Ecological Analysis and Ethnobotanical Evaluation of Plants in Khanthararat Public Benefit Forest, Kantarawichai District, Thailand
Abstract
1. Introduction
2. Materials and Methods
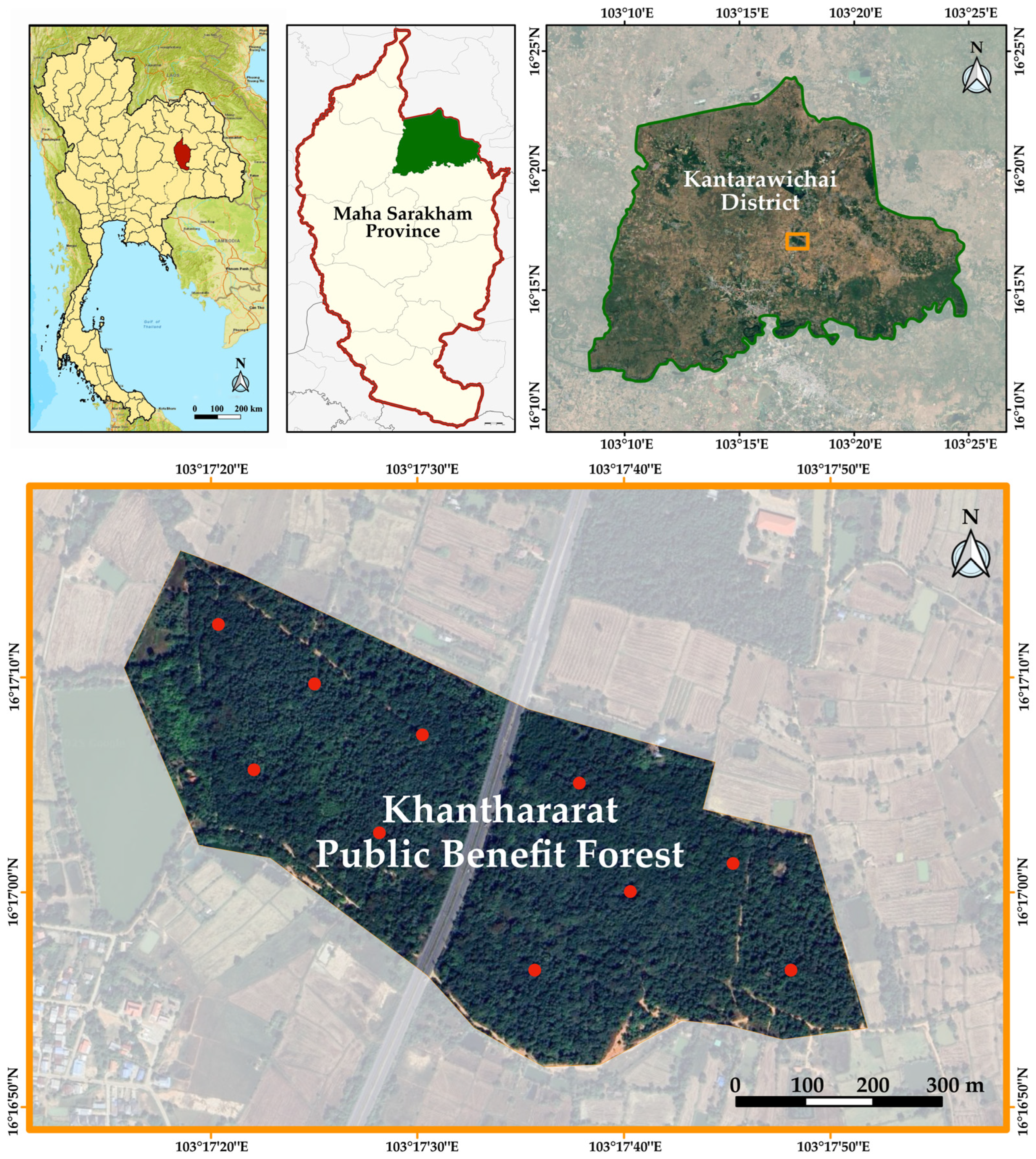
2.1. Study Area
2.2. Ethnobotanical Study
2.2.1. Ethnobotanical Data Collection
2.2.2. Ethnobotanical Analysis
- Use Value (UV)
- Informant consensus factor (Fic)
- Fidelity Level (FL)
2.3. Vegetation Sampling
2.3.1. Diversity Data Collection
- Tree Layer SurveyData on mature trees were collected from ten sampling plots, each measuring 40 × 40 m. Within each plot, individual trees with a diameter at breast height (DBH) of 4.5 centimeters or greater—measured at 1.30 m above ground level—were recorded. For each tree, the species name, DBH, and number of individuals per species were documented.
- Sapling Layer SurveyWithin the same ten plots (40 × 40 m), data were also collected for saplings. These were defined as woody plants with a DBH of less than 4.5 centimeters, measured at 1.30 m above ground. For each sapling encountered, the species name, DBH, and total number of individuals per species were recorded.
- Ground Flora SurveyGround-level vegetation was assessed by walking systematically throughout each plot and recording all woody plant species with a height of less than 1.30 m. For each species, presence within the plot was documented, providing insight into seedling composition and understory woody diversity. Non-woody herbaceous species (e.g., grasses, ferns, and forbs) were not systematically recorded in this study, as the focus was on ethnobotanically relevant woody flora.
2.3.2. Diversity Analysis
- Importance Value Index (IVI)
- Relative Frequency (RF%): The frequency of a species relative to the total frequency of all species in the community.
- Relative Density (RD%): The density of a species relative to the total density of all species.
- Relative Dominance (RDo%): The basal area of a species relative to the total basal area of all species.
- Species Diversity Index
- Pi = proportion of individuals of species relative to the total number of individuals in the community.
- S = number of individuals of one species;
- N = total number of all individuals in the sample.
3. Results
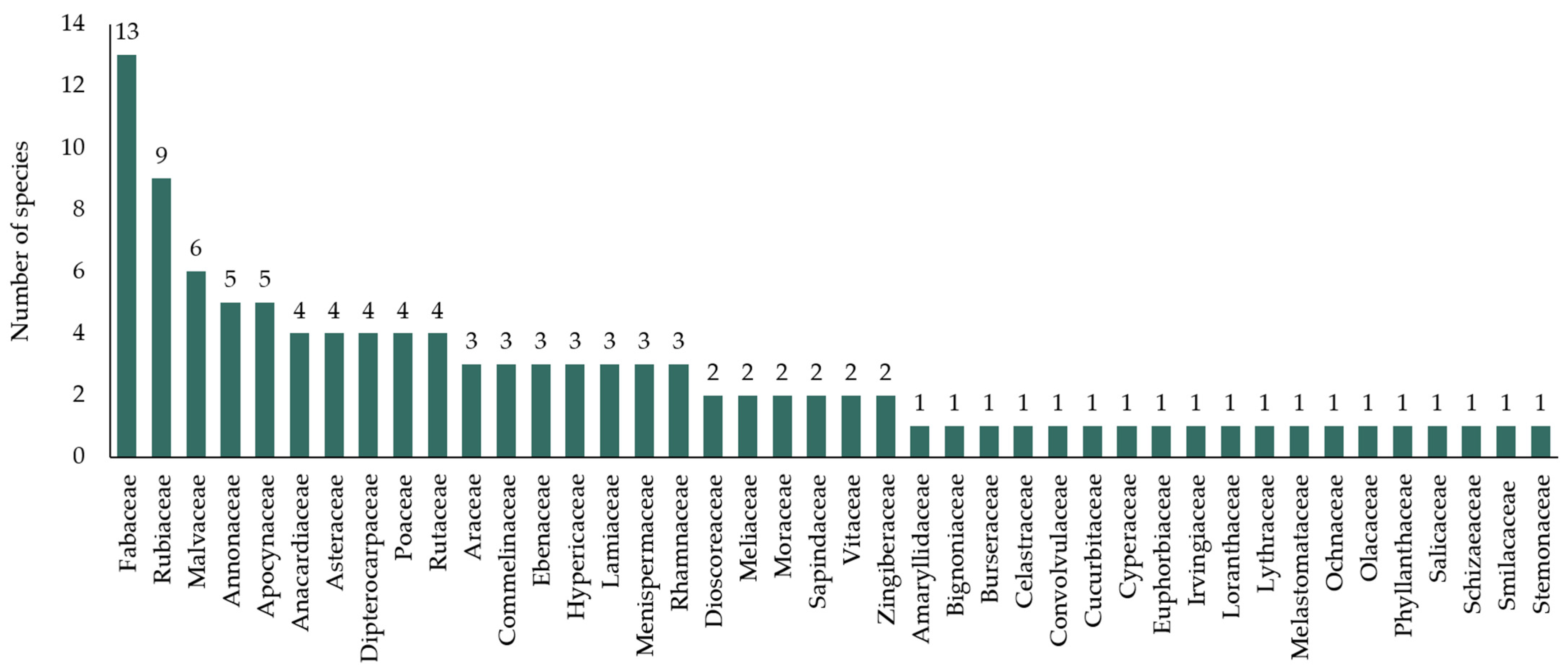
3.1. Diversity of Flora in Khanthararat Public Benefit Forest
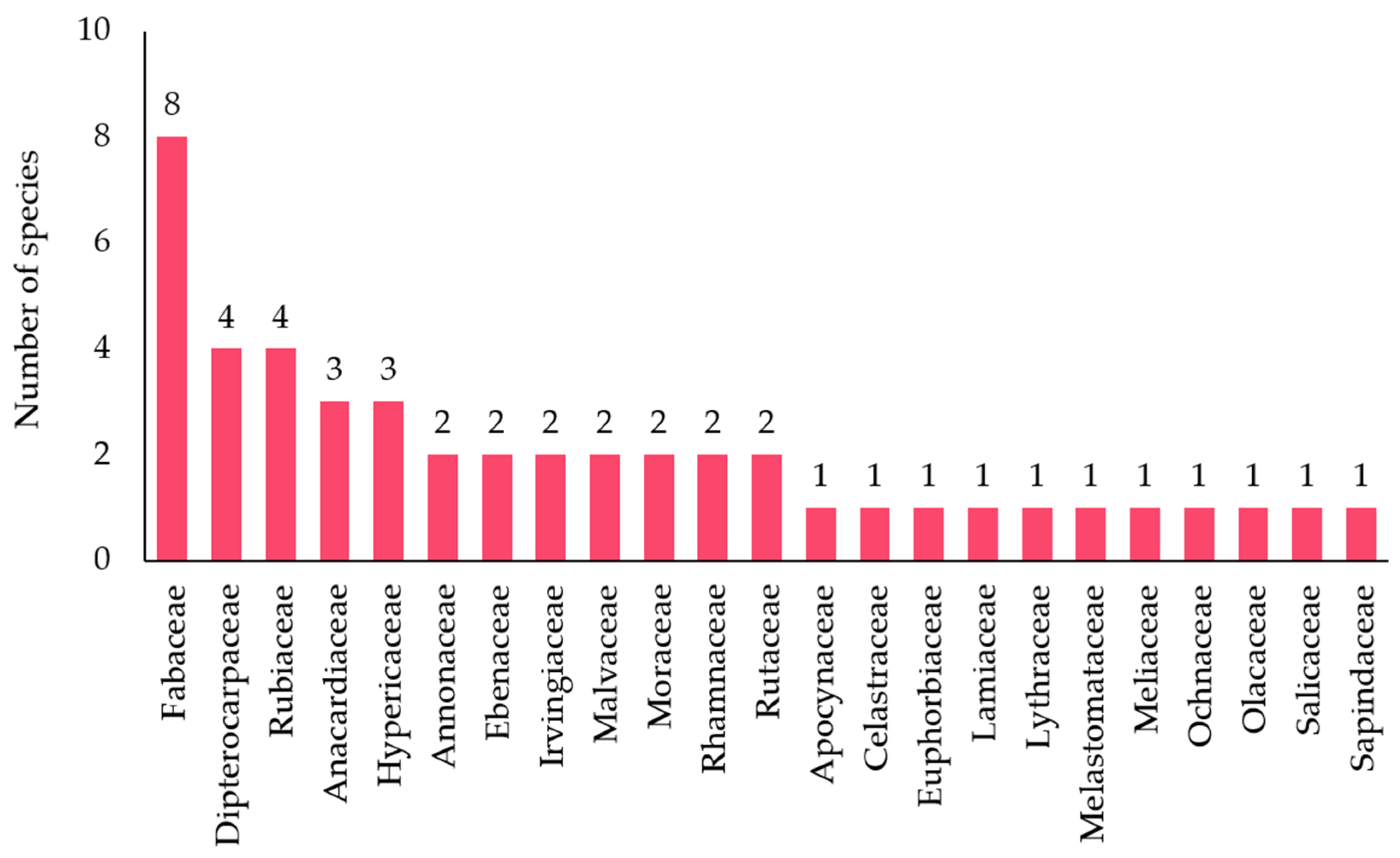
3.2. Ethnobotany: Traditional Uses of Plants by Khanthararat Public Benefit Forest Community
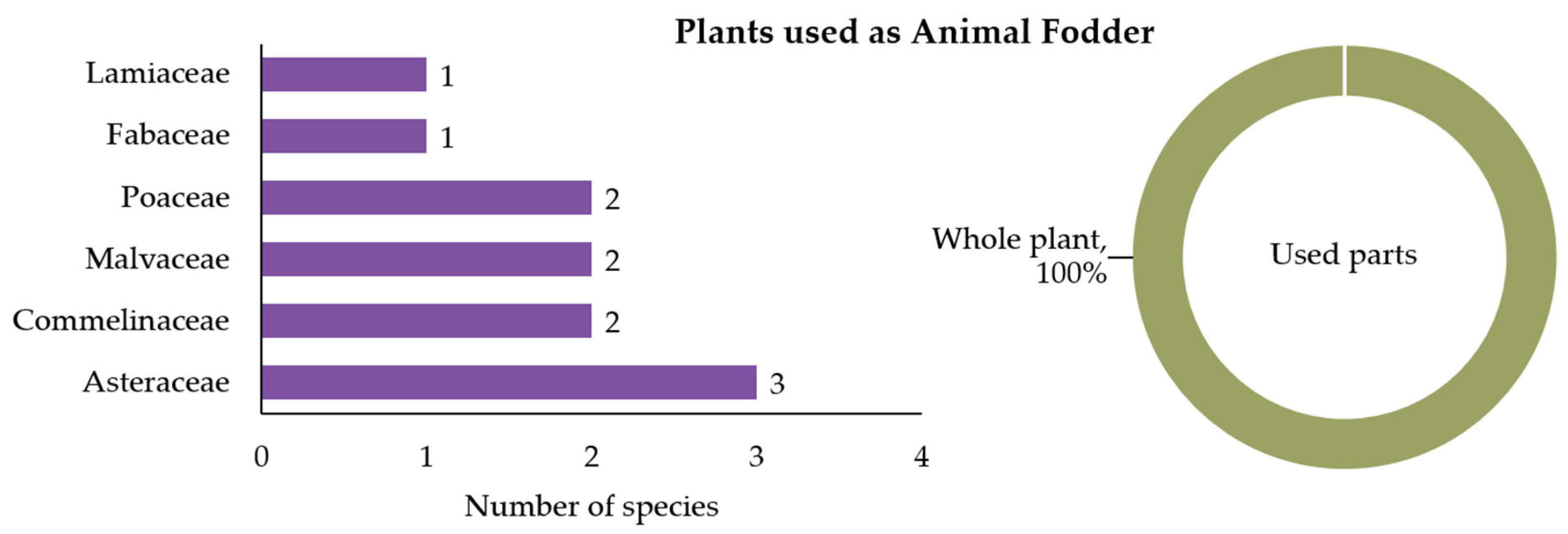
3.2.1. Plants Used as Animal Fodder
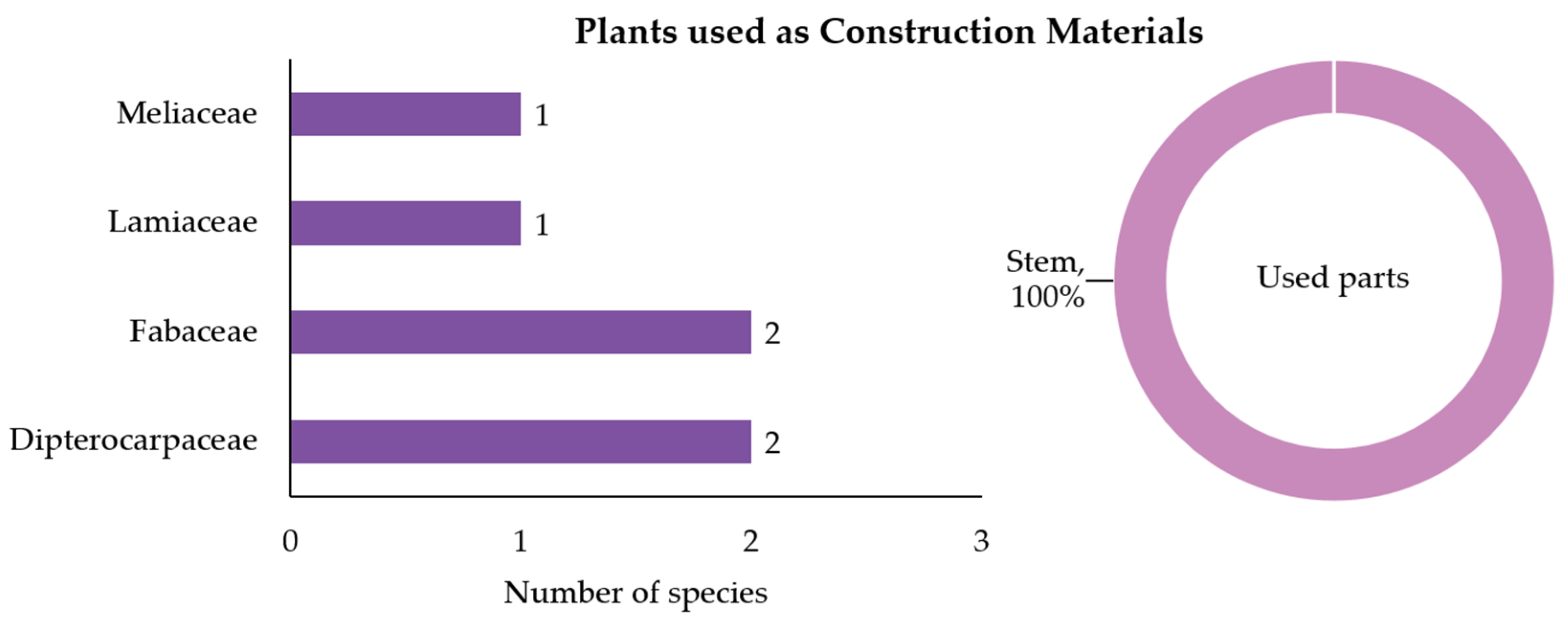
3.2.2. Plants Used as Construction Materials
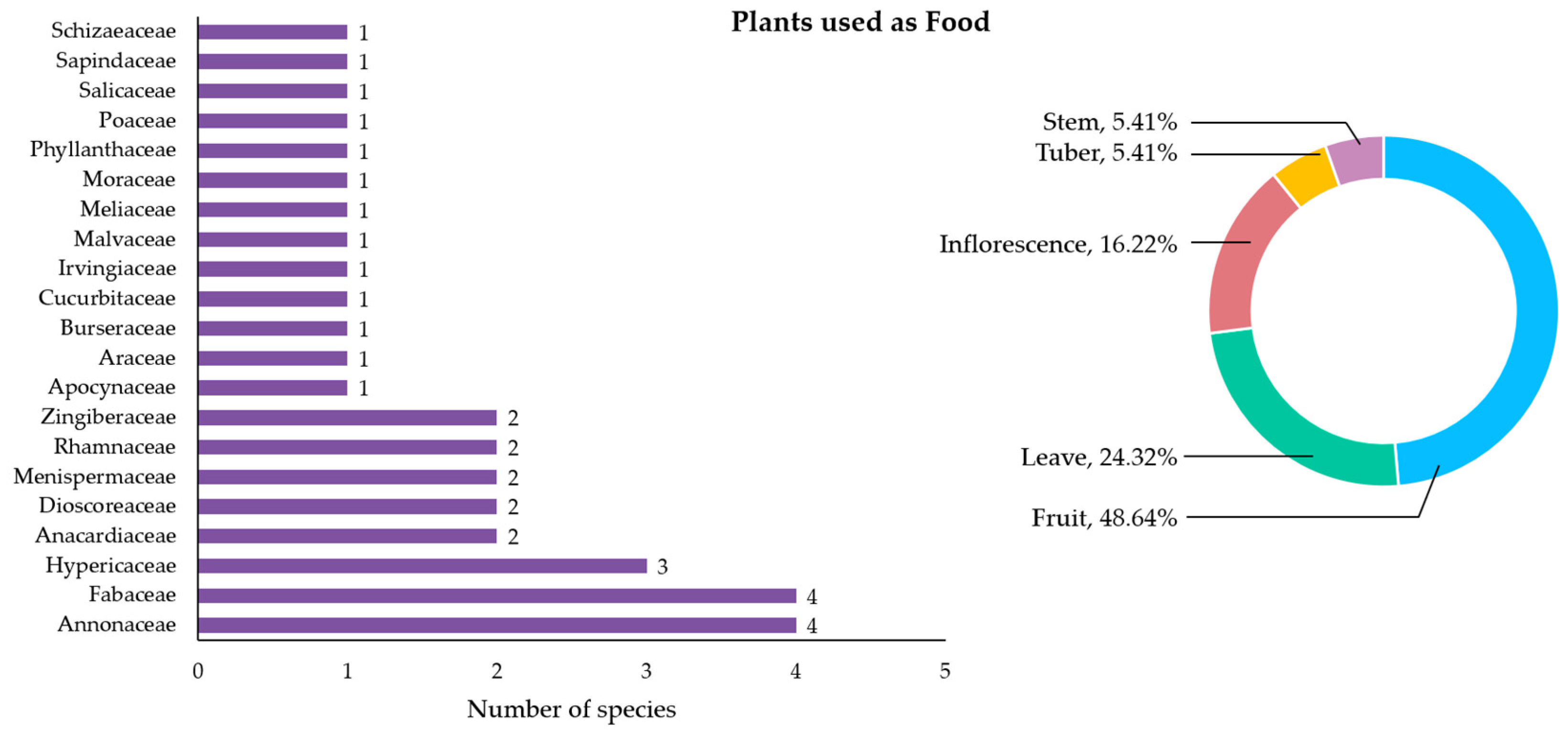
3.2.3. Plants Used as Food
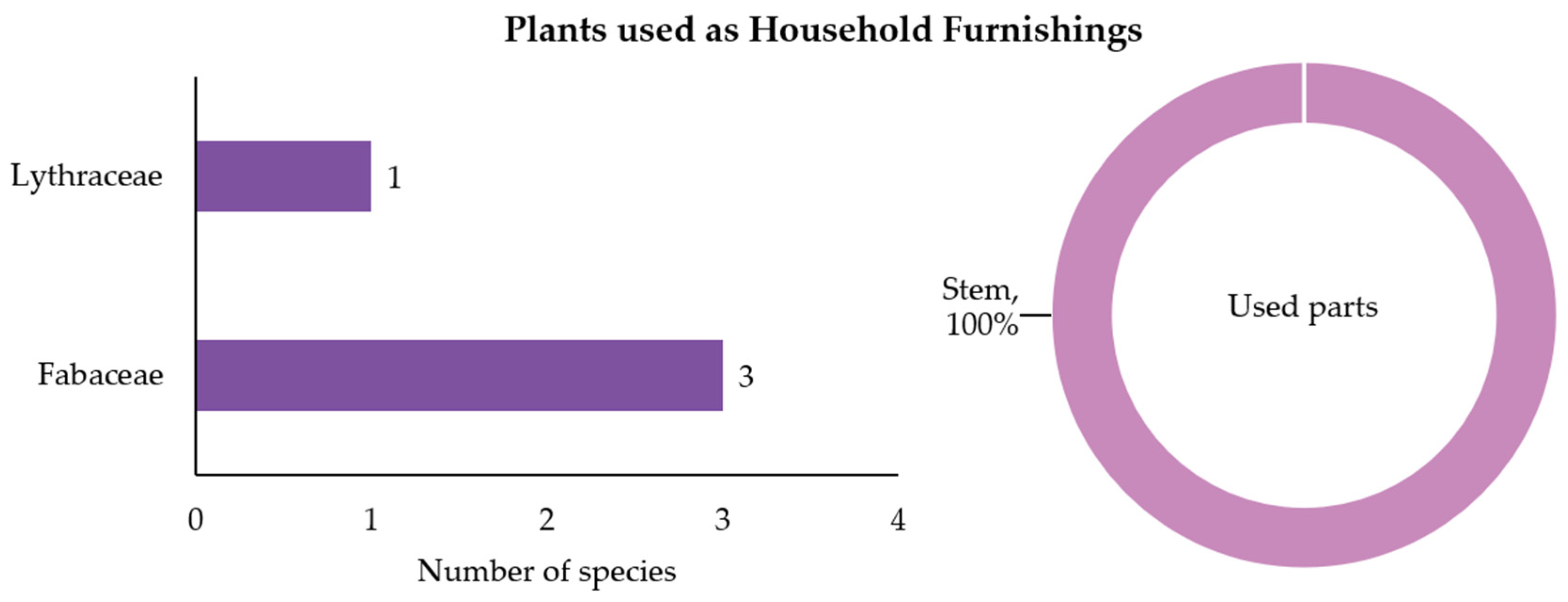
3.2.4. Plants Used as Household Furnishings
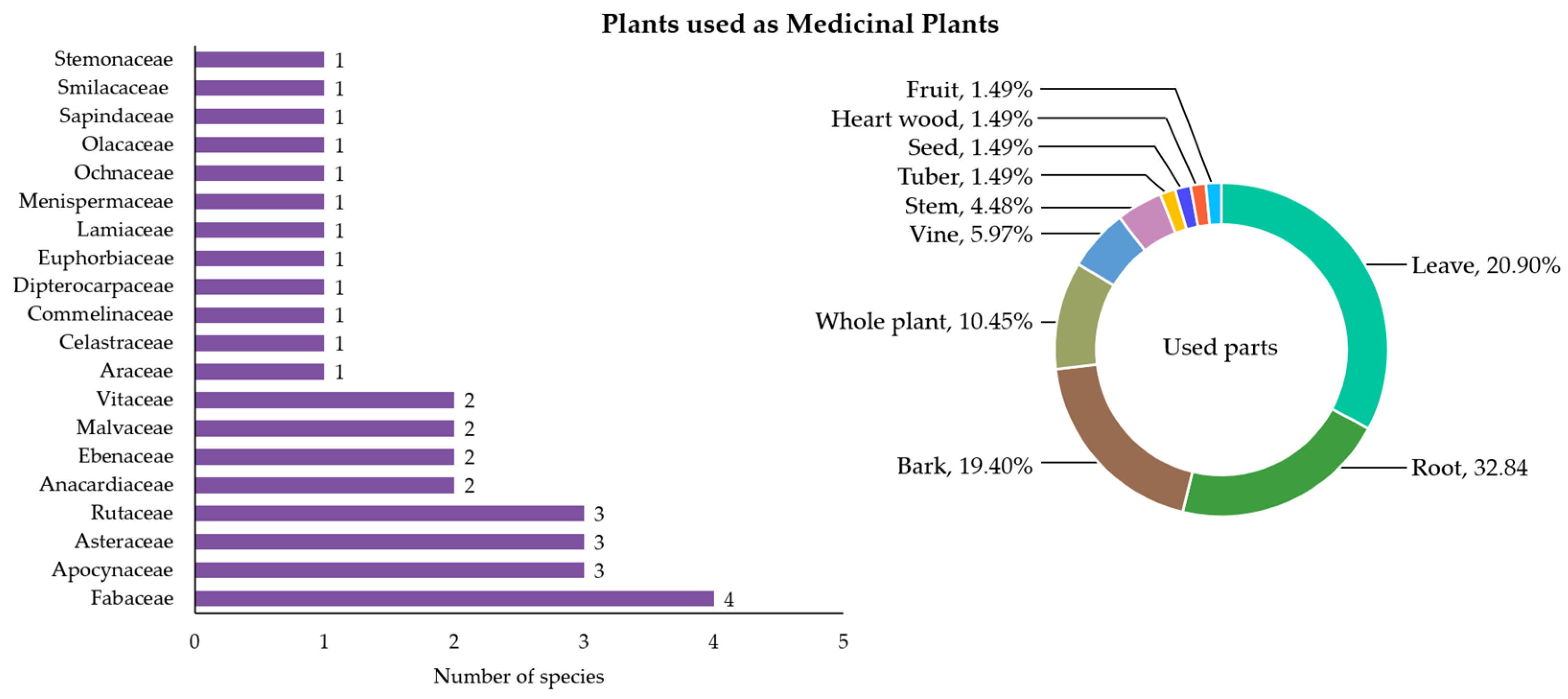
3.2.5. Plants Used as Medicinal Plants
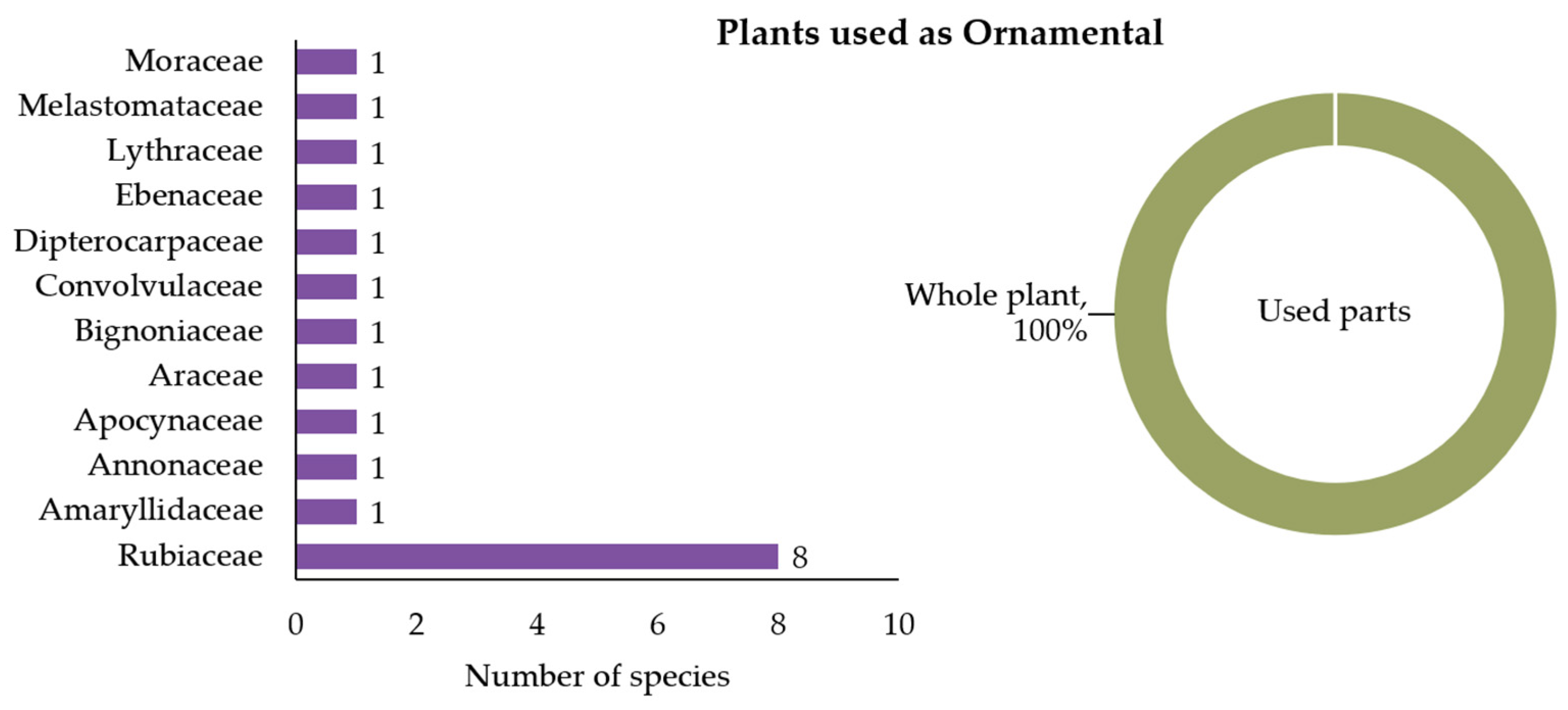
3.2.6. Plants Used as Ornamental
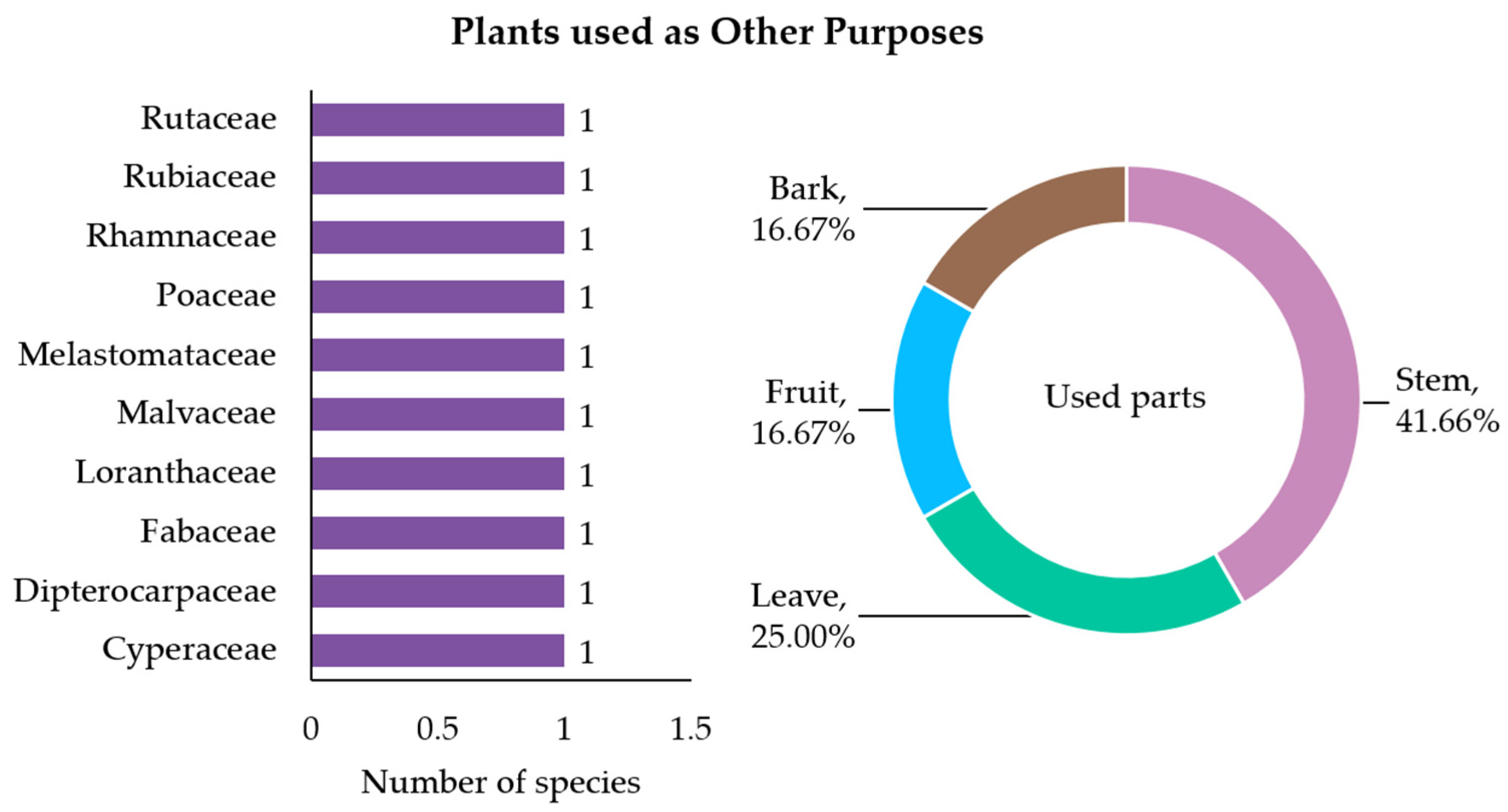
3.2.7. Plants Used for Other Purposes
3.3. Use Value (UV)
3.4. Informant Consensus Factor (Fic)
3.5. Fidelity Level (FL)
3.6. Ecological Study: Diversity of Tree Species in Khanthararat Public Benefit Forest
3.6.1. Relative Density (RD%) of Tree Species
3.6.2. Relative Dominance (RDo%) of Tree Species
3.6.3. Relative Frequency (RF%) of Tree Species
3.6.4. Importance Value Index (IVI) of Tree Species
3.6.5. Species Diversity Index of Tree Species
3.7. Ecological Study: Diversity of Sapling Species in Khanthararat Public Benefit Forest
3.7.1. Relative Density (RD%) of Sapling Species
3.7.2. Relative Dominance (RDo%) of Sapling Species
3.7.3. Relative Frequency (RF%) of Sapling Species
3.7.4. Importance Value Index (IVI) of Sapling Species
3.7.5. Species Diversity Index of Sapling Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaal, B. Plants and people: Our shared history and future. Plants People Planet 2018, 1, 14–19. [Google Scholar] [CrossRef]
