Improved Management of Verticillium Wilt in Smoke Trees Through the Use of a Combination of Fungicide and Bioagent Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Information on Experimental Sites
2.2. Agents Information and Application Methods
2.3. Experimental Design
2.4. Evaluation of the Control Effect
2.5. Statistical Analysis
3. Results
3.1. Effect of Different Treatments on Verticillium Wilt Disease Index
3.2. Effectiveness of Treatments in Controlling Different Wilt Severity
3.3. Protective and Preventive Effects Shown by the Treatment Group in the Following Year
3.4. Application of the Control Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, S.; Xiong, D.; Tian, C. Genetic transformation, infection process and qPCR quantification of V. dahliae on smoke-tree Cotinus coggygria. Australas. Plant Pathol. 2013, 42, 33–41. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, B. Management of the soil-borne fungal pathogen–V. dahliae Kleb. causing vascular wilt diseases. J. Plant Pathol. 2021, 103, 1185–1194. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tian, C. Quantitative detection of pathogen DNA of Verticillium wilt on smoke tree Cotinus coggygria. Plant Dis. 2013, 97, 1645–1651. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.; Wang, L.; Zhao, X.; Liang, L.; Chen, F. Wilt of shantung maple caused by V. dahliae in China. Plant Dis. 2018, 102, 249. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, M.; Li, T.; Wang, L.; Liao, C.; Liu, D.; Zhang, H.; Zhao, Y.; Liu, L.; Ge, X. Interactions between V. dahliae and cotton: Pathogenic mechanism and cotton resistance mechanism to Verticillium wilt. Front. Plant Sci. 2023, 14, 1174281. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Shang, J.-Y.; Niu, L.-X.; Sun, X.-R.; Su, Z.-H.; Dong, L.-H.; Guo, Q.-G.; Li, S.-Z.; Ma, P. First report of Verticillium Wilt of watermelon caused by V. dahliae in China. Plant Dis. 2021, 105, 2723. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Qian, X.; Li, G. First report of Verticillium wilt of grapevine (Vitis vinifera) caused by V. dahliae in China. Plant Dis. 2009, 93, 841. [Google Scholar] [CrossRef]
- Türkkan, M.; Şahin, N.; Özer, G.; Evgin, Z.; Yaman, M.; Erper, I. First report of V. dahliae causing Verticillium wilt on kiwifruit in Ordu, Turkey. J. Plant Pathol. 2020, 102, 221–222. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Keykhasaber, M.; Barjasteh, A.; Landa, B.B.; Rafiei, V. First report of Verticillium wilt caused by V. dahliae on Russian olive (Elaeagnus angustifolia) in Iran. Plant Dis. 2021, 105, 1213. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Carter, C.A.; Goodhue, R.E.; Lawell, C.-Y.C.L.; Subbarao, K.V. A review of control options and externalities for Verticillium wilts. Phytopathology 2018, 108, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Vallad, G.E.; Bhat, R.G.; Koike, S.T.; Ryder, E.J.; Subbarao, K.V. Weedborne reservoirs and seed transmission of V. dahliae in lettuce. Plant Dis. 2005, 89, 317–324. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D. Control of crop diseases through Integrated Crop Management to deliver climate-smart farming systems for low-and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Abada, K.; Attia, A.; Zyton, M. Management of pepper Verticillium wilt by combinations of inducer chemicals for plant resistance, bacterial bioagents and compost. J. Appl. Biotechnol. 2018, 5, 117–127. [Google Scholar] [CrossRef]
- Uppal, A.; El Hadrami, A.; Adam, L.; Tenuta, M.; Daayf, F. Biological control of potato Verticillium wilt under controlled and field conditions using selected bacterial antagonists and plant extracts. Biol. Control 2008, 44, 90–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, Q.-Y.; Xu, L.-L.; Xu, Q.; Lu, S.; Gu, C.; Guo, J.-H. A screening strategy of fungal biocontrol agents towards Verticillium wilt of cotton. Biol. Control 2011, 56, 209–216. [Google Scholar] [CrossRef]
