Phlebia formosana Strain SMF410-5-1 and Auricularia cornea Strain ME1-1 Display Potential in Wood Degradation and Forest Waste Reutilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Fungal Isolates and Culture Conditions
2.2. Screening of Potential Wood-Rotting Fungi
2.3. Enzyme Activity Assays of Laccase, Manganese Peroxidase, and Lignin Peroxidase
2.4. Assays of Wood Weight Loss
2.5. Degradation of Wood Lignin, Cellulose, and Hemicellulose
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
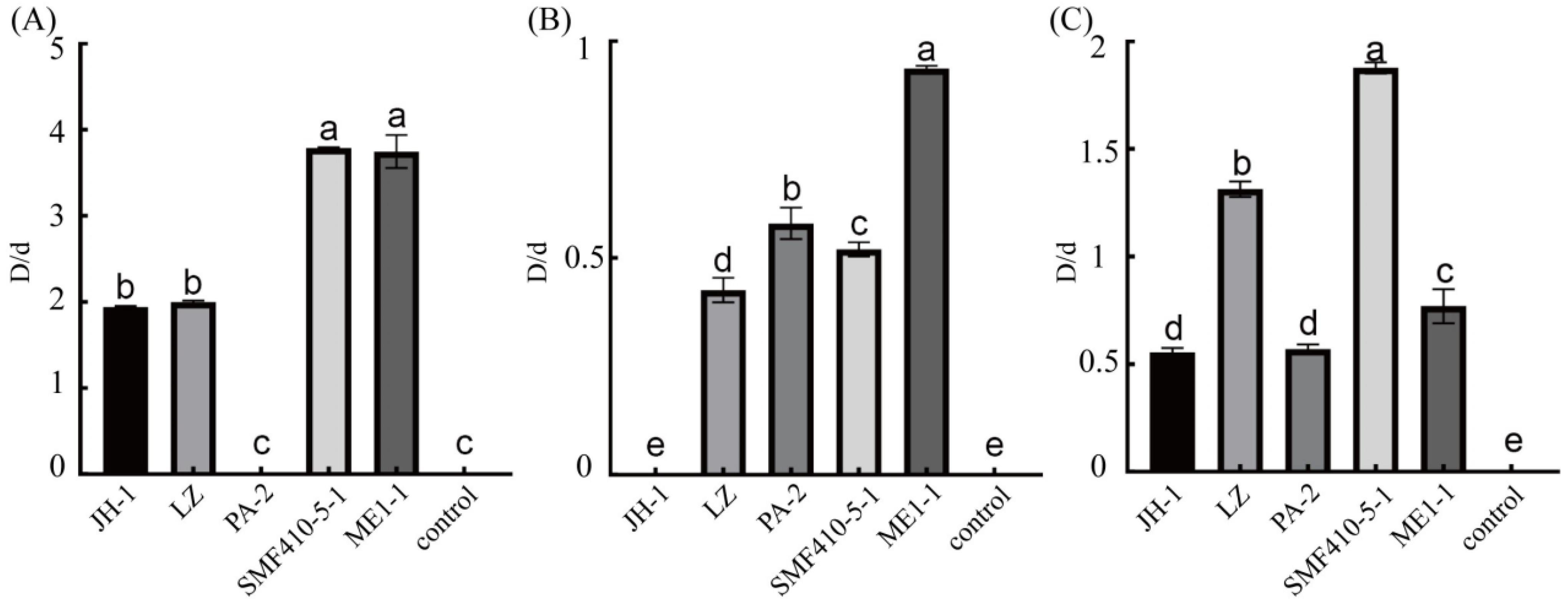
3.1. Isolation and Screening of Wood-Rotting Fungal Isolates
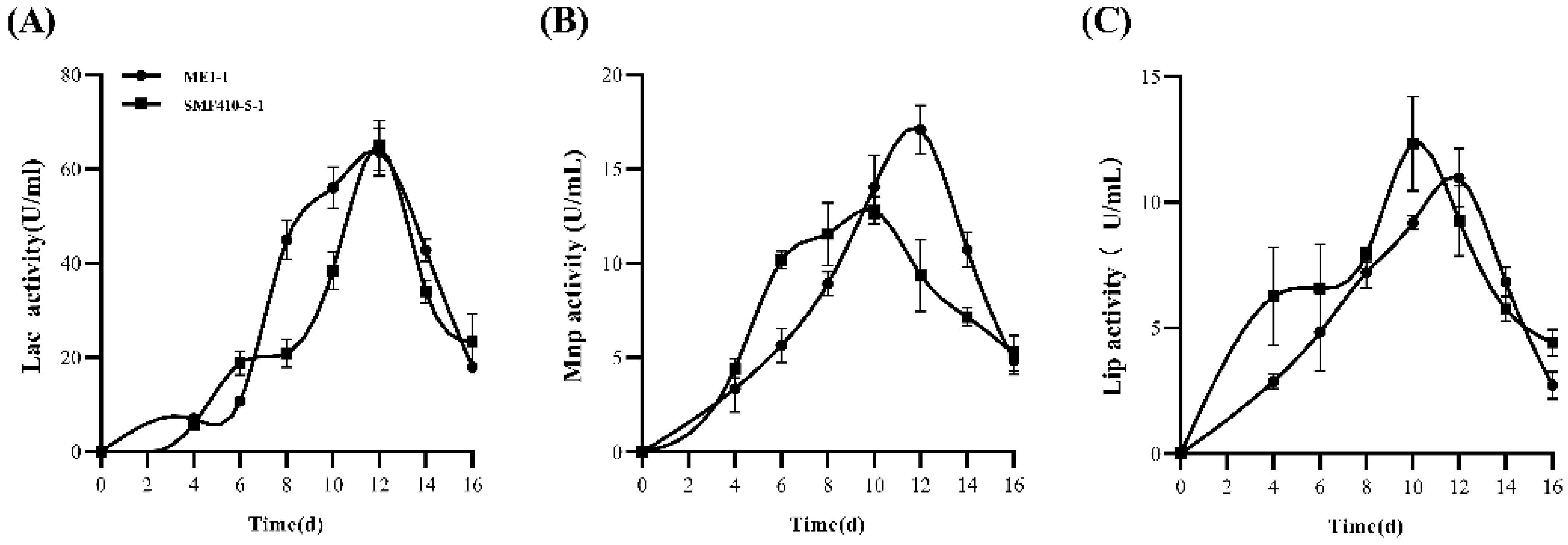
3.2. Quantitation of Laccase, Manganese Peroxidase, and Lignin Peroxidase of ME1-1 and SMF410-5-1
3.3. Wood Weight Loss Caused by ME1-1 and SMF410-5-1
3.4. Degradation of Wood Lignin, Cellulose, and Hemicellulose by M1E-1 and SMF410-5-1
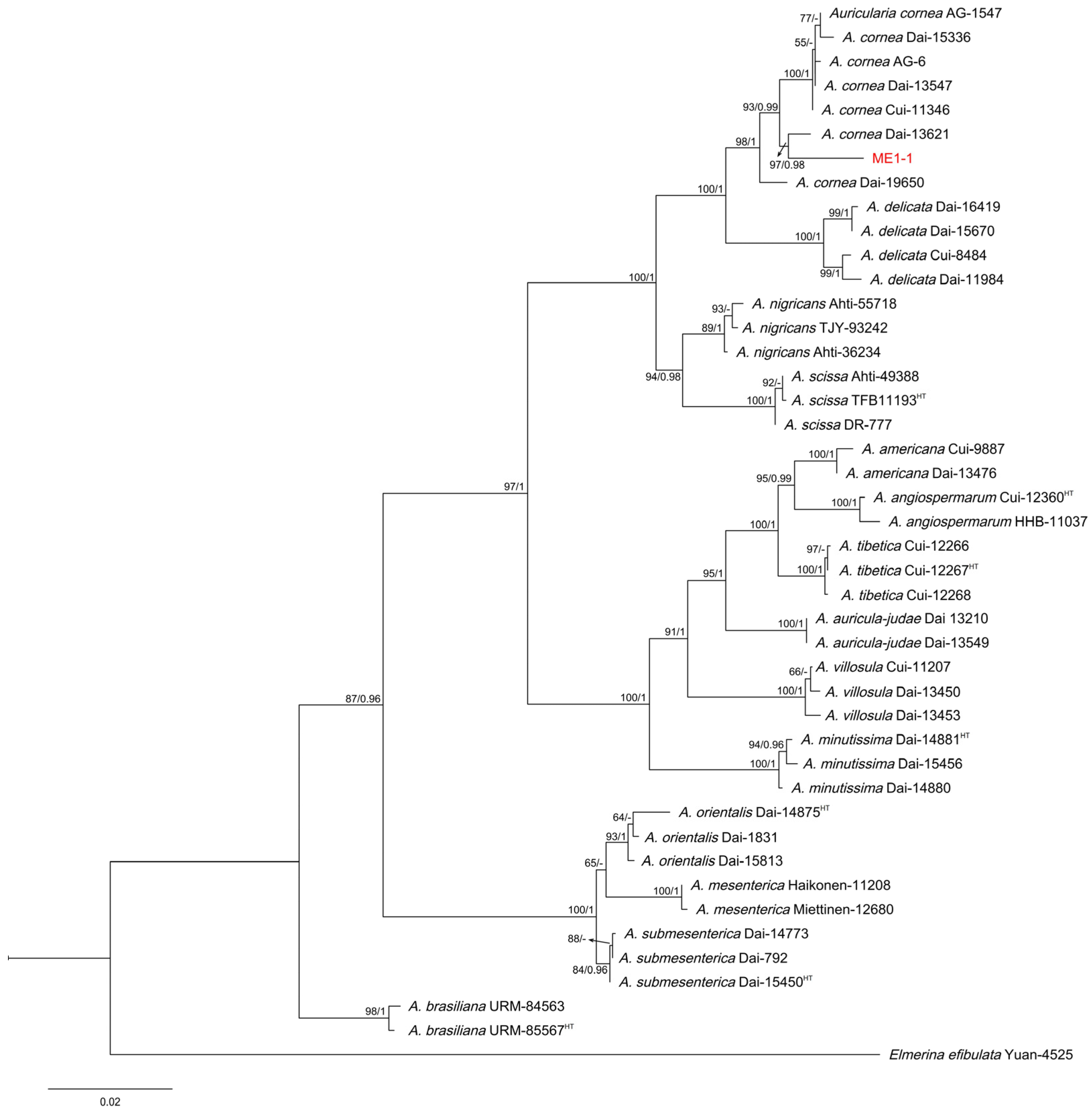
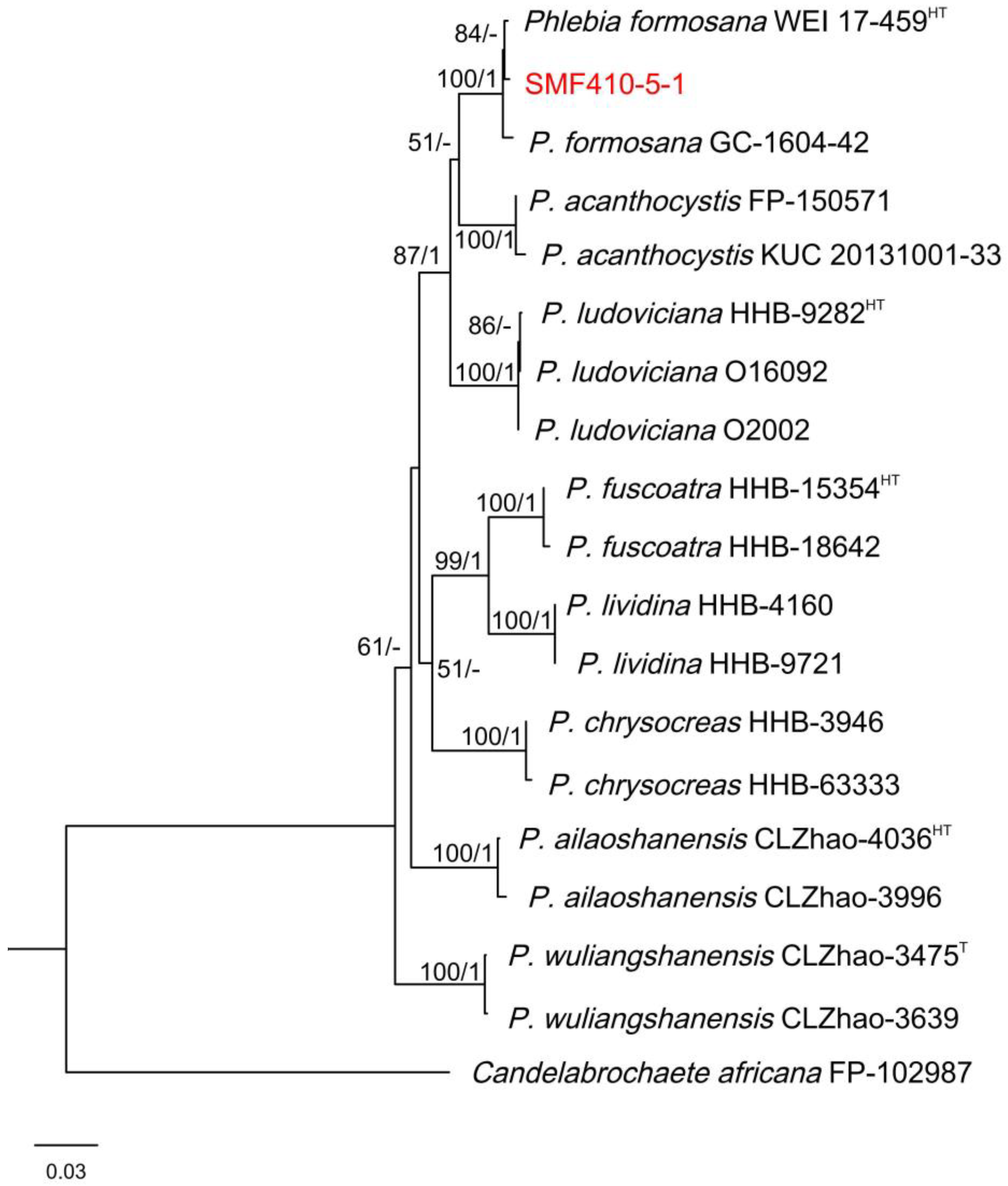
3.5. Molecular Identification of ME1-1 and SMF410-5-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mancini, M.; Rinnan, A. Classification of waste wood categories according to the best reuse using FT-NIR spectroscopy and chemometrics. Anal. Chim. Acta. 2023, 1275, 341564. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, A.; Gelosia, M.; Giannoni, T.; Fabbrizi, G.; Nicolini, A.; Castellani, B. Wood waste valorization: Ethanol based organosolv as a promising recycling process. Waste Manag. 2023, 170, 75–81. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood Waste from Fruit Trees: Biomolecules and Their Applications in Agri-Food Industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef]