- Constant, N.L.; Tshisikhawe, M.P. Hierarchies of knowledge: Ethnobotanical knowledge, practices and beliefs of the Vhavenda in South Africa for biodiversity conservation. J. Ethnobiol. Ethnomed. 2018, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, Y.Y.S.; Sujarwo, W.; Irsyam, A.S.D.; Dwiartama, A.; Rosleine, D. Exploring unconventional food plants used by local communities in a Rural Area of West Java, Indonesia: Ethnobotanical assessment, use trends, and potential for improved nutrition. J. Ethnobiol. Ethnomed. 2024, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Phatlamphu, N.; Saensouk, S.; Saensouk, P.; Jungsongduang, A. Ethnobotany of edible plants in Muang District, Kalasin Province, Thailand. Biodiversitas 2021, 22, 5425–5431. [Google Scholar] [CrossRef]
- Numpulsuksant, W.; Saensouk, S.; Sanesouk, P. Diversity and ethnobotanical study of medicinal plants in Ban Hua Kua, Kae Dam District, Thailand. Biodiversitas 2021, 22, 4349–4357. [Google Scholar] [CrossRef]
- Arjona-García, C.; Blancas, J.; Beltrán-Rodríguez, L.; López Binnqüist, C.; Colín Bahena, H.; Moreno-Calles, A.I.; Sierra-Huelsz, J.A.; López-Medellín, X. How does urbanization affect perceptions and traditional knowledge of medicinal plants? J. Ethnobiol. Ethnomed. 2021, 17, 48. [Google Scholar] [CrossRef]
- Rosero-Toro, J.H.; Romero-Duque, L.P.; Santos-Fita, D.; Ruan-Soto, F. Cultural significance of the flora of a tropical dry forest in the Doche Vereda (Villavieja, Huila, Colombia). J. Ethnobiol. Ethnomed. 2018, 14, 22. [Google Scholar] [CrossRef]
- Ramirez, C.R. Ethnobotany and the loss of traditional knowledge in the 21st century. Ethnobot. Res. Appl. 2007, 5, 245–247. [Google Scholar] [CrossRef]
- Gonfa, N.; Tulu, D.; Hundera, K.; Raga, D. Ethnobotanical study of medicinal plants, its utilization, and conservation by indigenous people of Gera District, Ethiopia. Cogent Food Agric. 2020, 6, 1852716. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Komal; Ramchiary, N.; Singh, P. Role of traditional ethnobotanical knowledge and indigenous communities in achieving sustainable development goals. Sustainability 2021, 13, 3062. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Biogeography, conservation status, and traditional uses of Zingiberaceae in Saraburi Province, Thailand, with Kaempferia chaveerachiae sp. nov. Horticulturae 2024, 10, 934. [Google Scholar] [CrossRef]
- Jitpromma, T.; Saensouk, S.; Saensouk, P.; Boonma, T. Diversity, traditional uses, economic values, and conservation status of Zingiberaceae in Kalasin Province, Northeastern Thailand. Horticulturae 2025, 11, 247. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Hein, K.Z.; Appamaraka, S.; Maknoi, C.; Souladeth, P.; Koompoot, K.; Sonthongphithak, P.; Boonma, T.; Jitpromma, T. Diversity, ethnobotany, and horticultural potential of local vegetables in Chai Chumphol Temple Community Market, Maha Sarakham Province, Thailand. Horticulturae 2025, 11, 243. [Google Scholar] [CrossRef]
- Banda, L.O.L.; Banda, C.V.; Banda, J.T.; Singini, T. Preserving cultural heritage: A community-centric approach to safeguarding the Khulubvi Traditional Temple, Malawi. Heliyon 2024, 10, e37610. [Google Scholar] [CrossRef]
- Panyadee, P.; Meunrew, J.; Balslev, H.; Inta, A. Ethnobotany and ecosystem services in a Tidal Forest in Thailand. Sustainability 2022, 14, 6322. [Google Scholar] [CrossRef]
- Phumthum, M.; Balslev, H.; Kantasrila, R.; Kaewsangsai, S.; Inta, A. Ethnomedicinal plant knowledge of the Karen in Thailand. Plants 2020, 9, 813. [Google Scholar] [CrossRef]
- Inta, A.; Kampuansai, J.; Kutanan, W.; Srikummool, M.; Pongamornkul, W.; Srisanga, P.; Panyadee, P. Women’s wellness in the mountains: An exploration of medicinal plants among Tibeto-Burman Groups in Thailand. Heliyon 2023, 9, e17722. [Google Scholar] [CrossRef]
- Thothela, S.N.S.; Kola, E.; Dalu, M.T.B.; Ndhlovu, P.T. Indigenous knowledge and utilization of Strychnos spinosa Lam. in Sub-Saharan Africa: A systematic review of its medicinal, nutritional, and cultural significance. Diversity 2025, 17, 228. [Google Scholar] [CrossRef]
- Reid, W.; Mooney, H.; Cropper, A.; Capistrano, D.; Carpenter, S.; Chopra, K. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- POWO. World Checklist of Selected Plant Families (WCSP). 2024. Available online: http://apps.kew.org/wcsp/ (accessed on 1 January 2025).
- Phillips, O.; Gentry, A.H.; Reynel, C.; Wilkin, P.; Galvez-Durand, B.C. Quantitative ethnobotany and Amazonian conservation. Conserv. Biol. 1994, 8, 225–248. [Google Scholar] [CrossRef]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Stohlgren, T.J.; Chong, G.W.; Kalkhan, M.A.; Schell, L.D. Multiscale sampling of plant diversity: Effects of minimum mapping unit size. Ecol. Appl. 1997, 7, 1064–1074. [Google Scholar] [CrossRef]
