Toward Safer Resin Tapping: Assessing Alternative Chemical Stimulants for Pinus pinaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Growth and Physiological Measurements
2.3. Resin Yield and Dry Weight
2.4. Resin Duct Density
2.5. Statistical Analysis
3. Results
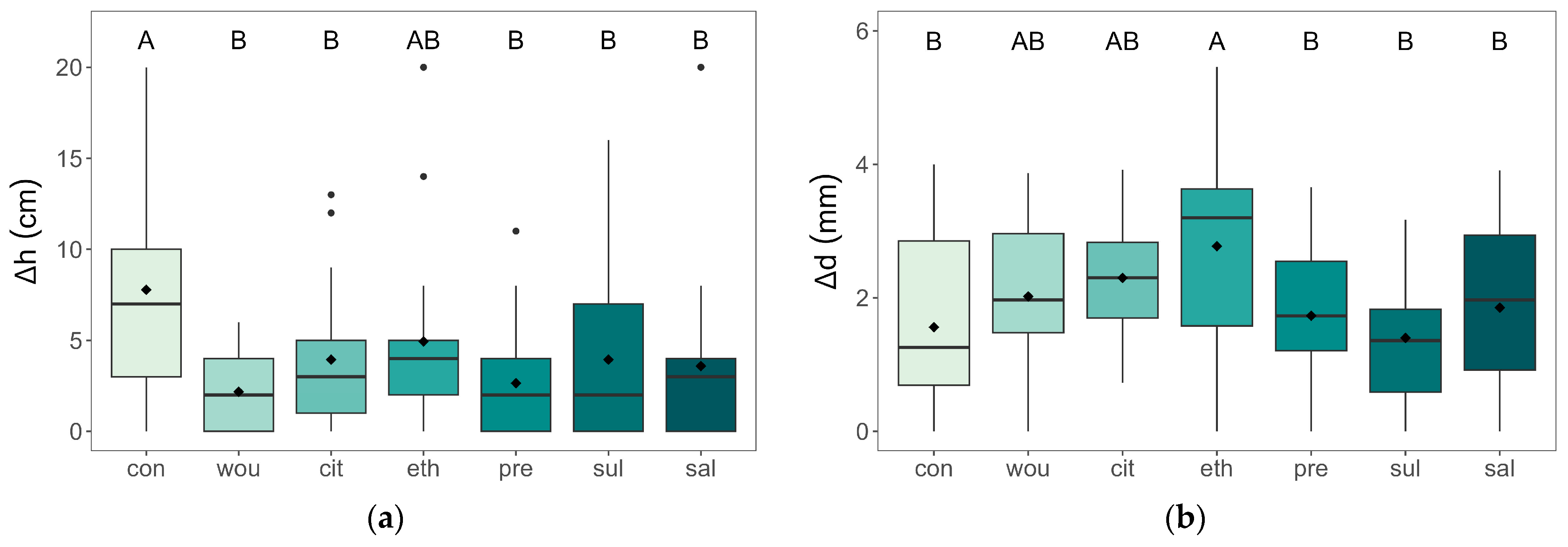
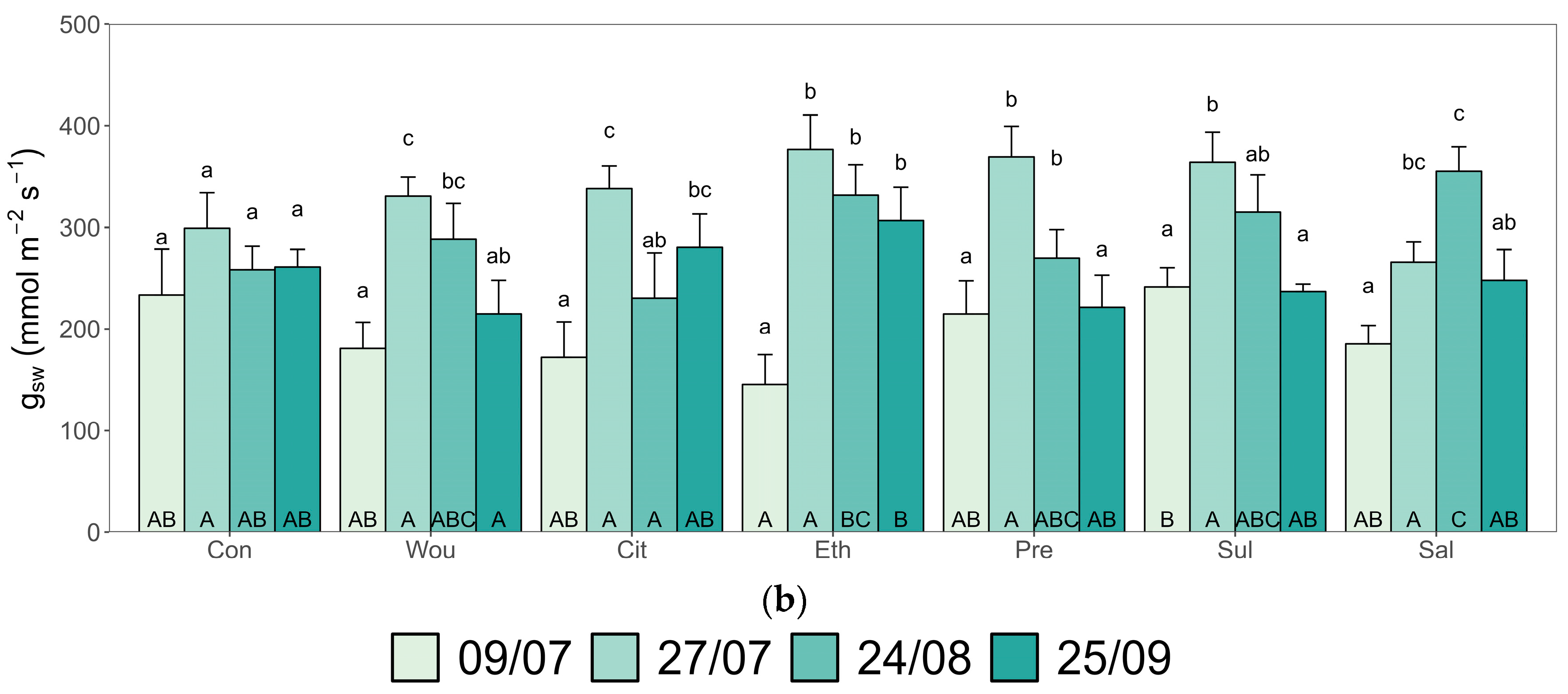
3.1. Growth and Physiological Traits
3.2. Resin Yield
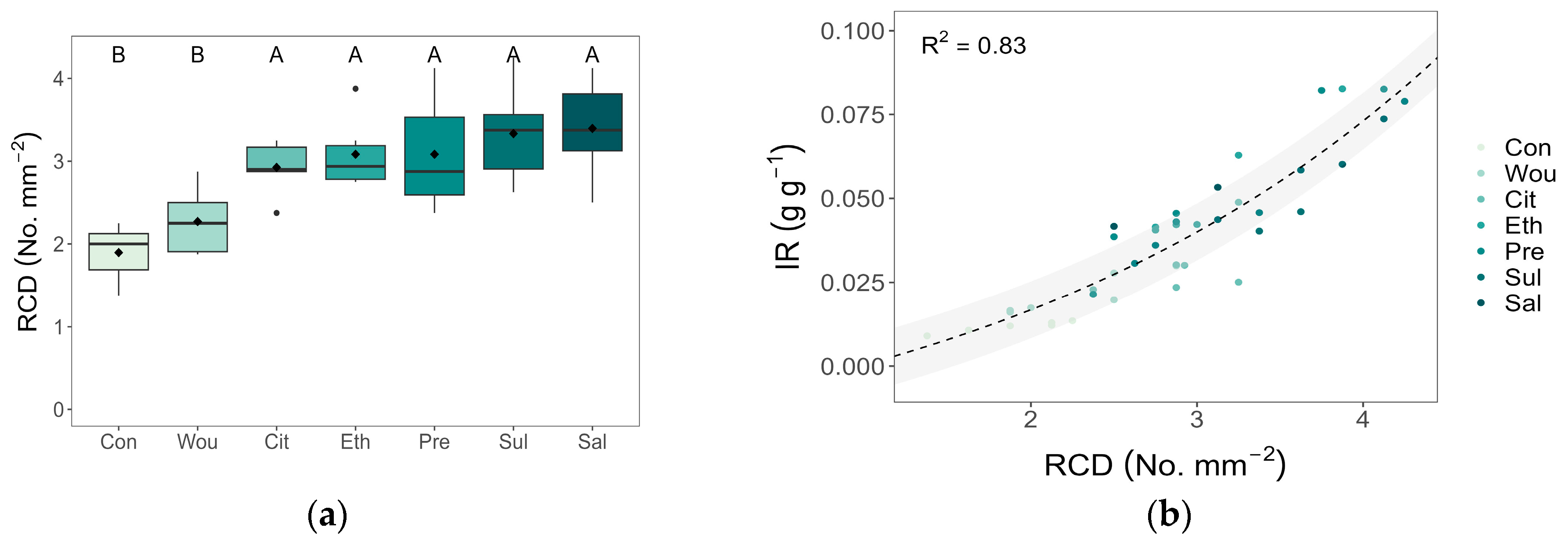
3.3. Resin Canal Density
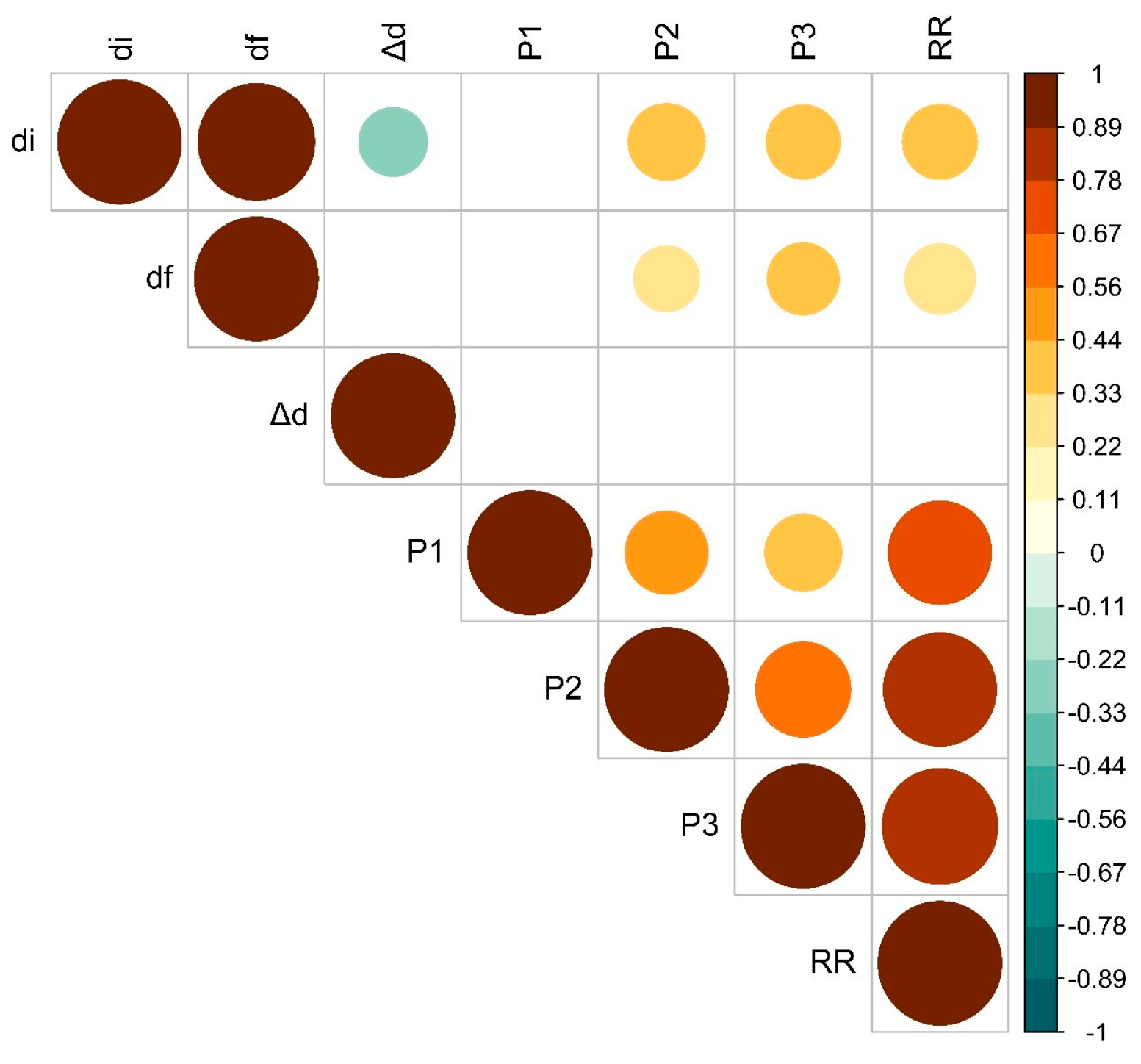
3.4. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Con | Control (plants without wounds or stimulant) |
| Wou | Wounded (plants tapped without stimulant) |
| Eth | Ethrel (stimulant paste containing ethephon) |
| Pre | Pretta paste (Brazilian stimulant paste) |
| Sul | Sulfuric acid-based stimulant paste |
| Sal | Salicylic acid-based stimulant paste |
| Cit | Citric acid-based stimulant paste |
| Hf | Height or diameter at the end of the experiment |
| Hi | Height or diameter at the beginning of the experiment |
| tf − ti | Duration of the tapping experiment |
| Ψmd | Midday needle water potential |
| gsw | Stomatal conductance |
| RR | Released resin |
| IR | Internal resin |
| TR | Total resin content (RR + IR) |
| RCD | Resin canal density |
| ANOVA | Analysis of variance |
| LME | Linear mixed-effects model |
| SE | Standard error |
Appendix A

References
- Phillips, M.A.; Croteau, R.B. Resin-Based Defenses in Conifers. Trends. Plant. Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef]
- Celedon, J.M.; Bohlmann, J. Oleoresin Defenses in Conifers: Chemical Diversity, Terpene Synthases and Limitations of Oleoresin Defense under Climate Change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef]
- Trapp, S.; Croteau, R. Defensive Resin Biosynthesis in Conifers. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef] [PubMed]
- Neis, F.A.; de Costa, F.; de Almeida, M.R.; Colling, L.C.; de Oliveira Junkes, C.F.; Fett, J.P.; Fett-Neto, A.G. Resin Exudation Profile, Chemical Composition, and Secretory Canal Characterization in Contrasting Yield Phenotypes of Pinus elliottii Engelm. Ind. Crops Prod. 2019, 132, 76–83. [Google Scholar] [CrossRef]
