Characteristic and Adaptive Strategy in Leaf Functional Traits of Giant Panda (Ailuropoda melanoleuca) Staple Bamboo Species
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Staple Bamboo Species
2.3. Survey Sites Setting
2.4. Sampling and Trait Measurement
2.5. Climate Data of Sampling Site
3. Statistical Analyses
4. Results
4.1. Trait Characteristics and Variation
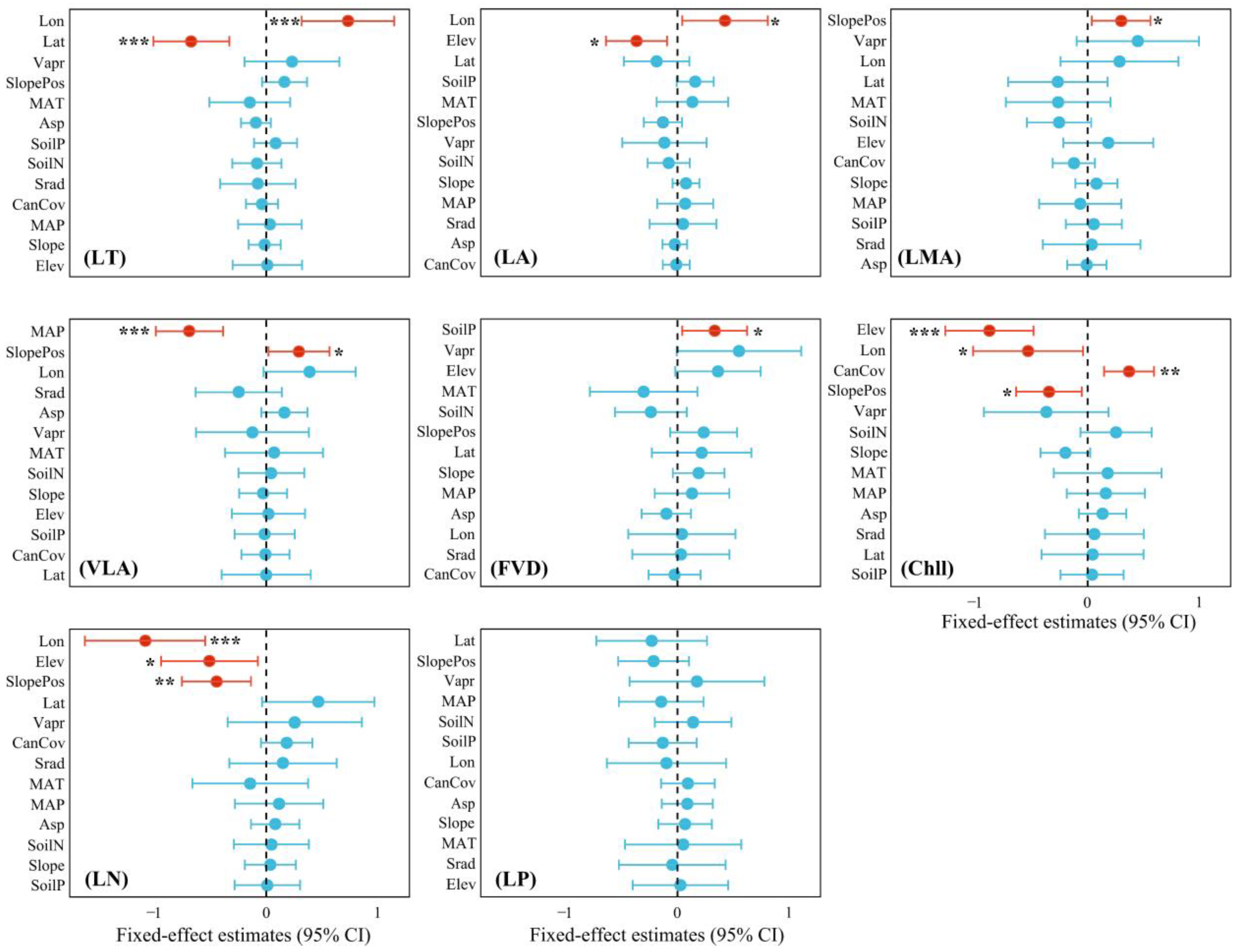
4.2. Factors Influencing Trait Variation
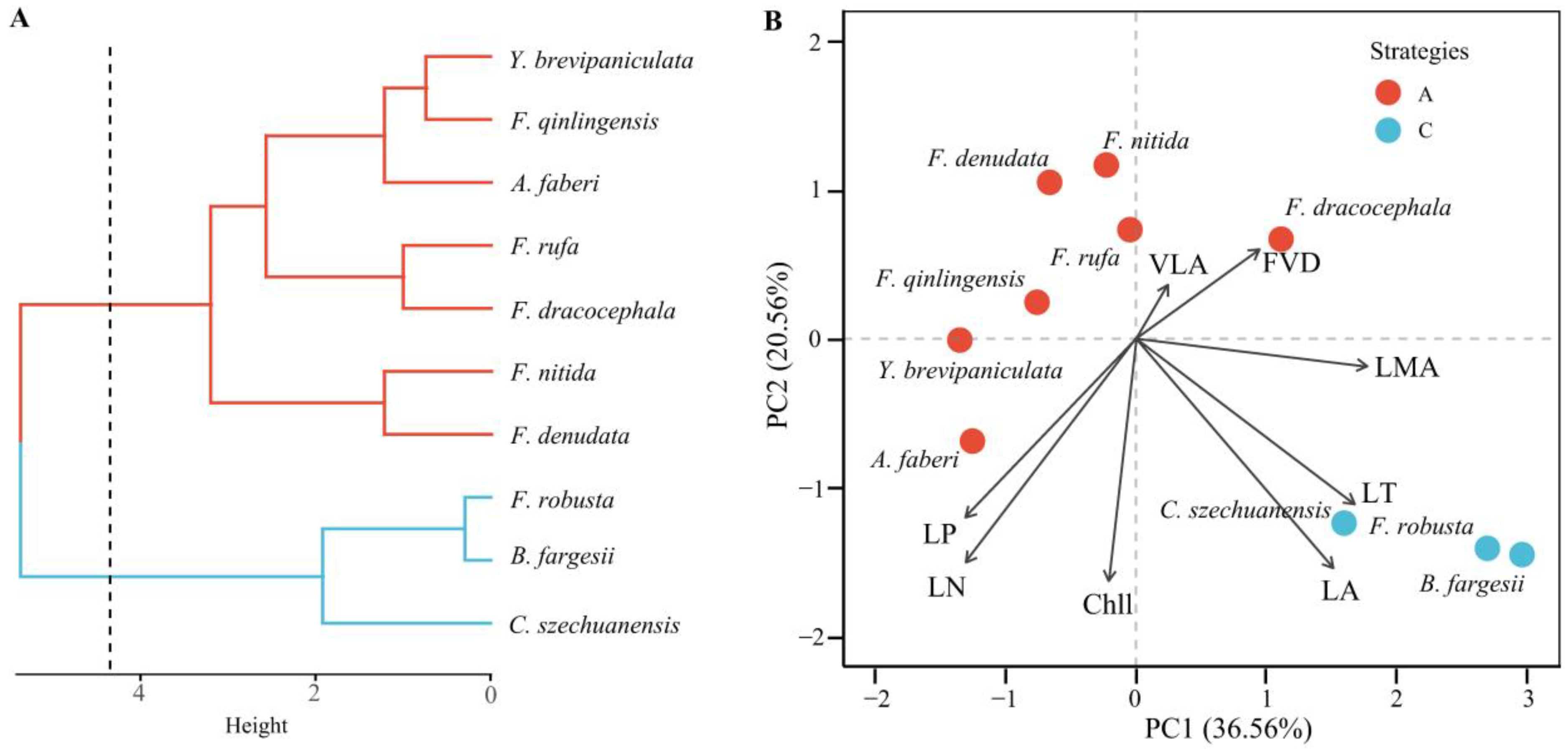
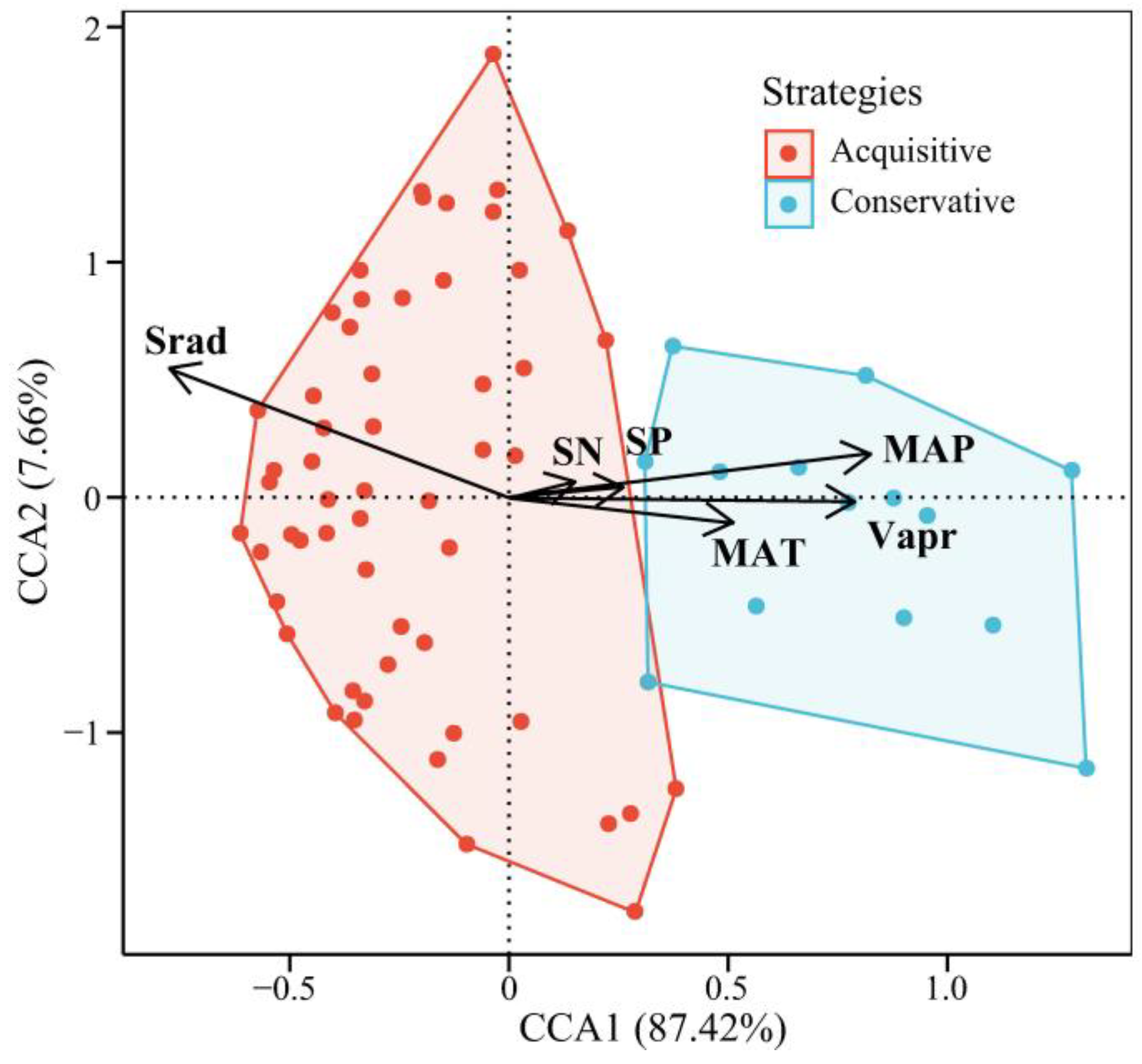
4.3. Trait-Based Strategies and Environmental Adaptation
5. Discussion
5.1. Trait Characteristics and Variation
5.2. Trait-Based Strategies and Environment Adaptation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, F.; Fan, H.; Hu, Y. Ailuropoda Melanoleuca (Giant Panda). In Trends in Genetics; Elsevier: Amsterdam, The Netherlands, 2020; Volume 36, pp. 68–69. [Google Scholar]
- Wei, F.; Swaisgood, R.; Hu, Y.; Nie, Y.; Yan, L.; Zhang, Z.; Qi, D.; Zhu, L. Progress in the Ecology and Conservation of Giant Pandas. Conserv. Biol. 2015, 29, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, J.R.; Xu, H.; Zhang, J. Pseudogenization of the Umami Taste Receptor Gene Tas1r1 in the Giant Panda Coincided with Its Dietary Switch to Bamboo. Mol. Biol. Evol. 2010, 27, 2669–2673. [Google Scholar] [CrossRef]
- Kang, D.; Lv, J.; Li, S.; Chen, X.; Wang, X.; Li, J. Relationship between Bamboo Growth Status and Woody Plants in a Giant Panda Habitat. Ecol. Indic. 2019, 98, 840–843. [Google Scholar] [CrossRef]
- Tang, J.; Swaisgood, R.R.; Owen, M.A.; Zhao, X.; Wei, W.; Pilfold, N.W.; Wei, F.; Yang, X.; Gu, X.; Yang, Z.; et al. Climate Change and Landscape-Use Patterns Influence Recent Past Distribution of Giant Pandas. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200358. [Google Scholar] [CrossRef]
- Kong, L.; Xu, W.; Xiao, Y.; Pimm, S.L.; Shi, H.; Ouyang, Z. Spatial Models of Giant Pandas under Current and Future Conditions Reveal Extinction Risks. Nat. Ecol. Evol. 2021, 5, 1309–1316. [Google Scholar] [CrossRef]
- Tuanmu, M.N.; Viña, A.; Winkler, J.A.; Li, Y.; Xu, W.; Ouyang, Z.; Liu, J. Climate-Change Impacts on Understorey Bamboo Species and Giant Pandas in China’s Qinling Mountains. Nat. Clim. Change 2013, 3, 249–253. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornellssen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global Climatic Drivers of Leaf Size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Osnas, J.L.D.; Katabuchi, M.; Kitajima, K.; Joseph Wright, S.; Reich, P.B.; Van Bael, S.A.; Kraft, N.J.B.; Samaniego, M.J.; Pacala, S.W.; Lichstein, J.W. Divergent Drivers of Leaf Trait Variation within Species, among Species, and among Functional Groups. Proc. Natl. Acad. Sci. USA 2018, 115, 5480–5485. [Google Scholar] [CrossRef]
