Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Treatments
2.3. Phenotypic, Physiological, and Nutritional Evaluation
2.3.1. Sprout Productivity
2.3.2. Rooting Performance
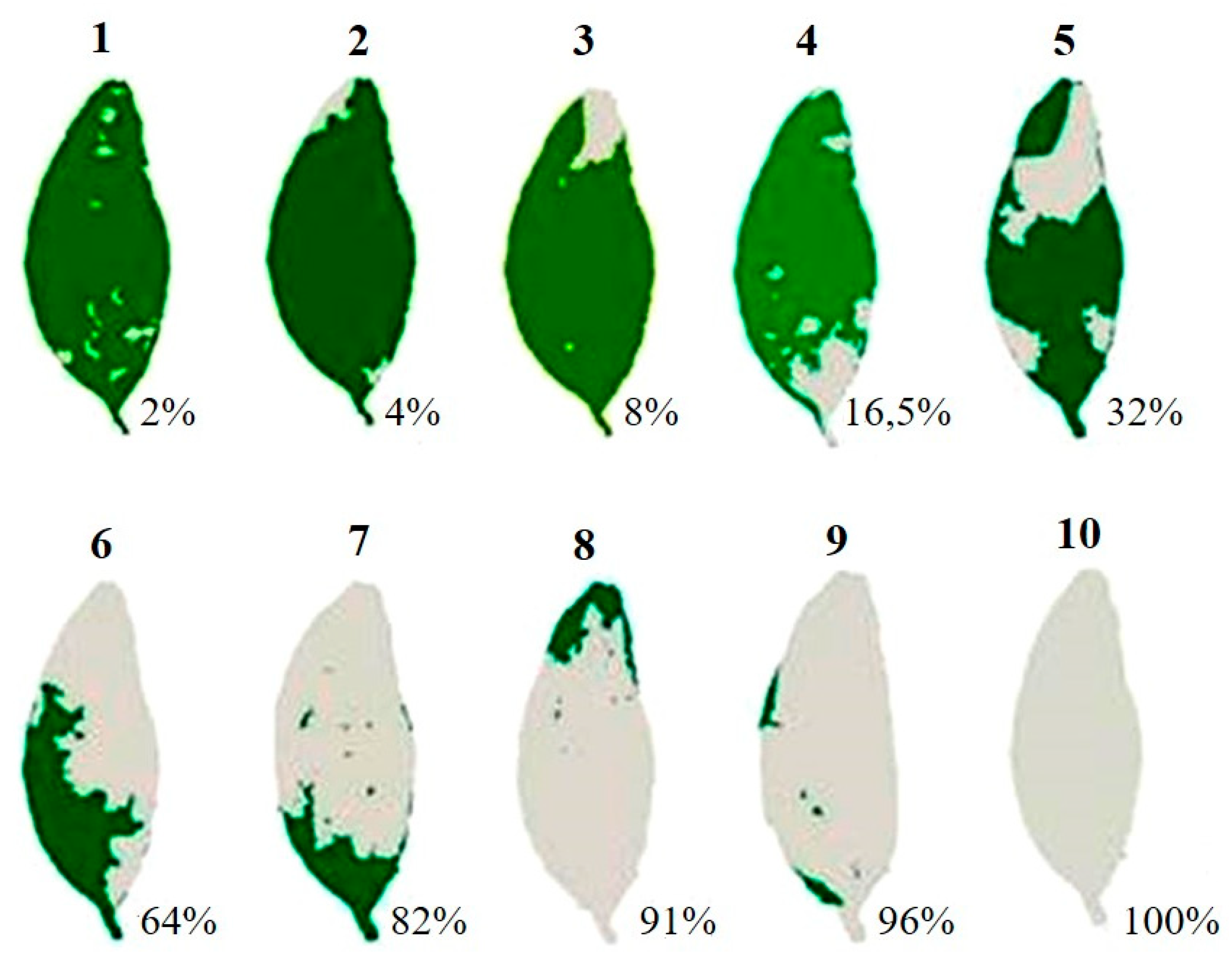
