Abstract
Dothistroma needle blight (DNB) is one of the most significant diseases of conifers, causing premature defoliation, growth reduction, and, in extreme cases, mortality. Histological analysis was undertaken on inoculated seedlings of three different seed sources of Pinus sylvestris L. to investigate the process of infection and degradation of needle tissue on this host species. Seedlings were inoculated using a single spore isolate of Dothistroma septosporum (Doroguine) M. Morelet (D636) from northern Scotland. Mesophyll degradation in the needles occurred by four weeks after inoculation; collapse of mesophyll, bundle sheath tissues, and tracheids by five weeks; and eruption of fruiting bodies in near proximity to stomatal openings by six weeks. Significantly greater collapse of mesophyll during the early stages of infection occurred in the Austrian provenance compared with the United Kingdom provenance, although in the later stages of infection, this difference disappeared. Furthermore, disease severity, assessed as the proportion of needles with D. septosporum conidiomata on each tree, was not significantly different between seed sources.
1. Introduction
Dothistroma needle blight (DNB) is caused by two foliar pathogens, Dothistroma septosporum and D. pini Hulbary [1]. Dothistroma septosporum has a global distribution with a wide range of coniferous hosts, including more than 100 species of pine (Pinus), several spruce (Picea) species, Douglas fir (Pseudotsuga menziesii (Mirbel) Franco), larch (Larix) [2,3,4,5], and others [3]. In contrast, D. pini has a more limited geographic distribution [3,6,7,8], though recent records of D. pini across Europe indicate a wider distribution than initially inferred [9,10,11].
The origin of D. septosporum was hypothesized to be Central America [12], the Himalayas [13], or parts of North America and Europe [14]. A recent analysis of historical scenarios [15] suggests that D. septosporum is an Old World species, native to Northeastern Europe and Western Asia.
DNB is one of the most economically important foliar diseases of pine species worldwide [1]. It has increased in incidence and severity in the Northern Hemisphere over the last three decades [3]. Symptoms include premature needle fall, reduced growth, and, in some cases, tree mortality [16,17].
Interspecific and intraspecific variation in susceptibility to D. septosporum infection and disease development have been observed in various hosts. Intraspecific variation is observed as variation among individuals of the same species within a stand, trees of different age classes, and among populations and provenances [18]. Continued planting of pine in Europe may depend on the identification of individuals, populations, and provenances with low susceptibility to DNB for use in forest establishment. Understanding the contribution of genetically controlled variation in susceptibility would allow the establishment of a breeding program for low susceptibility [19].
Susceptibility of Pinus sylvestris to DNB was rated as high by Gibson [20] but low by most authors [21,22,23,24,25]. Intraspecific variation in susceptibility of United Kingdom (UK) populations of P. sylvestris has been reported [19,26]. When comparing P. sylvestris seed sources from across Europe [18], including the seed sources used here, the impact of seed source on susceptibility varied between experiments. In one highly successful artificial inoculation experiment, in which severe disease developed, an Austrian provenance (AU1) was significantly more resistant than plants derived from two UK sources (UK1 and UK2). Although not always significant in other trials, lower disease severity consistently developed on the Austrian provenance than on the British one.
The incidence and severity of DNB are influenced by both host susceptibility [17,27] and environmental/climatic conditions [28,29,30]. DNB disease outbreaks are often linked to the occurrence of wet weather [29]. A crucial environmental variable known to impact the incidence and severity of DNB [29], along with some other needle diseases [31], is the occurrence of warm rain periods from May to September [32].
Histological studies are of great value in improving our understanding of host–pathogen interactions and disease progression. Kabir et al. [33] confirmed the hemibiotrophic lifestyle of D. septosporum through histological analysis of inoculated Pinus radiata D. Don needles. The fungus initially exhibits a biotrophic phase, growing on the needle surface, followed by penetrating stomata and colonizing epistomatal chambers. In the subsequent necrotrophic phase, hyphae invade the mesophyll tissue and trigger extensive cell necrosis, accompanied by a significant increase in dothistromin toxin production as lesions progress.
Histological studies contribute to pathogen diagnosis and understanding of disease progression [34] and reveal differences in infection patterns and tissue responses across pine species, helping explain why some species are more susceptible to Dothistroma than others [35]. By uncovering how Dothistroma interacts with host tissues, histological studies could contribute to the development of targeted management strategies, including optimal timing of fungicide applications and breeding programs developing resistant pine varieties [36,37]. Understanding how variability in physiological processes relates to variations in susceptibility across provenances (seed sources) remains limited.
The aim of the work reported here was to determine variations in morphological traits in pine needles and fungal infection and development processes that may relate to susceptibility to DNB. The paper reports an assessment of spore germination and mycelial growth on needles of inoculated UK seedlings from seed sources varying in putative resistance level. The processes of mesophyll degradation and sporulation by D. septosporum on these seed sources were also compared.
2. Materials and Methods
2.1. Plant Material and Inoculation
Seeds of Austrian provenance and two United Kingdom (UK) seed sources of P. sylvestris were obtained from the institutions shown in Table 1. Seedlings raised from seeds were maintained in the Cruickshank Botanic Gardens, University of Aberdeen [18,26,38]. In May 2015, two-year-old plants were potted into 1 L square-top pots (9 × 9 × 13 cm) with multipurpose compost (Bulrush Horticulture Ltd., Magherafelt, UK) and fertilized with a 1% solution of Universal Water Soluble Fertilizer Blue (18-11-18 N:P:K + 2·5% MgO + TE; Everris, Ipseich, UK)) according to the protocols of Fraser et al. [18,38].