- Heberling, J.M.; Fridley, J.D. Biogeographic Constraints on the World-Wide Leaf Economics Spectrum. Glob. Ecol. Biogeogr. 2012, 21, 1137–1146. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Yu, X.; Ji, R.; Li, M.; Xia, X.; Yin, W.; Liu, C. Geographical Variation in Functional Traits of Leaves of Caryopteris mongholica and the Role of Climate. BMC Plant Biol. 2023, 23, 394. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, J.; Wang, J.; Si, J.; Li, J.; Ge, X.; Li, Z. Interrelationships and Environmental Influences of Photosynthetic Capacity and Hydraulic Conductivity in Desert Species Populus pruinosa. Forests 2024, 15, 1094. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Schmid, B.; Kattge, J.; Fang, J.; Stocker, B.D. Environmental versus Phylogenetic Controls on Leaf Nitrogen and Phosphorous Concentrations in Vascular Plants. Nat. Commun. 2024, 15, 5346. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and Driving Factors of Leaf Functional Traits in the Dominant Desert Plant Species along an Environmental Gradient in the Drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Zhou, J.; Cieraad, E.; van Bodegom, P.M. Global Analysis of Trait–Trait Relationships within and between Species. New Phytol. 2022, 233, 1643–1656. [Google Scholar] [CrossRef]
- Bai, X.L.; Feng, T.; Zou, S.; He, B.; Chen, Y.; Li, W.J. Differences in Leaf Functional Traits of Quercus rehderiana Hand.-Mazz. in Forests with Rocky and Non-Rocky Desertification in Southwest China. Forests 2024, 15, 1439. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From Tropics to Tundra: Global Convergence in Plant Functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Liu, R.; Yang, X.; Gao, R.; Huang, Z.; Cornelissen, J.H.C. Coordination of Economics Spectra in Leaf, Stem and Root within the Genus Artemisia along a Large Environmental Gradient in China. Glob. Ecol. Biogeogr. 2023, 32, 324–338. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Anderson, C.B.; Martin, R.E.; Vaughn, N. Large-Scale Climatic and Geophysical Controls on the Leaf Economics Spectrum. Proc. Natl. Acad. Sci. USA 2016, 113, E4043–E4051. [Google Scholar] [CrossRef]
- Pan, Y.; Cieraad, E.; Armstrong, J.; Armstrong, W.; Clarkson, B.R.; Colmer, T.D.; Pedersen, O.; Visser, E.J.W.; Voesenek, L.A.C.J.; van Bodegom, P.M. Global Patterns of the Leaf Economics Spectrum in Wetlands. Nat. Commun. 2020, 11, 4519. [Google Scholar] [CrossRef] [PubMed]
- Muir, C.D.; Conesa, M.; Roldán, E.J.; Molins, A.; Galmés, J. Weak Coordination between Leaf Structure and Function among Closely Related Tomato Species. New Phytol. 2017, 213, 1642–1653. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-Species Patterns Challenge Our Understanding of the Leaf Economics Spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Weng, E.; Yan, E.; Xia, J. Robust Leaf Trait Relationships across Species under Global Environmental Changes. Nat. Commun. 2020, 11, 2999. [Google Scholar] [CrossRef]
