Abstract
This study aimed to assess the impact of different biochar (BCH) applications (0%, 10%, 20%, and 50%, v/v) on soil respiration in a European beech (Fagus sylvatica L.) forest located in the Tuscan-Emilian Apennines. The experiment was conducted over four months during summer 2023. Results revealed that BCH applications did not significantly affect overall soil respiration. On the other hand, soil respiration was positively influenced by soil temperature and soil moisture, the latter only for the 10% and 20% BCH amendments. At higher BCH concentrations (50%), soil respiration was not enhanced by moisture, probably due to soil water saturation and reduced oxygen availability. In conclusion, it appears that BCH does not directly stimulate soil respiration in field conditions, although specific concentrations may improve soil carbon sequestration. These findings highlight the potential for BCH amendments to be employed as a climate-smart forestry strategy in support of carbon sequestration and ecosystem stability in temperate forest ecosystems.
1. Introduction
The European beech (Fagus sylvatica L.) is widespread in Europe, from northern Spain to southern Ukraine, as well as from southern Scandinavia to southern Italy and Greece, where it predominantly thrives in locations above 1000 m a.s.l. [1]. In Italy, the European beech constitutes approximately 12% of the national forest area [2]. While European beech forest productivity is increasing in northern Europe, this trend is not observed in the south [3]. In fact, by the end of this century, a significant reduction in growth performance is expected in southern Italy [4], probably as a result of drought [5]. Increased xylem embolism and reduced water and nutrient uptake are compromising the growth and competitiveness of beech seedlings, posing a potential threat to future forest regeneration [6]. Data from 1986–2016 confirmed this trend, indicating a decline in European beech growth of up to 20% in southern Europe compared to the 1955–1985 period [7].
The reduction of European beech forest biomass will inevitably reduce the ecosystem services provided by these forests, in particular carbon (C) sequestration, which was recently estimated to be 4 to 6 tC ha−1 year−1 [1,8]. The development and implementation of strategies to mitigate the negative effects of climate change (CC) on these forests are therefore of crucial importance, especially those related to C sequestration, which is a key factor in combating CC. The promotion of practices that enhance C sequestration (see [9]) and the optimization of socio-economic benefits while, at the same time, maintaining biodiversity and ecosystem services, are core elements of “climate-smart forestry” (CSF; [10]), i.e., an integrated approach to forest management that seeks to maximize the contribution of forests to CC mitigation and adaptation.
Soil conditions are a “central indicator” of CSF effectiveness [10] and play a key role in its success. By improving soil properties such as pH, cation exchange capacity (CEC), carbon/nitrogen (C/N) ratio, and organic C content, soil fertility and plant development can be significantly enhanced [11,12], resulting in a positive effect on C sequestration [13]. Techniques such as careful site preparation (i.e., reducing soil compaction after the use of heavy machinery), adequate soil drainage, and the conservation of soil and water resources, along with the application of micronutrients or biosolids as fertilizers, are all crucial factors in maintaining healthy forest ecosystems [9,14]. Among biosolids, biochar (BCH) is fast gaining recognition as a “frontier technology” [15] due to its unique ability to simultaneously improve soil fertility and enhance C sequestration; in this context, BCH is particularly suitable for CSF [16]. In fact, due to its high aromatic C content (>65%), resistance to microbial degradation, CEC, and porosity [17,18,19,20], BCH can contribute to long-term C storage [21,22,23], improve soil structure, and enhance plant growth [24,25,26].
Regarding European beech forests, BCH applications appear to be an effective strategy aimed at increasing soil C stocks. Recent studies have shown that applications at rates of 10%, 20%, and 50% (v/v) do not hamper either forest regeneration or the early stages of litter decomposition [27,28], supporting the potential use of BCH in CSF for these under-researched forest ecosystems. However, there are still concerns about BCH effectiveness as a C sequestration strategy since BCH applications tested in a controlled mesocosm experiment (which excluded root respiration) have led to an increase in soil respiration (i.e., CO2 release from the soil; [29]). Further research is therefore needed to determine whether the observed increase in soil respiration could have been the result of either the priming effect of BCH on microbial activity, triggered by the presence of the labile fraction on the surface of the biochar [30], or the high soil temperatures experienced during the mesocosm experiment [29], which may have enhanced CO2 efflux, especially under the 20% BCH amendment. Field studies are thus called for in order to investigate the actual effects of BCH in forest ecosystems, where root respiration—the primary source of CO2 in forest ecosystems [31]—is also taken into account, and where soils are exposed to natural temperatures. Understanding the true impact of BCH on soil respiration in situ is crucial if an accurate assessment of its overall effectiveness as a C sequestration tool is to be achieved.