- Zobel, D.D.; Jha, P.K.; Behan, M.J.; Yadav, U.K.R. A Practical Manual for Ecology; Ratna Book Distributors: Kathmandu, Nepal, 1987. [Google Scholar]
- Shannon, C.E.; Weiner, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Aisor, N.; Prathepha, P.; Saensouk, S. Ethnobotanical study and utilization of plants in Khok Nhong Phok Forest, Kosum Phisai District, Northeastern Thailand. Biodiversitas 2021, 22, 4336–4348. [Google Scholar] [CrossRef]
- Eisah, J.S.; Nyumah, F.; Johnny, J.; Charles, J.F. Ethnobotanical studies on the use of medicinal plants among forest fringe communities around the Kasewe Forest in Moyamba District, Southern Sierra Leone. Am. J. Plant Sci. 2021, 12, 1989–2006. [Google Scholar] [CrossRef]
- Thorn, J.P.R.; Thornton, T.F.; Helfgott, A.; Willis, K.J. Indigenous uses of wild and tended plant biodiversity maintain ecosystem services in agricultural landscapes of the Terai Plains of Nepal. J. Ethnobiol. Ethnomed. 2020, 16, 33. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Baselga, A.; Sousa, R.; Vilà, M.; Doadrio, I.; de Sostoa, A. Fine-scale determinants of conservation value of river reaches in a hotspot of Native and Non-Native Species Diversity. Sci. Total Environ. 2017, 574, 455–466. [Google Scholar] [CrossRef]
- Vitousek, P.; D’Antonio, C.; Loope, L.; Rejmanek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. N. Z. J. Ecol. 1996, 21, 1–16. [Google Scholar]
- Nogué, S.; Rull, V.; Vegas-Vilarrúbia, T. Elevational gradients in the Neotropical Table Mountains: Patterns of endemism and implications for conservation. Divers. Distrib. 2013, 19, 676–687. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria Version 16. 2024. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf (accessed on 1 January 2025).
- United Nations. Convention on Biological Diversity; United Nations: Rio de Janeiro, Brazil, 1992. [Google Scholar]
- Murray, B.R.; Rice, B.L.; Keith, D.A.; Myerscough, P.J.; Howell, J.; Floyd, A.G.; Mills, K.; Westoby, M. Species in the tail of rank–abundance curves. Ecology 1999, 80, 1806–1816. [Google Scholar] [CrossRef]
- Haq, S.M.; Yaqoob, U.; Majeed, M.; Amjad, M.S.; Hassan, M.; Ahmad, R.; Waheed, M.; Bussmann, R.W.; Calixto, E.S.; Proćków, J.; et al. Quantitative ethnoveterinary study on plant resource utilization by indigenous communities in high-altitude regions. Front. Vet. Sci. 2022, 9, 944046. [Google Scholar] [CrossRef]
- Khan, S.M.R.; Akhter, T.; Hussain, M. Ethno-veterinary practice for the treatment of animal diseases in Neelum Valley, Kashmir Himalaya, Pakistan. PLoS ONE 2021, 16, e0250114. [Google Scholar] [CrossRef]
- Gao, H.; Huang, W.; Zhao, C.; Xiong, Y. An ethnoveterinary study on medicinal plants used by the Bai People in Yunlong County, Northwest Yunnan, China. J. Ethnobiol. Ethnomed. 2024, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Long, C. An ethnoveterinary study on medicinal plants used by the Buyi People in Southwest Guizhou, China. J. Ethnobiol. Ethnomed. 2020, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.; Wang, Z.; Maundu, P.; Hunter, D. The role of traditional knowledge and food biodiversity to transform modern food systems. Trends Food Sci. Technol. 2022, 130, 32–41. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Diversity and traditional utilization of the Zingiberaceae plants in Nakhon Nayok Province, Central Thailand. Diversity 2023, 15, 904. [Google Scholar] [CrossRef]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Galvão, M.L.; Rodrigues, T.N.M.; Santos, I.S.; Fernandes, M.E.B. Traditional ecological knowledge of mangrove wood use on the Brazilian Amazon Coast. Ethnobiol. Conserv. 2024, 13, 1–19. [Google Scholar] [CrossRef]
- Widiyono, W. Biological and economic values of Dipterocarpaceae, the main timber forest product of Indonesia. Indones. J. Appl. Environ. Stud. 2021, 2, 104–112. [Google Scholar] [CrossRef]
- Wang, R.; Haller, P. Applications of wood ash as a construction material in civil engineering: A review. Biomass Convers. Biorefin. 2022, 1–21. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal triterpenes in Meliaceae: Plant species, molecules and activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef]