2.3.3. Phytosanitary Status
2.3.4. Physiological Parameters
2.3.5. Nutritional Composition
2.4. Statistical Analyses
3. Results
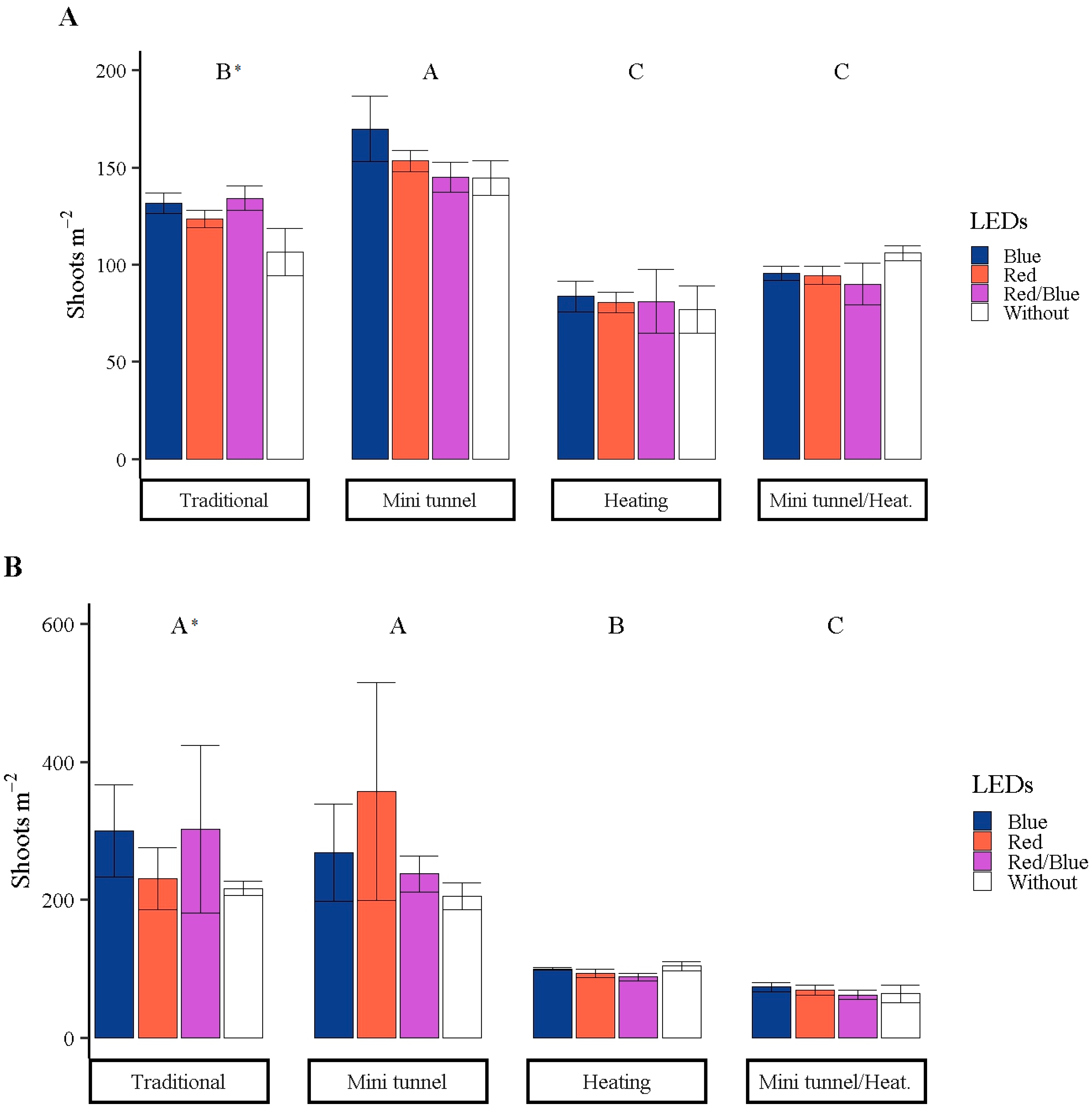
3.1. Sprout Productivity
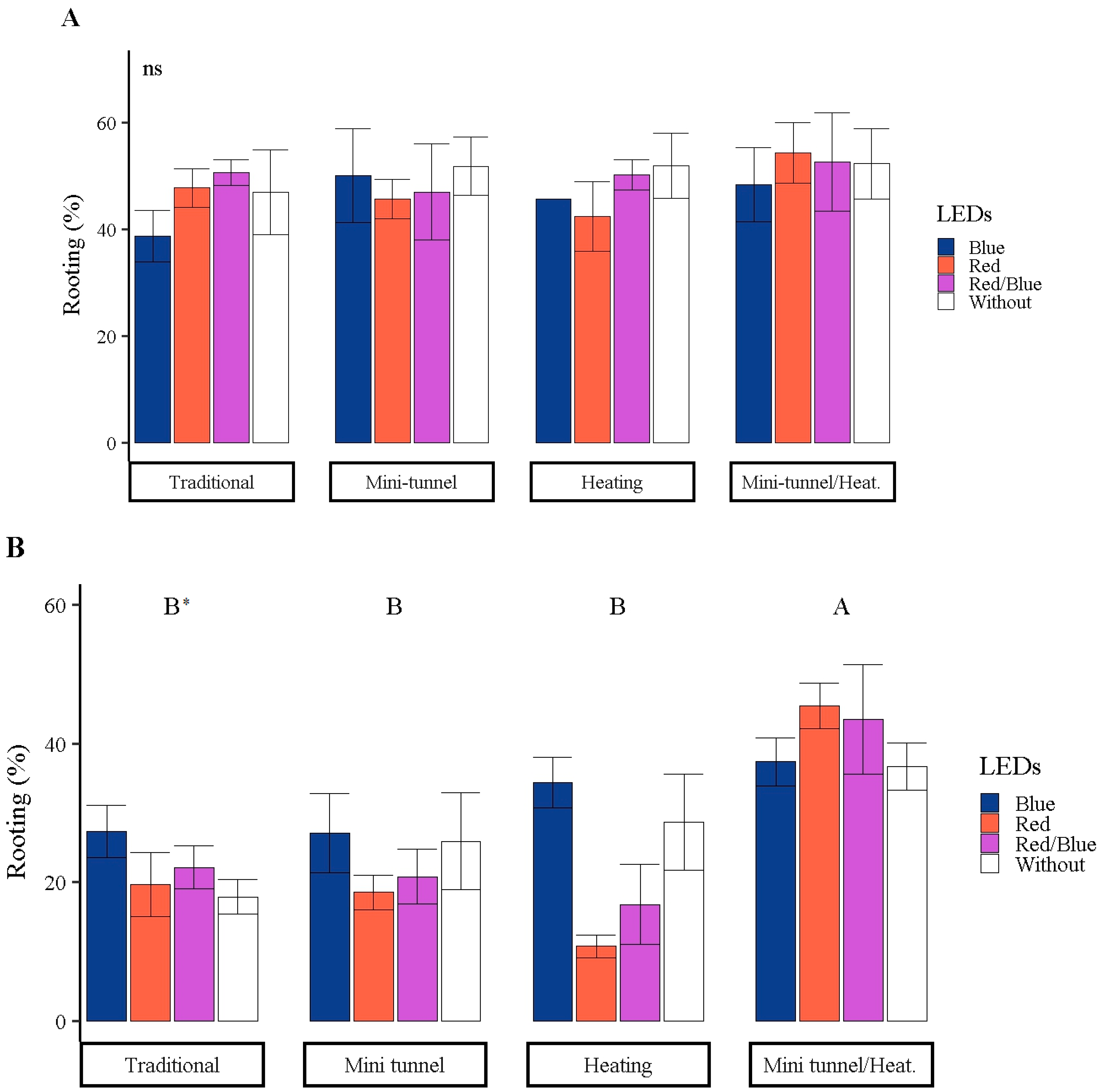