- López-Álvarez, Ó.; Zas, R.; Marey-Perez, M. Resin Tapping: A Review of the Main Factors Modulating Pine Resin Yield. Ind Crops Prod. 2023, 202, 117105. [Google Scholar] [CrossRef]
- da Silva Rodrigues-Corrêa, K.C.; de Lima, J.C.; Fett-Neto, A.G. Pine Oleoresin: Tapping Green Chemicals, Biofuels, Food Protection, and Carbon Sequestration from Multipurpose Trees. Food Energy Secur. 2012, 1, 81–93. [Google Scholar] [CrossRef]
- Justes, A.; Soliño, M. La Resina En Castilla y León (España): Preferencias de Resineros En Tiempos de Crisis Económica. Madera Bosques 2018, 24. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Martín, J.A.; López, R.; Sanz, A.; Gil, L. Effect of Four Tapping Methods on Anatomical Traits and Resin Yield in Maritime Pine (Pinus pinaster Ait.). Ind. Crops Prod. 2016, 86, 143–154. [Google Scholar] [CrossRef]
- Nájera y Angulo, F.; Rifé Lamprecht, M.P. Resinación Con Estimulantes Químicos. Estudio General y Experiencias Realizadas En Los Pinares Españoles. I. Acido Clorhídrico; Instituto Forestal de Investigaciones y Experiencias y Servicio de la Madera: Madrid, Spain, 1951. [Google Scholar]
- Solís, W.; Zamorano, J.L. Características y Utilización de La «Pasta IFIE» Como Estimulante de Resinación; Hoja Técnica INIA: Madrid, Spain, 1974. [Google Scholar]
- Lema, M.; Touza, R.; Feijoo, D.; Bustingorri, G.; Martínez, É.; Zas, R. Resin Tapping of Atlantic Pine Forests: Towards an Optimized Use of Stimulant Pastes over the Season. Eur. J. For. Res. 2024, 143, 1213–1224. [Google Scholar] [CrossRef]
- DeAngelis, J.D.; Nebeker, T.E.; Hodges, J.D. Influence of Tree Age and Growth Rate on the Radial Resin Duct System in Loblolly Pine (Pinus taeda). Can. J. Bot. 1986, 64, 1046–1049. [Google Scholar] [CrossRef]
- Münch, E. Naturwissenschaftliche Grundlagen der Kiefernharznutzung; Biologische Reichsanstalt für Land- und Forstwirtschaft: Berlin, Germany, 1921; Volume 10. [Google Scholar]
