Bark Stripping Damage Caused by Red Deer (Cervus elaphus L.): Inventory Design Using Hansen–Hurwitz and Horvitz–Thompson Approach
Abstract
1. Introduction
1.1. Bark Stripping Damage as Ecological and Economical Factor
1.2. Importance of Bark Stripping Assessments
1.3. Adaptive Sampling with Hansen–Hurwitz and Horvitz–Thompson Estimator
1.3.1. ACS and Network Development
1.3.2. Inclusion Probability in ACS; Hansen–Hurwitz and Horvitz–Thompson Estimator
- N: Number of all potential sample points
- mi: Number of sample points in the network Ai
- n1: number of the initial sample.
- αjk: second-order probability for the networks j and k.
- : Estimator for the mean
- wi: mean of yi-values in the mi observed networks
- nNW: number of sample points in the network m
- ya: observed y-values of the sample points in the network m.
- : sum of the y-values in the kth network
- αk: 1st order inclusion probability of the network k
- K: number of distinct networks intersected by the initial sample n1.
- πi and πj: 1st order prob. for the networks i and j to be in the sample S
- yi and yj: sum of the y-values in the jth and kth networks
- πij: 2nd order inclusion probability of the networks j and k.
1.4. Advantages of Adaptive Sampling
1.5. Disadvantages of Adaptive Sampling
1.6. Hypotheses
- Adaptive cluster sampling is more accurate for estimating bark stripping, summer bark stripping damage, and new bark stripping damage for both the Hansen–Hurwitz and Horvitz–Thompson estimators.
- Gains in precision are higher for new bark stripping damage than for summer bark stripping damage and total bark stripping damage because it is a rare event. Estimates from the Horvitz–Thompson estimator are computationally more demanding but more precise.
2. Materials and Methods
2.1. Study Area and Data
- Tree species;
- Diameter at breast height (DBH) [cm];
- Tree height calculated from height curves [m];
- Wound characteristics (visually classified):
- ○
- Damage type (summer or winter damage);
- ○
- Damage age (old damage or new damage);
- ○
- Wound length (max. vertical extent) [cm];
- ○
- Wound width (max. horizontal extent) [cm].
2.2. Software
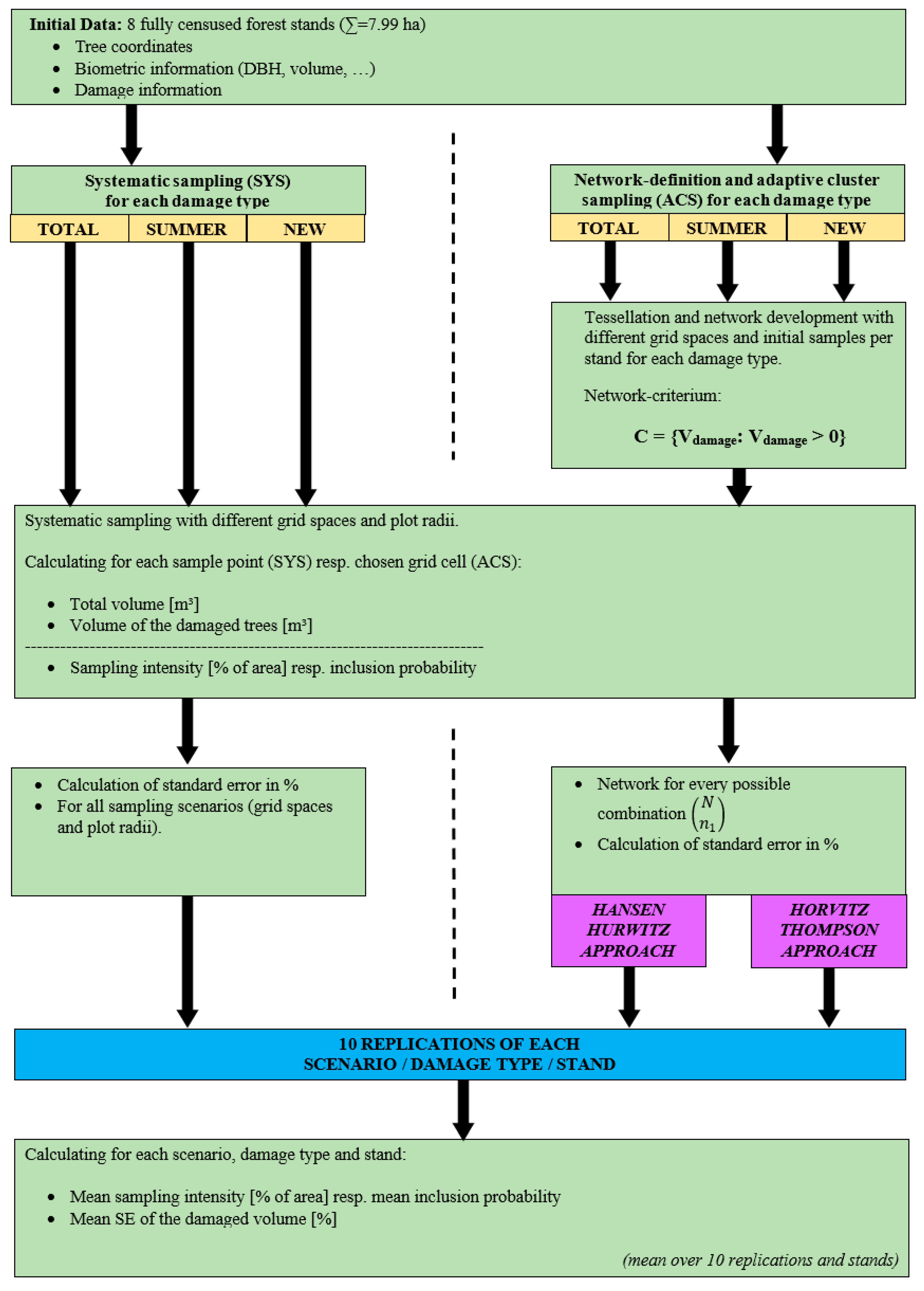
2.3. Tessellation and Sampling Scenarios
- Grid space of the potential sample points (GS) ranged from 10 to 50 m (step: 5 m).