- Deng, F.; Xiao, L.; Huang, J.; Luo, H.; Zang, R. Changes in Leaf Functional Traits Driven by Environmental Filtration in Different Monsoon Tropical Forest Types. Forests 2023, 14, 2101. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; He, B.; Lu, X. CSR Ecological Strategies and Functional Traits of the Co-Existing Species along the Succession in the Tropical Lowland Rain Forest. Forests 2022, 13, 1272. [Google Scholar] [CrossRef]
- Fadrique, B.; Baraloto, C.; Bravo-Avila, C.H.; Feeley, K.J. Bamboo Climatic Tolerances are Decoupled from Leaf Functional Traits across an Andean Elevation Gradient. Oikos 2022, 2022, e09229. [Google Scholar] [CrossRef]
- Yao, L.; Ding, Y.; Yao, L.; Ai, X.; Zang, R. Trait Gradient Analysis for Evergreen and Deciduous Species in a Subtropical Forest. Forests 2020, 11, 364. [Google Scholar] [CrossRef]
- Ahmad, Z.; Upadhyay, A.; Ding, Y.; Emamverdian, A.; Shahzad, A. Bamboo: Origin, Habitat, Distributions and Global Prospective. In Biotechnological Advances in Bamboo; Ahmad, Z., Ding, Y., Shahzad, A., Eds.; Springer: Singapore, 2021; pp. 1–31. ISBN 9789811613098. [Google Scholar]
- Clark, L.G.; Londoño, X.; Ruiz-Sanchez, E. Bamboo Taxonomy and Habitat. In Tropical Forestry Series; Liese, W., Köhl, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–30. ISBN 978-3-319-14133-6. [Google Scholar]
- Liu, X.; Zhou, S.; Hu, J.; Zou, X.; Tie, L.; Li, Y.; Cui, X.; Huang, C. Variations and Trade-Offs in Leaf and Culm Functional Traits among 77 Woody Bamboo Species. BMC Plant Biol. 2024, 24, 387. [Google Scholar] [CrossRef]
- Keenan, T.F.; Niinemets, Ü. Global Leaf Trait Estimates Biased due to Plasticity in the Shade. Nat. Plants 2016, 3, 16201. [Google Scholar] [CrossRef]
- Blonder, B.; Salinas, N.; Bentley, L.P.; Shenkin, A.; Chambi Porroa, P.O.; Valdez Tejeira, Y.; Boza Espinoza, T.E.; Goldsmith, G.R.; Enrico, L.; Martin, R.; et al. Structural and Defensive Roles of Angiosperm Leaf Venation Network Reticulation across an Andes–Amazon Elevation Gradient. J. Ecol. 2018, 106, 1683–1699. [Google Scholar] [CrossRef]
- Li, Y.; Viña, A.; Yang, W.; Chen, X.; Zhang, J.; Ouyang, Z.; Liang, Z.; Liu, J. Effects of Conservation Policies on Forest Cover Change in Giant Panda Habitat Regions, China. Land Use Policy 2013, 33, 42–53. [Google Scholar] [CrossRef]
- Lu, Z.; Franklin, S.B. Clonal Integration and Regeneration in Bamboo Bashania fargesii. For. Ecol. Manag. 2022, 523, 120504. [Google Scholar] [CrossRef]