This study aims to compare soil respiration (edaphon + roots) in control soil and soil with different BCH applications (10, 20, and 50%, v/v) in a European beech forest. We hypothesize that moderate BCH additions (10 and 20%) will increase CO2 soil emissions in the short term, at least in warmer and/or drier periods, as a result of increased soil water retention enhancing soil respiration under higher temperatures [29]. On the other hand, a higher dose of BCH (50%) may limit soil respiration rates due to (i) the reduced amount of organic substrate, (ii) excessive water retention hampering oxygen diffusion in the soil, or (iii) a strong increase in soil pH, which could make the system unsuitable for microorganisms adapted to acidic conditions.
2. Materials and Methods
2.1. Sampling and Preparation of Soil Samples
The experiment was carried out in a beech forest in the Tuscan-Emilian Apennines (Corniglio, Parma, Italy) within the Tuscan-Emilian Apennine National Park (coordinates: 44.414425, 10.021867; Figure A1) and lasted from 30 May 2023 to 10 November 2023. The target forest stand is characterized by the total dominance of Fagus sylvatica (canopy covering 60% of the light), is subject to regular management, and has an estimated age of approximately 30 years. Climatic, geological, and vegetation information on the study area can be found in Vannini et al. [28]. Six blocks 1 m2 in size (statistical replication) were selected within an area of 20 × 30 m2. The blocks were 10 m apart, and any soil characterized by stony material or potential rainwater runoff was avoided. After carefully removing the litter, four holes (depth 10 cm, diameter 13.5 cm) were dug in each block, approximately 30 cm apart, using random criteria. All the soil removed from each block was then pooled and sieved to 0.5 cm. This sieved soil was then used for the following four soil amendments: control, with 0% BCH (soil only; BC); 10% BCH (B10); 20% BCH (B20); and 50% BCH (B50, worst-case scenario; see [28]). It is important to note that since BCH properties depend on many factors, including the feedstock used and pyrolysis conditions [32,33]), we employed the same BCH used in other studies involving European beech forests [27,28,29]. After soil amendments were carried out, four plastic cylinders (diameter 13.5 cm, height 15 cm) were inserted into the ground in each block, with the first 10 cm buried and the remaining 5 cm positioned above the soil surface to avoid soil loss during heavy rainfall. Each cylinder was then randomly filled with one of the four prepared soil amendments. Disturbance by wild animals was avoided by covering each plot with an iron net raised 10 cm above the ground (Figure 1). Finally, an additional area was permanently marked in each block in order to perform soil respiration measurements under unaltered conditions, i.e., no soil mixing with only leaf litter being removed from the surface. Data gathered from this non-manipulated control was then used to evaluate treatment effects only during the period in which no significant variations in soil respiration were detected between BC and non-manipulated plots (see Section 2.4). This procedure was followed to avoid artifacts due to experimental manipulation, such as soil oxygenation and the absence of roots in the upper soil layer.
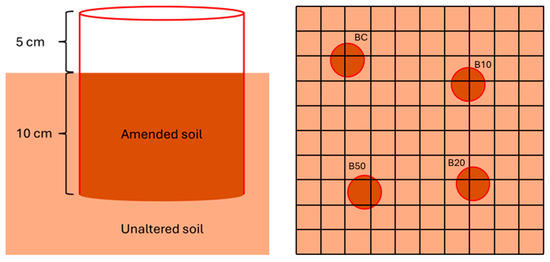
Figure 1.
(Left) Schematic representation of a pot with a plastic cylinder inserted into the soil which contains the amended soil. (Right) Illustration of a plot protected by a 10 × 10 cm2 iron net covering the four cylinders: BC (control; mixed soil only), B10 (10% biochar), B20 (20% biochar), and B50 (50% biochar).
2.2. Soil Respiration Measurements
The soil respiration measurement schedule is shown in Table 1.

Table 1.
Days on which soil respiration measurements were taken during the experiment in 2023.
Soil respiration was assessed on a fortnightly or monthly basis utilizing a CO2 meter EGM-4 (PP Systems) paired with a 1 L dark cylindrical chamber. Each assessment lasted for 60 s, capturing CO2 flux from the soil at 5 s intervals. This enabled the calculation of CO2 concentrations emitted (g m−2 h−1) according to the ideal gas law principles. To minimize diurnal variability and ensure methodological consistency, respiration measurements were always recorded at the end of the morning.
2.3. Soil Temperature and Moisture Measurements
Throughout the study period, soil temperature data was recorded hourly at a depth of −5 cm by data loggers (HOBO Pendant® Temperature/Light 64K Data Logger, Onset Computer Corporation, Bourne, MA, USA) installed in the four cylinders of two randomly selected blocks.