- Rundel, P.W.; Boonpragob, K.; Patterson, M. Seasonal water relations and leaf temperature in a deciduous dipterocarp forest in Northeastern Thailand. Forests 2017, 8, 368. [Google Scholar] [CrossRef]
- Vos, M.A.E.; den Ouden, J.; Hoosbeek, M.; Valtera, M.; de Vries, W.; Sterck, F. The sustainability of timber and biomass harvest in perspective of forest nutrient uptake and nutrient stocks. For. Ecol. Manag. 2023, 530, 120791. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Rakarcha, S.; Boonma, T.; Jitpromma, T.; Sonthongphithak, P.; Ragsasilp, A.; Souladeth, P. Diversity and local uses of the Convolvulaceae family in Udon Thani Province, Thailand, with notes on its potential horticultural significance. Horticulturae 2025, 11, 312. [Google Scholar] [CrossRef]
- Chamorro, M.; Ladio, A. Management of native and exotic plant species with edible fruits in a protected area of NW Patagonia. Ethnobiol. Conserv. 2021, 10, 1–24. [Google Scholar] [CrossRef]
- Padumanonda, T.; Gritsanapan, W. Barakol contents in fresh and cooked Senna siamea leaves. Southeast Asian J. Trop. Med. Public Health 2006, 37, 388–393. [Google Scholar]
- Heywood, V.H. Ethnopharmacology, food production, nutrition and biodiversity conservation: Towards a sustainable future for indigenous peoples. J. Ethnopharmacol. 2011, 137, 1–15. [Google Scholar] [CrossRef]
- Hailemariam, M.B.; Woldu, Z.; Asfaw, Z.; Lulekal, E. Ethnobotany of an indigenous tree Piliostigma thonningii (Schumach.) Milne-Redh. (Fabaceae) in the Arid and Semi-Arid Areas of South Omo Zone, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2021, 17, 44. [Google Scholar] [CrossRef]
- Nyemba, W.R.; Hondo, A.; Mbohwa, C.; Madiye, L. Unlocking economic value and sustainable furniture manufacturing through recycling and reuse of sawdust. Procedia Manuf. 2018, 21, 510–517. [Google Scholar] [CrossRef]
- Russell, J.D.; Huff, K.; Haviarova, E. Evaluating the cascading-use of wood furniture: How value-retention processes can contribute to material efficiency and circularity. J. Ind. Ecol. 2023, 27, 856–867. [Google Scholar] [CrossRef]
- Chanthavong, S.; Buot, I.E. Conservation status of plant diversity at Dong Na Tard Provincial protected area, Lao people’s democratic republic. Int. J. Conserv. Sci. 2021, 12, 393–402. [Google Scholar]
- Usman, M.; Khan, W.R.; Yousaf, N.; Akram, S.; Murtaza, G.; Kudus, K.A.; Ditta, A.; Rosli, Z.; Rajpar, M.N.; Nazre, M. Exploring the phytochemicals and anti-cancer potential of the members of Fabaceae family: A comprehensive review. Molecules 2022, 27, 3863. [Google Scholar] [CrossRef] [PubMed]
- Bhadane, B.S.; Patil, M.P.; Maheshwari, V.L.; Patil, R.H. Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytother. Res. 2018, 32, 1181–1210. [Google Scholar] [CrossRef] [PubMed]
- Mounce, R.; Smith, P.; Brockington, S. Ex Situ conservation of plant diversity in the World’s Botanic Gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Suhaeri, S.; Fulazzaky, M.A.; Husaini, H.; Dirhamsyah, M.; Hasanuddin, I. Application of Scirpus grossus fiber as a sound absorber. Heliyon 2024, 10, e28961. [Google Scholar] [CrossRef]
- Bisht, S.S.; Mishra, K.; Pandey, K.K.; Chandra, G.; Nayak, M.M.; Shashikala, S. Chromatographic and spectrophotometric analysis of heartwood extracts of four Pterocarpus species. Wood Sci. Technol. 2022, 56, 459–476. [Google Scholar] [CrossRef]
- Sinthumule, I. Traditional ecological knowledge and its role in biodiversity conservation: A systematic review. Front. Environ. Sci. 2023, 11, 1164900. [Google Scholar] [CrossRef]
- Swathi, S.; Lakshman, K. Phytopharmacological and biological exertion of Spondias pinnata: A review. Orient. J. Chem. 2022, 38, 268–277. [Google Scholar] [CrossRef]
- Uddin, M.Z.; Hassan, M.A. Determination of informant consensus factor of ethnomedicinal plants used in Kalenga Forest, Bangladesh. Bangladesh J. Plant Taxon. 2014, 21, 83–91. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Zidane, L. Ethnobotanical and ethnomedicinal study of medicinal and aromatic plants used against dermatological diseases by the people of Rif, Morocco. J. Herb. Med. 2022, 32, 100542. [Google Scholar] [CrossRef]
- Le, H.T.; Luu, T.N.; Nguyen, H.M.T.; Nguyen, D.H.; Le, P.T.Q.; Trịnh, N.N.; Le, V.S.; Nguyen, H.D.; Van, H.T. Antibacterial, antioxidant and cytotoxic activities of different fractions of acetone extract from flowers of Dipterocarpus intricatus Dyer (Dipterocarpaceae). Plant Sci. Today 2021, 8, 273–277. [Google Scholar] [CrossRef]