3.2. Rooting of Mini Cuttings
3.3. Phytosanitary Variable
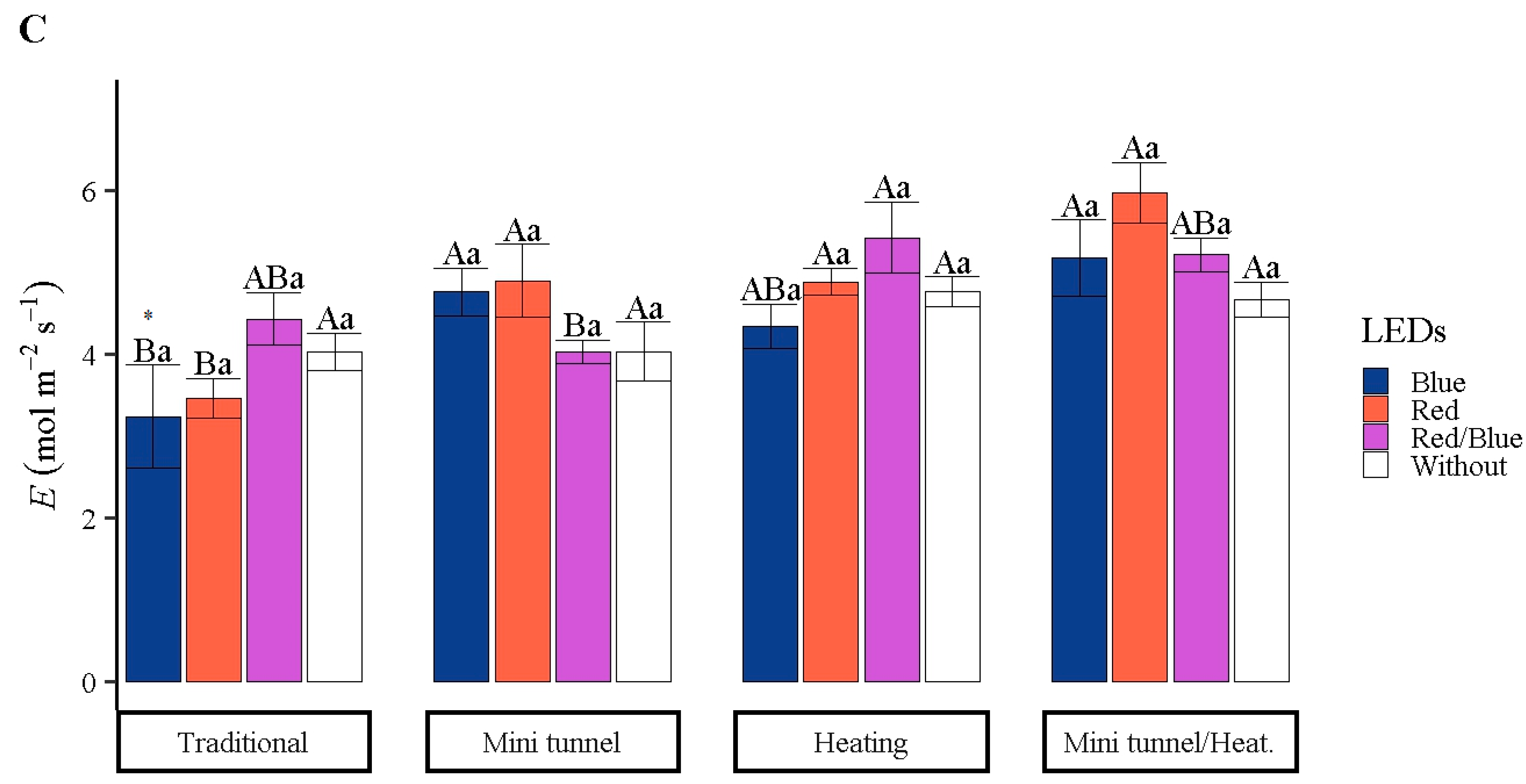
3.4. Physiologic and Nutritional Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indústria Brasileira de Árvores. Anuário Estatístico do IBÁ; Ano-Base 2023; Associação Brasileira de Árvores: São Paulo, Brazil, 2023; p. 88. Available online: https://iba.org/datafiles/publicacoes/relatorios/relatorio2024.pdf (accessed on 18 January 2025).