Table 1.
Sources of Scots pine seed used in DNB inoculations [18].
Eighteen plants in total, six from each of three seed sources (AU1—Austria, Hochwolkersdorf; UK1—UK, Roseisle Forest; UK2—UK, Kielder Forest District; Figure S1), were placed in randomized blocks. Another six plants, two of each provenance, acted as uninoculated controls.
Conidial suspensions were produced using isolate D636, provided by Martin Mullett, originally isolated from Scots pine needles collected in Aberdeenshire in October 2010. Molecular diagnosis with mating type primers [39] confirmed the isolate as D. septosporum mating type 2. For sporulation, D. septosporum was subcultured to pine needle minimal medium with glucose (PMMG). Procedures used for artificial inoculation and incubation methods were modified from the protocol of Kabir et al. [40].
In June 2014, plants were cleaned by removing dead needles before inoculation. Inoculum concentration was adjusted to 3 × 106 spores/mL, and each plant was sprayed with a handheld atomizer from all sides until large droplets formed on the needles (equivalent to an average of 38 mL of conidial suspension per plant). Control plants were sprayed with distilled water. Plants were left to dry and subsequently covered individually with clear plastic bags (moistened internally with distilled water). Plants were maintained in a Fitotron chamber (Weiss Technik UK Ltd., Loughborough, UK) with the following environmental conditions: 20 °C day and 12 °C night, 16 h light and 8 h dark, and a relative humidity of 99.9% with watering as necessary. Floating mist generators placed in a tub containing tap water ensured high relative humidity and needle surface wetness. After 4 days, the bags were removed. Each plant was also sprayed manually with distilled water three times per day.
2.2. Spore Germination, Fungal Colonization, and Histological Assessment of Tissue Degradation
Spore germination on needles was examined at 1 and 4 days after inoculation. Fungal growth/coverage of needles was assessed at 7 and 14 days after inoculation. Four needles were randomly collected from each treated pine seedling 1, 4, 7, and 14 days after inoculation and cut longitudinally before clearing by soaking in acetic acid/ethanol 1:3 v/v (solution A) for 16 h, followed by acetic acid/ethanol/glycerol 1:5:3 v/v/v (solution B) for 3 h with slow shaking. Needle sections were subsequently stained in trypan blue (0.01% in lactophenol) overnight (based on Mehrabi et al. [41]). One hundred randomly selected spores or stomata were examined microscopically for spore germination or fungal penetration from each of the four replicate needles per plant. Surface growth of hyphae was also examined on cleared needles using the same process. Tissue samples were examined under an Olympus BX61 light microscope.
Three symptomatic needles with chlorotic bands (4th week), red bands (5th), and lesions (6th) were collected from each tree. Small pieces of needles, including lesions (bands), were removed for histological analysis of the host–pathogen interaction between P. sylvestris and D. septosporum to observe changes in tissue and cell morphology. Samples were dehydrated in an ethanol series (20, 35, and 50%) at room temperature for 30 min. Following fixing in fresh FAA (50% ethanol, 3.7% formaldehyde, 5% acetic acid, and water to volume) for 30 min at room temperature, post-fixation and embedding were carried out at the Institute of Medical Sciences, University of Aberdeen. Samples were stained in aqueous toluidine blue and embedded in LR White resin. Sections (0.2 µm) were cut using a diamond knife in an Ultramicrotome EM UC7 (Leica Microsystems, Werzlar, Germany). Sections were transferred to glass slides for observation under the Olympus BX61 microscope (Olympus Corporation, Tokyo, Japan).
Infection progress, fungal behavior, and morphology of infection structures of D. septosporum were compared within the 4th, 5th, and 6th weeks after inoculation. Seed-source-specific traits examined included the number of resin canals, resin canal diameters, and thickness of the epidermis, hypodermis, and endodermis.
Degradation of needle tissue by D. septosporum was estimated by image analysis of transverse sections. At 4, 5, and 6 weeks after inoculation, 25 randomly selected transverse sections of each provenance were analyzed using ImageJ (version 1.53 [42]), selecting the color threshold approach to estimate the proportion of healthy tissue (stained blue) and degraded tissue in needles and chlorotic spots.
2.3. Severity Assessment
Nine weeks after inoculation, all remaining needles were removed from each plant and visually inspected for disease severity assessment. Needles were separated into different classes, counted, and categorized using the assessment system of Fraser et al. [18] as green healthy needles, chlorotic or necrotic needles with no apparent symptoms, and needles with D. septosporum conidiomata. Disease severity was measured as the proportion of needles with D. septosporum conidiomata on each tree.
2.4. Statistical Analyses
2.4.1. Spore Germination, Fungal Colonization, and Histological Assessment of Tissue Degradation
Statistical analysis examined the effects of time and provenance on spore germination and fungal coverage of needle surfaces. The analysis sought to identify significant changes across time points and to evaluate whether these patterns were consistent between pine seed sources. The experimental design included two independent variables: time and seed source. Time was categorized into four levels—T1, T4, T7, and T14—representing observations at increasing days after inoculation, while seed source included three levels—AU1, UK1, and UK2—indicating the geographical origin of the plants. The dependent variables spore germination (T1 and T4) and fungal coverage (T7 and T14) were expressed as percentages under the given conditions. Missing data were present in the dataset (n = 27 missing values). To assess the effects of time and provenance, a two-way ANOVA was performed. Assumptions for ANOVA were checked prior to analysis. The independence of observations was assumed based on the study design, the normality of residuals was confirmed using Q-Q plots, and variance homogeneity was assessed through diagnostic plots. All analyses were conducted using the R programming language, with key contributions from the “dplyr” and “tidyr” packages for data manipulation and the “ggplot2” package for visualization. ANOVA and residual diagnostics were performed using base R functions [43].