- Christian, A.L.; Knott, K.K.; Vance, C.K.; Falcone, J.F.; Bauer, L.L.; Fahey, G.C.J.; Willard, S.; Kouba, A.J. Nutrient and Mineral Composition during Shoot Growth in Seven Species of Phyllostachys and Pseudosasa Bamboo Consumed by Giant Panda. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1172–1183. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, D.; Winkler, J.A.; Huang, Q.; Zhang, Y.; Wu, P.; Liu, J.; Ouyang, Z.; Xu, W.; Chen, X.; et al. Field Experiment Reveals Complex Warming Impacts on Giant Pandas’ Bamboo Diet. Biol. Conserv. 2024, 294, 110635. [Google Scholar] [CrossRef]
- Tian, H.; Zeng, Y.; Zhang, Z.; Lu, M.; Wei, W. Grazing-Induced Habitat Degradation: Challenges to Giant Panda Survival Resulting from Declining Bamboo and Soil Quality. Animals 2025, 15, 202. [Google Scholar] [CrossRef]
- Chinese State Forestry Administration. The Fourth National Giant Panda Survey; Chinese Science Press: Beijing, China, 2021; pp. 1–125. [Google Scholar]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Migliavacca, M.; Musavi, T.; Mahecha, M.D.; Nelson, J.A.; Knauer, J.; Baldocchi, D.D.; Perez-Priego, O.; Christiansen, R.; Peters, J.; Anderson, K.; et al. The Three Major Axes of Terrestrial Ecosystem Function. Nature 2021, 598, 468–472. [Google Scholar] [CrossRef]
- Wei, W.; Swaisgood, R.R.; Pilfold, N.W.; Owen, M.A.; Dai, Q.; Wei, F.; Han, H.; Yang, Z.; Yang, X.; Gu, X.; et al. Assessing the Effectiveness of China’s Panda Protection System. Curr. Biol. 2020, 30, 1280–1286.e2. [Google Scholar] [CrossRef]
- Makita, A. The Significance of the Mode of Clonal Growth in the Life History of Bamboos. Plant Species Biol. 1998, 13, 85–92. [Google Scholar] [CrossRef]
- Li, Q.; Peng, C.; Zhang, J.; Li, Y.; Song, X. Nitrogen Addition Decreases Methane Uptake Caused by Methanotroph and Methanogen Imbalances in a Moso Bamboo Forest. Sci. Rep. 2021, 11, 5578. [Google Scholar] [CrossRef] [PubMed]
- Liese, W.; Weiner, G. Ageing of Bamboo Culms. A Review. Wood Sci. Technol. 1996, 30, 77–89. [Google Scholar] [CrossRef]
- Guo, W.; Cherubini, P.; Zhang, J.; Hu, X.; Li, M.H.; Qi, L. Soil Physicochemical Properties Determine Leaf Traits but Not Size Traits of Moso Bamboo (Phyllostachys edulis). Environ. Res. Lett. 2022, 17, 114061. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Assessing the Causes and Scales of the Leaf Economics Spectrum Using Venation Networks in Populus tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global Trait–Environment Relationships of Plant Communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Li, J.; Prentice, I.C. Global Patterns of Plant Functional Traits and Their Relationships to Climate. Commun. Biol. 2024, 7, 1136. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Lusk, C.H. Predicting Leaf Physiology from Simple Plant and Climate Attributes: A Global Glopnet Analysis. Ecol. Appl. 2007, 17, 1982–1988. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Bentley, L.P.; Shenkin, A.; Asner, G.P.; Atkin, O.K.; Díaz, S.; Enquist, B.J.; Farfan-Rios, W.; Gloor, E.; Guerrieri, R.; et al. Solar Radiation and Functional Traits Explain the Decline of Forest Primary Productivity along a Tropical Elevation Gradient. Ecol. Lett. 2017, 20, 730–740. [Google Scholar] [CrossRef]
- Salazar Zarzosa, P.; Diaz Herraiz, A.; Olmo, M.; Ruiz-Benito, P.; Barrón, V.; Bastias, C.C.; de la Riva, E.G.; Villar, R. Linking Functional Traits with Tree Growth and Forest Productivity in Quercus ilex Forests along a Climatic Gradient. Sci. Total Environ. 2021, 786, 147468. [Google Scholar] [CrossRef]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F. Intraspecific Variation in Phragmites australis: Clinal Adaption of Functional Traits and Phenotypic Plasticity Vary with Latitude of Origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Murphy, S. Analysis of Variance. In Translational Orthopedics; Eltorai, A.E.M., Bakal, J.A., Haglin, J.M., Abboud, J.A., Crisco, J.J., Eds.; Handbook for Designing and Conducting Clinical and Translational Research; Academic Press: New York, NY, USA, 2024; Chapter 30; pp. 151–154. ISBN 978-0-323-85663-8. [Google Scholar]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Asao, S.; Hayes, L.; Aspinwall, M.J.; Rymer, P.D.; Blackman, C.; Bryant, C.J.; Cullerne, D.; Egerton, J.J.G.; Fan, Y.; Innes, P.; et al. Leaf Trait Variation is Similar among Genotypes of Eucalyptus camaldulensis from Differing Climates and Arises in Plastic Responses to the Seasons Rather than Water Availability. New Phytol. 2020, 227, 780–793. [Google Scholar] [CrossRef]
- Mo, L.; Crowther, T.W.; Maynard, D.S.; van den Hoogen, J.; Ma, H.; Bialic-Murphy, L.; Liang, J.; de-Miguel, S.; Nabuurs, G.J.; Reich, P.B.; et al. The Global Distribution and Drivers of Wood Density and Their Impact on Forest Carbon Stocks. Nat. Ecol. Evol. 2024, 8, 2195–2212. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. Glmm.Hp: An R Package for Computing Individual Effect of Predictors in Generalized Linear Mixed Models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Lai, J.; Zhu, W.; Cui, D.; Mao, L. Extension of the Glmm.Hp Package to Zero-Inflated Generalized Linear Mixed Models and Multiple Regression. J. Plant Ecol. 2023, 16, rtad038. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- González, I.; Déjean, S.; Martin, P.G.P.; Baccini, A. CCA: An R Package to Extend Canonical Correlation Analysis. J. Stat. Softw. 2008, 23, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf Morphological Traits as Adaptations to Multiple Climate Gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 16 August 2020).