- de Klerk, S.; Ghaffariyan, M.R.; Miles, M. Leveraging the entrepreneurial method as a tool for the circular economy: The case of wood waste. Sustainability 2022, 14, 1559. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, N.; Liu, M.; Lin, Y.; Chang, G.; Deng, L.; Huang, H. Emissions of nitrogenous pollutants in chemical looping gasification of high nitrogen wood waste using a K-modified copper slag oxygen carrier. J. Therm. Anal. Calorim. 2022, 147, 9725–9735. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Chaverri, R.H.; Figueroa, J.B. Biomass: Biorefinery as a model to boost the bioeconomy in Costa Rica, a review. Agron. Mesoam. 2021, 32, 1047–1070. [Google Scholar]
- Ong, S.S.; Wickneswari, R. Expression profile of small RNAs in Acacia mangium secondary xylem tissue with contrasting lignin content—Potential regulatory sequences in monolignol biosynthetic pathway. BMC Genom. 2011, 12, S13. [Google Scholar] [CrossRef] [PubMed]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Kamperidou, V. Chemical and structural characterization of poplar and black pine wood exposed to short thermal modification. Drv. Ind. 2021, 72, 155–167. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Viceconte, F.R.; Bidegain, M.A.; Dietrich, J. Regulation of lignocellulose degradation in microorganisms. J. Appl. Microbiol. 2023, 134, lxac002. [Google Scholar] [CrossRef]
- Banu Jamaldheen, S.; Kurade, M.B.; Basak, B.; Yoo, C.G.; Oh, K.K.; Jeon, B.H.; Kim, T.H. A review on physico-chemical delignification as a pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2022, 346, 126591. [Google Scholar] [CrossRef]