Descriptive statistics were used for the main provenance-specific trait comparisons (number of resin canals, resin canal diameters, thickness of epidermis, hypodermis, and endodermis).
A mixed model was constructed using the generalized least squares framework (GLS; [44]) in order to explain the percentage of collapsed plant mesophyll based on “plant seed source” and “time”, while individual “plants” were treated as random factors. The significance of model terms was checked using analysis of variance (ANOVA). Pseudo-R2, which determines the proportion of explained variance, was obtained from a correlation between the dependent variable and squared predicted values. Subsequently, models were visualized as predicted values with corresponding 95% confidence intervals. Generalized least squares were analyzed with the “gls” command of the “nlme” package [45]. All graphics and analyses were processed in R 4.2.2 [43].
2.4.2. Severity Assessment
The needles from each tree were categorized by symptoms and counted. The resulting data included the number of symptomatic needles, total needles, and the calculated symptomatic percentage for each tree across different seed sources and blocks. Subsequently, a linear mixed-effects model was constructed to examine the effect of seed source on the symptomatic percentage of needles while accounting for the block design by incorporating it as a random effect. Given that trees with a larger total number of needles provide more precise estimates, we employed a weighted mixed-effects model where weights were inversely proportional to the total number of needles. Confidence intervals for fixed effect estimates were calculated based on the standard errors derived from the Fisher Information Matrix. Satterthwaite’s approximation was used to estimate the effective degrees of freedom. The 95% confidence intervals were computed as the fixed effect estimate plus or minus the critical value from the t-distribution for the approximated degrees of freedom, multiplied by the standard error of the estimate. All statistical computations were carried out using the R programming language (version 4.2.2, [43]) using the “lmerTest” package (version 3.1-3 [46]) for linear mixed-effects modelling.
3. Results
3.1. Light Microscopy Observations and Tissue Degradation
The seed source-specific traits, namely, the number of resin canals, differentiated between seed origins. Generally, AU1 needles had 3–4.8 (mean) −8 resin canals, whereas UK1 and UK2 had 3–5 (mean 3.3 and 3.7, respectively) resin canals adjoining the hypodermis (Table 2). Resin canals also differed in diameter: UK1 had a greater diameter and AU1 a lower diameter but with greater variability (Table 2). The dimensions of the remaining seed source-specific traits were similar among seed origins.

Table 2.
The seed source-specific traits, including descriptive statistics for key anatomical features in µm.
All seed sources had intermediate mesophyll cell folding. Needles collected in the early stage of infection (4th week) with chlorotic spots had mesophyll degradation (Figure 1a–c). The greatest mesophyll collapse was observed in needles of the AU1 provenance. Observations on needles with red bands five weeks post-inoculation showed extensive mesophyll disintegration in the necrotic region. Degradation of the endodermis and transfusion tissue cell collapse occurred in all seed sources (Figure 1d–f). Pathogen hyphae were observed throughout the entire needle transverse section (Figure 1h,i) by six weeks after inoculation, when fruiting bodies appeared on the needles. Microscope observations showed extensive breakdown of mesophyll, bundle sheath, and vascular cells. Fungal hyphae were seen through damaged mesophyll (Figure 1h,i). Large, coalesced masses of hyphae were present within the vascular bundles (phloem) in many samples. Fruiting body formation occurred in tissues where mycelium growth was the most extensive. Subsequently, fruiting bodies erupted through the epidermis.
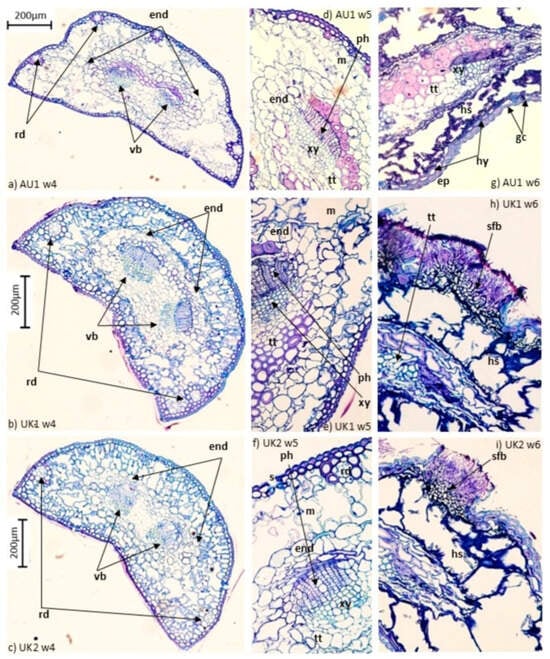
Figure 1.
Cross sections (CS) of Pinus sylvestris needles following inoculation with Dothistroma septosporum. (a–c) CS full needle in the fourth week after inoculation showing the early stage of collapsing mesophyll and endodermis with intact transfusion tissue and vascular bundles; (d–f) expanded cross-sectional views in the fifth week of infection showing collapsed mesophyll and endodermis and the outset of disintegrating transfusion tissue; (g–i) expanded cross-sectional views in the sixth week of infection, where the transfusion tissue is collapsed, with hyphae in intracellular spaces of the collapsed mesophyll and soporiferous fruiting body. end, endodermis; vb, vascular bundle; rd, resin duct; m, mesophyll; ph, phloem; xy, xylem; tt, transfusion tissue; ep, epidermis; hy, hypodermis; gc, guardian cell; hs, hyphae structure; sfb, soporiferous fruiting body.