Following each soil respiration measurement, soil moisture (volumetric water content, VWC, %) was measured within the 0–7.6 cm soil layer using a portable device (TDR 100; Spectrum Technologies Inc., Chicago, IL, USA). A single measurement was taken at the center of each cylinder. The small holes created by the rods during recordings were then carefully refilled with soil from the same cylinder.
Soil water content was also directly estimated by measuring water mass on a subset of cylinders. At the end of the experiment, cylinders containing soil from blocks where no soil temperature measurements had been recorded were removed from the ground and transported to the laboratory. After air-drying (with VWC values between 6% and 8%), soil samples were slowly rehydrated using deionized water, ensuring that no water was lost from the bottom of the cylinders. Following rehydration, the cylinders were weighed, and VWC was measured twice a week. When the soil samples reached VWC values between 6% and 8%, rehydration was repeated. Correlations between soil mass (excluding cylinder mass) and VWC were used to estimate the water mass (in grams) in both amended and unamended soils during the experiment.
2.4. Statistical Analysis
To avoid bias due to the type of manipulation, the analysis of treatment effects did not include all measurement dates. This approached was followed specifically to avoid any artifact effects of soil mixing and oxygenation at the beginning of the experiment, as well as the effects of the possible consumption of organic substrate over time in the pots with manipulated soil and/or lack of root compound input at the end of the experiment (see Section 2.1). Such data exclusion was carried out following the outcome of the non-parametric Wilcoxon–Mann–Whitney test (in which the block number was considered as a stratification factor) comparing soil respiration between BC (control, mixed soil only) and the additional non-manipulated control. As a result, we excluded the data from the first two measurement dates at the beginning of the experiment, where soil respiration in BC and non-manipulated plots significantly differed (DOY 150: Z = 2.13, p = 0.033 and DOY 170: Z = 1.98, p = 0.048, corresponding to May 30 and June 19, respectively), together with data from the last measurement date (DOY 314: Z = −2.22, p = 0.026, November 10). Consequently, the following analysis is based on the 6 measurement dates between DOY 178 (June 27, Z = −0.57, p = 0.566) and DOY 279 (October 6, Z = 1.55, p = 0.122).
To assess the overall effects of BCH amendments over time, a linear mixed-effect model was performed where soil respiration was considered as the response variable, whereas treatment (4-level factor: BC, B10, B20, and B50), time (6-level factor: measurement dates T2 to T7), and their interaction were included as fixed effects, with plot as the random effect. A variance structure allowing for different variation across measurement dates was included in the model to avoid heteroscedasticity.
To test whether interactions occurred between BCH amendment and soil temperature and moisture, a mixed-effect model was carried out. In this model, soil respiration was set as the response variable, whereas treatment, soil temperature (numerical variable), soil water mass (numerical variable), and their two-way interactions were considered as fixed factors, with plot as the random factor. Model residuals were allowed to have different variations, both across measurement dates and among treatments in order to prevent heteroscedasticity.
For all models, linear model assumptions were checked by means of visual inspection of residuals, whereas optimal random effect and variance structures were identified by comparing the Akaike information criterion of alternative models. Finally, minimal adequate models were selected by excluding non-significant terms from the model structure.
All analyses were performed in R 4.1.3 [34] with the package “coin” [35] for non-parametric tests and “nlme” [36] for mixed-effect models.
3. Results
Soil respiration was not affected by BCH applications (p = 0.6413) but was significantly influenced by time (p < 0.001; Figure 2). Additionally, no significant interaction was detected between time and treatment (p = 0.2735).
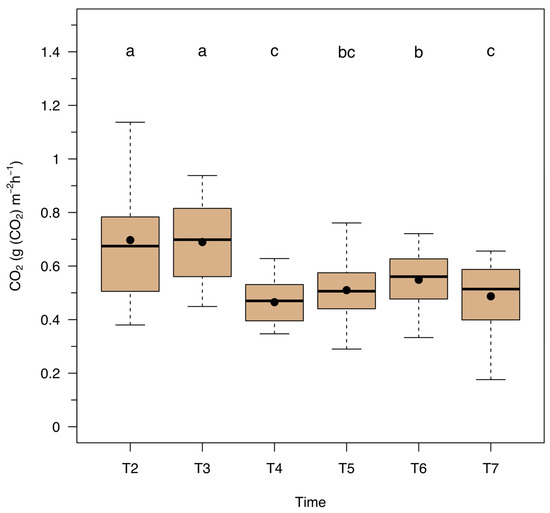
Figure 2.