- Bagnazari, M.; Saidi, M.; Sripathy, H.; Geetha, D. Establishment of an improved, efficient and eco-friendly micropropagation system in Salacia chinensis L., an endangered anti-diabetic medicinal plant. Agricult. Forest. 2017, 63, 167–176. [Google Scholar] [CrossRef]
- Azma, W.; Anwar, J.; Waseem, A.; Mohammad, F. SIRAS (Albizia lebbeck (L.) Benth.) and its medicinal uses in unani medicine—A review. CellMed 2020, 10, 12. [Google Scholar] [CrossRef]
- Demies, M.; Samejima, H.; Sayok, A.K.; Noweg, G.T. Tree diversity, forest structure and species composition in a logged-over mixed dipterocarp forest, Bintulu, Sarawak, Malaysia. Trans. Sci. Technol. 2019, 6, 102–110. [Google Scholar]
- Ismail, M.H.; Fuad, M.F.A.; Zaki, P.H.; Jemali, N.J.N. Analysis of importance value index of unlogged and logged peat swamp forest in Nenasi Forest Reserve, Peninsular Malaysia. Bonorowo Wetl. 2017, 7, 74–78. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Alard, D.; Saguez, R.; Wattez-Franger, A.; de Foucault, B.; Delelis-Dusollier, A.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Xu, Z.; Liu, X. Functional diversity and secondary production of macrofaunal assemblages can provide insights of biodiversity–ecosystem function relationships. Environ. Sci. Eur. 2024, 36, 62. [Google Scholar] [CrossRef]
- Danyagri, G.; Baral, S.K.; Pelletier, G. Effects of disturbance and site factors on sapling dynamics and species diversity in Northern Hardwood Stands. For. Ecol. Manag. 2019, 444, 225–234. [Google Scholar] [CrossRef]
- Malik, Z.; Bhatt, A.B. Regeneration status of tree species and survival of their seedlings in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 2016, 57, 677–690. [Google Scholar]
- Prestes, N.C.C.d.S.; Massi, K.G.; Silva, E.A.; Nogueira, D.S.; de Oliveira, E.A.; Freitag, R.; Marimon, B.S.; Marimon-Junior, B.H.; Keller, M.; Feldpausch, T.R. Fire effects on understory forest regeneration in Southern Amazonia. Front. For. Glob. Chang. 2020, 3, 10. [Google Scholar] [CrossRef]
- Rocha, G.P.E.; Vieira, D.L.M.; Simon, M.F. Fast natural regeneration in abandoned pastures in Southern Amazonia. For. Ecol. Manag. 2016, 370, 93–101. [Google Scholar] [CrossRef]


| No. | Family | Scientific Name | Vernacular Name | Distribution | Habits | Plant Clustering | Utilization | Used Parts | UV | Voucher Specimens |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Amaryllidaceae | Hippeastrum sp. | Wan Si Thit | Introduced | H | Un | on | wp | 0.100 | KTRR001 |
| 2. | Anacardiaceae | Buchanania lanzan Spreng. | Mamuang Hua Maeng Wan | Native | T | Tr, Sa, Un | fd | ft | 0.433 | KTRR002 |
| 3. | Anacardiaceae | Buchanania siamensis Miq. | Tha Non Chai | Native | T | Tr | md | ba | 0.233 | KTRR003 |
| 4. | Anacardiaceae | Lannea coromandelica (Houtt.) Merr. | Kok Kan | Native | T | Tr, Sa | md | ba, le, ro | 0.300 | KTRR004 |
| 5. | Anacardiaceae | Spondias pinnata (L.f.) Kurz | Makok | Native | T | Tr, Sa, Un | fd | ft | 0.867 | KTRR005 |
| 6. | Annonaceae | Polyalthia debilis (Pierre) Finet & Gagnep. | Kon Khrok | Native | S | Un | fd | ft | 0.167 | KTRR006 |
| 7. | Annonaceae | Polyalthia evecta (Pierre) Finet & Gagnep. | Nom Noi | Native | S | Un | fd | ft | 0.200 | KTRR007 |
| 8. | Annonaceae | Uvaria rufa (Dunal) Blume | Phi Phuan Noi | Native | C | Tr, Sa, Un | fd | ft | 0.367 | KTRR008 |
| 9. | Annonaceae | Uvaria siamensis (Scheff.) L.L.Zhou, Y.C.F.Su & R.M.K.Saunders | Lamduan | Native | T | Un | on | wp | 0.267 | KTRR009 |
| 10. | Annonaceae | Xylopia vielana Pierre | Kluai Noi | Native | T | Tr, Sa | fd | ft | 0.233 | KTRR010 |
| 11. | Apocynaceae | Amphineurion marginatum (Roxb.) D.J.Middleton | Khruea Sai Tan | Native | C | Un | md | le, ro | 0.067 | KTRR011 |
| 12. | Apocynaceae | Carissa spinarum L. | Nam Phrom | Native | S | Un | md | hw, ro | 0.100 | KTRR012 |
| 13. | Apocynaceae | Streptocaulon juventas (Lour.) Merr. | Thao Prasong | Native | C | Un | md | wp | 0.067 | KTRR013 |
| 14. | Apocynaceae | Urceola polymorpha (Pierre ex Spire) D.J.Middleton & Livsh. | Som Lom | Native | C | Un | fd | ft, le | 0.500 | KTRR014 |
| 15. | Apocynaceae | Wrightia religiosa (Teijsm. & Binn.) Benth. ex Kurz | Mok | Native | T | Tr, Sa | on | wp | 0.267 | KTRR015 |