- Schopmeyer, C.S.; Mergen, F.; Evans, T.C. Applicability of Poiseuille’s Law to Exudation of Oleoresin from Wounds on Slash Pine. Plant. Physiol. 1954, 29, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.S.; Akash, A. Production and Cost of Chir-Pine Resin Tapping by Bore-Hole Method in Narendranagar Forest Division. World J. Environ. Biosci. 2023, 12, 40–45. [Google Scholar] [CrossRef]
- Kossuth, S.V.; Koch, P. Paraquat and CEPA Stimulation of Oleoresin Production in Lodgepole Pine Central Stump-Root System. Wood Fiber Sci. 1989, 21, 263–273. [Google Scholar]
- Füller, T.; de Lima, J.; de Costa, F.; Rodrigues-Honda, K.; Fett-Neto, A. Stimulant Paste Preparation and Bark Streak Tapping Technique for Pine Oleoresin Extraction. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2016; Volume 1405, pp. 19–26. ISBN 978-1-4939-3391-4. [Google Scholar]
- Wolter, K.; Zinkel, D. Observations on the Physiological Mechanisms and Chemical Constituents of Induced Oleoresin Synthesis in Pinus resinosa. Can. J. For. Res. 2011, 14, 452–458. [Google Scholar] [CrossRef]
- McReynolds, R.D.; Kossuth, S.V. CEPA in Sulfuric Acid Paste Increases Oleoresin Yields. South. J. Appl. For. 1984, 8, 168–172. [Google Scholar] [CrossRef]
- Silverman, F.P.; Petracek, P.D.; Fledderman, C.M.; Ju, Z.; Heiman, D.F.; Warrior, P. Salicylate Activity. 1. Protection of Plants from Paraquat Injury. J. Agric. Food Chem. 2005, 53, 9764–9768. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; López, R.; Martín, J.A.; Pinillos, F.; Gil, L. Resin Yield in Pinus pinaster Is Related to Tree Dendrometry, Stand Density and Tapping-Induced Systemic Changes in Xylem Anatomy. For. Ecol. Manag. 2014, 313, 47–54. [Google Scholar] [CrossRef]
- Rissanen, K.; Hölttä, T.; Bäck, J.; Rigling, A.; Wermelinger, B.; Gessler, A. Drought Effects on Carbon Allocation to Resin Defences and on Resin Dynamics in Old-Grown Scots Pine. Environ. Exp. Bot. 2021, 185, 104410. [Google Scholar] [CrossRef]
- López, R.; Cano, F.J.; Rodríguez-Calcerrada, J.; Sangüesa-Barreda, G.; Gazol, A.; Camarero, J.J.; Rozenberg, P.; Gil, L. Tree-Ring Density and Carbon Isotope Composition Are Early-Warning Signals of Drought-Induced Mortality in the Drought Tolerant Canary Island Pine. Agric. For. Meteorol. 2021, 310, 108634. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Franceschi, V.R. Methyl Jasmonate-Induced Ethylene Production Is Responsible for Conifer Phloem Defense Responses and Reprogramming of Stem Cambial Zone for Traumatic Resin Duct Formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef]