- Vilasboa, J.; Da Costa, C.T.; Fett-Neto, A.G. Environmental modulation of mini-clonal gardens for cutting production and propagation of hard- and easy-to-root Eucalyptus spp. Plants 2022, 11, 3281. [Google Scholar] [CrossRef] [PubMed]
- Canguçu, V.d.S.; Titon, M.; Silva, L.F.M.; Pena, C.A.A.; Júnior, S.L.d.A.; dos Santos, P.H.R.; de Oliveira, M.L.R. Mini-tunnel models influence the productivity of eucalyptus mini-stumps? Bosque 2022, 43, 211–219. [Google Scholar] [CrossRef]
- de Lima, M.S.; Araujo, M.M.; Berghetti, Á.L.P.; Aimi, S.C.; Costella, C.; Griebeler, A.M.; Somavilla, L.M.; dos Santos, O.P.; dos Reis Teixeira Valente, B.M. Mini-cutting technique application in Corymbia and Eucalyptus: Effects of mini-tunnel use across seasons of the year. New For. 2022, 53, 161–179. [Google Scholar] [CrossRef]
- Navroski, M.C.; Schicora, L.; Pereira, M.d.O.; Silva, J.J.d.N.; Duarte, L.F.C.; Schilisting, T. Influence of shadowing in Sequoia sempervirens (D. Don) Endl. mini-stumps and mini-cuttings. Rev. Ceres 2022, 69, 443–448. [Google Scholar] [CrossRef]
- Assis, T.F. Hybrids and mini-cutting: A powerful combination that has revolutionized the Eucalyptus clonal forestry. In BMC Proceedings; BioMed Central: London, UK, 2011; Volume 5. [Google Scholar]
- Rocha, F.M.; Maravilha, L.F.; Titon, M.; De-Oliveira-Fernandes, S.J.; Mendonça-Machado, E.L.; De-Souza-Martins, N. Productivity of mini-cuttings of a hybrid clone of Eucalyptus urophylla x Eucalyptus pellita as a function of exposure time of mini-stumps to mini-tunnel. Bosque 2023, 44, 595–603. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E. Plant Propagation Principles and Practices, 1st ed.; Pearson Education Limited: London, UK, 2014; 927p. [Google Scholar]
- Jo, W.J.; Shin, J.H. Effect of root-zone heating using positive temperature coefficient film on growth and quality of strawberry (Fragaria x ananassa) in greenhouses. Hortic. Environ. Biotechnol. 2022, 63, 89–100. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Zhang, X. Effects of different root zone heating methods on the growth and photosynthetic characteristics of cucumber. Horticulturae 2022, 8, 1137. [Google Scholar] [CrossRef]
- Shin, J.; Lee, B.; Cui, M.; Lee, H.; Myung, J.; Na, H.; Chun, C. Effects of supplemental root-zone pipe heating systems on the growth and development of strawberry plants in a greenhouse during the winter season. N. Z. J. Crop Hortic. Sci. 2023. [Google Scholar] [CrossRef]
- Nhut, D.T.; Nam, N.B. Light-emitting diodes (LEDs): An artificial lighting source for biological studies. In The Third International Conference on the Development of Biomedical Engineering in Vietnam; Springer: Berlin/Heidelberg, Germany, 2010; pp. 134–139. [Google Scholar]
- Ramesh, T.; Hariram, U.; Srimagal, A.; Sahu, J.K. Applications of light emitting diodes and their mechanism for food preservation. J. Food Saf. 2023, 43, e13040. [Google Scholar] [CrossRef]
- Konzen, E.R.; Saudade de Aguiar, N.; Navroski, M.C.; Mota, C.S.; Miranda, L.; Estopa, R.A.; Tonett, E.L.; Pereira, M.D.O. Artificial light improves productivity of mini-cuttings in a clonal minigarden of E. benthamii and E. dunnii. South. For. J. For. Sci. 2022, 83, 310–320. [Google Scholar] [CrossRef]
- Ruiz, A.M.M. Escala diagramática para avaliação da severidade de oídio em eucalipto. Ciência Florest. 2021, 31, 1535–1546. [Google Scholar] [CrossRef]
- Navroski, M.C.; Pereira, M.d.O.; Konzen, E.R.; Miranda, L.; Estopa, R.A.; Mota, C.S. Photosynthetic light response curves in Eucalyptus benthamii and Eucalyptus dunnii clones. Aust. J. Crop Sci. 2022, 16, 949–954. [Google Scholar] [CrossRef]
- Rodrigues, R.C. Métodos de Análises Bromatológicas de Alimentos: Métodos Físicos, Químicos e Bromatológicos. 2010. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/884390 (accessed on 30 January 2025).