- The number of the initial sampling units (n1) ranged from 2 to 6 points per stand (step: 1).
- 1.
- The area of the eight stands was tessellated into squares based on the specified grid space.
- 2.
- For each square, both total tree volume and volume of damaged trees were calculated (formulas for this are provided in the Appendix A).
- 3.
- All possible combinations of n1 initial sample points were drawn without replacement.
- 4.
- Network development was carried out for all combinations using the criterion C = {Vdamage: Vdamage > 0}; meaning that if at least one bark-stripped tree was included in the sample, the four adjacent points (north, east, south, and west) were added and analysed. This process continued until no further neighbouring points met the criterion.
- 5.
- For each scenario and replication, both the Hansen–Hurwitz and Horvitz–Thompson estimators, as well as their respective standard errors (SE), were calculated.
2.4. Evaluation of Hansen–Hurwitz (HH) and Horvitz–Thompson (HT) Estimator
- N: Number of potential sample points derived from the grid;
- n1: Number of sample points of the initial sample;
- j: Number of possible combinations from n1 initial sample points from N potential sample points;
- pi: Probability of a network to be chosen by n1 initial sample points and subsequent network development;
- yi: value of the analysed variable. In our case, the volume of the damaged trees [m] in the grid cell; separately for TOTAL, SUMMER and NEW damage;
- nNW: Number of the sample points in the network(s);
- mean(n) mean number of sample points chosen through the network development starting from n1.
- SEHH: HH-standard error of the sampling scenario;
- SEHT: HT-standard error of the sampling scenario;
- mean_nNW: Mean sample points in the chosen networks;
- µHH resp. µHT: mean damaged volume calculated by HH estimator resp. HT estimator;
- SE(%)HH resp. SE(%)HT: Standard error of the HH estimator resp. HT estimator in percent of the mean damaged volume.
2.5. Systematic Sampling—Calculation
2.6. Graphical Overview
3. Results
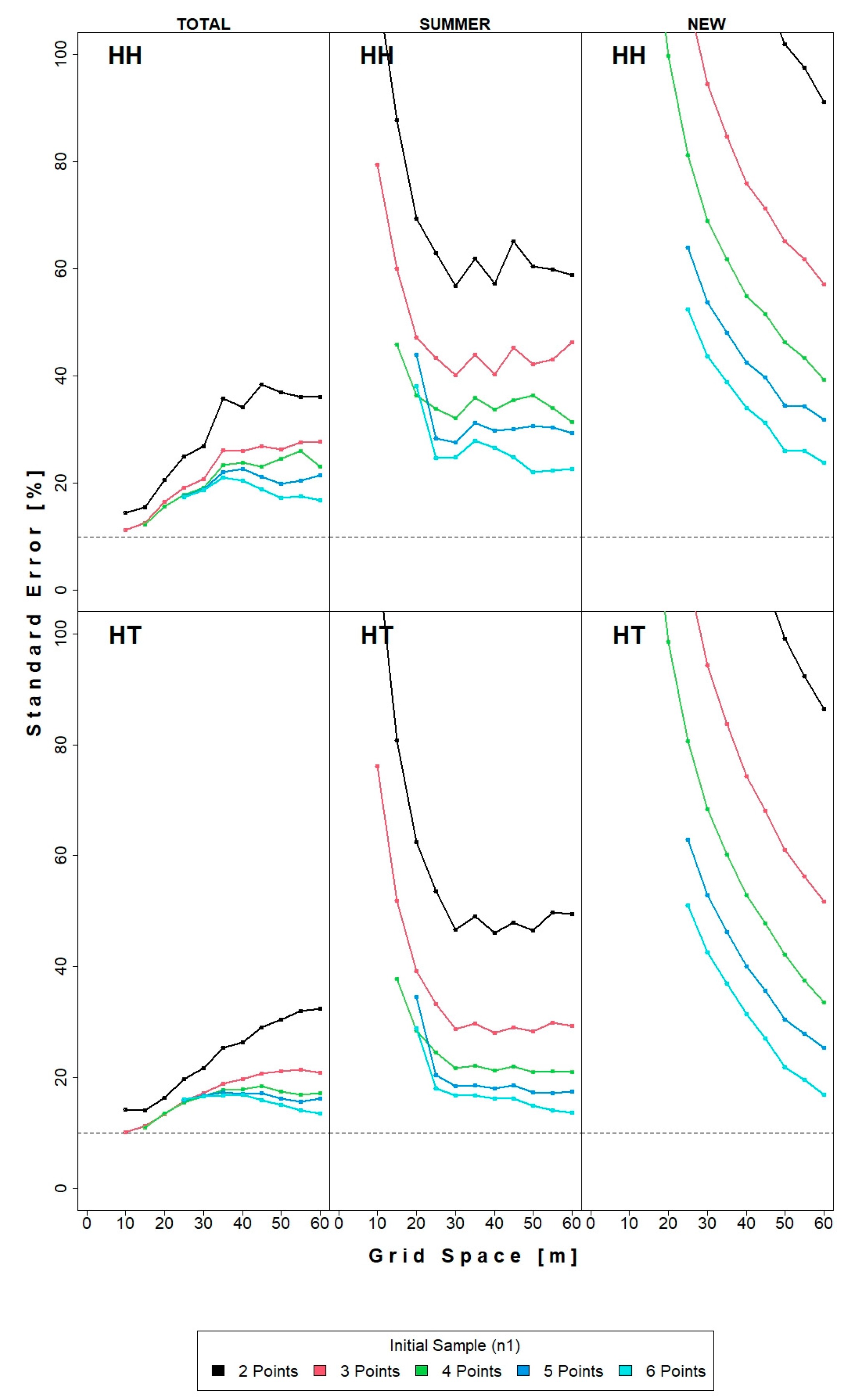
3.1. HH Estimator and HT Estimator
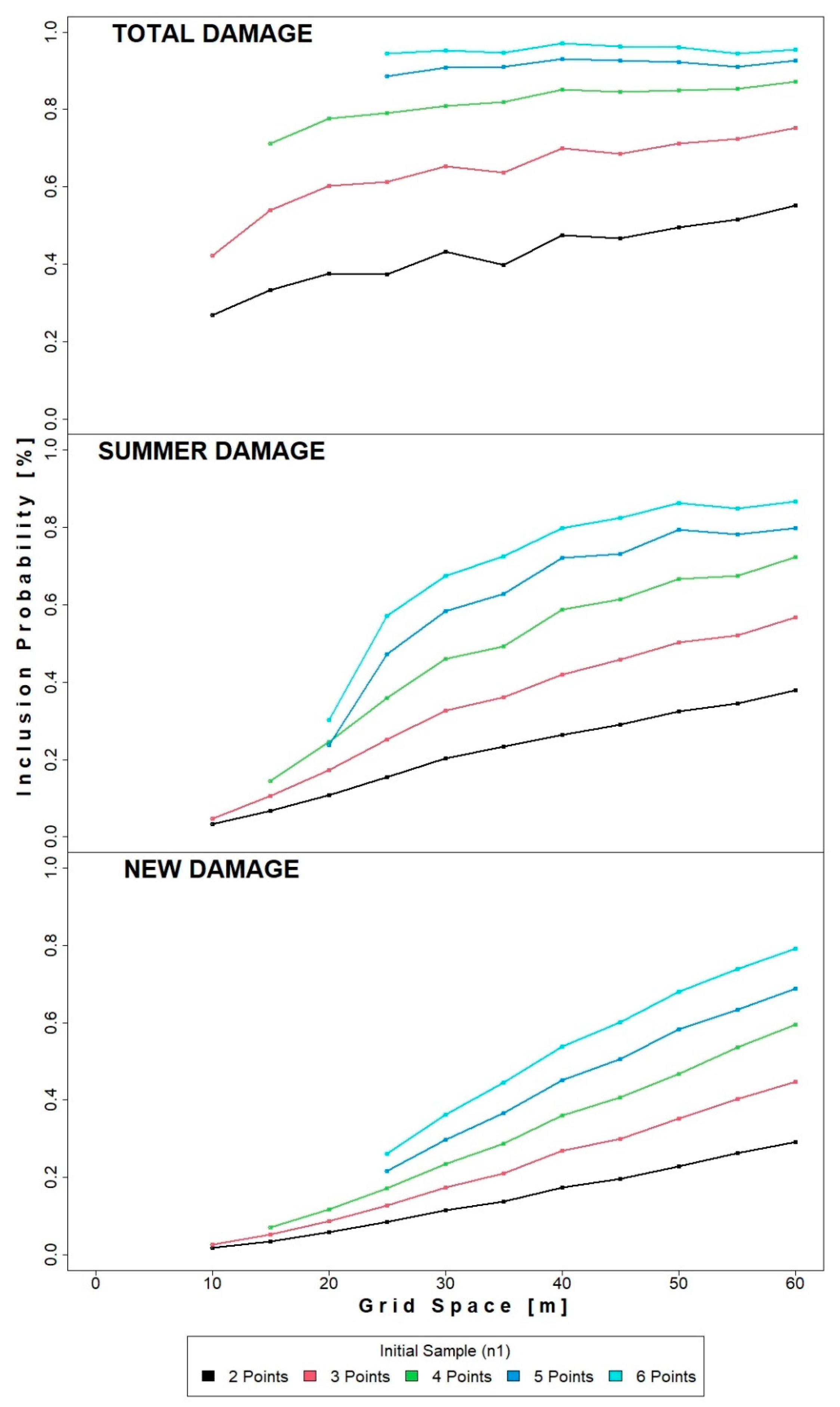
3.2. Inclusion Probability
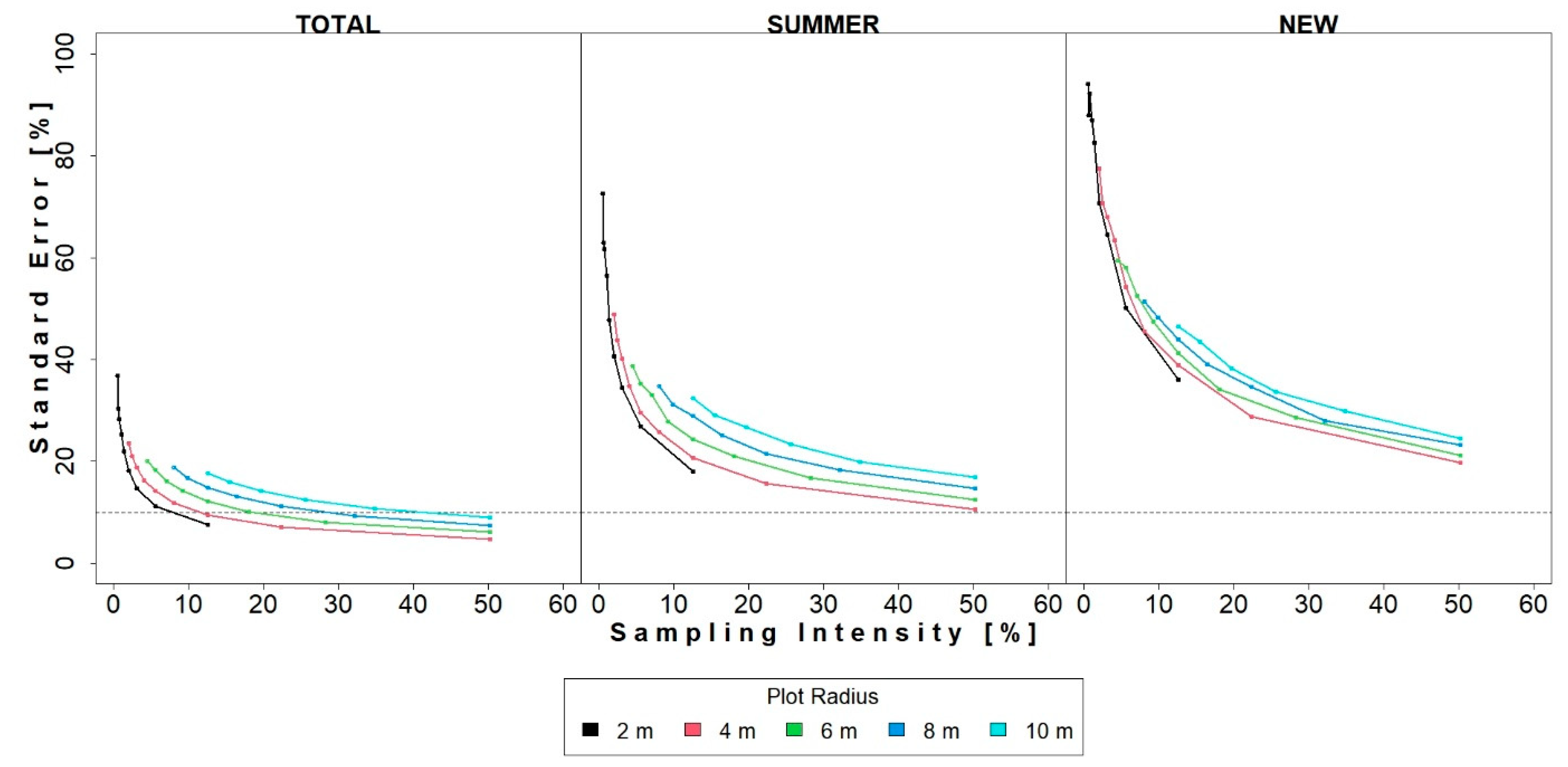
3.3. Systematic Sampling—Results
4. Discussion
4.1. Precision and Efficiency
4.2. Target Variable
4.3. Scale Level
5. Conclusions—Practical Considerations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HH | Hansen–Hurwitz |
| HT | Horvitz–Thompson |