- Datsomor, O.; Gou-Qi, Z.; Miao, L. Effect of ligninolytic axenic and coculture white-rot fungi on rice straw chemical composition and in vitro fermentation characteristics. Sci. Rep. 2022, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Rice straw, the role of silica and treatments to improve quality. Anim. Feed Sci. Tech. 2006, 130, 137–171. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic biomass transformations via greener oxidative pretreatment processes: Access to energy and value-added chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Characterization of the lignocellulosic and sugars composition of different olive leaves cultivars. Food Chem. 2020, 329, 127153. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Li, H.; Xiao, L.P.; Wang, T.P.; Ren, W.F.; Lu, Q.; Sun, R.C. Valorization of lignin into phenolic compounds via fast pyrolysis: Impact of lignin structure. Fuel 2022, 319, 123758. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Y.; Chen, P.; Li, Y. Contribution of Lignin Peroxidase, Manganese Peroxidase, and Laccase in Lignite Degradation by Mixed White-Rot Fungi. Waste Biomass Valor. 2021, 12, 3753–3763. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Dunn, K.; Unruh Snyder, L.; McCarter, J.; Frey, G.; Idassi, J.; Schnake, D.; Cubbage, F. Bioeconomic assessment of an alley cropping field trial in North Carolina, US: Tree density, timber production, and forage relationships. Sustainability 2021, 13, 11465. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Bio-pulping: An energy saving and environment-friendly approach. Phys. Sci. Rev. 2020, 5, 20190043. [Google Scholar] [CrossRef]
- Peng, L.; Ma, R.; Jiang, S.; Luo, W.; Li, Y.; Wang, G.; Xu, Z.; Wang, Y.; Qi, C.; Li, Y.; et al. Co-composting of kitchen waste with agriculture and forestry residues and characteristics of compost with different particle size: An industrial scale case study. Waste Manag. 2022, 149, 313–322. [Google Scholar] [CrossRef]