- Santos-Rufo, A.; Rodríguez-Jurado, D. Evaluation of chemical disinfestants in reducing V. dahliae conidia in irrigation water. Crop Prot. 2016, 79, 105–116. [Google Scholar] [CrossRef]
- Deketelaere, S.; Tyvaert, L.; França, S.C.; Höfte, M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Mulè, R.; Fodale, A.S.; Tucci, A. Control of olive verticillium wilt by trunk injection with different doses of fosetyl-al and benomyl. Acta Hortic. 2002, 586, 761–764. [Google Scholar] [CrossRef]
- Li, B.; Guo, R.; Zhao, Y.; Li, Q.; Song, L.; Shen, C.; Du, C.; Gu, Y.; Qiao, G.; Wang, L. Development of integrated control for Verticillium wilt of smoke trees in Beijing. Forests 2024, 15, 776. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Cardoni, M.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Villadas, P.J.; Fernández-López, M.; Mercado-Blanco, J. Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive. Microbiome 2020, 8, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Liu, J.; Li, X.; Wang, X.; Dai, C.; Zhang, T.; Carrión, V.J.; Wei, Z.; Cao, F. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat. Commun. 2023, 14, 8126. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist B. subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol. Fertil. Soils 2013, 49, 295–303. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.-Y.; Zhang, D.-D.; Li, R.; Kong, Z.-Q.; Zhu, H.; Dai, X.-F.; Han, D.; Wang, D. Genome resource of B. subtilis KRS015, a potential biocontrol agent for V. dahliae. PhytoFrontier 2024, 4, 443–448. [Google Scholar] [CrossRef]
- Guo, R.; Li, B.; Li, Q.; Klosterman, S.J.; Qiao, G.; Wang, Y. Belowground microbiota associated with the progression of Verticillium wilt of smoke trees. Plant Soil 2024, 500, 515–529. [Google Scholar] [CrossRef]
- Guo, R.; Li, B.; Zhao, Y.; Tang, C.; Klosterman, S.J.; Wang, Y. Rhizobacterial B. enrichment in soil enhances smoke tree resistance to Verticillium wilt. Plant Cell Environ. 2024, 47, 4086–4100. [Google Scholar] [CrossRef]
- Guo, R.; Shen, C.; Li, B.; Du, C.; Li, Q.; Wang, A.; Cui, Q.; Wang, Y. Control effect of Verticillium wilt on Cotinus coggygria inBadaling Forest Farm of Beijing. J. Beijing For. Univ. 2024, 46, 1–9. [Google Scholar] [CrossRef]
- Alkolaly, A.; Helal, M.R.; Mostafa, S. Infection suppression of verticillium wilt disease in eggplant as affected by some fungicides, biocides and salicylic acid. Zagazig J. Agric. Res. 2018, 45, 1675–1682. [Google Scholar] [CrossRef]
- Ju, C.; Li, X.; He, S.; Shi, L.; Yu, S.; Wang, F.; Xu, S.; Cao, D.; Fang, H.; Yu, Y. Root uptake of imidacloprid and propiconazole is affected by root composition and soil characteristics. J. Agric. Food Chem. 2020, 68, 15381–15389. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Wang, W.; Wan, Q.; Yu, X.; Sun, W. Uptake, translocation, and subcellular distribution of three triazole pesticides in rice. Environ. Sci. Pollut. Res. 2022, 29, 25581–25590. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, K.; Cai, L.; Zhang, Y.; Fu, Q.; Huang, S. Combination effects of tebuconazole with B. subtilis to control rice false smut and the related synergistic mechanism. Pest Manag. Sci. 2023, 79, 234–243. [Google Scholar] [CrossRef]
- Bynum, J.; Cothren, J.; Lemon, R.; Fromme, D.; Boman, R. Field evaluation of nitrophenolate plant growth regulator (Chaperone) for the effect on cotton lint yield. J. Cotton Sci. 2007, 11, 20–25. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20073278766 (accessed on 1 March 2025).