- Oliveira, A.S. Propagação Clonal de Eucalipto em Ambiente Protegido por Estufins: Produção, Ecofisiologia e Modelagem do Crescimento das Miniestacas. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2016. [Google Scholar]
- Pimentel, N.; Lencina, K.H.; Kielse, P.; Rodrigues, M.B.; Somavilla, T.M.; Bisognin, D.A. Produtividade de minicepas e enraizamento de miniestacas de clones de erva-mate (Ilex paraguariensis A. St.-Hil.). Cienc. Florest. 2019, 29, 559–570. [Google Scholar] [CrossRef]
- Cunha, A.C.M.C.M.; Paiva, H.N.; Leite, H.G.; Barros, N.F.; Leite, F.P. Relações entre variáveis climáticas com produção e enraizamento de miniestacas de eucalipto. Rev. Árvore 2009, 33, 195–203. [Google Scholar] [CrossRef]
- Konzen, E.R.; Navroski, M.C.; Pereira, M.O.; Nascimento, B.; Meneguzzi, A.; E Souza, P.F. Genetic variation for growth variables of Eucalyptus benthamii Maiden & Cambage and E. smithii R. T. Baker provenances in Southern Brazil. Cerne 2017, 23, 359–366. [Google Scholar]
- Kim, H.R.; You, Y.H. Effects of Red, Blue, White, and Far-red LED Source on Growth Responses of Wasabia japonica Seedlings in Plant Factory. Hortic. Sci. Technol. 2013, 31, 415–422. [Google Scholar]
- Fanwoua, J.; Vercambre, G.; Buck-Sorlin, G.; Dieleman, J.A.; de Visser, P.; Génard, M. Supplemental LED lighting affects the dynamics of tomato fruit growth and composition. Sci. Hortic. 2019, 256, 108571. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Li, C.X.; Chang, S.X.; Khalil Ur Rehman, M.; Xu, Z.G.; Tao, J.M. Effect of irradiating the leaf abaxial surface with supplemental light-emitting diode lights on grape photosynthesis. Aust. J. Grape Wine Res. 2017, 23, 58–65. [Google Scholar] [CrossRef]
- Brondani, G.E.; Wendling, I.; Grossi, F.; Dutra, L.F.; Araujo, M.A. Miniestaquia de Eucalyptus benthamii × Eucalyptus dunnii: Sobrevivência de minicepas e produção de miniestacas em função das coletas e estações do ano. Ciênc. Florest. 2010, 22, 11–21. [Google Scholar] [CrossRef]
- Rocha, P.S.G.; Oliveira, R.P.; Scivittaro, W.B. Uso de LEDs na multiplicação e enraizamento in vitro de framboeseiras. Pesqui. Agropecu. Gaúch. 2013, 19, 95–101. [Google Scholar]
- Baque, M.A.; Hahn, E.J.; Paek, K.Y. Induction mechanism of adventitious root from leaf explants of Morinda citrifolia as 673 affected by auxin and light quality. In Vitro Cell. Dev. Biol. Plant 2010, 46, 71–80. [Google Scholar] [CrossRef]
- Ou Yang, F.; Ou, Y.; Zhu, T.; Ma, J.; An, S.; Zhao, J.; Wang, J.; Kong, L.; Zhang, H.; Tigabu, M. Growth and Physiological 625 Responses of Norway Spruce (Picea abies (L.) H. Karst) Supplemented with Monochromatic Red, Blue and Far-Red 626 Light. Forests 2021, 12, 164. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, H.; Liu, B.; Song, S.; Zhang, X.; Song, Y.; Liu, B. Response of Morphological Plasticity of Quercus variabilis Seedlings to Different Light Quality. Forests 2024, 15, 2153. [Google Scholar] [CrossRef]
- Riikonen, J. Applications of Different Light Spectra in Growing Forest Tree Seedlings. Forests 2021, 12, 1194. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Rafiee, S.; Mousazadeh, H. Environmental impact assessment of open field and greenhouse strawberry production. Eur. J. Agron. 2013, 50, 29–37. [Google Scholar] [CrossRef]
- Alfenas, A.C.; Zauza, E.A.V.; Mafia, R.G.; Assis, T.F. Clonagem e Doenças do Eucalipto; Editora UFV: Viçosa, Brazil, 2009; Volume 2. [Google Scholar]
- Zauza, E.A.V.; Santos, Á.F.; Alfenas, A.C.; Barros, N.F.; Maia, M.L. Doenças em viveiros e em plantios de eucalipto. In Eucalyptus: Doenças e Pragas, 1st ed.; Alfenas, A.C., Ed.; UFV: Viçosa, Brazil, 2010; pp. 125–196. [Google Scholar]
- Martins, M.V.V.; Lima, J.S. Flutuação do Inóculo Nas Epidemias de Oídio do Cajueiro-Anão. Boletim de Pesquisa e Desenvolvimento. n. 256, 2025. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1172444 (accessed on 22 January 2025).
