Effects of Afforestation on Soil Aggregate Stability, Carbon, and Nitrogen in Alpine Sandy Lands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Soil Sampling
2.3. Measurement Parameters
2.4. Calculations and Statistical Analysis
3. Results
3.1. Changes in Distribution of Soil Aggregate Size at Different Restoration Years
3.2. Changes in Aggregate Stability at Different Restoration Years
3.3. Changes in SOC and TN Contents and Density at Different Restoration Years
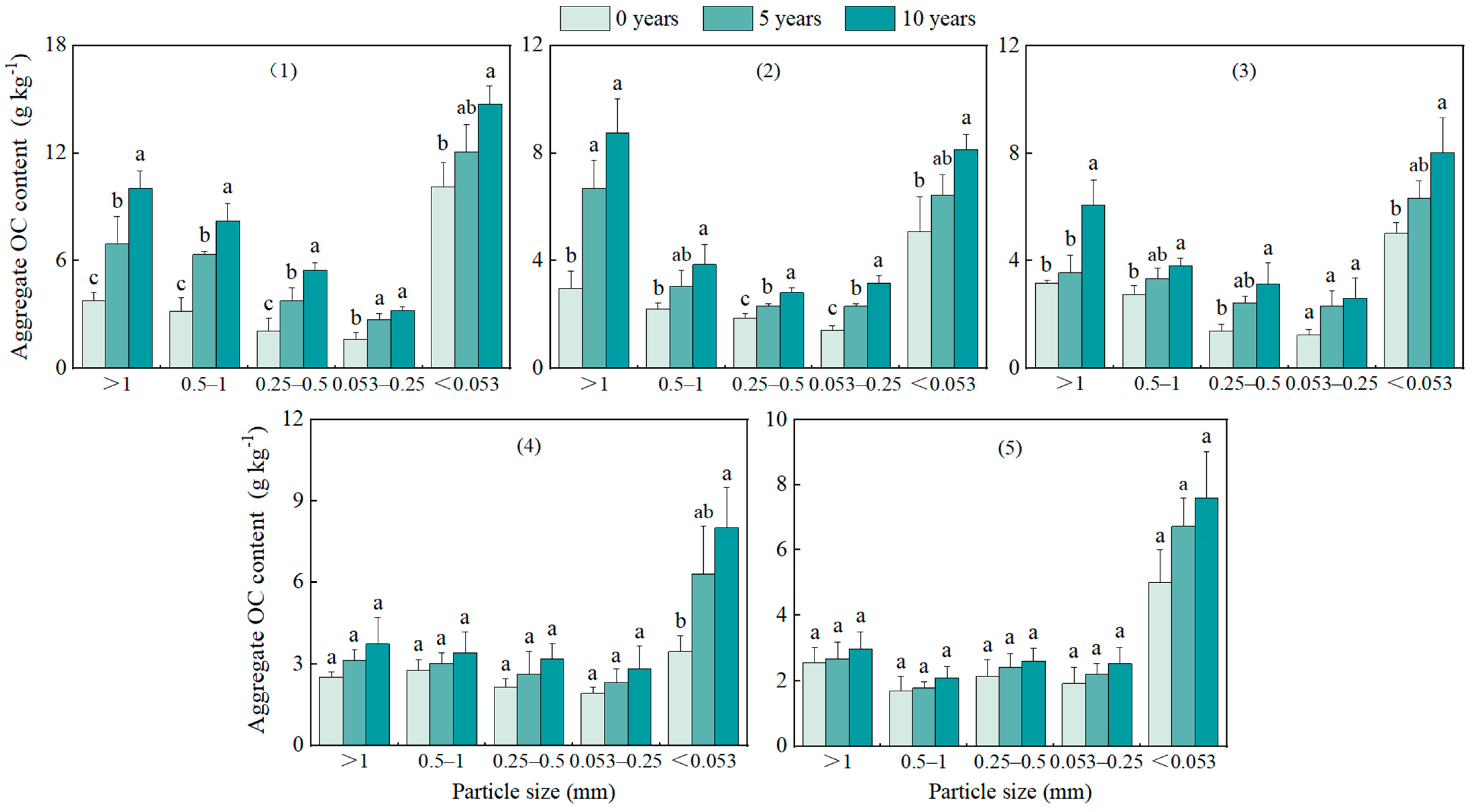
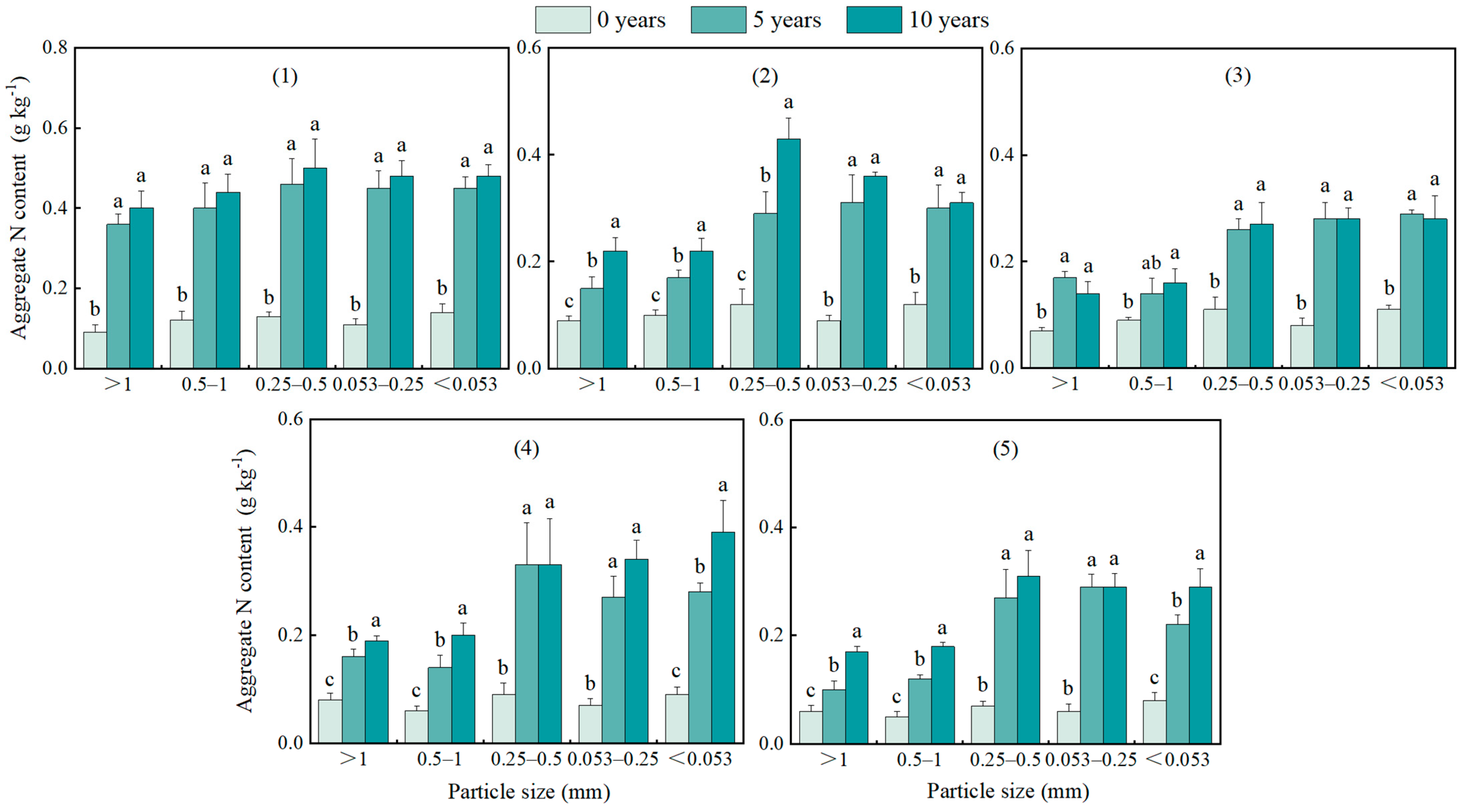