- Team, R. RStudio: Integrated Development Environment for R; RStudio Inc.: Boston, MA, USA, 2015; Volume 14. [Google Scholar]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and Coordination of Hydraulic, Leaf and Stem Traits along a Water Availability Gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Burton, J.I.; Perakis, S.S.; McKenzie, S.C.; Lawrence, C.E.; Puettmann, K.J. Intraspecific Variability and Reaction Norms of Forest Understorey Plant Species Traits. Funct. Ecol. 2017, 31, 1881–1893. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Kraft, T.S.; Wright, S.J.; Turner, I.; Lucas, P.W.; Oufiero, C.E.; Supardi Noor, M.N.; Sun, I.F.; Dominy, N.J. Seed Size and the Evolution of Leaf Defences. J. Ecol. 2015, 103, 1057–1068. [Google Scholar] [CrossRef]
- Lusk, C.H.; Falster, D.S.; Jara-Vergara, C.K.; Jimenez-Castillo, M.; Saldaña-Mendoza, A. Ontogenetic Variation in Light Requirements of Juvenile Rainforest Evergreens. Funct. Ecol. 2008, 22, 454–459. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf Venation: Structure, Function, Development, Evolution, Ecology and Applications in the Past, Present and Future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Westoby, M.; Yates, L.; Holland, B.; Halliwell, B. Phylogenetically Conservative Trait Correlation: Quantification and Interpretation. J. Ecol. 2023, 111, 2105–2117. [Google Scholar] [CrossRef]
- Firn, J.; McGree, J.M.; Harvey, E.; Flores-Moreno, H.; Schütz, M.; Buckley, Y.M.; Borer, E.T.; Seabloom, E.W.; La Pierre, K.J.; MacDougall, A.M.; et al. Leaf Nutrients, Not Specific Leaf Area, Are Consistent Indicators of Elevated Nutrient Inputs. Nat. Ecol. Evol. 2019, 3, 400–406. [Google Scholar] [CrossRef]
- Liu, R.H.; Bai, J.L.; Bao, H.; Nong, J.L.; Zhao, J.J.; Jiang, Y.; Liang, S.C.; Li, Y.J. Variation and Correlation in Functional Traits of Main Woody Plants in the Cyclobalanopsis glauca Community in the Karst Hills of Guilin, Southwest China. Chin. J. Plant Ecol. 2020, 44, 828–841. [Google Scholar] [CrossRef]
- Sardans, J.; Vallicrosa, H.; Zuccarini, P.; Farré-Armengol, G.; Fernández-Martínez, M.; Peguero, G.; Gargallo-Garriga, A.; Ciais, P.; Janssens, I.A.; Obersteiner, M.; et al. Empirical Support for the Biogeochemical Niche Hypothesis in Forest Trees. Nat. Ecol. Evol. 2021, 5, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, S.M.; Robson, T.M. The Roles of Species’ Relatedness and Climate of Origin in Determining Optical Leaf Traits over a Large Set of Taxa Growing at High Elevation and High Latitude. Front. Plant Sci. 2022, 13, 1058162. [Google Scholar] [CrossRef]
- Yang, S.J.; Sun, M.; Zhang, Y.J.; Cochard, H.; Cao, K.F. Strong Leaf Morphological, Anatomical, and Physiological Responses of a Subtropical Woody Bamboo (Sinarundinaria nitida) to Contrasting Light Environments. Plant Ecol. 2014, 215, 97–109. [Google Scholar] [CrossRef]