| 16. | Araceae | Amorphophallus brevispathus Gagnep. | I Rok | Endemic | H | Un | fd | st | 0.633 | KTRR016 |
| 17. | Araceae | Scindapsus officinalis (Roxb.) Schott | Phlu Chang | Native | C | Un | on | wp | 0.067 | KTRR017 |
| 18. | Araceae | Typhonium trilobatum (L.) Schott | Ut Ta Phit | Native | H | Un | md | ro, tu | 0.033 | KTRR018 |
| 19. | Asteraceae | Chromolaena odorata (L.) R.M.King & H.Rob. | Sap Suea | Introduced | H | Un | af, md | le, wp | 0.167 | KTRR019 |
| 20. | Asteraceae | Cyanthillium cinereum (L.) H.Rob. | Ya Dok Khao | Native | H | Un | af | wp | 0.200 | KTRR020 |
| 21. | Asteraceae | Elephantopus scaber L. | Do Mai Ru Lom | Native | H | Un | md | ro | 0.167 | KTRR021 |
| 22. | Asteraceae | Praxelis clematidea (Hieron. ex Kuntze) R.M.King & H.Rob. | Sap Muang | Introduced | H | Un | md | ro, wp | 0.167 | KTRR022 |
| 23. | Bignoniaceae | Millingtonia hortensis L.f. | Pib Khao | Native | T | Tr | on | wp | 0.333 | KTRR023 |
| 24. | Burseraceae | Canarium subulatum Guillaumin | Makok Kluean | Native | T | Tr | fd | ft | 0.333 | KTRR024 |
| 25. | Celastraceae | Salacia chinensis L. | Kamphaeng Chet Chan | Native | T | Tr, Sa | md | ro, st | 0.200 | KTRR025 |
| 26. | Commelinaceae | Commelina benghalensis L. | Phak Plap | Native | H | Un | af | wp | 0.167 | KTRR026 |
| 27. | Commelinaceae | Commelina diffusa Burm.f. | Plap Bai Khaep | Native | H | Un | af | wp | 0.167 | KTRR027 |
| 28. | Commelinaceae | Floscopa scandens Lour. | Phak Plap Chang | Native | H | Un | md | wp | 0.200 | KTRR028 |
| 29. | Convolvulaceae | Evolvulus nummularius (L.) L. | Tang Rian | Introduced | CrH | Un | on | wp | 0.133 | KTRR029 |
| 30. | Cucurbitaceae | Coccinia grandis (L.) Voigt | Tamlueng | Native | H | Un | fd | le | 0.833 | KTRR030 |
| 31. | Cyperaceae | Actinoscirpus grossus (L.f.) Goetgh. & D.A.Simpson | Kok Samliam | Native | H | Un | ot | le | 0.100 | KTRR031 |
| 32. | Dioscoreaceae | Dioscorea hispida Dennst. | Kloi | Native | H | Un | fd | tu | 0.433 | KTRR032 |
| 33. | Dioscoreaceae | Dioscorea pierrei Prain & Burkill | Man Nam | Native | H | Un | fd | tu | 0.467 | KTRR033 |
| 34. | Dipterocarpaceae | Anthoshorea roxburghii (G.Don) P.S.Ashton & J.Heck. | Phayom | Native | T | Tr, Sa | ct, on | st, wp | 0.433 | KTRR034 |
| 35. | Dipterocarpaceae | Dipterocarpus intricatus Dyer | Yang Krat | Native | T | Tr, Sa | md | ba | 0.133 | KTRR035 |
| 36. | Dipterocarpaceae | Dipterocarpus obtusifolius Teijsm. ex Miq. | Yang Hiang | Native | T | Tr, Sa, Un | ot | st | 0.233 | KTRR036 |
| 37. | Dipterocarpaceae | Pentacme siamensis (Miq.) Kurz | Rang | Native | T | Tr, Sa | ct | st | 0.200 | KTRR037 |
| 38. | Ebenaceae | Diospyros filipendula Pierre ex Lecomte | Lam Bit Dong | Native | T | Tr, Sa, Un | md | ba, ro | 0.133 | KTRR038 |
| 39. | Ebenaceae | Diospyros mollis Griff. | Ma Kluea | Native | T | Tr, Sa | md | ft, ro | 0.067 | KTRR039 |
| 40. | Ebenaceae | Diospyros rhodocalyx Kurz | Tako Na | Native | T | Tr | on | wp | 0.100 | KTRR040 |
| 41. | Euphorbiaceae | Suregada multiflora (A.Juss.) Baill. | Khan Thong Phayabat | Native | T | Tr, Sa, Un | md | ba, ro | 0.100 | KTRR041 |
| 42. | Fabaceae | Albizia lebbeck (L.) Benth. | Phruek | Introduced | T | Tr, Sa | md | ba, se | 0.200 | KTRR042 |
| 43. | Fabaceae | Clitoria ternatea L. | Anchan | Introduced | H | Un | fd | if | 0.333 | KTRR043 |
| 44. | Fabaceae | Erythrophleum succirubrum Gagnep. | Phan Sat | Native | T | Tr, Sa, Un | md | st | 0.067 | KTRR044 |
| 45. | Fabaceae | Leucaena leucocephala (Lam.) de Wit | Krathin | Introduced | T | Sa | fd | ft, le | 0.367 | KTRR045 |
| 46. | Fabaceae | Peltophorum dasyrhachis (Miq.) Kurz | A Rang | Native | T | Tr | ct, hf | st | 0.233 | KTRR046 |
| 47. | Fabaceae | Pithecellobium dulce (Roxb.) Benth. | Makhamthet | Introduced | T | Tr, Sa, Un | fd | ft | 0.300 | KTRR047 |
| 48. | Fabaceae | Pterocarpus macrocarpus Kurz | Pradu Pa | Native | T | Tr, Sa | hf, ot | ba, st | 0.167 | KTRR048 |
| 49. | Fabaceae | Senegalia comosa (Gagnep.) Maslin, Seigler & Ebinger | Nam Han | Native | C | Tr, Sa, Un | md | ro | 0.033 | KTRR049 |
| 50. | Fabaceae | Senna siamea (Lam.) H.S.Irwin & Barneby | Phak Khilek | Native | T | Tr | fd | le | 0.533 | KTRR050 |
| 51. | Fabaceae | Sindora siamensis Teijsm. ex Miq. | Ma Kha Tae | Native | T | Tr, Sa, Un | ct | st | 0.200 | KTRR051 |
