Effects of Mixed Addition of Fraxinus mandshurica Rupr. and Larix gmelinii (Rupr.) Kuzen. Litter on Nitrogen Mineralization in Dark Brown Soil of Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Collection and Preparation of Experimental Materials
2.3. Incubation Experiment
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
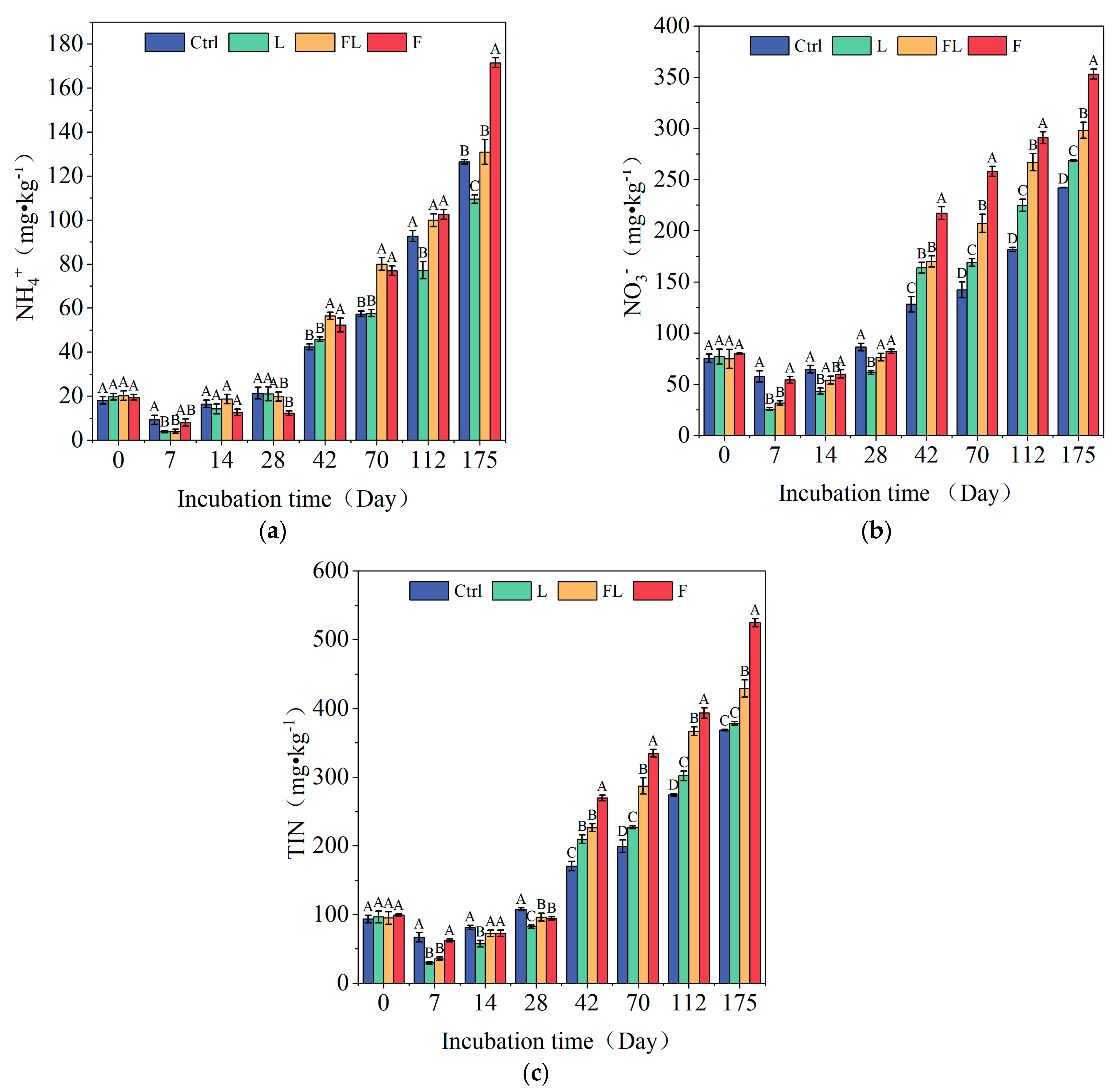
3.1. Soil Inorganic Nitrogen Under Different Litter Addition Treatments
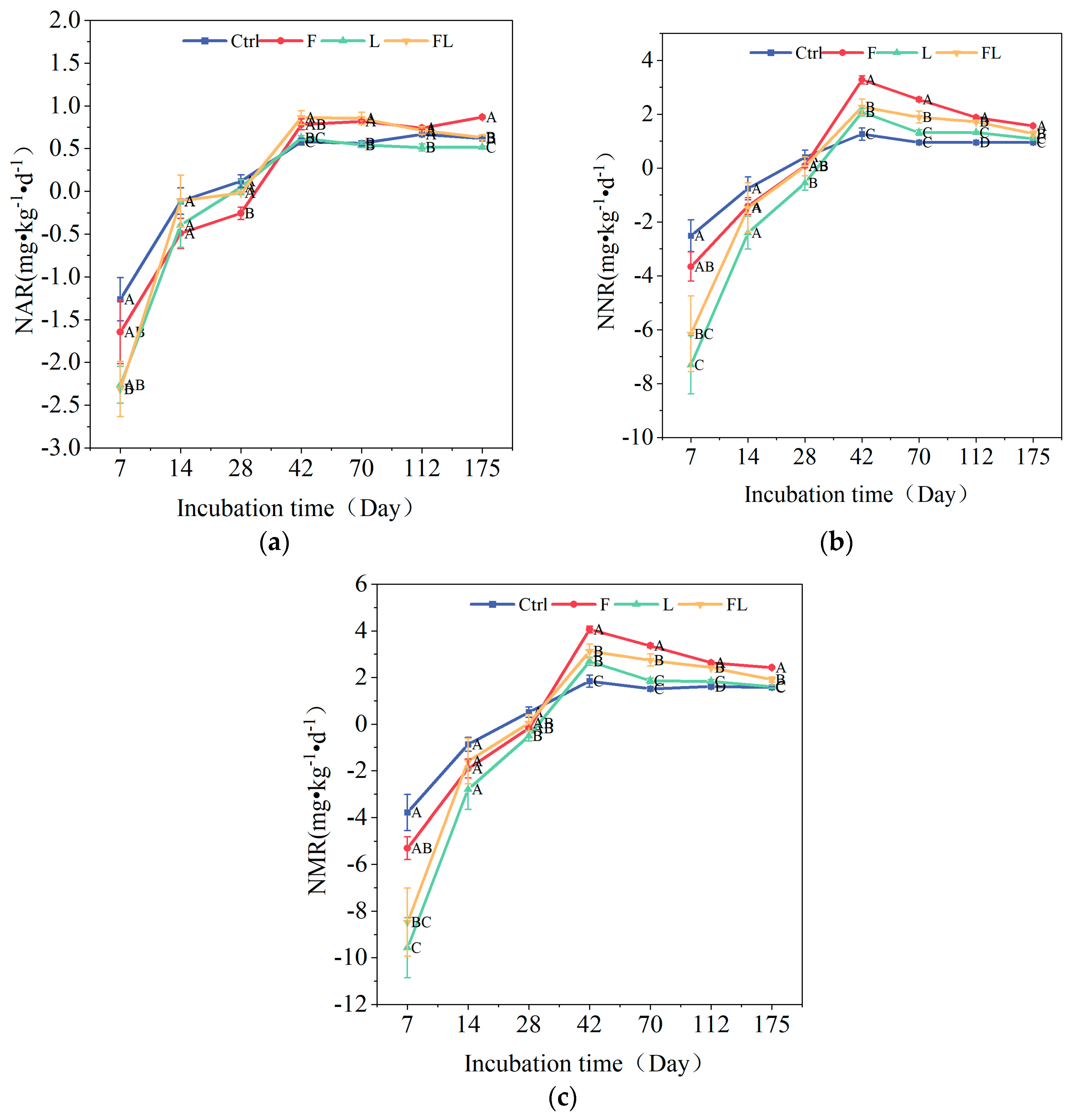
3.2. Soil Nitrogen Mineralization Rate Under Different Litter Addition Treatments
3.3. Soil Cumulative Nitrogen Mineralization Amount Under Different Litter Addition Treatments
3.4. Soil Microbial Biomass Carbon and Nitrogen Under Different Litter Addition Treatments
3.5. Factors Affecting Soil Inorganic Nitrogen and Net Nitrogen Mineralization
4. Discussion
4.1. Effects of Litter Addition on Soil Inorganic Nitrogen
4.2. Effects of Litter Addition on Soil Nitrogen Mineralization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.N.; Xiong, D.C.; Zhang, Y.H.; Xi, Y.Q.; Huang, J.X.; Chen, S.D.; Liu, X.F.; Yang, Z.J. Effects of warming and nitrogen addition on soil nitrogen mineralization and N2O emission in a mid-subtropical Cunninghamia lanceolata plantation. For. Res. 2023, 36, 22–31. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Sanford, R.L. Nutrient Cycling in Moist Tropical Forest. Annu. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Long, J.; Zhang, M.J.; Zhao, C.; Wu, Q.S.; Wu, J.N.; Huang, B.C.; Zhang, J.M. Effects of soil fauna on element release during litter decomposition in Maolan karst forest. Chin. J. Ecol. 2019, 38, 2671–2682. [Google Scholar] [CrossRef]
- Burton, J.; Chen, C.R.; Xu, Z.H.; Ghadiri, H. Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol. Biochem. 2007, 39, 426–433. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes:a meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef]
- Wang, G.J.; Tian, D.L.; Yan, W.D.; Zhu, F.; Fang, X.; Li, S.Z. Effects of litter-fall addition and removal on the nitrogen mineralization in soils of Cunninghamia lanceolata plantation. J. Cent. South Univ. For. Technol. 2009, 29, 6–10+32. [Google Scholar] [CrossRef]