- Krugner, T.L.; Auer, C.G. Doenças dos eucaliptos. In Manual de Fitopatologia: Doenças das Plantas 681 Cultivadas, 4th ed.; Kimati, H., Ed.; Agronômica CERES: São Paulo, Brazil, 2005; pp. 319–332. [Google Scholar]
- Flexas, J.; Carriquí, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2019, 101, 964–978. [Google Scholar] [CrossRef]
- Pacheco, F.; Lazzarini, L.; Alvarenga, I. Metabolismo relacionado com a fisiologia dos estômatos. Enciclopédia Biosf. 2021, 18, 186–206. [Google Scholar] [CrossRef]
- Shimazaki, K.I.; Doi, M.; Assman, S.M.; Kinoshita, T. Ligth regulations of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Higashi, E.N.; Silveira, R.L.V.A.; Gonçalves, A.N. Monitoramento nutricional e fertilização em macro, mini microjardim clonal de Eucalyptus. In Nutrição e Fertilização Florestal; Gonçalves, J.L.M., Benedetti, V., Eds.; Ipef: Piracicaba, Brazil, 2000; pp. 191–217. [Google Scholar]
- Schroeter-Zakrzewska, A.; Kleiber, T. The effect of light colour and type of lamps on rooting and nutrient status in cuttings of Michaelmas daisy. Bulg. J. Agric. Sci. 2014, 20, 1426–1434. [Google Scholar]

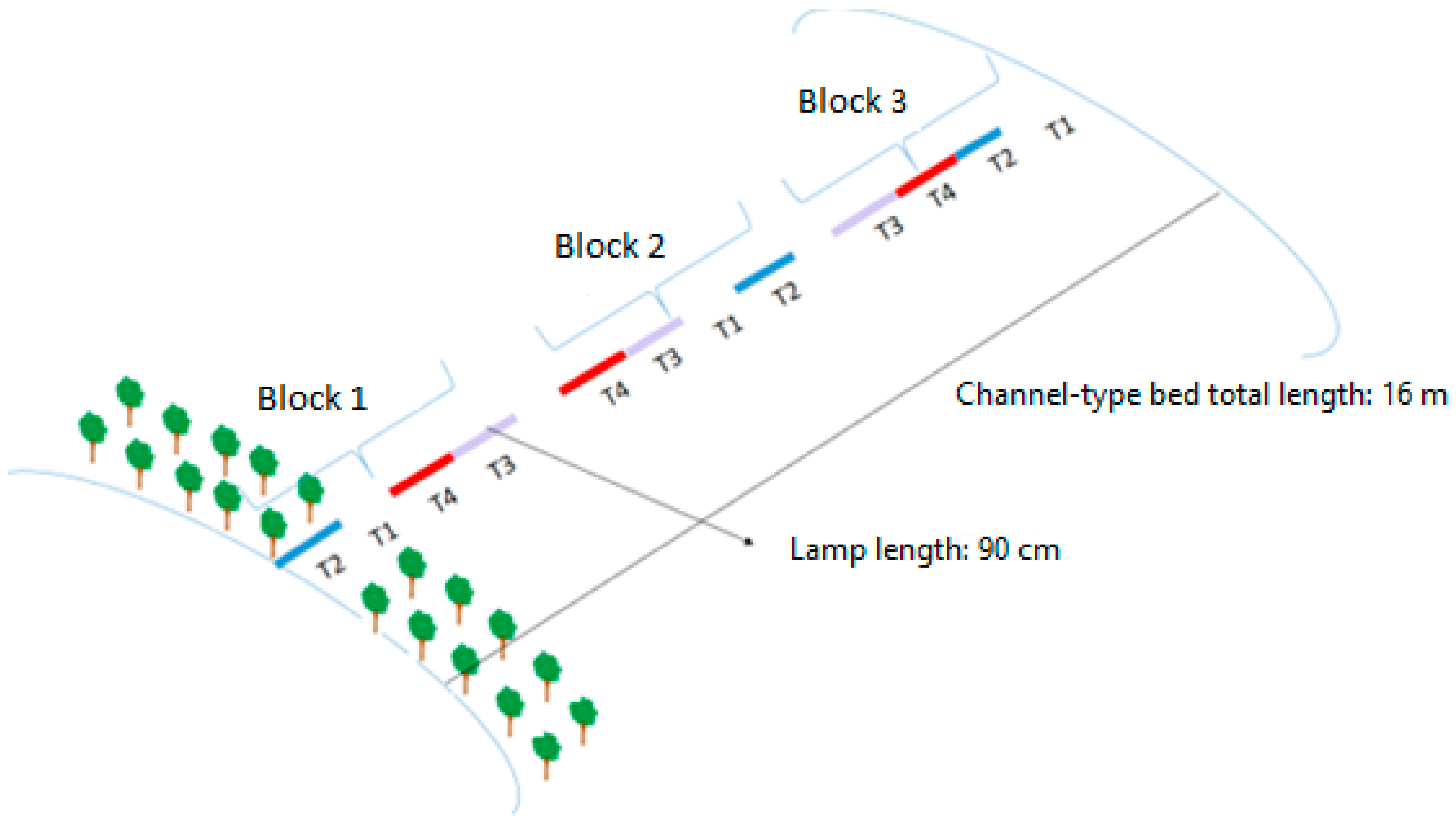


| Environment | Specification |
| Traditional | Without plastic cover and without heating of the sand bed |
| Mini tunnel | Covered with plastic (40 cm high) and without heating on the sand bed |
| Heating | Without plastic cover and with heating in the sand bed |
| Mini tunnel + heating | Covered with plastic (40 cm high) and with heating on the sand bed |
| Lamp | Specification |
| Without | Natural light (sunlight) |