| SE | Standard Error |
| IP | Inclusion Probability |
Appendix A
- z: Number of trees in the (quadratic) sample plot;
- DBHi: Diameter at breast height (1.3 m) of the ith sample tree [m];
- Hi: Height of the ith sample tree [m];
- fi: Form factor of the ith tree according to Pollanschütz [88];
- Di: Damage indicator;
- Vtotal: Total volume per ha [m³];
- Vdamage: Damaged volume per ha [m³].
References
- Apollonio, M.; Andersen, R.; Putman, R. (Eds.) European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Freudenschuss, A.; Zaunbauer, F.; Schadauer, K. Stammschäden—Ein Dauerbrenner. In BFW Praxisinformation: Zwischenauswertung der Waldinventur 2016/17; Federal Research and Training Centre for Forests, Natural Hazards and Landscape (BFW): Vienna, Austria, 2019; pp. 13–16. [Google Scholar]
- Randolph, K.C.; Dooley, K.; Shaw, J.D.; Morin, R.S.; Asaro, C.; Palmer, M.M. Past and present individual-tree damage assessments of the US national forest inventory. Environ. Monit. Assess. 2021, 193, 116. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, J.; Berglund, M.; Johansson, U.; Cleary, M. Incidence of Heterobasidion spp. following different thinning regimes in Norway spruce in southern Sweden. For. Ecol. Manag. 2013, 289, 409–415. [Google Scholar] [CrossRef]
- Čermák, P.; Jankovský, L.; Glogar, J. Progress of spreading Stereum sanguinolentum (Alb. et Schw.: Fr.) Fr. wound rot and its impact on the stability of spruce stands. J. For. Sci. 2004, 50, 360–365. [Google Scholar] [CrossRef]
- Butin, H. Krankheiten der Wald- und Parkbäume: Diagnose-Biologie-Bekämpfung, 4th ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 2011. [Google Scholar]
- Ligot, G.; Gheysen, T.; Perin, J.; Candaele, R.; de Coligny, F.; Licoppe, A.; Lejeune, P. From the simulation of forest plantation dynamics to the quantification of bark-stripping damage by ungulates. Eur. J. For. Res. 2023, 142, 899–916. [Google Scholar] [CrossRef]
- Völk, F. Schälschäden und Rotwildmanagement in Abhängigkeit von Jagdgesetz und Waldbau in Österreich. Ph.D. Thesis, Universität für Bodenkultur, Wien, Austria, 1997. [Google Scholar]
- Welch, D.; Staines, B.W.; Scott, D.; Catt, D.C. Bark stripping damage by red deer in a Sitka spruce forest in western Scotland: II. Wound size and position. Forestry 1988, 61, 245–254. [Google Scholar] [CrossRef]
- Månsson, J.; Jarnemo, A. Bark-stripping on Norway spruce by red deer in Sweden: Level of damage and relation to tree characteristics. Scand. J. For. Res. 2013, 28, 117–125. [Google Scholar] [CrossRef]
