Contrasting Drought Sensitivity in Silver Fir and Scots Pine Revealed Through Growth and Wood Density Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
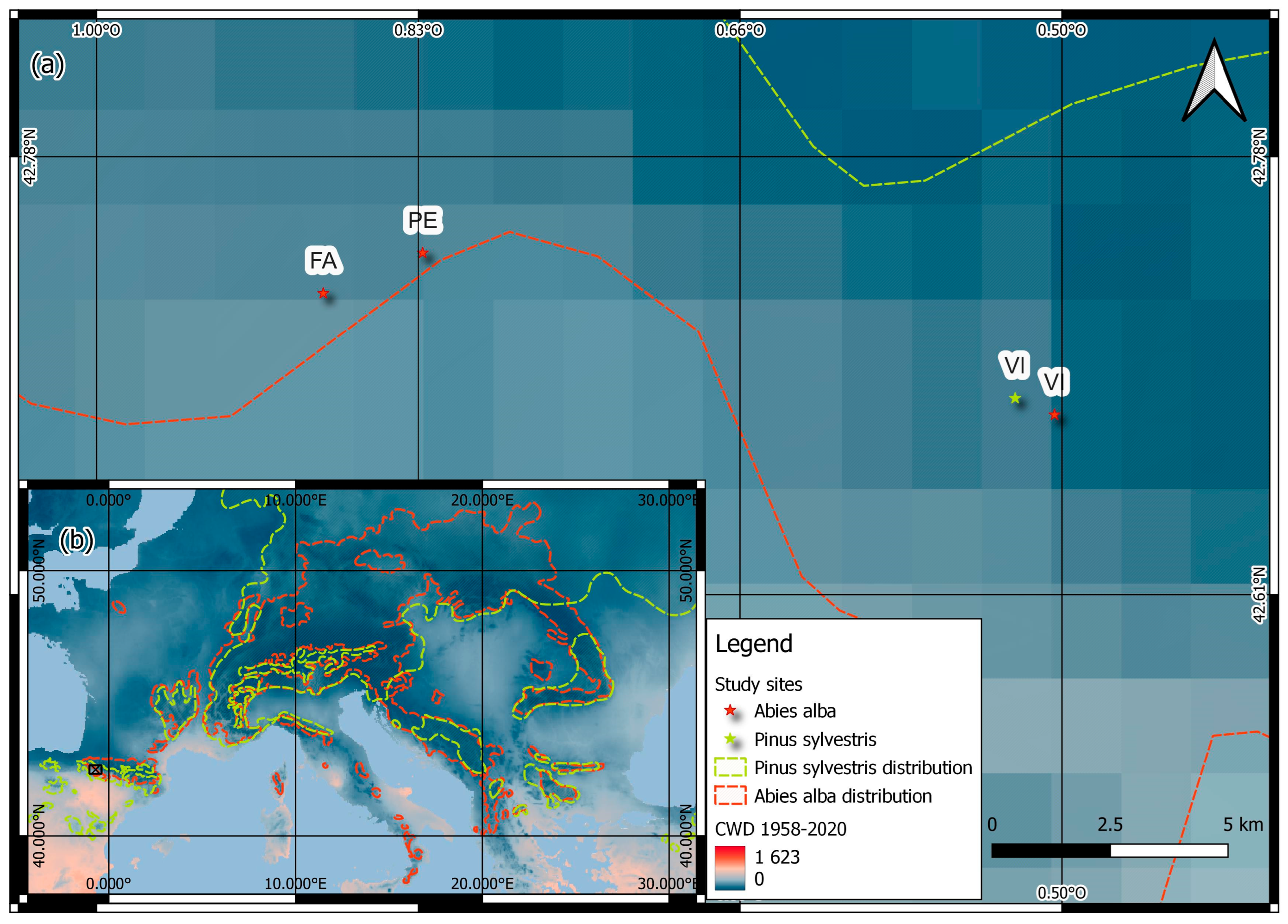
2.2. Climatological Data
2.3. Field Sampling and Dendrochronological Methods
2.4. Resilience, Resistance, and Recovery Indices
2.5. Statistical Analyses
3. Results
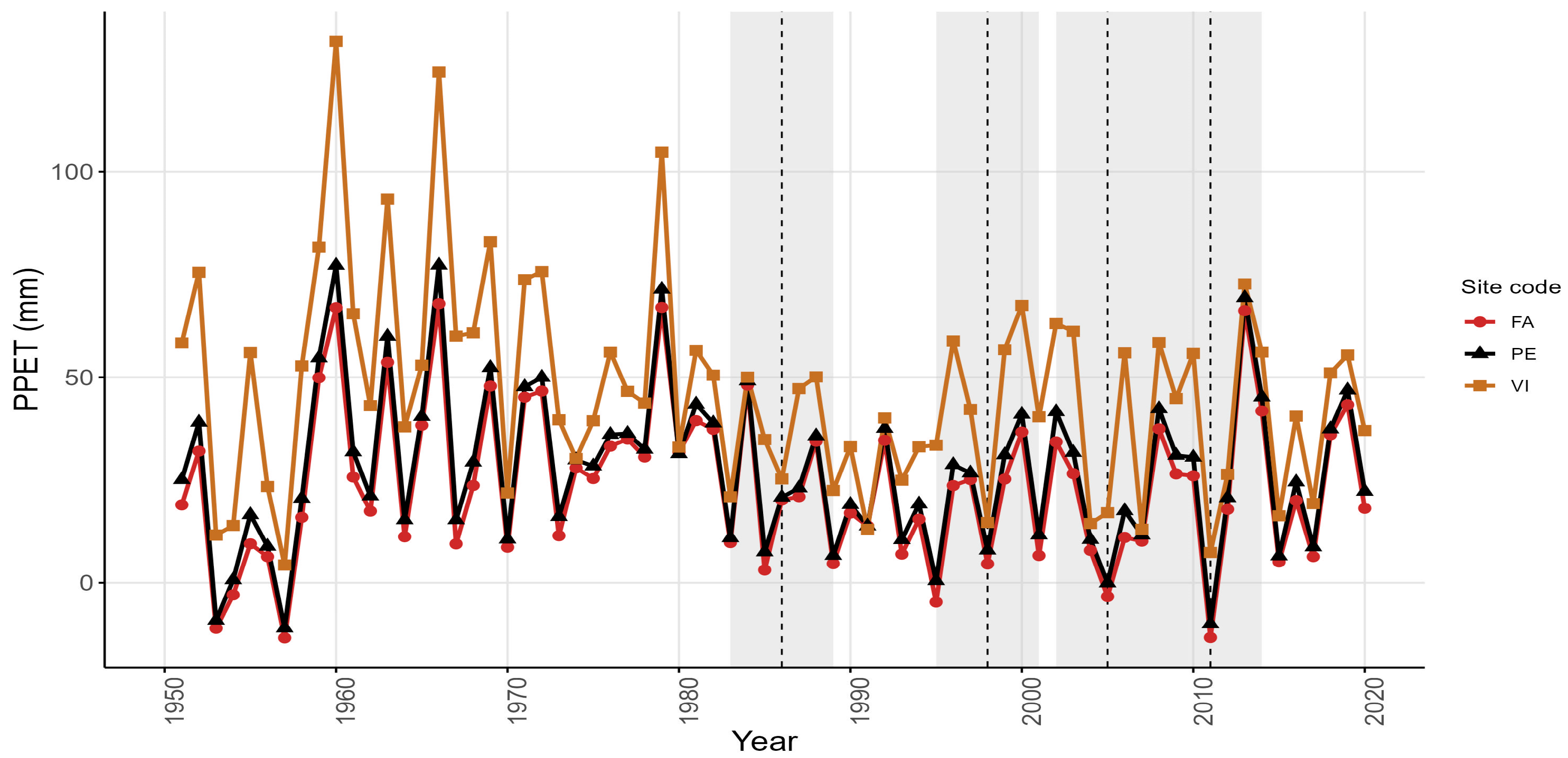
3.1. Climatic Trends and Drought Variability
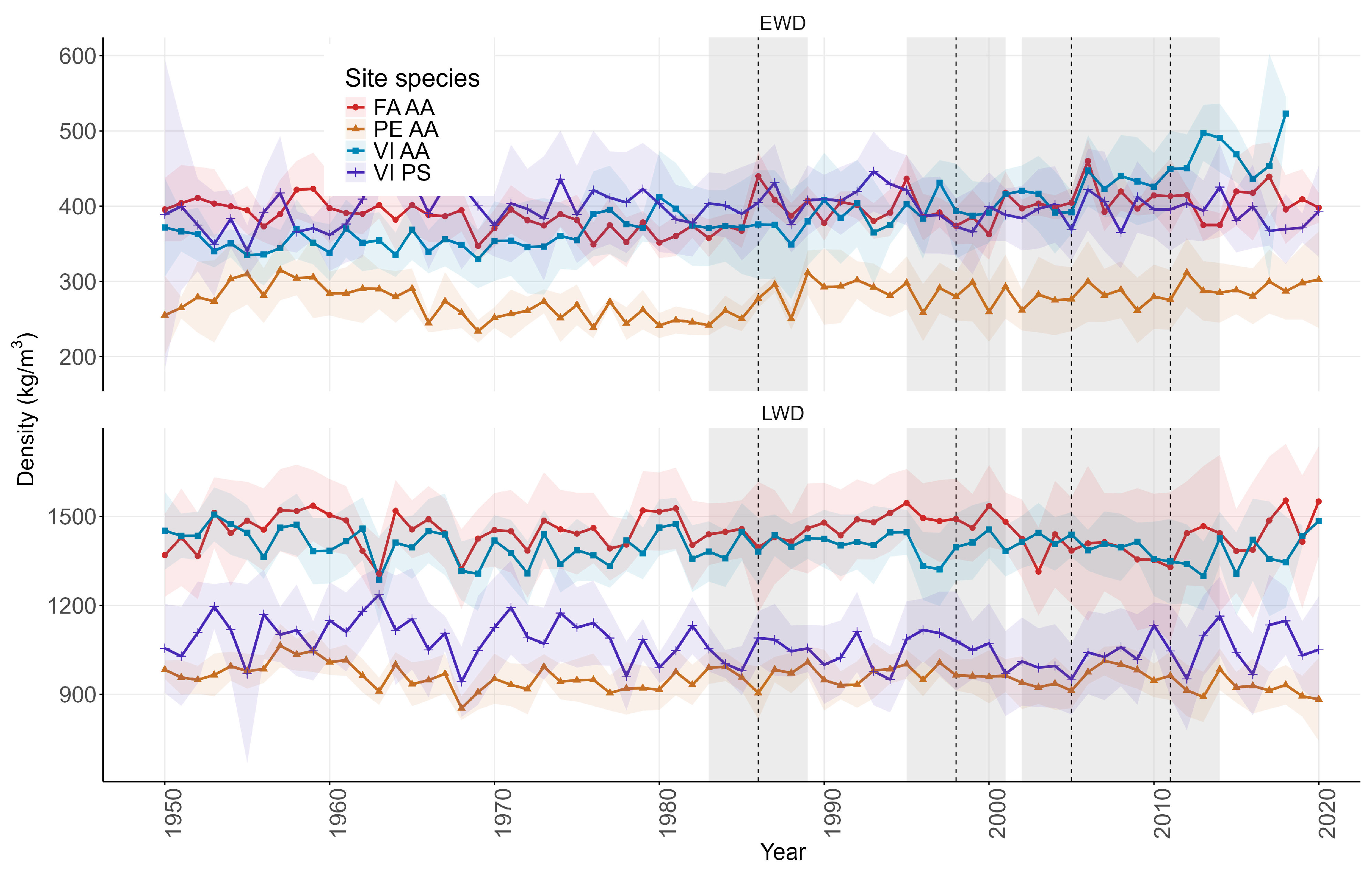
3.2. Growth and Wood Density Data
3.3. Growth and Wood Density Sensitivity to Climate
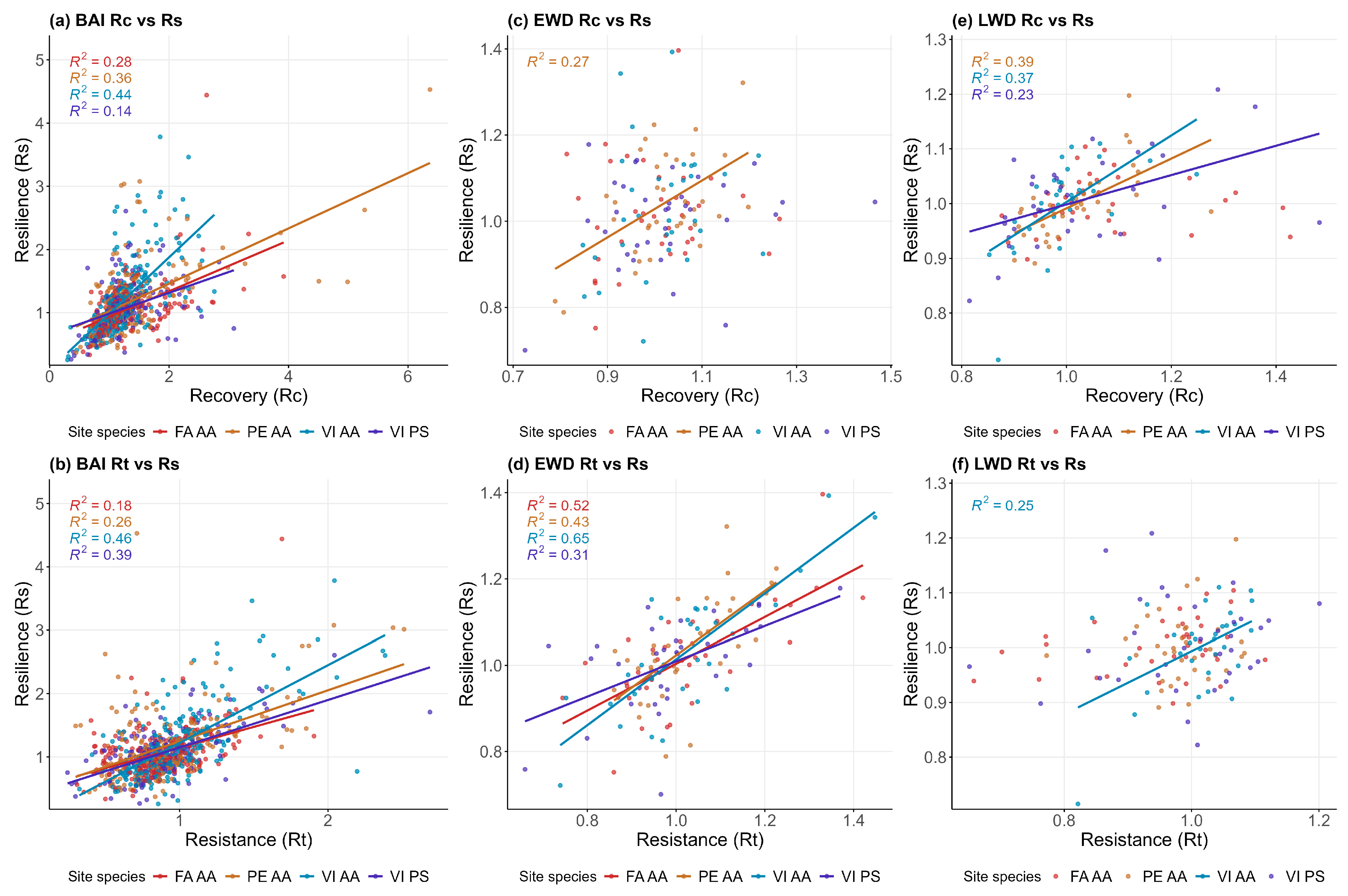
3.4. Resilience Indices
4. Discussion
4.1. Relationships Between Growth and Wood Density
4.2. Climatic Sensitivity of Radial Growth and Wood Density
4.3. Resilience Indices: Differences Between Growth and Wood Density
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebollo, P.; Moreno-Fernández, D.; Cruz-Alonso, V.; Gazol, A.; Rodríguez-Rey, M.; Astigarraga, J.; Zavala, M.A.; Gómez-Aparicio, L.; Andivia, E.; Miguel-Romero, S.; et al. Recent Increase in Tree Damage and Mortality and Their Spatial Dependence on Drought Intensity in Mediterranean Forests. Landsc. Ecol. 2024, 39, 38. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On Underestimation of Global Vulnerability to Tree Mortality and Forest Die-off from Hotter Drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global Field Observations of Tree Die-off Reveal Hotter-Drought Fingerprint for Earth’s Forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A Multi-Species Synthesis of Physiological Mechanisms in Drought-Induced Tree Mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Link, R.M.; Patthey, R.; Hoch, G.; Schuldt, B.; Kahmen, A. Rapid Hydraulic Collapse as Cause of Drought-Induced Mortality in Conifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025251118. [Google Scholar] [CrossRef]
- Björklund, J.; Von Arx, G.; Fonti, P.; Stridbeck, P.; De Mil, T.; Neycken, A.; Seftigen, K. The Utility of Bulk Wood Density for Tree-Ring Research. Dendrochronologia 2021, 69, 125880. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in Wood Density and Structure Are Linked to Prevention of Xylem Implosion by Negative Pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Olano, J.M.; Eugenio, M.; García-Cervigón, A.I.; Folch, M.; Rozas, V. Quantitative Tracheid Anatomy Reveals a Complex Environmental Control of Wood Structure in Continental Mediterranean Climate. Int. J. Plant Sci. 2012, 173, 137–149. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Reinhart, A.-L.; Bauhus, J. Increased Drought Mortality in Fast-Growing Silver Fir Trees in the Black Forest. For. Ecol. Manag. 2025, 578, 122441. [Google Scholar] [CrossRef]
- Bigler, C.; Bugmann, H. Growth-Dependent Tree Mortality Models Based on Tree Rings. Can. J. For. Res. 2003, 33, 210–221. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Engelbrecht, B.M.J.; Joswig, J.; Pereyra, G.; Schuldt, B.; Jansen, S.; Kattge, J.; Landhäusser, S.M.; Levick, S.R.; Preisler, Y.; et al. A Synthesis of Tree Functional Traits Related to Drought-induced Mortality in Forests across Climatic Zones. J. Appl. Ecol. 2017, 54, 1669–1686. [Google Scholar] [CrossRef]
- DeSoto, L.; De La Cruz, M.; Fonti, P. Intra-Annual Patterns of Tracheid Size in the Mediterranean Tree Juniperus Thurifera as an Indicator of Seasonal Water Stress. Can. J. For. Res. 2011, 41, 1280–1294. [Google Scholar] [CrossRef]