- Hallik, L.; Kull, O.; Niinemets, Ü.; Aan, A. Contrasting Correlation Networks between Leaf Structure, Nitrogen and Chlorophyll in Herbaceous and Woody Canopies. Basic Appl. Ecol. 2009, 10, 309–318. [Google Scholar] [CrossRef]
- Opedal, Ø.H.; Armbruster, W.S.; Graae, B.J. Linking Small-Scale Topography with Microclimate, Plant Species Diversity and Intra-Specific Trait Variation in an Alpine Landscape. Plant Ecol. Divers. 2015, 8, 305–315. [Google Scholar] [CrossRef]
- Delle Monache, D.; Martino, G.; Chiocchio, A.; Siclari, A.; Bisconti, R.; Maiorano, L.; Canestrelli, D. Mapping Local Climates in Highly Heterogeneous Mountain Regions: Interpolation of Meteorological Station Data vs. Downscaling of Macroclimate Grids. Ecol. Inform. 2024, 82, 102674. [Google Scholar] [CrossRef]
- Kemppinen, J.; Lembrechts, J.J.; Van Meerbeek, K.; Carnicer, J.; Chardon, N.I.; Kardol, P.; Lenoir, J.; Liu, D.; Maclean, I.; Pergl, J.; et al. Microclimate, an Important Part of Ecology and Biogeography. Glob. Ecol. Biogeogr. 2024, 33, e13834. [Google Scholar] [CrossRef]
- John, G.P.; Scoffoni, C.; Buckley, T.N.; Villar, R.; Poorter, H.; Sack, L. The Anatomical and Compositional Basis of Leaf Mass per Area. Ecol. Lett. 2017, 20, 412–425. [Google Scholar] [CrossRef]
- Xing, K.; Niinemets, Ü.; Rengel, Z.; Onoda, Y.; Xia, J.; Chen, H.Y.H.; Zhao, M.; Han, W.; Li, H. Global Patterns of Leaf Construction Traits and Their Covariation along Climate and Soil Environmental Gradients. New Phytol. 2021, 232, 1648–1660. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and Soil Factors Explain the Two-Dimensional Spectrum of Global Plant Trait Variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Berdanier, A.B.; Klein, J.A. Growing Season Length and Soil Moisture Interactively Constrain High Elevation Aboveground Net Primary Production. Ecosystems 2011, 14, 963–974. [Google Scholar] [CrossRef]
- Deng, C.; Bai, H.; Gao, S.; Zhao, T.; Ma, X. Differences and Variations in the Elevation-Dependent Climatic Growing Season of the Northern and Southern Slopes of the Qinling Mountains of China from 1985 to 2015. Theor. Appl. Climatol. 2019, 137, 1159–1169. [Google Scholar] [CrossRef]
- Kudo, G. Intraspecific Variation of Leaf Traits in Several Deciduous Species in Relation to Length of Growing Season. Écoscience 1996, 3, 483–489. [Google Scholar] [CrossRef]
- Blume-Werry, G.; Wilson, S.D.; Kreyling, J.; Milbau, A. The Hidden Season: Growing Season Is 50% Longer below than above Ground along an Arctic Elevation Gradient. New Phytol. 2016, 209, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Rathore, N.; Thakur, D.; Chawla, A. Seasonal Variations Coupled with Elevation Gradient Drives Significant Changes in Eco-Physiological and Biogeochemical Traits of a High Altitude Evergreen Broadleaf Shrub, Rhododendron Anthopogon. Plant Physiol. Biochem. 2018, 132, 708–719. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Niklas, K.J.; Sun, J.; Wang, Z.; Zhong, Q.; Hu, D.; Cheng, D. A Whole-Plant Economics Spectrum Including Bark Functional Traits for 59 Subtropical Woody Plant Species. J. Ecol. 2022, 110, 248–261. [Google Scholar] [CrossRef]