Respiration (g (CO2) m−2 h−1) of European beech forest soil samples amended in the field with different biochar application percentages according to monitoring times: T2 = 27 June 2023, DOY 178; T3 = 11 July 2023, DOY 192; T4 = 26 July 2023, DOY 207; T5 = 8 August 2023, DOY 220; T6 = 8 September 2023, DOY 251; T7 = 6 October 2023, DOY 279. Black dots indicate the average values. Different letters indicate significant differences between dates (post-hoc contrast, p < 0.05).
Soil respiration was positively affected by soil temperature (p < 0.001; Figure 3a) but was not influenced by water content (p = 0.4736). However, a positive effect of water content on soil respiration was observed following 10% and 20% BCH treatments (p = 0.0335; Figure 3b).
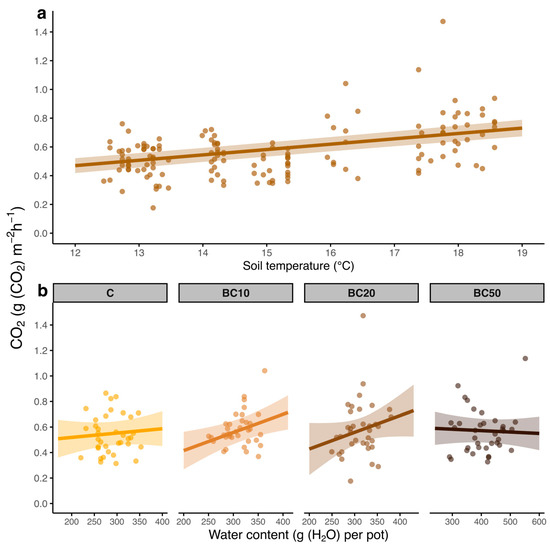
Figure 3.
Respiration (g (CO2) m−2 h−1) of European beech forest soil samples amended with different biochar application percentages (a) in response to soil temperature and (b) in interaction with water content and biochar applications: 0% biochar (BC), 10% biochar (B10), 20% biochar (B20), and 50% biochar (B50).
4. Discussion
The increase in soil respiration following BCH applications has been well documented in temperate forests [37], with more pronounced effects observed in laboratory incubations or pot experiments compared to field studies. These findings are consistent with those reported by Vannini et al. [29], as well as with the results obtained in the present study. Specifically, while BCH significantly affected soil respiration in a mesocosm experiment, its effect was not significant in the current field study—a difference that is probably due to the contribution of root respiration to total forest soil respiration, which can range from 30% to 80% [31]. Although we were unable to quantify the effect of root respiration in the present study, this factor could have played a significant role in masking the priming effect of BCH on soil organic matter decomposition [38] that is usually observed in soil respiration measurements [39,40]. However, without direct estimates, the magnitude of this effect on total soil respiration cannot be gauged.
Another explanation for the absence of a priming effect of BCH applications on soil respiration could be the lower temperatures experienced by amended soils during measurements, i.e., temperatures lower than those recorded during the mesocosm experiment in which the priming effect of BCH was found [29]. Temperature plays a crucial role in regulating soil respiration, particularly in Fagus spp. and mixed temperate forests [41,42], where higher respiration rates typically occur during the warmer months [42,43]. In this study, data recorded by the HOBO pendants indicated that both amended and unamended soils were characterized by lower temperatures (13–18 °C) than those recorded by the HOBO pendants during the mesocosm study in which a priming effect of BCH application on soil respiration was observed (10–30 °C; most readings 15–25 °C; [29]). Lower temperatures generally limit the action of soil microorganisms on soil organic matter, a factor that probably reduces the BCH priming effect on total soil respiration [44,45]. Despite these observations, a significant positive correlation between soil temperature and soil respiration was observed in this study, highlighting the influence of temperature fluctuations on soil respiration. This is supported by the findings of Hereș et al. [42] and Babur et al. [46], where a positive correlation between soil temperature and soil respiration within the same range of recorded temperatures was detected—a further indication that the temperature–soil respiration relationship is consistent across different studies and environments.
In line with the findings described above, soil respiration was significantly influenced by time in the present study. Higher respiration rates were recorded during the first two measurement dates, while lower rates were observed in the last four. Such temporal variation can primarily be attributed to a decline in soil temperature after the third measurement date (Figure A2), as opposed to changes in water content (Figure A3). Overall, the drop in temperature appears to be the dominant factor in reducing soil respiration over time, further emphasizing the critical role temperature plays in regulating soil microbial activity.