- Verma, S.; Midha, V.K.; Choudhary, A.K. Optimization of parameters for alkali pretreatment on coir fiber for biomass production using TOPSIS. J. Nat. Fibers 2022, 19, 3038–3050. [Google Scholar] [CrossRef]
- Lee, B.H.; Kwon, W.J.; Kim, J.Y.; Park, J.S.; Eom, A.H. Differences among endophytic fungal communities isolated from the roots of Cephalanthera longibracteata collected from different sites in Korea. Mycobiology 2017, 45, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Thrimothi, D.; Sujatha, E.; Swetha, K.G.; Krishna, G. Isolation, Screening, Identification, and Assessment of Laccase-Producing Fungi Isolated From Different Environmental Samples. Biosci. Biotechnol. Res. Asia 2023, 20, 1303–1315. [Google Scholar] [CrossRef]
- Ali, N.S.; Huang, F.; Qin, W.; Yang, T.C. A high throughput screening process and quick isolation of novel lignin-degrading microbes from large number of natural biomasses. Biotechnol. Rep. 2023, 39, e00809. [Google Scholar] [CrossRef]
- Helal, G.A.; Khalil, R.R.; Galal, Y.G.; Soliman, S.M.; Abd Elkader, R.S. Studies on cellulases of some cellulose-degrading soil fungi. Arch. Microbiol. 2022, 204, 65. [Google Scholar] [CrossRef]
- Sharma, S.; Murty, D.S. Enhancement of laccase production by optimizing the cultural conditions for Pleurotus sajor-caju in solid-state fermentation. J. Pure Appl. Microbiol. 2021, 15, 958–967. [Google Scholar] [CrossRef]
- Nurika, I.; Aristya, Y.I.; Azizah, N.; Sunyoto, N.M.; Suhartini, S.; Bugg, T.D.; Barker, G.C. Enhancement of the ligninolytic activity of Lysinibacillus sphaericus by the addition of MnSO4 and its impact on subsequent methane production from Oil Palm Empty Fruit Bunches (OPEFB). Bioresour. Technol. Rep. 2023, 22, 101394. [Google Scholar] [CrossRef]