| Blue | 36″, 1 W, AC85—265 V, blue = 450 = 2:1, LED quantity: 36 pcs |
| Red/Blue | 36″, 1 W, AC85—265 V, red/blue = 660:450 = 2:1, LED quantity: 36 pcs |
| Red | 36″, 1 W, AC85—265 V, red/rose = 660:730 = 1:1, LED quantity: 36 pcs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilisting, T.; Sá, A.C.S.; da Silva Filho, D.P.; da Silva, V.M.; Navroski, M.C.; de Oliveira Pereira, M.; Nascimento, B.; Moraes, C.; de Andrade, R.S.; Estopa, R.A.; et al. Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden. Forests 2025, 16, 901. https://doi.org/10.3390/f16060901
Schilisting T, Sá ACS, da Silva Filho DP, da Silva VM, Navroski MC, de Oliveira Pereira M, Nascimento B, Moraes C, de Andrade RS, Estopa RA, et al. Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden. Forests. 2025; 16(6):901. https://doi.org/10.3390/f16060901
Chicago/Turabian StyleSchilisting, Thalia, Alexandra Cristina Schatz Sá, Daniel Pereira da Silva Filho, Valéria Martel da Silva, Marcio Carlos Navroski, Mariane de Oliveira Pereira, Bruno Nascimento, Carolina Moraes, Ramon Silveira de Andrade, Regiane Abjaud Estopa, and et al. 2025. "Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden" Forests 16, no. 6: 901. https://doi.org/10.3390/f16060901
APA StyleSchilisting, T., Sá, A. C. S., da Silva Filho, D. P., da Silva, V. M., Navroski, M. C., de Oliveira Pereira, M., Nascimento, B., Moraes, C., de Andrade, R. S., Estopa, R. A., & Miranda, L. (2025). Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden. Forests, 16(6), 901. https://doi.org/10.3390/f16060901








