions in future environments. eLife 2018, 7, e41228. [Google Scholar] [CrossRef]
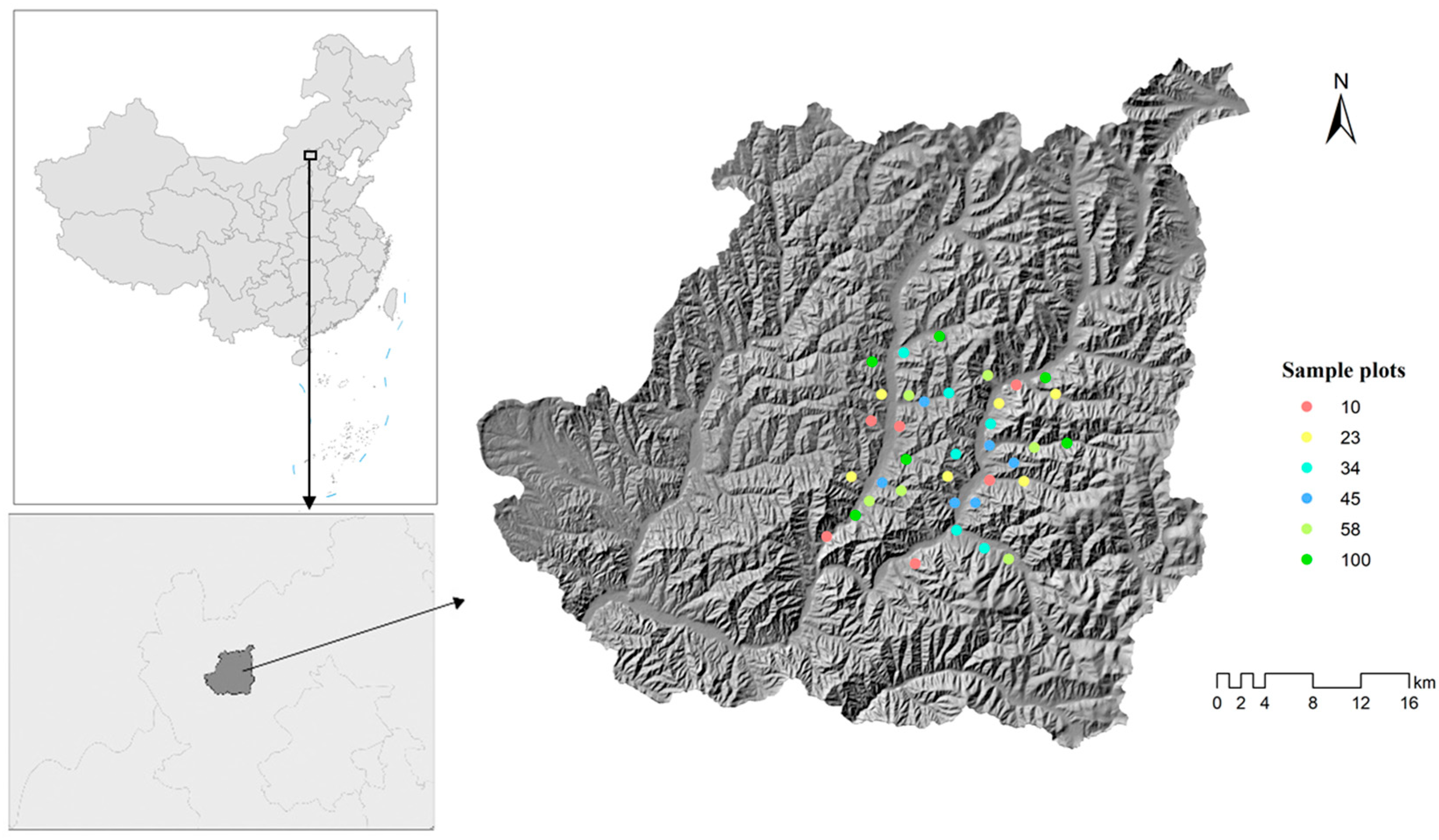


| Age | Number of Plots | Age of Midpoint |
|---|---|---|
| 6~12 | 6 | 10 |
| 21~26 | 6 | 23 |
| 33~36 | 6 | 34 |
| 43~46 | 6 | 45 |
| 53~63 | 6 | 58 |
| >100 | 6 | >100 |
| Plant Trait | Trait Characteristics | Data Sources | Related Ecosystem Services |
|---|---|---|---|
| Life form | arbor, shrub, herbaceous | field observations | soil conservation, climate change |
| Growth cycle | annual, perennial | database query 1 | community stability, soil retention |
| Dominance in situ | scattered, small groups, larger groups, larger stands, dominating large areas | field observations | regulation, support |
| Flower color | none (too small to see), green, white, yellow, violet, purple, orange, red, blue, pink, mix | field observations | biological control, culture |
| Red List species | yes, no | database query 2 | biodiversity |
| Edible or healing effect | yes, no | database query 3 | supply |
| Nectariferous plant | yes, no | database query 4 | pollination |
| Independent Variables | Coefficient | Standard Error | Z-Value | p-Value |
|---|---|---|---|---|
| Age | 1.179 | ±0.185 | 2.061 | 0.039 * |
| Soil total nitrogen | 0.369 | ±0.215 | 1.717 | 0.085 |
| Soil organic matter | 0.003 | ±0.007 | 0.463 | 0.643 |
| Landscape richness | 0.416 | ±0.318 | 1.907 | 0.060 |
| Patch size | 2.319 | ±1.172 | 1.979 | 0.047 * |
| Landscape fragmentation | −0.648 | ±0.315 | −2.055 | 0.039 * |
| Soil total N × Soil organic matter | −0.005 | ±0.003 | −1.964 | 0.049 * |
| Independent Variables | Coefficient | Standard Error | Z-Value | p-Value |
|---|---|---|---|---|
| Age | 3.367 | ±0.001 | 2.850 | 0.003 ** |
| Soil total nitrogen | 0.069 | ±0.034 | 1.085 | 0.085 |
| Soil organic matter | 0.003 | ±0.151 | 0.463 | 0.643 |
| Landscape richness | 0.806 | ±0.068 | 1.907 | 0.050 * |
| Patch size | 0.319 | ±0.026 | 1.979 | 0.077 |
| Landscape fragmentation | 2.368 | ±0.049 | 2.964 | 0.022 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Chen, Y.; Sardar, M.F.; Li, X. Restoration of Understory Plant Species and Functional Diversity in Temperate Plantations Along Successional Stages. Forests 2025, 16, 956. https://doi.org/10.3390/f16060956
Zhao W, Chen Y, Sardar MF, Li X. Restoration of Understory Plant Species and Functional Diversity in Temperate Plantations Along Successional Stages. Forests. 2025; 16(6):956. https://doi.org/10.3390/f16060956
Chicago/Turabian StyleZhao, Weiwei, Yanting Chen, Muhammad Fahad Sardar, and Xiang Li. 2025. "Restoration of Understory Plant Species and Functional Diversity in Temperate Plantations Along Successional Stages" Forests 16, no. 6: 956. https://doi.org/10.3390/f16060956
APA StyleZhao, W., Chen, Y., Sardar, M. F., & Li, X. (2025). Restoration of Understory Plant Species and Functional Diversity in Temperate Plantations Along Successional Stages. Forests, 16(6), 956. https://doi.org/10.3390/f16060956