In European beech forests, soil respiration is also positively influenced by water availability [42], especially during warmer periods [29], again highlighting the complex interaction between temperature, moisture, and soil microbial activity in these ecosystems. However, the results of this study did not show a general correlation between soil respiration and water content across all conditions. Interestingly, soil respiration appeared to be positively correlated with soil water content under specific BCH amendment conditions, i.e., BCH 10% and BCH 20%. This may be because these amendments provide the optimal water concentration and soil porosity balance, creating favorable conditions for microbial activity [47]. The study by Dissanayake et al. [48] supports this hypothesis, showing that in biochar-amended soils, the activity of key soil enzymes is tightly controlled by the water-holding capacity of soil, rising under moderate moisture but plateauing—or even declining—when moisture falls out the optimal range. In contrast, the absence or excess of BCH, particularly at BCH 50%, could have inhibited this effect. In all likelihood, the higher concentration of BCH led to water oversaturation in the soil, hindering microbial processes by reducing oxygen availability and limiting microbial respiration. This suggests that the BCH priming effect is susceptible to amendment percentages, with an optimum range maximizing microbial activity but avoiding waterlogging.
Overall, the results of this study provide valuable insights into the potential role of BCH as a soil amendment in temperate forest ecosystems, such as those in the Tuscan-Emilian Apennines. While the direct impact of BCH on soil respiration may be influenced by other factors, such as root respiration and temperature, the appropriate BCH application rates could promote C sequestration, especially when BCH is produced locally through circular economy. Identifying optimal BCH concentrations, i.e., those not enhancing soil microbial activity, could serve as an effective strategy for increasing soil C stocks and contribute to both soil health and CC mitigation. Among the application percentages tested in the present study, we found that 50% BCH was the dose at which soil respiration was not stimulated by increasing soil moisture levels. This concentration could, at first, appear to be the most effective option for short-term C sequestration purposes in temperate forest ecosystems, such as those found in the Tuscan-Emilian Apennines. However, economic and logistical constraints, together with potential environmental issues (e.g., impact on biodiversity) associated with high biochar concentrations, make this solution difficult to implement in the field. Furthermore, the use of 10% and 20% BCH applications, especially the latter, should also be approached with caution. As demonstrated by [29], the C released under the 20% BCH concentration tends to be higher in response to rising soil temperature.
Lastly, the potential of hydrogen (pH) can enhance C sequestration. When pH values move from acidic to neutral, the activity of soil fungal microorganisms is limited [49], which reduces the decomposition of lignin-rich litter [28] and therefore furthers soil C sequestration through increased organic C content. The 50% BCH amendment, on the other hand, increased the pH of the control soil from an acidic value of 5.6 to a neutral one of 7.6 (see Appendix A for methods). This pH shift was also extended to the layer immediately below the amended soil (between −10 and −15 cm from the surface, where the pH was 6.3) suggesting that the hypothesized reduction in the decomposition of lignin-rich litter may also extend to the subsurface. Higher BCH application rates, therefore, may further slow the decomposition of lignin-rich litter below the amended layer, which could enhance soil C sequestration. However, as noted by Brtnicky et al. [50], a careful evaluation of the effects of excessive BCH applications—i.e., applications exceeding the sensitivity threshold of the local soil community—is necessary to identify ecologically safe application ranges for the target ecosystem.
Notwithstanding these insights, the results of the present study are constrained by various factors: (i) the single four-month observation period, (ii) the investigation of a single beech forest and a single beech-derived biochar, and (iii) the use of a 50% (v/v) dose that exceeds that usually applied in the field. In order to verify and extend our observations, future studies will have to implement multi-season measurements and multi-site trials that separate autotrophic from heterotrophic respiration, with further investigations also being needed to test locally sourced BCHs at realistic application rates.
5. Conclusions
This study assessed the short-term impacts of biochar (BCH) applications on soil respiration in a European beech forest, providing valuable insights into its potential role in climate-smart forestry. Results showed that BCH amendments had a minimal effect on total soil respiration under field conditions, confirming the critical role of environmental factors, such as temperature and soil moisture, in modulating BCH effects.
Interestingly, soil respiration increased with higher soil water content under intermediate BCH applications (10% and 20%), suggesting that the use of BCH amendments at these percentages could increase microbial activity. On the other hand, the 50% BCH application did not affect soil respiration, probably due to the increase in soil pH and soil moisture, which may have led to less favorable conditions for the microbial breakdown of lignin-rich organic matter.
In conclusion, while the use of BCH offers a promising strategy for soil health improvement and carbon storage enhancement in temperate forest ecosystems, potential negative consequences, such as alterations in soil microbial communities and nutrient dynamics, must be carefully considered. Biochar applications should be tailored to local site conditions, and further long-term research is needed to better assess both the benefits and possible ecological trade-offs involved in its use for climate change mitigation.