- Maithani, K.; Arunachalam, A.; Tripathi, R.S.; Pandey, H.N. Influence of leaf litter quality on N mineralization in soils of subtropical humid forest regrowths. Biol. Fertil. Soils 1998, 27, 44–50. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.X.; Tu, F.L.; Xu, E.L.; Zhang, L.; Guo, J.F. Effects of litter inputs on soil nitrogen mineralization in a natural Castanopsis carlesii forest. Chin. J. Ecol. 2022, 41, 1916–1922. [Google Scholar] [CrossRef]
- Inagaki, Y.; Miura, S.; Kohzu, A. Effects of forest type and stand age on litterfall quality and soil N dynamics in Shikoku district, southern Japan. For. Ecol. Manag. 2004, 202, 107–117. [Google Scholar] [CrossRef]
- Wei, X.Y.; Ni, X.Y.; Shen, Y.; Chen, Z.H.; Yang, Y.L.; Wu, F.Z. Effects of litterfall on soil humification input from in three subalpine forests. Acta Ecol. Sin. 2021, 41, 8266–8275. [Google Scholar] [CrossRef]
- Zhou, M.T.; Liu, L.; Fu, R.X.; Li, X.G. Effects of litter decomposition of Cunninghamia lanceolata and Schima superba on soil carbon and nitrogen contents and enzyme activities in Cunninghamia lanceolata plantation. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 131–138. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pan, K.W.; Wu, N.; Luo, P.; Wang, J.C.; Xian, J.K. Effect of litter quality and decomposition on N mineralization in soil of Castanopsis platyacantha-Schima sinensis forest. Chin. J. Appl. Environ. Biol. 2005, 11, 146–151. [Google Scholar]
- Wang, Y.K.; Fang, S.Z.; Tian, Y.; Tang, L.Z. Effects of mixed residues on carbon and nitrogen mineralization in the soil of poplar-crops agroforestry system. J. Soil Water Conserv. 2012, 26, 150–154+164. [Google Scholar] [CrossRef]
- Gong, W.; Hu, T.X.; Wang, J.Y.; Gong, Y.B.; Ran, H. Impacts of litter on soil nitrogen supply potential of the natural evergreen broadleaved forests after artificial regeneration in southern Sichuan Province. J. Beijing For. Univ. 2006, S2, 64–72. [Google Scholar] [CrossRef]
- Guo, W.G.; Zhang, Q.Z.; Wang, C.K.; Wang, Y. Effect of forest management on the secondary forest vegetation carbon density and its distribution in Northeast of China. Acta Ecol. Sin. 2024, 44, 8651–8660. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wu, W.H.; Liang, Y.M. Prediction of potential suitable distribution of shoot blight of larch (Neofusicoccum laricinum) in China. Acta Ecol. Sin. 2024, 44, 3027–3037. [Google Scholar] [CrossRef]