- Candaele, R.; Lejeune, P.; Licoppe, A.; Malengreaux, C.; Brostaux, Y.; Morelle, K.; Latte, N. Mitigation of bark stripping on spruce: The need for red deer population to control. Eur. J. For. Res. 2021, 140, 227–240. [Google Scholar] [CrossRef]
- Hahn, C.; Vospernik, S. Position, size, and spatial patterns of bark stripping wounds inflicted by red deer (Cervus elaphus L.) on Norway spruce using generalized additive models in Austria. Ann. For. Sci. 2022, 79, 13. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Linda, R.; Vacek, S.; Šimůnek, V.; Brichta, J.; Gallo, J.; Prokůpková, A. Bark stripping, the crucial factor affecting stem rot development and timber production of Norvay spruce forests in Central Europe. For. Ecol. Manag. 2020, 474, 118360. [Google Scholar] [CrossRef]
- Vasiliauskas, R.; Stenlid, J. Discoloration following bark stripping wounds on Fraxinus excelsior. Eur. J. For. Pathol. 1998, 28, 383–390. [Google Scholar] [CrossRef]
- Arhipova, N.; Jansons, A.; Zaļuma, A.; Gaitnieks, T.; Vasaitis, R. Bark stripping of Pinus contorta caused by moose and deer: Wounding patterns, discoloration of wood and associated fungi. Can. J. For. Res. 2015, 45, 1434–1438. [Google Scholar] [CrossRef]
- Čermák, P.; Strejček, M. Stem decay by Stereum sanguinolentum after red deer damage in the Českomoravská vrchovina Highland. J. For. Sci. 2007, 53, 567–572. [Google Scholar] [CrossRef]
- Cukor, J.; Vacek, Z.; Linda, R.; Vacek, S.; Marada, P.; Šimůnek, V.; Havránek, F. Effects of Bark Stripping on Timber Production and Structure of Norway Spruce Forests in Relation to Climatic Factors. Forests 2019, 10, 320. [Google Scholar] [CrossRef]
- Kershaw, J.A., Jr.; Ducey, M.J.; Beers, T.W.; Husch, B. Forest Mensuration, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Simon, O.; Petrak, M. Zur Methodik der Linientaxation bei der Erhebung von Schälereignissen. Z. Für Jagdwiss. 1998, 44, 113–122. [Google Scholar] [CrossRef]
- Sessa, R.; Dolman, H. (Eds.) Terrestrial Essential Climate Variables: For Clemate Change Assessmant, Mitigation and Adaption; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2008. [Google Scholar]
- Conn, P.B.; Thorson, J.T.; Johnson, D.S. Confronting preferential sampling and analyzing population distributions: Diagnosis and model-based triage. Mehtods Ecol. Evol. 2017, 8, 1535–1546. [Google Scholar] [CrossRef]
- Roesch, F.A. Adaptive Cluster Sampling for Forest Inventories. For. Sci. 1993, 39, 655–669. [Google Scholar] [CrossRef]