- Arora, D.S.; Gill, P.K. Comparison of two assay procedures for lignin peroxidase. Enzyme Microb. Technol. 2001, 28, 602–605. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.Y.; Fang, Y.L.; Song, Q.; Sun, M.L.; Yang, J.Y.; Ju, Y.W.; Li, D.W.; Huang, L. The fungal endophyte Epicoccum dendrobii as a potential biocontrol agent against Colletotrichum gloeosporioides. Phytopathology 2021, 111, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.L.; Lu, X.Q.; Bian, J.Y.; Qi, X.L.; Li, D.W.; Huang, L. Curvularia spicifera and Curvularia muehlenbeckiae causing leaf blight on Cunninghamia lanceolata. Plant Pathol. 2020, 69, 1139–1147. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol. Lett. 2003, 220, 235–240. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Valorisation of lignocellulosic biomass to value-added products: Paving the pathway towards low-carbon footprint. Fuel 2021, 313, 122678. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Dickwella Widanage, M.C.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 2019, 10, 347. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Osono, T.; Takeda, H. Decomposition of Japanese beech wood by diverse fungi isolated from a cool temperate deciduous forest. Mycoscience 2005, 46, 97–101. [Google Scholar] [CrossRef]
- Rustøen, F.; Høiland, K.; Heegaard, E.; Boddy, L.; Gange, A.C.; Kauserud, H.; Andrew, C. Substrate affinities of wood decay fungi are foremost structured by wood properties not climate. Fungal Ecol. 2023, 63, 101231. [Google Scholar] [CrossRef]
- Veloz Villavicencio, E.; Mali, T.; Mattila, H.K.; Lundell, T. Enzyme Activity Profiles Produced on Wood and Straw by Four Fungi of Different Decay Strategies. Microorganisms 2020, 8, 73. [Google Scholar] [CrossRef]