| 52. | Fabaceae | Stylosanthes humilis Kunth | Ya Satai Lo | Introduced | S | Un | af | wp | 0.067 | KTRR052 |
| 53. | Fabaceae | Tephrosia vestita Vogel | Dan Ratchasi | Native | H | Un | md | le | 0.067 | KTRR053 |
| 54. | Fabaceae | Xylia xylocarpa (Roxb.) W.Theob. | Daeng | Native | T | Tr, Sa, Un | hf | st | 0.167 | KTRR054 |
| 55. | Hypericaceae | Cratoxylum cochinchinense (Lour.) Blume | Tio Kliang | Native | T | Tr, Sa, Un | fd | if | 0.367 | KTRR055 |
| 56. | Hypericaceae | Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer subsp. formosum | Tio Khao | Native | T | Sa, Un | fd | if | 0.400 | KTRR056 |
| 57. | Hypericaceae | Crotoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer subsp. pruniflorum (Kurz) Gogelein | Tio Khon | Native | T | Tr, Sa, Un | fd | if | 0.333 | KTRR057 |
| 58. | Irvingiaceae | Irvingia malayana Oliv. ex A.W.Benn. | Kra Bok | Native | T | Tr, Sa | fd | ft | 0.267 | KTRR058 |
| 59. | Lamiaceae | Hymenopyramis parvifolia Moldenke | Kha Pia | Endemic | S | Tr, Sa, Un | md | ba | 0.033 | KTRR059 |
| 60. | Lamiaceae | Mesosphaerum suaveolens (L.) Kuntze | Maenglak Kha | Introduced | H | Un | af | wp | 0.067 | KTRR060 |
| 61. | Lamiaceae | Vitex pinnata L. | Tin Nok | Native | T | Un | ct | st | 0.133 | KTRR061 |
| 62. | Loranthaceae | Dendrophthoe pentandra (L.) Miq. | Kafak Mamuang | Native | C | Un | ot | st | 0.067 | KTRR062 |
| 63. | Lythraceae | Lagerstroemia balansae Koehne | Ta Baek Kriap | Native | T | Tr, Sa, Un | hf, on | st, wp | 0.200 | KTRR063 |
| 64. | Malvaceae | Bombax anceps Pierre | Ngao | Native | T | Tr, Sa | ot | ft | 0.133 | KTRR064 |
| 65. | Malvaceae | Helicteres angustifolia L. | Po Khi Tun | Native | H | Un | md | le, ro | 0.100 | KTRR065 |
| 66. | Malvaceae | Microcos tomentosa Sm. | Phlapphla | Native | S | Tr, Sa, Un | fd | ft | 0.167 | KTRR066 |
| 67. | Malvaceae | Sida cordifolia L. | Ya Khat Bai Pom | Native | S | Un | md | le, ro | 0.100 | KTRR067 |
| 68. | Malvaceae | Urena rigida Wall. ex Mast. | Khi On | Native | H | Un | af | wp | 0.067 | KTRR068 |
| 69. | Malvaceae | Waltheria indica L. | Ya Hua Nok Khao | Introduced | S | Un | af | wp | 0.067 | KTRR069 |
| 70. | Melastomataceae | Memecylon edule Roxb. | Phlong Mueat | Native | S/ST | Tr, Sa, Un | on, ot | le, st, wp | 0.300 | KTRR070 |
| 71. | Meliaceae | Azadirachta indica A.Juss. | Sadao | Native | T | Tr, Sa | fd | if, le | 0.667 | KTRR071 |
| 72. | Meliaceae | Chukrasia tabularis A.Juss. | Yom Hin | Native | T | Tr | ct | st | 0.233 | KTRR072 |
| 73. | Menispermaceae | Cissampelos pareira L. | Khruea Ma Noi | Native | H | Un | fd | le | 0.600 | KTRR073 |
| 74. | Menispermaceae | Tiliacora triandra (Colebr.) Diels | Yanang | Native | H | Un | fd | le | 0.633 | KTRR074 |
| 75. | Menispermaceae | Tinospora baenzigeri Forman | Chingcha Chali | Endemic | H | Un | md | vi | 0.133 | KTRR075 |
| 76. | Moraceae | Ficus benjamina L. | Sai | Native | T | Sa | on | wp | 0.133 | KTRR076 |
| 77. | Moraceae | Streblus asper Lour. | Khoi | Native | S/ST | Tr, Sa | fd | ft | 0.100 | KTRR077 |
| 78. | Ochnaceae | Ochna integerrima (Lour.) Merr. | Chang Nao | Native | S/ST | Tr, Sa | md | ba, ro | 0.133 | KTRR078 |
| 79. | Olacaceae | Olax scandens Roxb. | Namchai Khrai | Native | S/C | Tr, Sa, Un | md | ba, ro | 0.167 | KTRR079 |
| 80. | Phyllanthaceae | Antidesma puncticulatum Miq. | Ma Mao | Native | T | Tr | fd | ft | 0.567 | KTRR080 |
| 81. | Poaceae | Imperata cylindrica (L.) Raeusch. | Ya Kha | Introduced | H | Un | ot | le | 0.133 | KTRR081 |
| 82. | Poaceae | Microstegium fasciculatum (L.) Henrard | Ya Khom Kha | Native | H | Un | af | wp | 0.033 | KTRR082 |
| 83. | Poaceae | Urochloa mutica (Forssk.) T.Q.Nguyen | Ya Khon | Introduced | H | Un | af | wp | 0.033 | KTRR083 |
| 84. | Poaceae | Vietnamosasa pusilla (A.Chev. & A.Camus) T.Q.Nguyen | Phek | Native | H | Un | fd | st | 0.300 | KTRR084 |
| 85. | Rhamnaceae | Ziziphus cambodiana Pierre | Nham Ta Khrong | Native | S | Tr, Sa, Un | ot | ba | 0.067 | KTRR085 |
| 86. | Rhamnaceae | Ziziphus mauritiana Lam. | Phutsa | Native | T | Tr | fd | ft | 0.333 | KTRR086 |
| 87. | Rhamnaceae | Ziziphus oenopolia (L.) Mill. | Lep Yiao | Native | S/C | Sa, Un | fd | ft | 0.300 | KTRR087 |
| 88. | Rubiaceae | Canthium berberidifolium E.T.Geddes | Ngiang Duk | Native | S | Un | on | wp | 0.100 | KTRR088 |