Mesophyll that did not collapse declined in apparent health during the progress of infection, although, based on the model diagnostics, it was concluded that the proportion of intact mesophyll generally did not change significantly with time (Table 3), with one exception. With plants of the UK2 seed source, a significant decrease in the number of apparently healthy mesophyll cells was observed between 4 and 5 weeks after inoculation. Plants also showed a certain degree of variation in terms of tissue colonization dynamics over time with respect to seed source (Table 4, Figure 2). During the fourth week after inoculation, the highest amount of intact mesophyll was present in needles from plants of UK1 and UK2 and the lowest in AU1; this difference gradually disappeared by the sixth week after inoculation when the intact mesophyll was the same regardless of seed source (Table 4 and Figure 2). The model explained approximately 23.5% of the variability of the dependent variable (% healthy, non-collapsed plant tissue).

Table 3.
Diagnostic table of constructed GLS model aimed to explain the percentage of non-collapsed plant tissue. Level of significance is indicated for every term (two main factors and one double interaction).

Table 4.
Estimated percentage of non-collapsed pine needle tissue for a given combination of seed source and time.
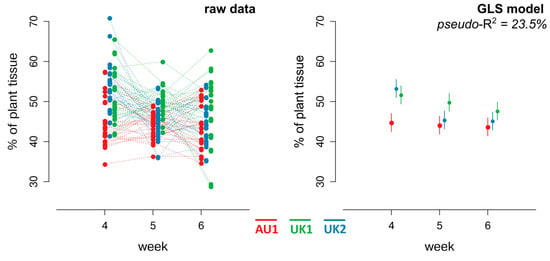
Figure 2.
Graphical representation of raw data and constructed GLS model. The left panel displays raw data for the percentage of healthy (non-degraded) plant tissue across three seed sources (AU1—red, UK1—green, UK2—blue) at weeks 4, 5, and 6 post-inoculation. Each dot represents an individual plant needle section; dots are connected to illustrate temporal changes. The right panel shows the corresponding predicted values from a generalized least squares model. Colored points and vertical lines represent estimated mean values with corresponding 95% confidence intervals for each seed source × time interaction. Overlapping intervals indicate the absence of a significant difference.
3.2. Disease Severity Assessment
The fixed effect of seed source had no significant influence on disease severity (expressed as a percentage of symptomatic needles). Specifically, provenance AU1 had a symptomatic needle percentage of 4.6% (SE = 2.6, df = 15, t-value = 1.790, p = 0.094; Figure 3 and Table 5). Compared to AU1, seed source UK1 showed a reduction in symptomatic needles by −1.5% (n.s.; SE = 3.9, df = 15, t-value = −0.381, p = 0.709; Figure 3 and Table 5). In contrast, symptoms on needles from the UK2 seed source increased by 7.1%, compared with AU1 (n.s.; SE = 3.8, df = 15, t-value = 1.854, p = 0.084; Figure 3 and Table 5). The model constructed with these data, therefore, cannot be used to make conclusive assertions about the role of seed source in determining disease severity. The random effect associated with Block yielded a variance of almost zero, indicating that the inclusion of this term did not explain a significant proportion of the observed variability in symptomatic needle percentages.
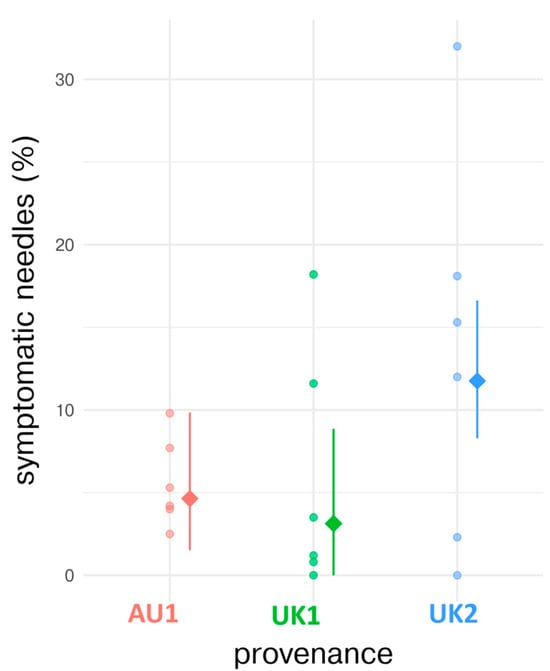
Figure 3.
Comparison of observed and model-estimated values of symptomatic needles across tree seed sources. The model-estimated values are represented by filled diamond markers, while the raw observed percentages (individuals) are denoted by full circles. Additionally, 95% confidence intervals for the model-estimated values are delineated by vertical line ranges, providing an envelope for statistical uncertainty.

Table 5.
Summary of the linear mixed-effects model assessing differences in disease severity (percentage of symptomatic needles) among Scots pine seed sources.
3.3. Spore Germination and Fungal Coverage of Needle Surfaces
Seed source of the plants had no significant effect on spore germination (F = 0.721, p = 0.487), although germination increased with time from inoculation (1 to 4 days). Hyphal coverage of needles, however, decreased sharply between days 7 and 14 (F = 165.353, p < 0.001). The decrease at day 14 after inoculation was common to all seed sources (F = 0.385, p = 0.888) (Table 6). Time, therefore, played a critical role in determining plant spore coverage. The lack of significant differences among plants of the three seed sources suggested that geographical origin did not influence the overall pattern of spore germination or changes in hyphal coverage.