- Park, G.W.; Gong, G.T.; Joo, J.C.; Song, J.; Lee, J.; Lee, J.; Kim, H.T.; Ryu, M.H.; Sirohi, R.; Zhuang, X.; et al. Recent progress and challenges in biological degradation and biotechnological valorization of lignin as an emerging source of bioenergy: A state-of-the-art review. Renew. Sustain. Energy Rev. 2022, 157, 112025. [Google Scholar] [CrossRef]
- Chai, Y.Z.; Bai, M.; Chen, A.W.; Peng, L.; Shao, J.H.; Luo, S.; Deng, Y.C.; Yan, B.H.; Peng, C. Valorization of waste biomass through fungal technology: Advances, challenges, and prospects. Ind. Crops Prod. 2022, 188, 115608. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Yuliana, T.; Putri, N.Z.; Komara, D.Z.; Mardawati, E.; Lanti, I.; Rahimah, S. Study of Ganoderma lucidum in Laccase Production using Corncob and Paddies Straw Substrates on Submerged Fermentation System. Pak. J. Biol. Sci. 2020, 23, 1060–1065. [Google Scholar] [CrossRef]
- Cui, T.; Yuan, B.; Guo, H.; Tian, H.; Wang, W.; Ma, Y.Q.; Li, C.Z.; Fei, Q. Enhanced lignin biodegradation by consortium of white rot fungi: Microbial synergistic effects and product mapping. Biotechnol. Biofuels 2021, 14, 162. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.Y.; Sun, Y.F.; He, X.L.; Song, C.G.; Si, J.; Liu, D.M.; Gates, G.; Cui, B.K. Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. 2023, 118, 1–94. [Google Scholar] [CrossRef]
- Phithakrotchanakoon, C.; Mayteeworakoon, S.; Siriarchawatana, P.; Kitikhun, S.; Harnpicharnchai, P.; Wansom, S.; Eurwilaichitr, L.; Ingsriswang, S. Beneficial bacterial-Auricularia cornea interactions fostering growth enhancement identified from microbiota present in spent mushroom substrate. Front. Microbiol. 2022, 13, 1006446. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Q.; Xie, N.; Yuan, G.; Liao, M.; Gui, Q.; Ding, G. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef]