| 89. | Rubiaceae | Catunaregam tomentosa (Blume ex DC.) Tirveng. | Nam Thaeng | Native | S/ST | Tr, Sa | ot | ft | 0.100 | KTRR089 |
| 90. | Rubiaceae | Gardenia sootepensis Hutch. | Kham Mok Luang | Native | T | Tr, Sa, Un | on | wp | 0.133 | KTRR090 |
| 91. | Rubiaceae | Hymenodictyon orixense (Roxb.) Mabb. | Som Kop | Native | T | Un | on | wp | 0.067 | KTRR091 |
| 92. | Rubiaceae | Ixora cibdela Craib | Khem Pa | Native | S | Un | on | wp | 0.100 | KTRR092 |
| 93. | Rubiaceae | Mitragyna diversifolia (Wall. ex G.Don) Havil. | Kra Thum Na | Native | T | Tr | on | wp | 0.100 | KTRR093 |
| 94. | Rubiaceae | Morinda pubescens Sm. | Yo Pa | Native | S/ST | Tr, Sa | on | wp | 0.067 | KTRR094 |
| 95. | Rubiaceae | Mussaenda uniflora Wall. ex G.Don | Mali Lueai | Native | H | Un | on | wp | 0.167 | KTRR095 |
| 96. | Rubiaceae | Pavetta tomentosa Roxb. ex Sm. | Khaosan Pa | Native | S/ST | Tr, Sa, Un | on | wp | 0.167 | KTRR096 |
| 97. | Rutaceae | Clausena excavata Burm.f. | Phia Fan | Native | H | Un | md | wp | 0.100 | KTRR097 |
| 98. | Rutaceae | Clausena wallichii Oliv. | Song Fa | Native | S | Un | md | le | 0.067 | KTRR098 |
| 99. | Rutaceae | Micromelum minutum (G.Forst.) Wight & Arn. | Samat Noi | Native | ST | Sa | md | le | 0.167 | KTRR099 |
| 100. | Rutaceae | Naringi crenulata (Roxb.) Nicolson | Krachae | Native | T | Tr, Sa, Un | ot | st | 0.100 | KTRR100 |
| 101. | Salicaceae | Flacourtia indica (Burm.f.) Merr. | Ta Khop Pa | Native | S | Sa, Un | fd | ft | 0.233 | KTRR101 |
| 102. | Sapindaceae | Lepisanthes rubiginosa (Roxb.) Leenh. | Ma Huat | Native | ST | Sa, Un | fd | ft | 0.400 | KTRR102 |
| 103. | Sapindaceae | Zollingeria dongnaiensis Pierre | Khi Non Daeng | Native | H | Un | md | ba, le | 0.133 | KTRR103 |
| 104. | Schizaeaceae | Lygodium flexuosum (L.) Sw. | Kut Ngotngaet | Native | H | Un | fd | le | 0.067 | KTRR104 |
| 105. | Smilacaceae | Smilax prolifera Roxb. | Khruea Khueang Sayam | Native | H | Un | md | ba | 0.067 | KTRR105 |
| 106. | Stemonaceae | Stemona tuberosa Lour. | Nhon Tai Yak | Native | H | Un | md | ro | 0.200 | KTRR106 |
| 107. | Vitaceae | Causonis trifolia (L.) Mabb. & J.Wen | Thao Khan Khao | Native | H | Un | md | le, vi | 0.067 | KTRR107 |
| 108. | Vitaceae | Leea thorelii Gagnep. | Ka Tang Bai Tia | Native | H | Un | md | ro, st | 0.133 | KTRR108 |
| 109. | Zingiberaceae | Curcuma singularis Gagnep. | Kra Chiao | Native | H | Un | fd | if | 0.833 | KTRR109 |
| 110. | Zingiberaceae | Kaempferia marginata Carey ex Roscoe | Tup Mup | Native | H | Un | fd | le | 0.600 | KTRR110 |
| Group of Ailments | Number of Use Report | Number of Taxa | Fic |
|---|---|---|---|
| Endocrine system | 5 | 2 | 0.75 |
| Gastro-intestinal system | 47 | 14 | 0.72 |
| Infections | 67 | 19 | 0.73 |
| Musculoskeletal and joint diseases | 6 | 2 | 0.80 |
| Nutrition and blood | 16 | 5 | 0.73 |
| Obstetrics, gynaecology and urinary-tract disorders | 22 | 5 | 0.81 |
| Respiratory system | 24 | 6 | 0.78 |
| Skin | 32 | 9 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saensouk, P.; Saensouk, S.; Boonma, T.; Hanchana, K.; Rakarcha, S.; Maknoi, C.; Chanthavongsa, K.; Jitpromma, T. Ecological Analysis and Ethnobotanical Evaluation of Plants in Khanthararat Public Benefit Forest, Kantarawichai District, Thailand. Forests 2025, 16, 1012. https://doi.org/10.3390/f16061012
Saensouk P, Saensouk S, Boonma T, Hanchana K, Rakarcha S, Maknoi C, Chanthavongsa K, Jitpromma T. Ecological Analysis and Ethnobotanical Evaluation of Plants in Khanthararat Public Benefit Forest, Kantarawichai District, Thailand. Forests. 2025; 16(6):1012. https://doi.org/10.3390/f16061012
Chicago/Turabian StyleSaensouk, Piyaporn, Surapon Saensouk, Thawatphong Boonma, Kasan Hanchana, Sarayut Rakarcha, Charun Maknoi, Khamfa Chanthavongsa, and Tammanoon Jitpromma. 2025. "Ecological Analysis and Ethnobotanical Evaluation of Plants in Khanthararat Public Benefit Forest, Kantarawichai District, Thailand" Forests 16, no. 6: 1012. https://doi.org/10.3390/f16061012
APA StyleSaensouk, P., Saensouk, S., Boonma, T., Hanchana, K., Rakarcha, S., Maknoi, C., Chanthavongsa, K., & Jitpromma, T. (2025). Ecological Analysis and Ethnobotanical Evaluation of Plants in Khanthararat Public Benefit Forest, Kantarawichai District, Thailand. Forests, 16(6), 1012. https://doi.org/10.3390/f16061012