Table 6.
Spore germination (T1 and T4, % of germinated spores) and fungal coverage (T7 and T14, % mycelium coverage on needle surface).
4. Discussion
There was little variation in needle structure between the three different seed sources of P. sylvestris investigated. The number and diameter of resin canals differed between the Austrian (AU1) and the two United Kingdom seed sources (UK1 and UK2). By week four after inoculation, the greatest abundance of collapsed mesophyll tissue was observed on plants from UK seed sources. However, any differences progressively disappeared in the 5th and 6th weeks after inoculation when symptoms had developed further, and similar amounts of collapsed mesophyll were found, regardless of the seed source. Ultimately, therefore, there were no differences between the three seed sources in DNB severity.
The pathogenicity of fungi lies in responding to the local environment in a way that promotes growth inside the host despite the presence of defense mechanisms. Pathogenic fungi that invade aboveground tissues recognize the plant surface, the cuticle, based on hydrophobicity and chemical composition [47]. Several observations suggest that defense mechanisms against DNB infection that act within needles may be more important than those acting on the needle surface [48]. Muir and Cobb [49] suggested the importance of post-penetration mechanisms in defense against the pathogen by demonstrating that there were no correlations between stomatal penetration of Pinus muricata D. Don by D. septosporum and the resulting disease severity during artificial inoculation experiments.
Few differences in needle structure were observed between the three Scots pine populations examined in this work; the thickness of the epidermis, hypodermis, and endodermis was the same regardless of provenance. Plants of the AU1 provenance differed from UK1 and UK2 only in the number and diameter of needle resin canals. Examination of samples by light microscopy in the 4th week after inoculation showed that the pathogen germ tube grew through stomatal openings by penetrating between guard cells, with no evidence for direct penetration of the epidermis by the fungus, as reported previously for P. radiata [49]. No hyphae were seen with light microscopy in the intercellular spaces by the 4th–5th week, deviating from the findings of Kabir et al. [50]. Similarly, no evidence for colonization of resin canals was found during this period, contrasting with previous reports by Gadgil [51] and Peterson and Walla [35], who each reported the presence of hyphae in resin canals in other pine hosts. Nevertheless, intracellular colonization by pathogenic structures was obvious by the 6th week after inoculation, when hyphae expanded into vascular bundles in all seed sources. Furthermore, the fruiting bodies had developed in this period regardless of the seed source. The disease cycle of D. septosporum is usually approximately 6–12 weeks under controlled conditions [33,50], which corresponds with the observations here, as the artificial inoculation tests enabled D. septosporum to complete a life cycle from infection to production of conidiomata.
Overall, the results reported here suggest that the fungus managed to overcome host resistance by the fourth week after inoculation, colonizing the mesophyll. At this time, the infection proceeded similarly in all seed sources. Collapsed mesophyll cells were proposed as a significant resistance mechanism, reducing the number of active infections that have the ability to reach other tissues [52].
After successful colonization of needle mesophyll tissues by the fungus and subsequent development of fruiting bodies, the needles were desiccating and generally decreased in dimension regardless of seed source. This effect, therefore, led to a decrease in the total needle area; thus, measurements of collapsed tissue may have been less accurate by the 6th week after inoculation compared with earlier time points.
The highest DNB severity nine weeks after inoculation was recorded on Scots pine population UK2 and the lowest on Scots pine population UK1, although the differences were not significant. Perry et al. [53] also found no significant differences in susceptibility of P. sylvestris seed sources to D. septosporum in an artificial inoculation trial, but there was high heritability and significant differences in susceptibility to D. septosporum among families, in contrast to the findings of Fraser et al. [26,38]. This variation in the host response could be a result of the use of different pathotypes of D. septosporum or inoculum loads and/or different Scots pine seed sources [51]. In naturally inoculated plants in the field, however, large variations in levels of susceptibility to D. septosporum were found in different Scots pine populations [38,53]. Observed variation in susceptibility to D. septosporum among Scots pine populations in natural inoculations revealed a significant relationship between climatic conditions at the origin and relative susceptibility to the pathogen [19].
The achievement of reliable and consistent levels of DNB infection by artificial inoculation under controlled conditions has long proven difficult [40]. The behavior of D. septosporum on pine seedlings in environmental chambers can deviate markedly from that in natural (forest) conditions [49]. Inoculum from cultures may become less virulent with serial subculturing [53,54,55,56], and Muir & Cobb [49] preferred fresh field inoculum for DNB inoculation. Dothistroma septosporum D626, the isolate used in the current and other published studies, was revived from a 5-year-old culture first made in October 2010 in Aberdeenshire [18,26,38]. Perry et al. [53] also used a single isolate of the pathogen, collected in May 2013. Continual leaf wetness is crucial for controlled inoculation and for hyphal penetration of host stomata [57]. Extremely high conidial densities used for pathogen challenge in the artificial inoculations may also have masked differences in the response among pine populations that were otherwise evident in the natural environment [53].
Ivory [13] suggested that Dothistroma was well adapted to the acid condition of the pine needle internal environment, which is normally below pH 4, and the spores of the fungus can germinate only if sufficient liquid water is present [14]. The needle environment of the seed sources used in the present investigations appeared to be similar, as there was no effect of seed source on spore germination at day 0 and day 4 after inoculation. Spore germination is the initial phase of fungal development and is followed by differentiation and growth. For D. septosporum on P. sylvestris, the process led to coverage of the needle by mycelium, although this particular fungal tissue decreased markedly from the 7th to the 14th day after inoculation on all seed sources; it is likely, however, that D. septosporum had successfully colonized the interior of the needle by that time, where nutrients were obtained to complete the life cycle.