- Ma, C.W. Soil Fertility Research on the Saihanba Larch Artificial Pure Forest. For. Investig. Des. 2017, 4, 86–88. [Google Scholar] [CrossRef]
- Peng, G.Z.; Liu, J.F.; Wang, Q.X.; Fu, K.Z.; Zhang, Y.; Zhang, C.X.; Zhang, Y.G. Effects of cycle rejuvenation on growth, reproduction and physiology of Fraxinus mandshurica. Bull. Bot. Res. 2024, 44, 721–729. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.C.; Lu, A.J.; Yu, S.H.; Zheng, Y.; Chen, D.S. Plant diversity and soil characteristics of larch-manchurian ash mixed stand in eastern Liaoning. J. Northwest A F Univ. (Nat. Sci. Ed.) 2021, 49, 27–37. [Google Scholar] [CrossRef]
- Jia, S.X.; Wang, Z.Q.; Li, X.P.; Zhu, S.P.; Mclaughlin, N. Effect of nitrogen fertilizer, root branch order and temperature on respiration and tissue N concentration of fine roots in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2011, 31, 718–726. [Google Scholar] [CrossRef]
- Ji, C.H.; Yang, N.; Guo, C.; Guan, J.Z.; Gu, J.C. Effects of mixed coniferous tree plantation on the morphology of absorption and transportation roots and vertical distribution of biomass in Fraxinus mandshurica. J. Northeast. For. Univ. 2024, 52, 25–30. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Guo, D.L.; Wang, X.R.; Li, M.; Gu, J.C. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Liu, H.L.; Yuan, X.J.; Liu, B.R.; Zhai, P.; Kong, J.R.; Ma, R.S. Nutrient composition and stoichiometric characteristics of plant species across different altitudes in Helan Mountain. Bull. Soil Water Conserv. 2024, 44, 107–116. [Google Scholar]
- Song, M.L.; Wang, Y.Q.; Wang, H.S.; Bao, G.S. Effect of Epichloë endophyte on the litter decomposition of Stipa purpurea in alpine grassland. Acta Pratacult. Sin. 2021, 30, 150–158. [Google Scholar] [CrossRef]
- Zou, W.A.; Jiang, B.; Gu, L.H. Measurement of soil moisture constants. J. China Hydrol. 2015, 35, 5. [Google Scholar] [CrossRef]