- Gheysen, T.; Brostaux, Y.; Hébert, J.; Ligot, G.; Rondeux, J.; Lejeune, P. A regional inventory and monitoring setup to evaluate bark peeling damage by red deer (Cervus elaphus) in coniferous plantations in Southern Belgium. Environ. Monit. Assess. 2011, 181, 335–345. [Google Scholar] [CrossRef]
- Hauk, E.; Niese, G.; Schadauer, K. Instruktion für die Feldarbeit der Österreichischen Waldinventur 2016 +. In Dienstanweisung des Bundesforschungs- und Ausbildungszentrums für Wald; Naturgefahren und Landschaft (BFW): Wien, Austria, 2020. [Google Scholar]
- Tomppo, E.; Toumainen, G.; Lawrence, M.; McRoberts, R.E. (Eds.) Individual country reports: Finland. In National Forest Inventories—Pathways for Common Reporting; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Tokola, T.; Shrestha, S. Comparision of cluster-sampling techniques for forest inventory in southern Nepal. For. Ecol. Manag. 1999, 116, 219–231. [Google Scholar] [CrossRef]
- Ritter, T.; Saborowski, J. Point transect sampling of deadwood: A Comparison with well esteblished sampling techniques for the estimation of volume and carbon storage in managed forests. Eur. J. For. Res. 2012, 131, 1845–1856. [Google Scholar] [CrossRef]
- Bäuerle, H.; Nothdurft, A. Spatial modeling of gabitat trees based on line transect sampling and point pattern reconstruction. Can. J. For. Res. 2011, 41, 715–727. [Google Scholar] [CrossRef]
- Bäuerle, H.; Nothdurft, A.; Kändler, G.; Bauhus, J. Monitoring habitat trees and coarse woody debris bases on sampling schemes. Allg. Forst Jagdztg. 2009, 180, 249–260. [Google Scholar]
- Kissa, D.; Sheil, D. Visual detection based distance sampling offers efficient density estimation for distinctive low abundance tropical forest tree species in comlex terrain. For. Ecol. Manag. 2012, 263, 114–121. [Google Scholar] [CrossRef][Green Version]
- Ritter, T.; Saborowski, J. Efficient integration of a deadood inventory into an existing forest inventory carried out as two-frase sampling stratifikation. Forestry 2014, 87, 571–581. [Google Scholar] [CrossRef]
- Hahn, C.; Vospernik, S. Stand-level sampling disigns for bark stripping caused by red deer (Cervus elaphus L.): Simulatin studies based on nine fully censused stands. Eur. J. For. Res. 2024, 143, 1069–1082. [Google Scholar] [CrossRef]
- Bürgerservice HESSEN. Hessisches Jagdgesetz (HJagdG) in der Fassung vom 5. Juni 2001; § 26: Grundsätze der Abschussregelung. Available online: https://www.rv.hessenrecht.hessen.de/bshe/document/jlr-JagdGHEV5P26a (accessed on 28 April 2023).