- Rodrigues, K.C.S.; Azevedo, P.C.N.; Sobreiro, L.E.; Pelissari, P.; Fett-Neto, A.G. Oleoresin Yield of Pinus elliottii Plantations in a Subtropical Climate: Effect of Tree Diameter, Wound Shape and Concentration of Active Adjuvants in Resin Stimulating Paste. Ind. Crops Prod. 2008, 27, 322–327. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhao, F.; Zeng, M.; Li, F.; Chen, L.; Wu, H.; Che, X.; Li, Y.; Deng, L.; et al. Efficient Resin Production Using Stimulant Pastes in Pinus elliottii × P. caribaea Families. Sci. Rep. 2022, 12, 13129. [Google Scholar] [CrossRef]
- Rodrigues, K.C.S.; Fett-Neto, A.G. Oleoresin Yield of Pinus elliottii in a Subtropical Climate: Seasonal Variation and Effect of Auxin and Salicylic Acid-Based Stimulant Paste. Ind. Crops Prod. 2009, 30, 316–320. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional Machineries in Jasmonate-Elicited Plant Secondary Metabolism. Trends. Plant. Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Christiansen, E.; Franceschi, V.R. Induction of Anatomically Based Defense Responses in Stems of Diverse Conifers by Methyl Jasmonate: A Phylogenetic Perspective. Tree Physiol. 2004, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junkes, C.F.; Duz, J.V.V.; Kerber, M.R.; Wieczorek, J.; Galvan, J.L.; Fett, J.P.; Fett-Neto, A.G. Resinosis of Young Slash Pine (Pinus elliottii Engelm.) as a Tool for Resin Stimulant Paste Development and High Yield Individual Selection. Ind. Crops Prod. 2019, 135, 179–187. [Google Scholar] [CrossRef]
- Michavila Puente-Villegas, S.; Rodríguez-García, A.; Rubio, F.; Gil, L.; López, R. Salicylic and Citric Acid as Promising New Stimulants for Resin Tapping in Maritime Pine (Pinus pinaster Ait.). For. Syst. 2021, 29, eSC07. [Google Scholar] [CrossRef]