Author Contributions
Conceptualization, A.V., M.C. and A.P.; data curation, A.V. and D.T.; formal analysis, F.G. and M.C.; funding acquisition, A.V. and A.P.; investigation, A.V.; methodology, T.G.W.F., M.C. and A.P.; project administration, A.V.; resources, A.P.; supervision, M.C. and A.P.; validation, M.C. and A.P.; visualization, F.G.; writing—original draft, A.V., T.G.W.F., M.C. and A.P.; writing—review and editing, A.V., D.T., F.G., T.G.W.F., M.C. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Parma, Bando di Ateneo 2021 per la Ricerca (Action: B-Progetti di ricerca per giovani ricercatori), and co-funded by the MIUR—the Italian Ministry for University and Research—D.M. (737/2021-PNR-PNRR—NextGenerationEU). The publication was produced by a researcher with a research contract that has been co-funded by the European Union-PON Research and Innovation 2014–2020 in accordance with Article 24, paragraph 3, letter a), of Law no. 240 of 30 December 2010, as amended and Ministerial Decree no. 10 August 2021 no. 1062.
Data Availability Statement
The dataset is available upon request from the authors.
Acknowledgments
This work has benefited from the equipment and framework of the COMP-HUB and COMP-R Initiatives, funded by the “Departments of Excellence” program of the MIUR (MIUR, 2018–2022 and MIUR, 2023–2027). We thank professor Alessio Malcevschi for providing the biochar employed in this study and Rebecca Whittingham for the English revision of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BCH | Biochar |
| C | Carbon |
| CEC | Cation exchange capacity |
| CC | Climate change |
| CSF | Climate-smart forestry |
| VWC | Volumetric water content |
Appendix A
Soil pH measurement methods: Measurements were performed on 2 pots, i.e., those which did not undergo weekly water manipulations. Additionally, soil measurements were also taken below the amended layer. Soil samples below the cylinder were collected at a depth of −15, −20, and −25 cm from the soil surface, when possible, i.e., when soil was not characterized by large roots or stones. In the laboratory, samples were dried at air temperature and then sieved at 2 mm. Soil pH was measured according to the protocol reported in [29] and the methodology published in the Gazzetta Ufficiale della Repubblica Italiana [51].
Appendix B
Figure A1.
Site location and European beech forest in which the experiment was carried out; in the right corner, a detail of the plot is reported, showing the four cylinders with the amended soil treatments (background photo by A. Petraglia; photo on the right by D. Tarasconi).
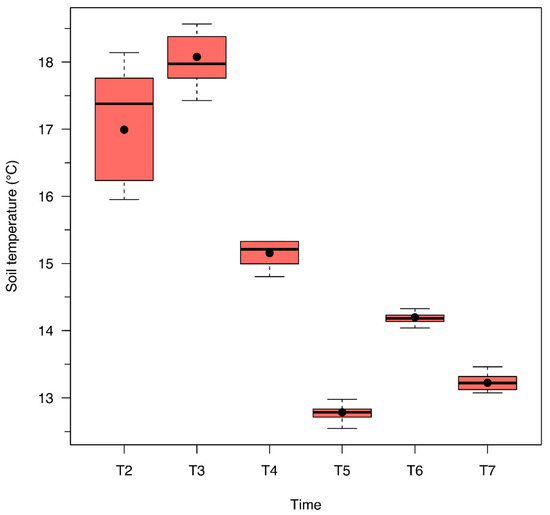
Figure A2.
Soil temperature (°C) for European beech forest soil samples amended with different BCH application percentages according to monitoring times: T2 = 27 June 2023, DOY 178; T3 = 11 July 2023, DOY 192; T4 = 26 July 2023, DOY 207; T5 = 08 August 2023, DOY 220; T6 = 08 September 2023, DOY 251; T7 = 06 October 2023, DOY 279.
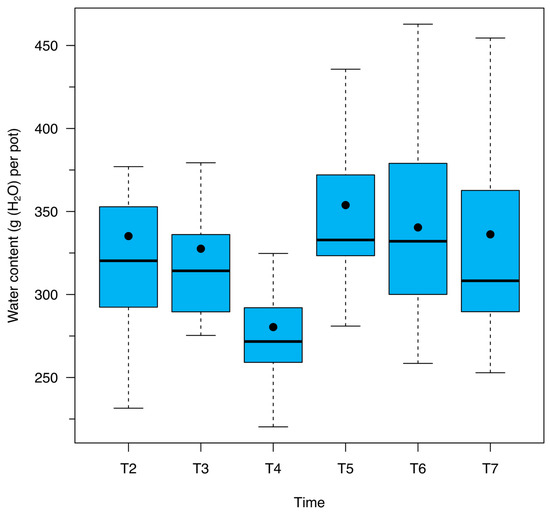
Figure A3.