- Peng, T.-W.; Xie, H.-Y.; Li, S.-J.; Liu, Y.-X.; Shuai, K.-F.; Peng, Y.-Y.; Wang, Q.; Li, D.-Q. Effects of sodium dinitrate with B. sbutilis complex on growth and physiological indexes of tobacco seedlings. J. Agric. Sci. Technol. 2022, 24, 154–161. [Google Scholar] [CrossRef]
- He, M.; Xie, X.; Zhang, N. Synergistic effect of sodium nitrophenolate on paclobutrazol and dimethylpiperidinium choride in foxtail millet germination. J. Shanxi Agric. Sci. 2018, 46, 339–343. [Google Scholar] [CrossRef]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by B. subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, B. subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Long, Y.; Ai, Q.; Su, Y.; Lei, Y. Oligosaccharins used together with tebuconazole enhances resistance of kiwifruit against soft rot disease and improves its yield and quality. Horticulturae 2022, 8, 624. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Tao, Q.; Sun, Q.; Zheng, Y.; Yin, D.; Yang, Y. A possible but unrecognized risk of acceptable daily intake dose triazole pesticides exposure—Bile acid disturbance induced pharmacokinetic changes of oral medication. Chemosphere 2023, 322, 138209. [Google Scholar] [CrossRef]
- Elad, Y.; Zimand, G.; Zaqs, Y.; Zuriel, S.; Chet, I. Use of Trichoderma harzianum in combination or alternation with fungicides to control cucumber grey mould (Botrytis cinerea) under commercial greenhouse conditions. Plant Pathol. 1993, 42, 324–332. [Google Scholar] [CrossRef]
- Tejada, M.; Gómez, I.; García-Martínez, A.M.; Osta, P.; Parrado, J. Effects of Prochloraz fungicide on soil enzymatic activities and bacterial communities. Ecotox. Environ. Safety 2011, 74, 1708–1714. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.n.; Ding, C.; Xu, W.; Wang, X. Temporal patterns of cotton Fusarium and Verticillium wilt in Jiangsu coastal areas of China. Sci. Rep. 2017, 7, 12581. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Dong, J.; Yang, X.; Sui, A. Interactions between V. dahliae and its host: Vegetative growth, pathogenicity, plant immunity. Appl. Microbiol. Biotechnol. 2014, 98, 6921–6932. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Land, C.; Lawrence, K.; Burmester, C.; Meyer, B. Cultivar, irrigation, and soil contribution to the enhancement of Verticillium wilt disease in cotton. Crop Prot. 2017, 96, 1–6. [Google Scholar] [CrossRef]
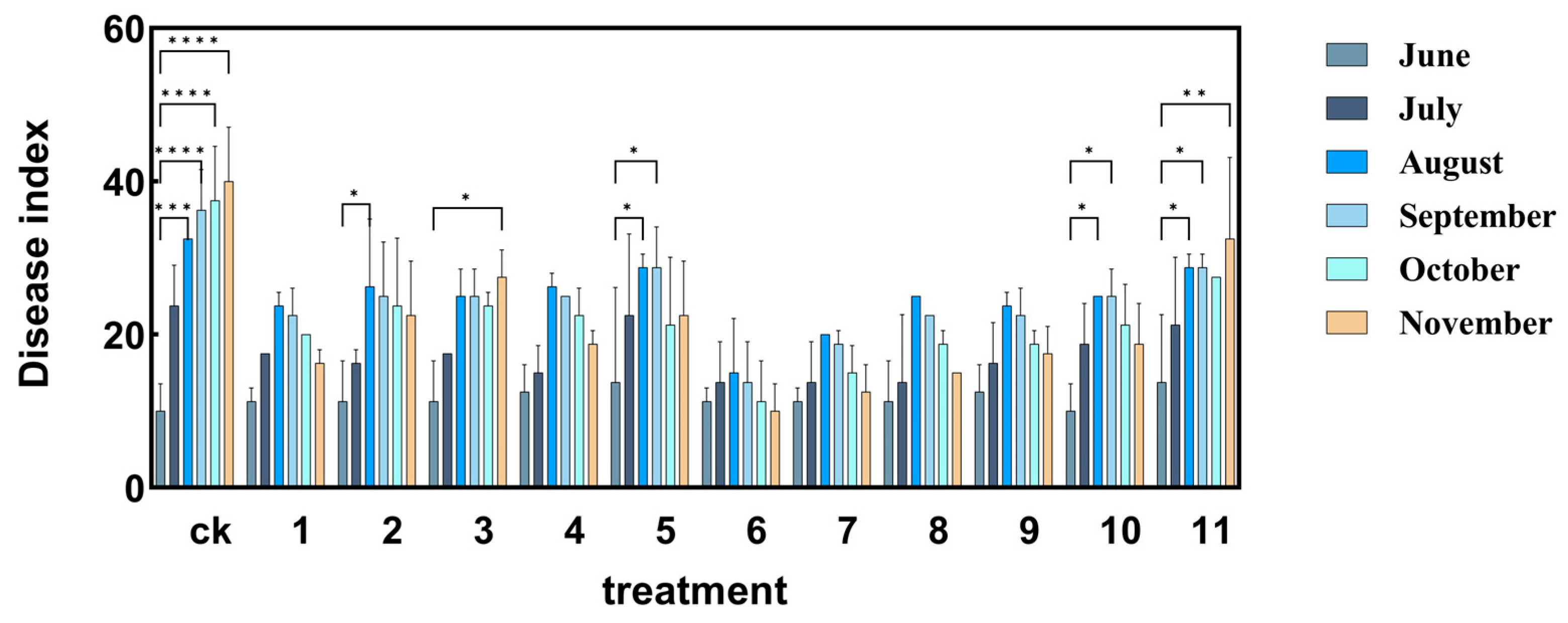


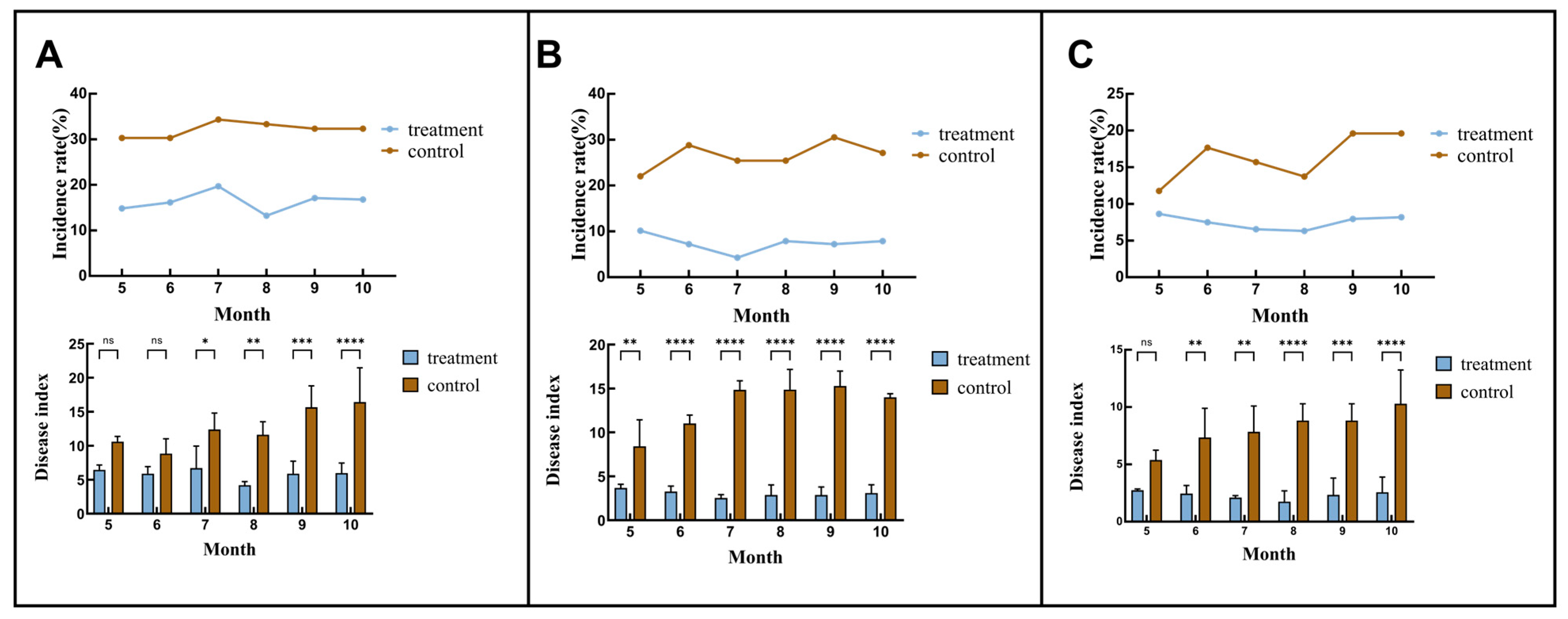

| Treatment | Way of Agents Application | Root Irrigation Agents | Spray Agents |
|---|---|---|---|
| CK | Blank | ||
| 1 | Root irrigation | B. subtilis | |
| 2 | 156 g/L propiconazole | ||
| 3 | 50% azoxystrobin | ||
| 4 | Trunk spraying | 25% tebuconazole, 1.4% sodium nitrophenolate | |
| 5 | 45% prochloraz, 1.4% sodium nitrophenolate | ||
| 6 | Combined treatment | B. subtilis | 25% tebuconazole, 1.4% sodium nitrophenolate |
| 7 | B. subtilis | 45% prochloraz, 1.4% sodium nitrophenolate | |
| 8 | 156 g/L propiconazole | 25% tebuconazole, 1.4% sodium nitrophenolate | |
| 9 | 156 g/L propiconazole | 45% prochloraz, 1.4% sodium nitrophenolate | |
| 10 | 50% azoxystrobin | 25% tebuconazole, 1.4% sodium nitrophenolate | |
| 11 | 50% azoxystrobin | 45% prochloraz, 1.4% sodium nitrophenolate |
| Wilt Grade | Method of Agent Application | Agents |
|---|---|---|
| I | Root irrigation | B. subtilis |
| Trunk spraying | 1.4% sodium nitrophenolate | |
| II–III | Root irrigation | B. subtilis |
| Trunk spraying | 45% prochloraz, 1.4% sodium nitrophenolate | |
| IV–V | Root irrigation | 156 g/L propiconazole |
| Trunk spraying | 45% prochloraz, 1.4% sodium nitrophenolate |