- Rodrigues-Corrêa, K.; Fett-Neto, A. Seasonality and Chemical Elicitation of Defense Oleoresin Production in Field-Grown Slash Pine under Subtropical Climate. Theor. Exp. Plant. Physiol. 2013, 25, 56–61. [Google Scholar] [CrossRef]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin Ducts as Resistance Traits in Conifers: Linking Dendrochronology and Resin-Based Defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef]
- Fernández-Blas, C.; Ruiz-Benito, P.; Gazol, A.; Granda, E.; Samblás, E.; Granado-Díaz, I.; Zavala, M.A.; Valeriano, C.; Camarero, J.J. Historical Forest Use Constrains Tree Growth Responses to Drought: A Case Study on Tapped Maritime Pine (Pinus pinaster). Trees For. People 2024, 18, 100699. [Google Scholar] [CrossRef]
- Moreira, X.; Zas, R.; Sampedro, L. Quantitative Comparison of Chemical, Biological and Mechanical Induction of Secondary Compounds in Pinus pinaster Seedlings. Trees 2012, 26, 677–683. [Google Scholar] [CrossRef]
- Moura, M.; Campelo, F.; Carvalho, A.; Nabais, C.; Garcia-Forner, N. Growth and Climate Drive Resin Production in Pinus pinaster and Pinus pinea. Trees 2025, 39, 22. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; López, R.; Rodríguez-Calcerrada, J.; Gil, L. Stress and Tree Mortality in Mediterranean Pine Forests: Anthropogenic Influences. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Osem, Y., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 141–181. ISBN 978-3-030-63625-8. [Google Scholar]
- Moreira, X.; Sampedro, L.; Zas, R. Defensive responses of Pinus pinaster seedlings to exogenous application of methyl jasmonate: Concentration effect and systemic response. Environ. Exp. Bot. 2009, 67, 94–100. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; Martín, J.A.; López, R.; Mutke, S.; Pinillos, F.; Gil, L. Influence of Climate Variables on Resin Yield and Secretory Structures in Tapped Pinus pinaster Ait. in Central Spain. Agric. For. Meteorol. 2015, 202, 83–93. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M. Ethylene in Plant Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Yamamoto, F.; Iwanaga, F.; Al-Busaidi, A.; Yamanaka, N. Roles of Ethylene, Jasmonic Acid, and Salicylic Acid and Their Interactions in Frankincense Resin Production in Boswellia sacra Flueck. Trees. Sci. Rep. 2020, 10, 16760. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive Oxygen Species in Plants: Their Generation, Signal Transduction, and Scavenging Mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Gershenzon, J. Metabolic Costs of Terpenoid Accumulation in Higher Plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Sharma, S. Effect of Chemical Stimulants on Oleoresin Production and Wood Quality of Pinus roxburghii Sargent. Ph.D. Thesis, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Solan, India, 2023. [Google Scholar]
- Neis, F.A.; de Costa, F.; Füller, T.N.; de Lima, J.C.; da Silva Rodrigues-Corrêa, K.C.; Fett, J.P.; Fett-Neto, A.G. Biomass Yield of Resin in Adult Pinus elliottii Engelm. Trees Is Differentially Regulated by Environmental Factors and Biochemical Effectors. Ind. Crops Prod. 2018, 118, 20–25. [Google Scholar] [CrossRef]
- da Silva Rodrigues-Corrêa, K.C.; Apel, M.A.; Henriques, A.T.; Fett-Neto, A.G. Efficient Oleoresin Biomass Production in Pines Using Low Cost Metal Containing Stimulant Paste. Biomass Bioenergy 2011, 35, 4442–4448. [Google Scholar] [CrossRef]
- Lukmandaru, G.; Sunarta, S.; Pujiarti, R.; Listyanto, T.; Widyorini, R. The Effect of Stimulants and Environmental Factors on Resin Yield of Pinus merkusii Tapping. Bioresources 2021, 16, 163–175. [Google Scholar] [CrossRef]
- Lukmandaru, G.; Amri, S.; Sunarta, S.; Listyanto, T.; Pujiarti, R.; Widyorini, R. Oleoresin Yield of Pinus merkusii Trees from East Banyumas. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 16–17 October 2019; IOP Publishing: Bristol, UK, 2020; Volume 449, p. 012024. [Google Scholar]
- Jakubowski, M.; Dobroczyński, M. Long-Term Climate Sensitivity of Resin-Tapped and Non-Resin-Tapped Scots Pine Trees Based on Tree Ring Width and Blue Intensity. Forests 2023, 14, 593. [Google Scholar] [CrossRef]
- Bhattarai, B.; Gaire, N.P.; Maraseni, T.; Devkota, B.P.; Bhattarai, B.; Tripathi, S.; Aryal, K.R.; Adhikari, H. Impact of Resin Tapping on the Radial Growth and Climate Sensitivity of Naturally-Regenerated Pinus roxburghii (Chir Pine) in Western Nepal. Trees For. People 2025, 19, 100795. [Google Scholar] [CrossRef]