- Camarero, J.J.; Fernández-Pérez, L.; Kirdyanov, A.V.; Shestakova, T.A.; Knorre, A.A.; Kukarskih, V.V.; Voltas, J. Minimum Wood Density of Conifers Portrays Changes in Early Season Precipitation at Dry and Cold Eurasian Regions. Trees Struct. Funct. 2017, 31, 1423–1437. [Google Scholar] [CrossRef]
- George, J.-P.; Schueler, S.; Karanitsch-Ackerl, S.; Mayer, K.; Klumpp, R.T.; Grabner, M. Inter- and Intra-Specific Variation in Drought Sensitivity in Abies spec. and Its Relation to Wood Density and Growth Traits. Agric. For. Meteorol. 2015, 214–215, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Rigling, A.; Gessler, A.; Hagedorn, F.; Brunner, I.; Feichtinger, L.; Bigler, C.; Egli, S.; Etzold, S.; Gossner, M.M.; et al. Lessons Learned from a Long-term Irrigation Experiment in a Dry Scots Pine Forest: Impacts on Traits and Functioning. Ecol. Monogr. 2022, 92, e1507. [Google Scholar] [CrossRef]
- Bose, A.K.; Gessler, A.; Büntgen, U.; Rigling, A. Tamm Review: Drought-Induced Scots Pine Mortality—Trends, Contributing Factors, and Mechanisms. For. Ecol. Manag. 2024, 561, 121873. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct Effects of Climate Warming on Populations of Silver Fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of Tree Resilience: Effects of Successive Low-Growth Episodes in Old Ponderosa Pine Forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- González De Andrés, E.; Gazol, A.; Querejeta, J.I.; Colangelo, M.; Camarero, J.J. Mistletoe-Induced Carbon, Water and Nutrient Imbalances Are Imprinted on Tree Rings. Tree Physiol. 2024, 44, tpae106. [Google Scholar] [CrossRef] [PubMed]
- Caudullo, G. Chorological Data for the Main European Woody Species. 2024. Available online: https://doi.org/10.17632/HR5H2HCGG4.18 (accessed on 31 March 2025).
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a High-Resolution Global Dataset of Monthly Climate and Climatic Water Balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; New York, NY, USA, 1976. [Google Scholar]
- Cottam, G.; Curtis, J.T. The Use of Distance Measures in Phytosociological Sampling. Ecology 1956, 37, 451–460. [Google Scholar] [CrossRef]
- Larsson, L. CDendro & CooRecorder Program Package. 2022. Available online: https://www.cybis.se/forfun/dendro (accessed on 31 March 2025).