- Haakana, H.; Heikkinen, J.; Katila, M.; Kangas, A. Efficiency of post-stratification for a large-scale forest inventory—Case Finnish NFI. Ann. For. Sci. 2019, 67, 9. [Google Scholar] [CrossRef]
- Borkowski, J.J. Network inclusion probabilities and Horvitz-Thompson estimation for adaptive simple Latin square sampling. Environ. Ecol. Stat. 1999, 6, 291–311. [Google Scholar] [CrossRef]
- Thompson, S.K. Adaptive cluster sampling. J. Am. Stat. Assoc. 1990, 85, 1050–1059. [Google Scholar] [CrossRef]
- Salehi, M. Comparison between Hansen-Hurwitz and Horvitz-Thompson estimators for adaptive cluster sampling. Environ. Ecol. Stat. 2003, 10, 115–127. [Google Scholar] [CrossRef]
- Hansen, M.H.; Hurwitz, W.N. On the Theory of Sampling from Finite Populations. Ann. Math. Stat. 1943, 14, 333–362. [Google Scholar] [CrossRef]
- Thompson, S.; Seber, G. Adaptive Sampling; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Horvitz, D.G.; Thompson, D.J. A Generalization of Sampling Without Replacement from a Finite Universe. J. Am. Stat. Assoc. 1952, 47, 663–685. [Google Scholar] [CrossRef]
- Packard, K.C.; Radtke, P.J. Forest sampling combining fixed- and variable-radius sample plots. Can. J. For. Res. 2007, 37, 1460–1471. [Google Scholar] [CrossRef]
- Münnich, R. Varianzschätzung in komplexen Erhebungen. Austrian J. Stat. 2007, 37, 319–334. [Google Scholar]
- Deville, J.-C. Variance Estimation for complex statistics and estimators: Linearization and residual techniques. Surv. Methodol. 1999, 25, 193–203. [Google Scholar]
- Berger, Y.G. A Simple Variance Estimator for Unequal Probability Sampling without Replacement. J. Appl. Stat. 2004, 31, 305–315. [Google Scholar] [CrossRef]
- Salehi, M.M.; Seber, G.A. Adaptive cluster sampling with netzworks selected without replacement. Biometrika 1997, 84, 209–219. [Google Scholar] [CrossRef]
- Hahn, C.; Vospernik, S.; Gollob, C.; Ritter, T. Bark stripping damage by red deer (Cervus elaphus L.): Assessing the spatial distribution on the stand level using generalised additive models. Eur. J. For. Res. 2023, 142, 611–626. [Google Scholar] [CrossRef]
- Thompson, S.K. Stratified adaptive cluster sampling. Biometrica 1991, 78, 389–391. [Google Scholar] [CrossRef]
- Christman, M.C.; Lan, F. Inverse Adaptive Cluster Sampling. Biometrics 2004, 57, 1096–1105. [Google Scholar] [CrossRef]
- Muttlak, H.A.; Khan, A. Adjusted two-stage adaptive cluster sampling. Environ. Ecol. Stat. 2002, 9, 111–120. [Google Scholar] [CrossRef]
- Félix-Medina, M.H.; Thompson, S.K. Adaptive cluster double sampling. Biometrika 2004, 91, 877–891. [Google Scholar] [CrossRef]
- Yang, H.; Kleinn, C.; Fehrmann, L.; Tang, S.; Magnussen, S. A new design for sampling with adaptive sample plots. Environ. Ecol. Stat. 2011, 18, 223–237. [Google Scholar] [CrossRef]
- Bitterlich, W. Die Winkelzählprobe—Ein optisches Meßverfahren zur raschen Aufnahme besonders gearteter Probeflächen für die Bestimmung der Kreisflächen pro Hektar an stehenden Waldbeständen. Forstwiss. Cent. 1952, 71, 215–225. [Google Scholar] [CrossRef]
- Bitterlich, W. The Relascope Idea: Relative Measurements in Forestry; Slough Commonwealth Agricultural Bureaux: Slough, UK, 1984. [Google Scholar]
- Smith, D.R.; Conroy, M.J.; Brakhage, D.H. Efficiency of Adaptive Cluster Sampling for Estimating Density of Wintering Waterfowl. Biometrics 1995, 51, 777–788. [Google Scholar] [CrossRef]
- Smith, S.J.; Lundy, M.J. Improving the precision of design-based scallop drag surveys using adaptive allocation methods. Can. J. Fish. Aquat. Sci. 2006, 63, 7. [Google Scholar] [CrossRef]
- Sullivan, W.P.; Morrison, B.J.; Beamish, F.W. Adaptive Cluster Sampling: Estimating Density of Spatially Autocorrelated Larvae of the Sea Lamprey with Improved Precision. J. Great Lakes Res. 2008, 34, 86–97. [Google Scholar] [CrossRef]