Water content (g (H2O) per pot) for European beech forest soil samples amended with different BCH application percentages according to monitoring times: T2 = 27 June 2023, DOY 178; T3 = 11 July 2023, DOY 192; T4 = 26 July 2023, DOY 207; T5 = 08 August 2023, DOY 220; T6 = 08 September 2023, DOY 251; T7 = 06 October 2023, DOY 279.
References
- Bayat, A.T. Carbon Stock in an Apennine Beech Forest. Master’s Thesis, Faculty of Geo-Information and Earth Observation (ITC), University of Twente, Enschede, The Netherlands, 2011. [Google Scholar]
- INFC, Inventario Nazionale Delle Foreste e Dei Serbatoi Forestali Di Carbonio. Superficie forestale e Tipi Forestali. Available online: https://www.sian.it/inventarioforestale/jsp/01tabelle_superficie.jsp#:~{}:text=La%20superficie%20 (accessed on 15 February 2025).
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-Change-Driven Growth Decline of European Beech Forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Mette, T.; Falk, W.; Poschenrieder, W.; Fridman, J.; Skudnik, M. Modelling Dominant Tree Heights of Fagus sylvatica L. Using Function-on-Scalar Regression Based on Forest Inventory Data. Forests 2023, 14, 304. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus Sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus Sylvatica L.) in a Changing Climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- European Commission. Europe’s Beech Forests Threatened by Climate Change. 26 October 2022. Available online: https://environment.ec.europa.eu/news/europes-beech-forests-threatened-climate-change-2022-10-26_en (accessed on 1 February 2025).
- Augustynczik, A.L.D.; Yousefpour, R. Assessing the Synergistic Value of Ecosystem Services in European Beech Forests. Ecosyst. Serv. 2021, 49, 101264. [Google Scholar] [CrossRef]
- von Hedemann, N.; Wurtzebach, Z.; Timberlake, T.J.; Sinkular, E.; Schultz, C.A. Forest Policy and Management Approaches for Carbon Dioxide Removal. Interface Focus. 2020, 10, 20200001. [Google Scholar] [CrossRef]
- Antonucci, S.; Santopuoli, G.; Marchetti, M.; Tognetti, R.; Chiavetta, U.; Garfì, V. What Is Known about the Management of European Beech Forests Facing Climate Change? A Review. Curr. For. Rep. 2021, 7, 321–333. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility: A Review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a Means to Improve Soil Fertility and Crop Productivity: A Review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive Effects of Composted Biochar on Plant Growth and Soil Fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Lal, R. Forest Soils and Carbon Sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- Franco, C.R.; Page-Dumroese, D.S.; Archuleta, J. Forest Management and Biochar for Continued Ecosystem Services. J. Soil. Water Conserv. 2022, 77, 60A–64A. [Google Scholar] [CrossRef]
- Lal, R. Biochar and Soil Carbon Sequestration. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; John Wiley & Sons, Ltd.: Madison, WI, USA, 2015; pp. 175–197. ISBN 978-0-89118-967-1. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar Increases Soil Microbial Biomass but Has Variable Effects on Microbial Diversity: A Meta-Analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon. Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Pumpanen, J. Chapter 17—Biochar Use in Global Forests: Opportunities and Challenges. In Developments in Soil Science; Global Change and Forest Soils; Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 427–453. [Google Scholar]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar Increases Soil Carbon Pools: Evidence from a Global Meta-Analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of Biochar Application in Forest Ecosystems on Soil Properties and Greenhouse Gas Emissions: A Review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and Forest Restoration: A Review and Meta-Analysis of Tree Growth Responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Schaffert, E.; Lukac, M.; Percival, G.; Rose, G. The Influence of Biochar Soil Amendment on Tree Growth and Soil Quality: A Review for the Arboricultura Industry. Arboric. Urban For. (AUF) 2022, 48, 176–202. [Google Scholar] [CrossRef]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.G.W.; Lumiero, F.; Malcevschi, A.; Rodolfi, M.; Ganino, T.; Petraglia, A. Effects of Wood-Derived Biochar on Germination, Physiology, and Growth of European Beech (Fagus Sylvatica L.) and Turkey Oak (Quercus Cerris L.). Plants 2022, 11, 3254. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.G.W.; Rodolfi, M.; Ganino, T.; Petraglia, A. Biochar Effects on Early Decomposition of Standard Litter in a European Beech Forest (Northern Italy). Sci. Total Environ. 2023, 903, 166224. [Google Scholar] [CrossRef]
- Vannini, A.; Tarasconi, D.; Pietropoli, F.; Forte, T.G.W.; Grillo, F.; Carbognani, M.; Petraglia, A. Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest under Current Climate and Simulated Climate Change. Forests 2025, 16, 474. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A Meta-Analysis on Pyrogenic Organic Matter Induced Priming Effect. GCB Bioenergy 2015, 7, 577–590. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier Academic Press: Amsterdam, The Netherlands, 2006; ISBN 978-0-12-088782-8. [Google Scholar]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; Version 10.0 from 1 January 2022; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012; Available online: http://european-biochar.org (accessed on 1 October 2024).