A particular limitation of the current study was that only a small selection of P. sylvestris genetic sources could be included in the work. Similar to some of the less successful trials [21,41], disease severity was not great enough to observe differences. Further work on this subject should, therefore, include a greater range of seed sources and provenances of pines, particularly as it was not possible to work with clonal (or even family) level variation or with greater numbers of individual plants.
5. Conclusions
This study showed that despite minor anatomical differences among seed sources, DNB severity and infection patterns caused by Dothistroma septosporum did not differ significantly under controlled conditions. Histological analysis revealed similar progression across seed sources, suggesting that once host defenses mechanisms are overcome, disease development follows a common trajectory. The results suggest similar susceptibility levels under controlled conditions and highlight the value of histological approaches for understanding host–pathogen interactions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f16060973/s1, Figure S1: Geographic distribution of Pinus sylvestris seed sources investigated in the study.
Author Contributions
Conceptualization, Z.J., K.A., E.O., S.W. and S.F.; methodology, S.F., Z.J. and S.W.; validation, S.F.; formal analysis, Z.J.; investigation, S.F. and Z.J.; resources, S.W.; data curation, Z.J. and R.O.; writing—original draft preparation, Z.J.; writing—review and editing, S.F., S.W., E.O., K.A. and R.O.; visualization, R.O.; supervision, S.W. and K.A.; project administration, Z.J.; funding acquisition, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research, and Sport of the Slovak Republic and the Slovak Academy of Sciences, grant number VEGA 2/0132/22 and MVTS COST 20132 (grant holder: Zuzana Jánošíková). Stuart Fraser (SF) was supported by funding from the Scottish Forestry Trust, Forestry Commission, and Forest Enterprise Scotland.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Gilian Milne for helping with the histological preparation and the collaboration facilitated through the COST Action FP1102—Determining Invasiveness and Risk of Dothistroma (DIAROD)—within the framework of a Short-Term Scientific Mission (STSM) granted to Zuzana (Hečková) Jánošíková (COST STSM Reference Number: COST-STSM-FP1102-18658).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barnes, I.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Multigene phylogenies reveal that red band needle blight is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Stud. Mycol. 2004, 50, 551–565. [Google Scholar]
- Barnes, I.; Kirisits, T.; Akulov, A.; Chhetri, D.B.; Wingfield, B.D.; Bulgakov, T.S.; Wingfield, M.J. New host and country records of the Dothistroma needle blight pathogens from Europe and Asia. For. Pathol. 2008, 38, 178–195. [Google Scholar] [CrossRef]
- Drenkhan, R.; Tomešová-Haataja, V.; Fraser, S.; Bradshaw, R.E.; Vahalík, P.; Mullett, M.S.; Martín-García, J.; Bulman, L.S.; Wingfield, M.J.; Kirisits, T.; et al. Global geographic distribution and host range of Dothistroma species: A comprehensive review. For. Pathol. 2016, 46, 408–442. [Google Scholar] [CrossRef]
- Markovskaja, S.; Raitelaitytė, K.; Kačergius, A.; Kolmakov, P.; Vasilevich, V. Occurrence of Dothistroma needle blight in Lithuania and Belarus: The risk posed to native Scots Pine forests. For. Pathol. 2020, 50, e12626. [Google Scholar] [CrossRef]
- Watt, M.S.; Kriticos, D.J.; Alcaraz, S.; Brown, A.V.; Leriche, A. The hosts and potential geographic range of Dothistroma needle blight. For. Ecol. Manag. 2009, 257, 1505–1519. [Google Scholar] [CrossRef]
- Fabre, B.; Ioos, R.; Piou, D.; Marcais, B. Is the emergence of Dothistroma needle blight of pine in France caused by the cryptic species Dothistroma pini? Phytopathology 2012, 102, 47–54. [Google Scholar] [CrossRef]
- Adamson, K.; Mullett, M.S.; Solheim, H.; Barnes, I.; Müller, M.M.; Hantula, J.; Vuorinen, M.; Kačergius, A.; Markovskaja, S.; Musolin, D.L.; et al. Looking for relationships between the populations of Dothistroma septosporum in northern Europe and Asia. Fungal Genet. Biol. 2018, 110, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Davydenko, K.; Baturkyn, D.; Hnoievyi, I.; Shcherbak, O. New Data on Host Range and Geographical Distribution of Dothistroma Needle Blight in Ukraine. Environ. Sci. Proc. 2021, 3, 89. [Google Scholar] [CrossRef]
- Mullett, M.S.; Adamson, K.; Bragança, H.; Bulgakov, T.S.; Georgieva, M.; Henriques, J.; Jurisoo, L.; Laas, M.; Drenkhan, R. New country and regional records of the pine needle blight pathogens Lecanosticta acicola, Dothistroma septosporum and Dothistroma pini. For. Pathol. 2018, 48, e12440. [Google Scholar] [CrossRef]