- Lo, N.C.H.; Griffith, D.; Hunter, J.R. Using retricted adaptive cluster sampling to estimate Pacific hake larval abundance. Calif. Coop. Ocean. Fish. Investig. Rep. 1997, 37, 160–174. [Google Scholar]
- Arabkhedri, M.; Lai, F.S.; Noor-Akma, I.; Mohamad-Roslan, M.K. An application of adaptive cluster sampling for estimating total suspended sediment load. Hydrol. Res. 2010, 41, 63–73. [Google Scholar] [CrossRef][Green Version]
- Conners, M.E.; Schwager, S.J. The use of adaptive cluster sampling for hydroacoustic surveys. ICES J. Mar. Sci. 2002, 59, 1314–1325. [Google Scholar] [CrossRef]
- Philippi, T. Adaptive Cluster Sampling for Estimation of Abundances Within Local Populations of Low-Abundance Plants. Ecology 2005, 86, 1091–1100. [Google Scholar] [CrossRef]
- Goldberg, N.A.; Heine, J.N.; Brown, J.A. The application of adaptive cluster sampling for rare subtidal macroalgae. Mar. Biol. 2007, 151, 1343–1348. [Google Scholar] [CrossRef]
- Noon, B.R.; Ishwar, N.M.; Vasudevan, K. Efficiency of Adaptive Cluster and Random Sampling in Detecting Terrestrial Herpetofauna in a Tropical Rainforest. Wildl. Soc. Bull. 2010, 34, 59–68. [Google Scholar] [CrossRef]
- Magnussen, S.; Kurz, W.; Leckie, D.G.; Paradine, D. Adaptive cluster sampling for estimation of deforestation rates. Eur. J. For. Res. 2005, 124, 207–220. [Google Scholar] [CrossRef]
- Talvitie, M.; Leino, O.; Holopainen, M. Inventory of Sparse Forest Populations Using Adaptive Cluster Sampling. Sivla Fenn. 2006, 40, 101–108. [Google Scholar] [CrossRef]
- Brown, J.; Manly, B. Restricted Adaptive Cluster Sampling. Ecol. Stat. 1998, 5, 49–63. [Google Scholar] [CrossRef]
- Su, Z.; Quinn, T.J. Estimator bias and efficiency for adaptive cluster sampling with order statistics and a stopping rule. Environ. Ecol. Stat. 2003, 10, 17–41. [Google Scholar] [CrossRef]
- Gattone, S.A.; Di Battista, T. Adaptive cluster sampling with a data driven stopping rule. Stat. Methods Appl. 2011, 20, 1–21. [Google Scholar] [CrossRef]
- Xu, Q.; Ståhl, G.; McRoberts, R.E.; Li, B.; Tokola, T.; Hou, Z. Generalizing systematic adaptive cluster sampling for forest ecosystem inventory. For. Ecol. Manag. 2021, 489, 119051. [Google Scholar] [CrossRef]
- Ritter, T.; Schwarz, M.; Tockner, A.; Leisch, F.; Nothdurft, A. Automatic Mapping of Forest Stands Based on Three-Dimensional Point Clouds Derived from Terrestrial Laser-Scanning. Forests 2017, 8, 265. [Google Scholar] [CrossRef]
- Gollob, C.; Ritter, T.; Wassermann, C.; Nothdurft, A. Influence of Scanner Position and Plot Size on theAccuracy of Tree Detection and Diameter EstimationUsing Terrestrial Laser Scanning on ForestInventory Plots. Remote Sens. 2019, 11, 1602. [Google Scholar] [CrossRef]
- Gollob, C.; Ritter, T.; Nothdurft, A. Forest Inventory with Long Range and High-SpeedPersonal Laser Scanning (PLS) and SimultaneousLocalization and Mapping (SLAM) Technology. Remote Sens. 2020, 12, 1509. [Google Scholar] [CrossRef]
- Reimoser, F.; Reimoser, S. Richtiges Erkennen von Wildschäden am Wald; Zentralstelle Österreichsicher Landesjagdverbände: Vienna, Austria, 2017. [Google Scholar]
- Turk, P.; Borkowski, J.J. A review of adaptive cluster sampling: 1990–2003. Environ. Ecol. Stat. 2005, 12, 55–95. [Google Scholar] [CrossRef]
- Ritter, T.; Nothdurft, A.; Saborowski, J. Correcting the nondetection bias of angle count sampling. Can. J. For. Res. 2013, 43, 344–354. [Google Scholar] [CrossRef]
- Vasiliauskas, R. Damage to trees due to forestry operations and its pathological significance in temperate forests: A literature review. Forestry 2001, 74, 319–336. [Google Scholar] [CrossRef]
- Burneviča, N.; Jansons, Ā.; Zaļuma, A.; Kļaviņa, D.; Jansons, J.; Gaitnieks, T. Fungi inhabiting bark stripping wounds made by large game on stems of Picea abies (L.) Karst. in Latvia. Balt. For. 2016, 22, 2–7. [Google Scholar]