- Cairns, S.; Sigmund, G.; Robertson, I.; Haine, R. Engineered Biochar as Adsorbent for the Removal of Contaminants from Aqueous Medium. In Engineered Biochar: Fundamentals, Preparation, Characterization and Applications; Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T., Eds.; Springer Nature: Singapore, 2022; pp. 353–381. ISBN 978-981-19-2488-0. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2022. [Google Scholar]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a Class of Permutation Tests: The Coin Package. J. Stat. Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Versione 3.1-155. Available online: https://CRAN.R-project.org/package=nlme (accessed on 16 October 2023).
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar Increased Soil Respiration in Temperate Forests but Had No Effects in Subtropical Forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-Induced Priming Effects in Soil via Modifying the Status of Soil Organic Matter and Microflora: A Review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Q.; Durenkamp, M.; Kuzyakov, Y. Does Repeated Biochar Incorporation Induce Further Soil Priming Effect? J. Soils Sediments 2018, 18, 128–135. [Google Scholar] [CrossRef]
- Whitman, T.; Singh, B.P.; Zimmerman, A.R. Priming Effects in Biochar-Amended Soils: Implications of Biochar-Soil Organic Matter Interactions for Carbon Storage. In Biochar for Environmental Management; Routledge: Abingdon, UK, 2015; ISBN 978-0-203-76226-4. [Google Scholar]
- Klimek, B.; Chodak, M.; Niklińska, M. Soil Respiration in Seven Types of Temperate Forests Exhibits Similar Temperature Sensitivity. J. Soils Sediments 2021, 21, 338–345. [Google Scholar] [CrossRef]
- Hereș, A.-M.; Bragă, C.; Petritan, A.M.; Petritan, I.C.; Curiel Yuste, J. Spatial Variability of Soil Respiration (Rs) and Its Controls Are Subjected to Strong Seasonality in an Even-Aged European Beech (Fagus sylvatica L.) Stand. Eur. J. Soil. Sci. 2021, 72, 1988–2005. [Google Scholar] [CrossRef]
- Barba, J.; Cueva, A.; Bahn, M.; Barron-Gafford, G.A.; Bond-Lamberty, B.; Hanson, P.J.; Jaimes, A.; Kulmala, L.; Pumpanen, J.; Scott, R.L.; et al. Comparing Ecosystem and Soil Respiration: Review and Key Challenges of Tower-Based and Soil Measurements. Agric. For. Meteorol. 2018, 249, 434–443. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.; Singh, B.P. Effect of Temperature on Biochar Priming Effects and Its Stability in Soils. Soil. Biol. Biochem. 2015, 80, 136–145. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Pettersson, M.; Bååth, E. Comparison of Temperature Effects on Soil Respiration and Bacterial and Fungal Growth Rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Solaiman, Z.M.; Battaglia, M.L. Microbial Respiration, Microbial Biomass and Activity Are Highly Sensitive to Forest Tree Species and Seasonal Patterns in the Eastern Mediterranean Karst Ecosystems. Sci. Total Environ. 2021, 775, 145868. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Riaz, M.; Uslu, O.S. Seasonal Variations in Litter Layers’ Characteristics Control Microbial Respiration and Microbial Carbon Utilization under Mature Pine, Cedar, and Beech Forest Stands in the Eastern Mediterranean Karstic Ecosystems. Microb. Ecol. 2022, 84, 153–167. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Palansooriya, K.N.; Sang, M.K.; Oh, D.X.; Park, J.; Hwang, S.Y.; Igalavithana, A.D.; Gu, C.; Ok, Y.S. Combined Effect of Biochar and Soil Moisture on Soil Chemical Properties and Microbial Community Composition in Microplastic-Contaminated Agricultural Soil. Soil. Use Manag. 2022, 38, 1446–1458. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A Critical Review of the Possible Adverse Effects of Biochar in the Soil Environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Serie Generale n° 248 21/10/1999. Approvazione dei “Metodi Ufficiali di An[alisi Chimica del Suolo”, Gazzetta Ufficiale della Repubblica Italiana, 21 October 1999.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).