- Thippayarugs, S.; Toomsan, B.; Vityakon, P.; Patanothai, A.; Cadisch, G.; Limpinuntana, V. Interactions in decomposition and N mineralization between tropical legume residue components. Agrofor. Syst. 2008, 72, 137–148. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jekinson, D.S. Chloroform fumigation and release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.H.; Chen, Y.L.; Du, T.; You, C.M.; Zhang, L.; Tan, B.; Xu, Z.F.; Li, H. Effects of strip clearcutting with different bandwidths on soil nitrogen mineralization characteristics of Cryptomeria japonica Plantations in Rainy Area of Western China. J. Sichuan Agric. Univ. 2023, 41, 863–872. [Google Scholar] [CrossRef]
- Ge, X.M.; Chen, X.D.; Tang, L.Z.; Wang, R.H.; Wu, L.; Shen, L.X. Preliminary study on the effects of litter addition on nitrogen and phosphorus mineralization of soil in poplar plantations. J. Soil Water Conserv. 2013, 27, 189–193. [Google Scholar] [CrossRef]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sarker, T.C.; Zotti, M.; Mazzoieni, S.; Allevato, E.; Cesarano, G. Predicting nitrogen mineralization from organic amendments: Beyond C/N ratio by 13C-CPMAS NMR approach. Plant Soil 2019, 441, 129–146. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Goek, M.; Kara, E.E. Rates of decomposition of plant residues and available N in soil, related to residue composition through simulation of carbon and N turnover. Soil Biol. Biochem. 2004, 36, 255–266. [Google Scholar] [CrossRef]
- Miao, R.; Ma, J.; Liu, Y.; Liu, Y.; Yang, Z.; Guo, M. Variability of Aboveground Litter Inputs Alters Soil Carbon and Nitrogen in a Coniferous–Broadleaf Mixed Forest of Central China. Forests 2019, 10, 188. [Google Scholar] [CrossRef]
- Fu, M.J.; Wang, C.K.; Wang, Y.; Liu, S. Temporal and spatial patterns of soil nitrogen mineralization and nitrification in four temperate forests. Acta Ecol. Sin. 2009, 29, 3747–3758. [Google Scholar] [CrossRef]
- Li, R.; Feng, J.G.; Zhu, B. Effects of Nitrogen and Phosphorus Addition on Soil Carbon and Nitrogen Mineralization in Temperate Forest and Subtropical Forest. Acta Sci. Nat. Univ. Pekin. 2022, 58, 730–738. [Google Scholar] [CrossRef]
- Fyles, J.W.; Fyles, I.H.; Feller, M.C. Comparison of nitrogen mineralization in forest floor materials using aerobic and anaerobic incubation and bioassay techniques. Can. J. Soil Sci. 1990, 70, 73–81. [Google Scholar] [CrossRef]
- Redin, M.; Recous, S.; Aita, C.; Dietrich, G.; Schmatz, R.; Giacomini, S.J.; Skolaude, A.C.; Ludke, W.H. How the chemical composition and heterogeneity of crop residue mixtures decomposing at the soil surface affects C and N mineralization. Soil Biol. Biochem. 2014, 78, 65–75. [Google Scholar] [CrossRef]