- Ripullone, F.; Guerrieri, M.R.; Nole’, A.; Magnani, F.; Borghetti, M. Stomatal Conductance and Leaf Water Potential Responses to Hydraulic Conductance Variation in Pinus pinaster Seedlings. Trees 2007, 21, 371–378. [Google Scholar] [CrossRef]
- Zlobin, I.E.; Kartashov, A.V.; Ivanov, Y.V.; Ivanova, A.I.; Kuznetsov, V. V Stem Notching Decreases Stem Hydraulic Conductance but Does Not Influence Drought Impacts and Post-Drought Recovery in Scots Pine and Norway Spruce. Physiol. Plant. 2022, 174, e13813. [Google Scholar] [CrossRef]
- Zeng, X.; Ni, P.; Li, Y.; Wang, W.; Sun, S.; Wang, Y.; Chang, Y.; Tao, X.; Hou, M.; Liu, X. Short-Term Resin Tapping Activities Had a Minor Influence on Physiological Responses Recorded in the Tree-Ring Isotopes of Chinese Pine (Pinus tabuliformis). Dendrochronologia 2021, 70, 125895. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A. Ponderosa Pine Resin Defenses and Growth: Metrics Matter. Tree Physiol. 2015, 35, 1223–1235. [Google Scholar] [CrossRef]
- Krokene, P.; Nagy, N. Anatomical Aspects of Resin-Based Defences in Pine. In Pine Resin: Biology, Chemistry and Applications; Research SignPost: Ahmedabad, India, 2012; pp. 67–86. ISBN 978-81-308-0493-4. [Google Scholar]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound Signalling in Plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef]
- Garcia-Forner, N.; Campelo, F.; Carvalho, A.; Vieira, J.; Rodríguez-Pereiras, A.; Ribeiro, M.; Salgueiro, A.; Silva, M.E.; Louzada, J.L. Growth-Defence Trade-Offs in Tapped Pines on Anatomical and Resin Production. For. Ecol. Manag. 2021, 496, 119406. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Rasmann, S.; Petry, W.K.; Carrillo-Gavilán, A.; Zas, R.; Sampedro, L. Trade-Offs Between Constitutive and Induced Defences Drive Geographical and Climatic Clines in Pine Chemical Defences. Ecol. Lett. 2014, 17, 537–546. [Google Scholar] [CrossRef]
- Zas, R.; Touza, R.; Sampedro, L.; Lario, F.J.; Bustingorri, G.; Lema, M. Variation in Resin Flow Among Maritime Pine Populations: Relationship with Growth Potential and Climatic Responses. For. Ecol. Manag. 2020, 474, 118351. [Google Scholar] [CrossRef]