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Klesse, S.; Mérian, P.; Qeadan, F.; Zang, C.; Buras, A.; Cecile, A.; et al. dplR: Dendrochronology Program Library in R. 2025. Available online: https://cran.r-project.org/web/packages/dplR/index.html (accessed on 25 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 31 March 2025).
- Kasambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 31 March 2025).
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R Package for the Numerical Calibration of Proxy-Climate Relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Fonti, P.; Von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying Global Change through Investigation of the Plastic Responses of Xylem Anatomy in Tree Rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- De Micco, V.; Campelo, F.; De Luis, M.; Bräuning, A.; Grabner, M.; Battipaglia, G.; Cherubini, P. Intraannual density fluctuations in tree rings: How, when, where, and why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- Gindl, W.; Grabner, M.; Wimmer, R. The Influence of Temperature on Latewood Lignin Content in Treeline Norway Spruce Compared with Maximum Density and Ring Width. Trees Struct. Funct. 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Pellizzari, E.; Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Carrer, M. Wood Anatomy and Carbon-isotope Discrimination Support Long-term Hydraulic Deterioration as a Major Cause of Drought-induced Dieback. Glob. Chang. Biol. 2016, 22, 2125–2137. [Google Scholar] [CrossRef]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The Response of Pinus sylvestris to Drought: Stomatal Control of Transpiration and Hydraulic Conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef]
- Aussenac, G. Ecology and Ecophysiology of Circum-Mediterranean Firsin the Context of Climate Change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- Domec, J.-C.; Warren, J.M.; Meinzer, F.C.; Lachenbruch, B. Safety Factors for Xylem Failure by Implosion and Air-Seeding Within Roots, Trunks and Branches of Young and Old Conifer Trees. IAWA J. 2009, 30, 101–120. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; León-Gómez, C.; Dawson, T.; Camarero Martínez, J.J.; Castorena, M.; et al. Plant Height and Hydraulic Vulnerability to Drought and Cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gutiérrez, E. Wood Density of Silver Fir Reflects Drought and Cold Stress across Climatic and Biogeographic Gradients. Dendrochronologia 2017, 45, 101–112. [Google Scholar] [CrossRef]
- Ziaco, E.; Biondi, F.; Rossi, S.; Deslauriers, A. Climatic Influences on Wood Anatomy and Tree-ring Features of Great Basin Conifers at a New Mountain Observatory. Appl. Plant Sci. 2014, 2, 1400054. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Beeckman, H.; Cañellas, I. Influence of Drought on Tree Rings and Tracheid Features of Pinus nigra and Pinus sylvestris in a Mesic Mediterranean Forest. Eur. J. Forest Res. 2013, 132, 33–45. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Fonti, P.; Rigling, A. Drought-Induced Adaptation of the Xylem in Scots Pine and Pubescent Oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.-I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of Increasing Drought Severity Caused by Temperature Rise in Southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]


| Abbreviation | Definition | Abbreviation | Definition | Abbreviation | Definition |
|---|---|---|---|---|---|
| TRW | Tree-Ring Width | BAI | Basal Area Increment | CWD | Climatic Water Deficit |