- Aber, J.D.; Magill, A.; Boone, R.; Melillo, J.M.; Steudler, P. Plant and soil responses to chronic nitrogen additions at the Harvard Forest, Massachusetts. Ecol. Appl. 1993, 3, 156–166. [Google Scholar] [CrossRef]
- Wang, L.G.; Zhang, Q.Q.; Zhao, M.S.; Miao, D.N.; Li, Y.F.; Teng, Q.M.; Li, Y.C. Effects of litter extracts from Moso Bamboo (Phyllostachys edulis) and broadleaved forests on soil microbes and nitrogen mineralization. J. Agric. Biotechnol. 2023, 31, 1053–1063. [Google Scholar]
- Blagodatskaya, E.; Khomyakov, N.; Myachina, O.; Bogomolova, I.; Blagodatsky, S.; Kuzyakov, Y. Microbial interactions affect sources of priming induced by cellulose. Soil Biol. Biochem. 2014, 74, 39–49. [Google Scholar] [CrossRef]
- Trofymow, J.A.; Morley, C.R.; Coleman, D.C.; Anderson, R.V. Mineralization of cellulose in the presence of chitin and assemblages of microflora and fauna in soil. Oecologia 1983, 60, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Suo, P.H.; Xuan, H.F.; Wei, Q.; Hu, Y.L. Seasonal dynamics of soil nitrogen availability and microbial biomass carbon, nitrogen along restoration ages of Pinus massoniana plantations in red soils erosion region at southern China. Acta Ecol. Sin. 2025, 45, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.J.; Duan, X.Q.; Li, W.Q.; Chen, F.S.; Liu, W.Q.; Fang, X.M. Effects of tree mixture on rhizosphere soil nitrogen mineralization and microbial characteristics of coniferous trees in subtropical plantations. Acta Ecol. Sin. 2022, 42, 8414–8424. [Google Scholar] [CrossRef]

| Litter | C (g/kg) | N (g/kg) | P (g/kg) | Lignin (%) | Cellulose (%) | C/N | Lignin/N |
|---|---|---|---|---|---|---|---|
| Fraxinus mandshurica | 422.75 | 22.27 | 2.34 | 11.52 | 16.89 | 18.98 | 0.52 |
| Larix gmelinii | 357.68 | 10.08 | 1.75 | 11.54 | 24.42 | 35.55 | 1.14 |
| Factor | F Value | |||||
|---|---|---|---|---|---|---|
| NH4+ | NO3− | TIN | NAR | NNR | NMR | |
| Litter | 54.78 * | 164.92 * | 188.30 * | 3.02 * | 9.12 * | 10.02 * |
| Time | 1598.08 * | 1330.38 * | 2197.34 * | 164.01 * | 120.98 * | 186.59 * |
| Litter × Time | 21.15 * | 24.84 * | 31.01 * | 2.53 * | 4.00 * | 4.89 * |
| Indicator | Ctrl | F | L | FL |
|---|---|---|---|---|