| Treatment | Method of Application | Jun 2023 | Nov 2023 | Corrective Disease Index | Corrective Disease Index | Corrective Control Effects | ||
|---|---|---|---|---|---|---|---|---|
| Disease Severity | Number | Disease Severity | Number | |||||
| CK | Blank | I | 12 | I | 6 | 20.83 | 30.00 ± 3.54 a | - |
| II–III | 6 | |||||||
| II–III | 8 | II–III | 3 | 43.75 | ||||
| IV–V | 5 | |||||||
| 1 | Rootirrigation | I | 11 | I | 9 | 4.55 | 5.00 ± 3.54 cde | 83.92 ± 9.89 abc |
| II–III | 2 | |||||||
| II–III | 9 | II–III | 9 | 5.56 | ||||
| 2 | I | 13 | I | 6 | 17.31 | 11.25 ± 12.37 bcd | 65.38 ± 38.07 abc | |
| II–III | 7 | |||||||
| II–III | 7 | II–III | 7 | 0 | ||||
| 3 | I | 12 | I | 8 | 12.5 | 16.25 ± 1.77 bc | 50.00 ± 5.44 bc | |
| II–III | 4 | |||||||
| II–III | 8 | II–III | 5 | 25 | ||||
| IV–V | 3 | |||||||
| 4 | Trunkspraying | I | 11 | I | 9 | 4.55 | 6.25 ± 1.77 cde | 79.37 ± 3.46 abc |
| II-III | 2 | |||||||
| II–III | 9 | I | 2 | 8.33 | ||||
| II–III | 5 | |||||||
| IV–V | 2 | |||||||
| 5 | I | 10 | I | 8 | 5 | 8.75 ± 5.30 bcde | 69.58 ± 21.26 abc | |
| II–III | 2 | |||||||
| II–III | 10 | I | 1 | 12.5 | ||||
| II–III | 7 | |||||||
| IV–V | 2 | |||||||
| 6 | Combined treatment | I | 13 | I | 12 | 1.92 | −1.25 ± 1.77 e | 104.55 ± 6.43 a |
| II–III | 1 | |||||||
| II–III | 7 | I | 2 | −7.14 | ||||
| II–III | 5 | |||||||
| 7 | I | 13 | I | 12 | 1.92 | 1.25 ± 5.30 de | 94.76 ± 18.30 ab | |
| II–III | 1 | |||||||
| II–III | 7 | I | 1 | 0 | ||||
| II–III | 5 | |||||||
| IV–V | 1 | |||||||
| 8 | I | 12 | I | 10 | 4.17 | 3.75 ± 5.30 de | 86.36 ± 19.29 abc | |
| II–III | 2 | |||||||
| II–III | 8 | I | 3 | 3.12 | ||||
| II–III | 5 | |||||||
| 9 | I | 11 | I | 11 | 0 | 5.00 ± 0 cde | 83.22 ± 1.98 abc | |
| II–III | 9 | I | 1 | 11.11 | ||||
| II–III | 6 | |||||||
| IV–V | 2 | |||||||
| 10 | I | 12 | I | 6 | 12.5 | 8.75 ± 8.84 bcde | 68.88 ± 33.13 abc | |
| II–III | 6 | |||||||
| II–III | 8 | I | 2 | 3.125 | ||||