| TRWi | Tree-Ring Width Index | P | Precipitation | Rt | Resistance Index |
| EWD | Earlywood Density | Tmax | Maximum Temperature | Rc | Recovery Index |
| EWDi | Earlywood Density Index | Tmin | Minimum Temperature | Rs | Resilience Index |
| LWD | Latewood Density | Tmean | Mean Temperature | FA | Fago (site code) |
| LWDi | Latewood Density Index | PET | Potential Evapotranspiration | PE | Paco Ezpela (site code) |
| DBH | Diameter at Breast Height | P-PET | Climatic Water Balance (P minus PET) | VI | Villanúa (site code) |
| Site | Species | Latitude N (°) | Longitude W (°) | Elevation (m a.s.l.) | Temperature (°C) | Precipitation (mm) |
|---|---|---|---|---|---|---|
| Fago (FA) | Abies alba (AA) | 42°43′34″ N | 0°52′41″ W | 915 | 10.3 ± 0.1 | 1248 ± 25 |
| Paco Ezpela (PE) | Abies alba (AA) | 42°49′45″ N | 0°42′15″ W | 1285 | 10.1 ± 0.1 | 1302 ± 26 |
| Villanúa (VI) | Abies alba (AA) | 42°42′36″ N | 0°38′51″ W | 1320 | 6.1 ± 0.1 | 1580 ± 40 |
| Villanúa (VI) | Pinus sylvestris (PS) | 42°41′11″ N | 0°31′18″ W | 1140 | 7.3 ± 0.1 | 1465 ± 34 |
| Variable | Fago (FA) | Paco Ezpela (PE) | Villanúa (VI) | |
| Silver Fir (AA) | Silver Fir (AA) | Silver Fir (AA) | Scots Pine (PS) | |
| DBH (cm) | 36.7 ± 1.9 a | 25.5 ± 0.7 b | 33.1 ± 1.2 a | 25.6 ± 1.33 b |
| Tree age (years) * | 82.9 ± 5.5 b | 102 ± 5.2 a | 106 ± 4.0 a | 69.1 ± 2.3 b |
| BAI (cm2 × year−1) | 13.6 ± 0.2 a | 5.1 ± 0.1 c | 8.6 ± 0.1 b | 8.0 ± 0.1 b |
| BAI 1981–2000 (cm2 × year−1) | 14.6 ± 0.3 a | 6.6 ± 0.2 c | 9.7 ± 0.3 b | 9.2 ± 0.2 b |
| BAI 2001–2020 (cm2 × year−1) | 14.0 ± 0.4 a | 11.2 ± 0.2 b | 12.3 ± 0.3 ab | 6.5 ± 0.2 c |
| EWD (kg × m−3) ** | 393.16 ± 1.81 a | 284.94 ± 1.95 c | 378.58 ± 2.42 b | 398.57 ± 3.15 a |
| EWD 1981–2000 (kg × m−3) | 388.27 ± 3.35 a | 275.00 ± 3.64 b | 384.00 ± 6.32 a | 401.00 ± 5.34 a |
| EWD 2001–2020 (kg × m−3) | 408.29 ± 3.66 b | 285.00 ± 4.62 c | 437.06 ± 6.14 a | 392.76 ± 5.39 b |
| LWD (kg × m−3) ** | 1423.27 ± 7.33 a | 954.85 ± 3.67 c | 1423.21 ± 5.11 a | 1060.40 ± 7.93 b |
| LWD 1981–2000 (kg × m−3) | 1469.77 ± 14.5 a | 966.57 ± 7.47 d | 1405.74 ± 11.2 b | 1052.41 ± 12.8 c |
| LWD 2001–2020 (kg × m−3) | 1415.86 ± 21.0 a | 942.34 ± 8.15 c | 1386.51 ± 12.8 a | 1040.23 ± 15.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Antia, J.P.; Gazol, A.; González de Andrés, E.; Valeriano, C.; Rubio-Cuadrado, Á.; Altman, J.; Doležal, J.; Linares, J.C.; Camarero, J.J. Contrasting Drought Sensitivity in Silver Fir and Scots Pine Revealed Through Growth and Wood Density Data. Forests 2025, 16, 921. https://doi.org/10.3390/f16060921
Crespo-Antia JP, Gazol A, González de Andrés E, Valeriano C, Rubio-Cuadrado Á, Altman J, Doležal J, Linares JC, Camarero JJ. Contrasting Drought Sensitivity in Silver Fir and Scots Pine Revealed Through Growth and Wood Density Data. Forests. 2025; 16(6):921. https://doi.org/10.3390/f16060921
Chicago/Turabian StyleCrespo-Antia, Juan Pablo, Antonio Gazol, Estér González de Andrés, Cristina Valeriano, Álvaro Rubio-Cuadrado, Jan Altman, Jiří Doležal, Juan Carlos Linares, and J. Julio Camarero. 2025. "Contrasting Drought Sensitivity in Silver Fir and Scots Pine Revealed Through Growth and Wood Density Data" Forests 16, no. 6: 921. https://doi.org/10.3390/f16060921
APA StyleCrespo-Antia, J. P., Gazol, A., González de Andrés, E., Valeriano, C., Rubio-Cuadrado, Á., Altman, J., Doležal, J., Linares, J. C., & Camarero, J. J. (2025). Contrasting Drought Sensitivity in Silver Fir and Scots Pine Revealed Through Growth and Wood Density Data. Forests, 16(6), 921. https://doi.org/10.3390/f16060921