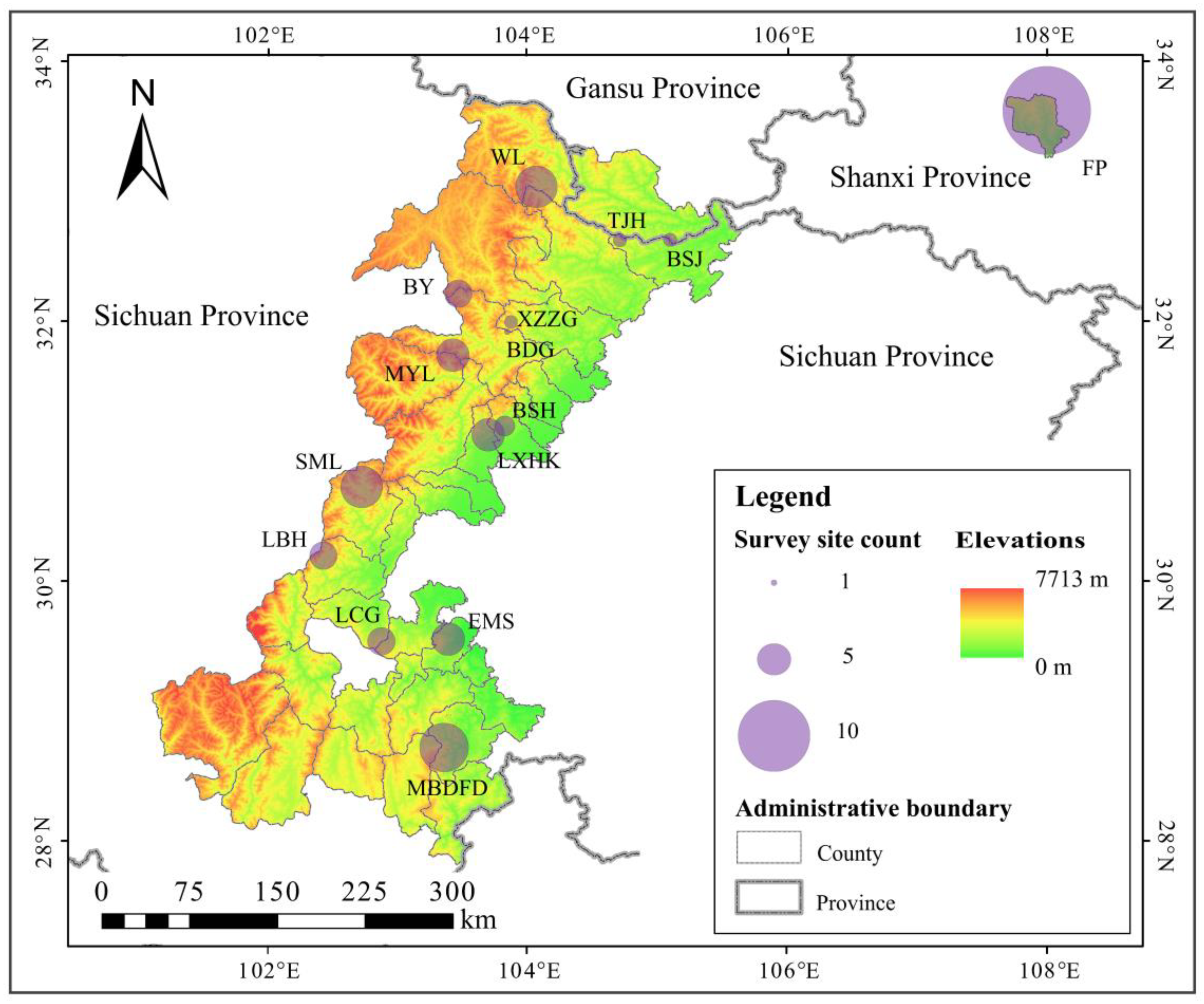



| ID | Species | Protected Area and the Number of Survey Site | Sum |
|---|---|---|---|
| 1 | A. faberi | EMS (4), MBDFD (3), LBH (3), LCG (2), LXHK (2), BSH (1) | 15 |
| 2 | B. fargesii | FP (2), TJH (1), BSJ (1) | 4 |
| 3 | C. szechuanensis | MBDFD (3), LCG (2), EMS (1) | 6 |
| 4 | F. denudata | WL (6), BY (2), BDG (1) | 9 |
| 5 | F. dracocephala | FP (6) | 6 |
| 6 | F. qinlingensis | FP (5) | 5 |
| 7 | F. robusta | LXHK (3), BSH (1) | 4 |
| 8 | F. rufa | XZZG (2), LBH (1), TJH (1), BSJ (1) | 5 |
| 9 | F. nitida | MYL (5), BY (2), SML (1), MBDFD (1) | 9 |
| 10 | Y. brevipaniculata | SML (5), BSH (1) | 6 |
| Sum | — | — | 69 |
| LT (mm) | LA (cm2) | LMA (g m−2) | VLA (cm cm−2) | FVD (μm) | Chll (mg g−1) | LN (mg g−1) | LP (mg g−1) | |
|---|---|---|---|---|---|---|---|---|
| Min | 0.05 | 4.96 | 24.69 | 82.09 | 6.72 | 4.75 | 16.70 | 0.77 |
| Max | 0.15 | 57.57 | 66.56 | 166.86 | 17.63 | 7.98 | 40.57 | 2.59 |
| Median | 0.08 | 10.52 | 40.01 | 129.29 | 10.76 | 7.00 | 27.29 | 1.54 |
| Mean ± SD | 0.08 ± 0.03 | 16.06 ± 12.71 | 41.28 ± 10.22 | 127.96 ± 20.07 | 11.07 ± 2.82 | 6.86 ± 0.66 | 27.78 ± 5.29 | 1.56 ± 0.38 |
| CVs (%) | 31.87 | 79.16 | 24.76 | 15.69 | 25.45 | 9.58 | 19.05 | 24.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, Y.; Zou, X.; Zhu, W.; Wan, R.; Liang, Z.; Hu, J.; Tie, L.; Cui, X.; Zhang, Y.; et al. Characteristic and Adaptive Strategy in Leaf Functional Traits of Giant Panda (Ailuropoda melanoleuca) Staple Bamboo Species. Forests 2025, 16, 954. https://doi.org/10.3390/f16060954
Liu X, Zhou Y, Zou X, Zhu W, Wan R, Liang Z, Hu J, Tie L, Cui X, Zhang Y, et al. Characteristic and Adaptive Strategy in Leaf Functional Traits of Giant Panda (Ailuropoda melanoleuca) Staple Bamboo Species. Forests. 2025; 16(6):954. https://doi.org/10.3390/f16060954
Chicago/Turabian StyleLiu, Xiong, Yilin Zhou, Xingcheng Zou, Weiyu Zhu, Renping Wan, Zhengchuan Liang, Junxi Hu, Liehua Tie, Xinglei Cui, Yuanbin Zhang, and et al. 2025. "Characteristic and Adaptive Strategy in Leaf Functional Traits of Giant Panda (Ailuropoda melanoleuca) Staple Bamboo Species" Forests 16, no. 6: 954. https://doi.org/10.3390/f16060954
APA StyleLiu, X., Zhou, Y., Zou, X., Zhu, W., Wan, R., Liang, Z., Hu, J., Tie, L., Cui, X., Zhang, Y., Zhou, S., Sardans, J., Huang, C., & Peñuelas Reixach, J. (2025). Characteristic and Adaptive Strategy in Leaf Functional Traits of Giant Panda (Ailuropoda melanoleuca) Staple Bamboo Species. Forests, 16(6), 954. https://doi.org/10.3390/f16060954