| CNA | 108.45 ± 1.18 B | 152.09 ± 2.8 A | 89.85 ± 2.36 C | 110.75 ± 5.34 B |
| CNN | 166.65 ± 4.26 C | 273.18 ± 5.1 A | 191.73 ± 7.54 C | 223.31 ± 14.92 B |
| CNM | 275.1 ± 4.24 C | 425.26 ± 7.33 A | 281.58 ± 9.48 C | 334.06 ± 19.41 B |
| Litter | N (g/kg) | Lignin (%) | Cellulose (%) | C/N | Lignin/N |
|---|---|---|---|---|---|
| Fraxinus mandshurica | 30.31 | 28.08 | 6.02 | 13.54 | 9.27 |
| Larix gmelinii | 12.36 | 48.59 | 17.81 | 33.18 | 39.39 |
| mixed litter | 22.94 | 39.56 | 13.81 | 17.47 | 17.25 |
| MBC | MBN | N | Lignin | Cellulose | Lignin/N | C/N | |
|---|---|---|---|---|---|---|---|
| NH4+ | 0.896 ** | 0.825 ** | 0.946 ** | −0.949 ** | −0.953 ** | −0.874 ** | −0.839 ** |
| NO3− | 0.868 ** | 0.835 ** | 0.940 ** | −0.942 ** | −0.909 ** | −0.873 ** | −0.843 ** |
| TIN | 0.883 ** | 0.834 ** | 0.946 ** | −0.949 ** | −0.924 ** | −0.877 ** | −0.844 ** |
| NAR | 0.912 ** | 0.833 ** | 0.942 ** | −0.954 ** | −0.945 ** | −0.870 ** | −0.833 ** |
| NNR | 0.869 ** | 0.828 ** | 0.896 ** | −0.891 ** | −0.837 ** | −0.834 ** | −0.805 ** |
| NMR | 0.869 ** | 0.838 ** | 0.924 ** | −0.927 ** | −0.891 ** | −0.858 ** | −0.825 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Miao, X.; Zhang, Y.; Sun, H. Effects of Mixed Addition of Fraxinus mandshurica Rupr. and Larix gmelinii (Rupr.) Kuzen. Litter on Nitrogen Mineralization in Dark Brown Soil of Northeast China. Forests 2025, 16, 842. https://doi.org/10.3390/f16050842
Han S, Miao X, Zhang Y, Sun H. Effects of Mixed Addition of Fraxinus mandshurica Rupr. and Larix gmelinii (Rupr.) Kuzen. Litter on Nitrogen Mineralization in Dark Brown Soil of Northeast China. Forests. 2025; 16(5):842. https://doi.org/10.3390/f16050842
Chicago/Turabian StyleHan, Shixing, Xuesong Miao, Yandong Zhang, and Hailong Sun. 2025. "Effects of Mixed Addition of Fraxinus mandshurica Rupr. and Larix gmelinii (Rupr.) Kuzen. Litter on Nitrogen Mineralization in Dark Brown Soil of Northeast China" Forests 16, no. 5: 842. https://doi.org/10.3390/f16050842
APA StyleHan, S., Miao, X., Zhang, Y., & Sun, H. (2025). Effects of Mixed Addition of Fraxinus mandshurica Rupr. and Larix gmelinii (Rupr.) Kuzen. Litter on Nitrogen Mineralization in Dark Brown Soil of Northeast China. Forests, 16(5), 842. https://doi.org/10.3390/f16050842