- Ortíz de Urbina, E.; Mesanza, N.; Aragonés, A.; Raposo, R.; Elvira-Recuenco, M.; Boqué, R.; Patten, C.; Aitken, J.; Iturritxa, E. Emerging Needle Blight Diseases in Atlantic Pinus Ecosystems of Spain. Forests 2017, 8, 18. [Google Scholar] [CrossRef]
- Wartalska, P.; Oszako, T.; Bakier, S.; Belbahri, L.; Malewski, T.; Hsiang, T.; Popowska-Nowak, E.; Nowakowska, J. Dothistroma septosporum not detected in Pinus sylvestris seed trees from investigated stands in Southern Poland. Forests 2021, 12, 1323. [Google Scholar] [CrossRef]
- Evans, H.C. The Genus Mycosphaerella and Its Anamorphs Cercoseptoria, Dothistroma and Lecanosticta on Pines; CABI: Wallingford, UK, 1984; 102p. [Google Scholar]
- Ivory, M.H. Records of foliage pathogens of Pinus species in tropical countries. Plant Pathol. 1994, 43, 511–518. [Google Scholar] [CrossRef]
- Gibson, I.A.S. Impact and control of Dothistroma blight of pines. Eur. J. For. Pathol. 1974, 4, 89–100. [Google Scholar] [CrossRef]
- Mullett, M.S.; Drenkhan, R.; Adamson, K.; Boroń, P.; Lenart-Boroń, A.; Barnes, I.; Tomšovský, M.; Jánošíková, Z.; Adamčíková, K.; Ondrušková, E.; et al. Worldwide genetic structure elucidates the Eurasian origin and invasion pathways of Dothistroma septosporum, causal agent of Dothistroma needle blight. J. Fungi 2021, 7, 111. [Google Scholar] [CrossRef]
- Brown, A.; Webber, J. Red Band Needle Blight of Conifers in Britain; Edinburgh, Research Note—Forestry Commission, Bulletin (No. 002); Forestry Commission: Bristol, UK, 2008. [Google Scholar]
- Rodas, C.A.; Wingfield, M.J.; Granados, G.M.; Barnes, I. Dothistroma needle blight: An emerging epidemic caused by Dothistroma septosporum in Colombia. Plant Pathol. 2016, 65, 53–63. [Google Scholar] [CrossRef]
- Fraser, S.; Woodward, S.; Brown, A.V. Inter- and intraspecific variation in susceptibility to Dothistroma needle blight in Britain. How susceptible are Pinus sylvestris and Pinus contorta? For. Pathol. 2016, 46, 534–546. [Google Scholar] [CrossRef]
- Perry, A.; Brown, A.V.; Cavers, S.; Cottrell, J.E.; Ennos, R.A. Has Scots pine (Pinus sylvestris) co-evolved with Dothistroma septosporum in Scotland? Evidence for spatial heterogeneity in the susceptibility of native provenances. Evol. Appl. 2016, 9, 982–993. [Google Scholar] [CrossRef]
- Gibson, I.A.S. Diseases of Forest Trees Widely Planted as Exotics in the Tropics and Southern Hemisphere. Part II. The Genus Pinus; Commonwealth Mycological Institute/Commonwealth Forestry Institute: Kew, UK, 1979. [Google Scholar]
- Peterson, G.W. Dothistroma needle blight of Austria and ponderosa pines: Epidemiology and control. Phytopathology 1967, 57, 437–441. [Google Scholar]
- Gilmour, J.W. Distribution and significance of the needle blight of pines caused by Dothistroma pini in New Zealand. Plant Dis. Rep. 1967, 51, 727–730. [Google Scholar]
- Lang, K.J. Dothistroma pini on young spruces (Picea, Abies). Eur. J. For. Pathol. 1987, 17, 316–317. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Burnes, T.A.; David, A.J.; Blanchette, R.A. Histopathology of primary needles and mortality associated with white pine blister rust in resistant and susceptible Pinus strobus. For. Pathol. 2009, 39, 361–376. [Google Scholar] [CrossRef]
- Karadzic, D. The mechanism of some fungal infections of the needles of Austrian pine and scotch pine. Zast. Bilja 1989, 40, 35–46. [Google Scholar]
- Fraser, S.; Brown, A.V.; Woodward, S. Intraspecific variation in susceptibility to Dothistroma needle blight within native Scottish Pinus sylvestris. Plant Pathol. 2015, 64, 864–870. [Google Scholar] [CrossRef]
- Ivory, M.H. Reaction of pines in Kenya to attack by Dothistroma pini var. keniensis. East Afr. Agric. For. J. 1968, 33, 236–244. [Google Scholar] [CrossRef]
- Peterson, G.W. Infection of Austrian and ponderosa pines by Dothistroma pini in Eastern Nebraska. Phytopathology 1973, 63, 1060–1063. [Google Scholar] [CrossRef]
- Woods, A.J.; Coates, K.D.; Haman, A. Is an unprecedented Dothistroma needle blight epidemic related to climate change? Bioscience 2005, 55, 761–769. [Google Scholar] [CrossRef]
- Woods, A.J.; Martín-García, J.; Bulman, L.; Vasconcelos, M.W.; Boberg, J.; La Porta, N.; Peredo, H.; Vergara, G.; Ahumada, R.; Brown, A.; et al. Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. For. Pathol. 2016, 46, 443–452. [Google Scholar] [CrossRef]
- Hoff, R.J. Susceptibility of Lodgepole Pine to the Needle Cast Fungus Lophodermella concolor; Intermountain Forest and Range Experiment Station, US Department of Agriculture, Forest Service: Ogden, UT, USA, 1985; Volume 349, pp. 1–5. [Google Scholar]
- Ondrušková, E.; Ostrovský, R.; Jánošíková, Z.; Adamčíková, K.; Kobza, M. Selected climatic variables in Slovakia are favourable to the development of Dothistroma needle blight. Folia Oecologica 2020, 47, 144–152. [Google Scholar] [CrossRef]