- Snepsts, G.; Kitenberga, M.; Elferts, D.; Donis, J.; Jansons, A. Stem Damage Modifies the Impact of Wind on Norway Spruces. Forests 2020, 11, 463. [Google Scholar] [CrossRef]
- Vasaitis, R.; Lygis, V.; Vailiauskaite, I.; Valiliauskas, A. Wound occlusion and decay in Picea abies. Eur. J. For. Res. 2012, 131, 1211–1216. [Google Scholar] [CrossRef]
- Krisans, O.; Saleniece, R.; Rust, S.; Elferts, D.; Kapostins, R.; Jansons, A.; Matisons, R. Effects of Bark-Stripping on Mechanical Stability of Norway Spruce. Forests 2020, 11, 357. [Google Scholar] [CrossRef]
- Nagaike, T. Effects of heavy, repeated bark stripping by Cervus neppon on survival of Abies vietchii in a subalpine coniferous forest in central Japan. J. For. Res. 2020, 31, 1139–1145. [Google Scholar] [CrossRef]
- StF: LGBl. Nr. 20/2024; Landesgesetz über die Regelung des Jagdwesens in Oberösterreich. Amt der Oö. Landesregierung: Linz, Austria, 2024.
- Milner, J.M.; Bonenfant, C.; Mysterud, A.; Gaillard, J.-M.; Csányi, S.; Stenseth, N.C. Temporal and spatial development of red deer harvesting in Europe: Biological and cultural factors. J. Appl. Ecol. 2006, 43, 721–734. [Google Scholar] [CrossRef]
- Henttonen, H.M.; Kangas, A. Optimal plot design in a multiporpose forest inventory. For. Ecosyst. 2015, 2, 31. [Google Scholar] [CrossRef]
- Gill, R.M.A. A Review of Damage by Mammals: 1. Deer. Forestry 1992, 65, 145–169. [Google Scholar] [CrossRef]
- Gerhardt, P.; Arnold, J.M.; Hackländer, K.; Hochbichler, E. Determinants of deer impact in European forests—A systematic literature analysis. For. Ecol. Manag. 2013, 310, 173–186. [Google Scholar] [CrossRef]
- Vospernik, S. Probability of Bark Stripping Damage by Red Deer (Cervus elaphus) in Austria. Silva Fenn. 2006, 40, 589–601. [Google Scholar] [CrossRef][Green Version]
- Prentius, W.; Grafström, A. Two-pahse adaptive cluster sampling with circular field plots. Environmetrics 2022, 33, e2729. [Google Scholar] [CrossRef]
- Pollanschütz, J. Formzahlfunktionen der Hauptbaumarten Österreichs. Allg. Forstztg. 1974, 85, 341–343. [Google Scholar]
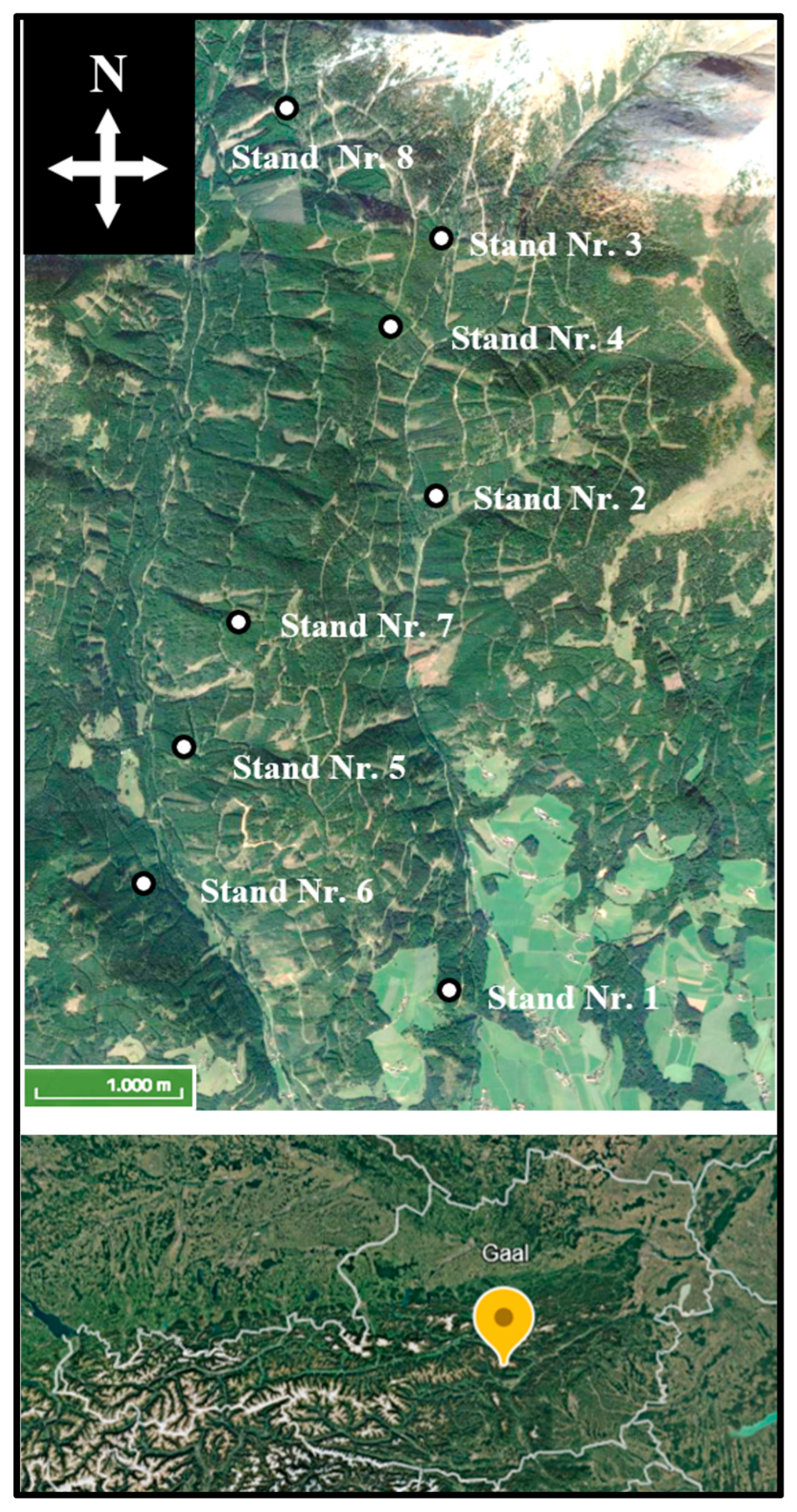
| Stand | Area [ha] | N [ha−1] | BA [m2·ha−1] | V [m3·ha−1] | Damage Percentage [% of Volume] | ||
|---|---|---|---|---|---|---|---|
| TOTAL | NEW | SUMMER | |||||
| 1 | 0.65 | 930 | 63.5 | 731 | 56.2 | 0.2 | --- |
| 2 | 0.31 | 1195 | 52.3 | 489 | 13.4 | --- | --- |
| 3 | 0.85 | 1041 | 39.0 | 329 | 11.1 | 0.3 | --- |
| 4 | 1.26 | 1507 | 41.8 | 362 | 33.9 | 0.9 | 0.5 |
| 5 | 0.54 | 1116 | 38.6 | 423 | 28.2 | 0.2 | 2.5 |
| 6 | 1.77 | 495 | 34.7 | 393 | 13.8 | 0.2 | 3.4 |
| 7 | 1.22 | 1370 | 39.2 | 318 | 17.8 | 0.5 | 6.9 |
| 8 | 1.39 | 864 | 45.7 | 413 | 15.9 | 0.2 | 4.9 |
| TOTAL | 7.99 | 1015 | 42.2 | 407 | 22.1 | 0.4 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, C.; Vospernik, S. Bark Stripping Damage Caused by Red Deer (Cervus elaphus L.): Inventory Design Using Hansen–Hurwitz and Horvitz–Thompson Approach. Forests 2025, 16, 890. https://doi.org/10.3390/f16060890
Hahn C, Vospernik S. Bark Stripping Damage Caused by Red Deer (Cervus elaphus L.): Inventory Design Using Hansen–Hurwitz and Horvitz–Thompson Approach. Forests. 2025; 16(6):890. https://doi.org/10.3390/f16060890
Chicago/Turabian StyleHahn, Christoph, and Sonja Vospernik. 2025. "Bark Stripping Damage Caused by Red Deer (Cervus elaphus L.): Inventory Design Using Hansen–Hurwitz and Horvitz–Thompson Approach" Forests 16, no. 6: 890. https://doi.org/10.3390/f16060890
APA StyleHahn, C., & Vospernik, S. (2025). Bark Stripping Damage Caused by Red Deer (Cervus elaphus L.): Inventory Design Using Hansen–Hurwitz and Horvitz–Thompson Approach. Forests, 16(6), 890. https://doi.org/10.3390/f16060890