| II–III | 5 | |||||||
| IV–V | 1 | |||||||
| 11 | I | 10 | I | 7 | 7.5 | 18.75 ± 1.77 ab | 37.41 ± 1.48 c | |
| II–III | 3 | |||||||
| II–III | 10 | I | 0 | 30 | ||||
| II–III | 7 | |||||||
| IV–V | 3 | |||||||
| Area | Category | Severity Based on Disease Grade | ||
|---|---|---|---|---|
| I | II–III | IV–V | ||
| Xiangshan Park | treatment | 2.27 | −11.8 | −32.5 |
| ck | 4.71 | 17.3 | 18.75 | |
| Xishan Park | treatment | 1.73 | −18.26 | −35 |
| ck | 3.26 | 15 | 8.33 | |
| Pofengling Park | treatment | 1.85 | −20.59 | −33.33 |
| ck | 3.89 | 12.5 | 12.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Guo, R.; Zheng, B.; Yuan, F.; Song, X.; Zhang, M.; Guo, J.; Liu, K.; Liu, W.; Zhou, X.; et al. Improved Management of Verticillium Wilt in Smoke Trees Through the Use of a Combination of Fungicide and Bioagent Treatments. Forests 2025, 16, 914. https://doi.org/10.3390/f16060914
Zhao Y, Guo R, Zheng B, Yuan F, Song X, Zhang M, Guo J, Liu K, Liu W, Zhou X, et al. Improved Management of Verticillium Wilt in Smoke Trees Through the Use of a Combination of Fungicide and Bioagent Treatments. Forests. 2025; 16(6):914. https://doi.org/10.3390/f16060914
Chicago/Turabian StyleZhao, Yize, Ruifeng Guo, Bo Zheng, Fei Yuan, Xi Song, Mengfei Zhang, Jinzi Guo, Kexin Liu, Weijia Liu, Xiaoran Zhou, and et al. 2025. "Improved Management of Verticillium Wilt in Smoke Trees Through the Use of a Combination of Fungicide and Bioagent Treatments" Forests 16, no. 6: 914. https://doi.org/10.3390/f16060914
APA StyleZhao, Y., Guo, R., Zheng, B., Yuan, F., Song, X., Zhang, M., Guo, J., Liu, K., Liu, W., Zhou, X., Ren, Y., Liu, Z., Zhang, X., & Wang, Y. (2025). Improved Management of Verticillium Wilt in Smoke Trees Through the Use of a Combination of Fungicide and Bioagent Treatments. Forests, 16(6), 914. https://doi.org/10.3390/f16060914