- Kabir, M.S.; Ganley, R.J.; Bradshaw, R.E. Dothistromin toxin is a virulence factor in Dothistroma needle blight of pines. Plant Pathol. 2014, 64, 225–234. [Google Scholar] [CrossRef]
- Peterson, R.L.; Enstone, D.E.; Basu, C. Histological techniques for plant pathology research. Can. J. Bot. 2011, 89, 122–131. [Google Scholar]
- Peterson, G.W.; Walla, J.A. Development of Dothistroma pini upon and within needles of Austrian and ponderosa pines in eastern Nebraska. Phytopathology 1978, 68, 1422–1430. [Google Scholar] [CrossRef]
- Bradshaw, R.E. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: A review. For. Pathol. 2004, 34, 163–185. [Google Scholar] [CrossRef]
- Bulman, L.S.; Bradshaw, R.E.; Fraser, S.; Martín-García, J.; Barnes, I.; Musolin, D.L.; La Porta, N.; Woods, A.J.; Diez, J.J.; Koltay, A.; et al. A worldwide perspective on the management and control of Dothistroma needle blight. For. Pathol. 2016, 46, 472–488. [Google Scholar] [CrossRef]
- Fraser, S.; Mullett, M.S.; Woodward, S.; Brown, A.V. Between-site and -year variation in the relative susceptibility of native Scottish Pinus sylvestris populations to Dothistroma needle blight. Plant Pathol. 2016, 65, 369–379. [Google Scholar] [CrossRef]
- Groenewald, M.; Barnes, I.; Bradshaw, R.E.; Brown, A.; Dale, A.; Groenewald, J.Z.; Lewis, K.J.; Wingfield, B.D.; Wingfield, M.J.; Crous, P.W. Characterization and worldwide distribution of the mating type genes in the Dothistroma needle blight pathogens. Phytopathology 2007, 97, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Ganley, R.J.; Bradshaw, R.E. An improved artificial pathogenicity assay for Dothistroma needle blight on Pinus radiata. Australas. Plant Pathol. 2013, 42, 503–510. [Google Scholar] [CrossRef]
- Mehrabi, R.; Zwiers, L.H.; De Waard, M.; Kema, G.H.J. MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola. Mol. Plant Microbe Interact. 2006, 19, 1262–1269. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: Berlin/Heidelberg, Germany, 2000; pp. 100, 461. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1–118. 2014. Available online: http://CRAN.R-project.org/package=nlme (accessed on 24 February 2024).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: http://www.R-project.org/ (accessed on 24 February 2024).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- van der Does, H.C.; Rep, M. Adaptation to the host environment by plant-pathogenic fungi. Annu. Rev. Phytopathol. 2017, 4, 427–450. [Google Scholar] [CrossRef]
- Fraser, S.; Martín-García, J.; Perry, A.; Kabir, M.S.; Owen, T.; Solla, A.; Brown, A.V.; Bulman, L.S.; Barnes, I.; Hale, M.D.; et al. A review of Pinaceae resistance mechanisms against needle and shoot pathogens with a focus on the Dothistroma–Pinus interaction. For. Pathol. 2016, 46, 453–471. [Google Scholar] [CrossRef]
- Muir, J.A.; Cobb, F.W. Infection of radiata and bishop pine by Mycosphaerella pini in California. Can. J. For. Res. 2005, 35, 2529–2538. [Google Scholar] [CrossRef]
- Kabir, M.S.; Ganley, R.J.; Bradshaw, R.E. The hemibiotrophic lifestyle of the fungal pine pathogen Dothistroma septosporum. For. Pathol. 2015, 45, 190–202. [Google Scholar] [CrossRef]
- Gadgil, P.D. Infection of Pinus radiata needles by Dothistroma pini. N. Z. J. Bot. 1967, 5, 498–503. [Google Scholar] [CrossRef]
- Jurgens, J.A.; Blanchette, R.A.; Zambino, P.J.; David, A. Histology of white pine blister rust in needles of resistant and susceptible eastern white pine. Plant Dis. 2003, 87, 1026–1030. [Google Scholar] [CrossRef]
- Perry, A.; Wachowiak, W.; Brown, A.V.; Ennos, R.A.; Cottrell, J.E.; Cavers, S. Substantial heritable variation for susceptibility to Dothistroma septosporum within populations of native British Scots pine (Pinus sylvestris). Plant Pathol. 2016, 65, 987–996. [Google Scholar] [CrossRef]
- Devey, M.E.; Groom, K.A.; Nolan, M.F.; Bell, J.C.; Dudzinski, M.J.; Old, K.M.; Matheson, A.C.; Moran, G.F. Detection and verification of quantitative trait loci for resistance to Dothistroma needle blight in Pinus radiata. Theor. Appl. Genet. 2004, 108, 1056–1063. [Google Scholar] [CrossRef]
- Bradshaw, R.E.; Jin, H.P.; Morgan, B.S.; Schwelm, A.; Teddy, O.R.; Young, C.A.; Zhang, S. A polyketide synthase gene required for biosynthesis of the aflatoxin-like toxin, dothistromin. Mycopathologia. 2006, 161, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Schwelm, A.; Barron, N.J.; Baker, J.; Dick, M.; Long, P.G.; Zhang, S.; Bradshaw, R.E. Dothistromin toxin is not required for Dothistroma needle blight in Pinus radiata. Plant Pathol. 2009, 58, 293–304. [Google Scholar] [CrossRef]
- Gadgil, P.D. Duration of leaf wetness periods and infection of Pinus radiata by Dothistroma pini. N. Z. J. For. 1977, 7, 83–90. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).