- Ding, X.; Guo, Y.; Ye, J.; Wu, X.; Lin, S.; Chen, F.; Zhu, L.; Huang, L.; Song, X.; Zhang, Y.; et al. Population differentiation and epidemic tracking of Bursaphelenchus xylophilus in China based on chromosome-level assembly and whole-genome sequencing data. Pest Manag. Sci. 2022, 78, 1213–1226. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.-L.; Ren, Y.; Huang, J.-H.; Ren, J.-L.; Yang, J.; He, J.; Li, D.-W.; Huang, L. Phlebia formosana Strain SMF410-5-1 and Auricularia cornea Strain ME1-1 Display Potential in Wood Degradation and Forest Waste Reutilization. Forests 2025, 16, 795. https://doi.org/10.3390/f16050795
Qin H-L, Ren Y, Huang J-H, Ren J-L, Yang J, He J, Li D-W, Huang L. Phlebia formosana Strain SMF410-5-1 and Auricularia cornea Strain ME1-1 Display Potential in Wood Degradation and Forest Waste Reutilization. Forests. 2025; 16(5):795. https://doi.org/10.3390/f16050795
Chicago/Turabian StyleQin, Hao-Long, Yi Ren, Jin-Hua Huang, Jian-Ling Ren, Jiyun Yang, Jiao He, De-Wei Li, and Lin Huang. 2025. "Phlebia formosana Strain SMF410-5-1 and Auricularia cornea Strain ME1-1 Display Potential in Wood Degradation and Forest Waste Reutilization" Forests 16, no. 5: 795. https://doi.org/10.3390/f16050795
APA StyleQin, H.-L., Ren, Y., Huang, J.-H., Ren, J.-L., Yang, J., He, J., Li, D.-W., & Huang, L. (2025). Phlebia formosana Strain SMF410-5-1 and Auricularia cornea Strain ME1-1 Display Potential in Wood Degradation and Forest Waste Reutilization. Forests, 16(5), 795. https://doi.org/10.3390/f16050795