- Caglayan, İ.; Dolu, A.Ö.; Kabak, Ö.; Rodríguez-García, A.; Demirel, T.; Özkan, U.Y.; Makineci, E.; Yeşil, A.; Ayberk, H. Dynamics of Resin Yield in Pinus brutia: A Quantitative Analysis Using Bark Streak Tapping. Ind. Crops Prod. 2024, 221, 119344. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E. Importance of Resin Ducts in Reducing Ponderosa Pine Mortality from Bark Beetle Attack. Oecologia 2010, 164, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.J.; Ayres, M.P.; Lorio, P.L., Jr.; Ruel, J.J. Environmental Effects on Constitutive and Inducible Resin Defences of Pinus taeda. Ecol. Lett. 2000, 3, 329–339. [Google Scholar] [CrossRef]
- Ruel, J.J.; Ayres, M.P.; Lorio, P.L., Jr. Loblolly Pine Responds to Mechanical Wounding with Increased Resin Flow. Can. J. For. Res. 1998, 28, 596–602. [Google Scholar] [CrossRef]



| Stimulant Paste | Acid (%) | [H3O+] En Moles/Litro | pH |
|---|---|---|---|
| Citric | 40% | 0.039 | 1.4 |
| Ethrel | 13.5% | 0.189 | 0.72 |
| Salicylic | 24.5% | 0.6125 | 0.21 |
| Sulfuric | 33.5% | 1.139 | −0.06 |
| Pretta | 49.4% | 2.47 | −0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio Pérez, F.; Rodríguez-García, A.; Michavila, S.; Rodríguez, A.; Gil, L.; López, R. Toward Safer Resin Tapping: Assessing Alternative Chemical Stimulants for Pinus pinaster. Forests 2025, 16, 849. https://doi.org/10.3390/f16050849
Rubio Pérez F, Rodríguez-García A, Michavila S, Rodríguez A, Gil L, López R. Toward Safer Resin Tapping: Assessing Alternative Chemical Stimulants for Pinus pinaster. Forests. 2025; 16(5):849. https://doi.org/10.3390/f16050849
Chicago/Turabian StyleRubio Pérez, Faustino, Aida Rodríguez-García, Santiago Michavila, Ana Rodríguez, Luis Gil, and Rosana López. 2025. "Toward Safer Resin Tapping: Assessing Alternative Chemical Stimulants for Pinus pinaster" Forests 16, no. 5: 849. https://doi.org/10.3390/f16050849
APA StyleRubio Pérez, F., Rodríguez-García, A., Michavila, S., Rodríguez, A., Gil, L., & López, R. (2025). Toward Safer Resin Tapping: Assessing Alternative Chemical Stimulants for Pinus pinaster. Forests, 16(5), 849. https://doi.org/10.3390/f16050849