3.4. Changes in Aggregates OC and N Contents and Density at Different Restoration Years
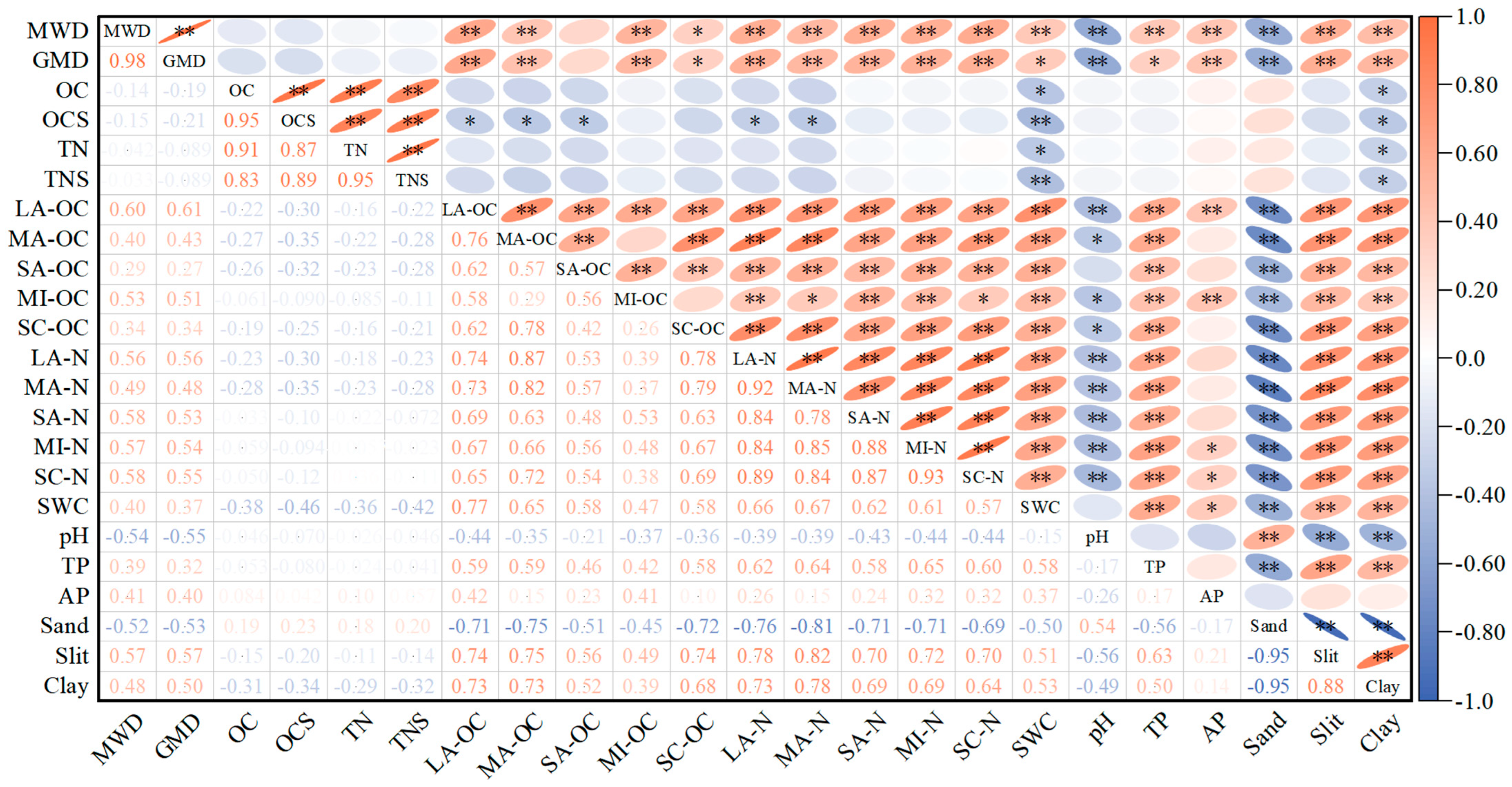
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, P.L.; Billings, S.A.; Hirmas, D.; Li, L.; Zhang, X.; Ziegler, S.; Murenbeeld, K.; Ajami, H.; Guthrie, A.; Singha, K.; et al. Embracing the dynamic nature of soil structure: A paradigm illuminating the role of life in critical zones of the Anthropocene. Earth-Sci. Rev. 2022, 225, 103873. [Google Scholar] [CrossRef]
- Han, C.; Song, M.X.; Tang, Q.L.; Wei, J.; He, X.B.; Collins, A.L. Post-farming land restoration schemes exhibit higher soil aggregate stability and organic carbon: Evidence in the Three Gorges Reservoir Area, China. Catena 2023, 227, 107099. [Google Scholar] [CrossRef]
- Deng, L.; Kim, D.G.; Peng, C.; Shangguan, Z. Controls of soil and aggregate associated organic carbon variations following natural vegetation restoration on the Loess Plateau in China. Land Degrad. Dev. 2018, 29, 3974–3984. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Jia, L.; Yu, K.X.; Li, Z.B.; Li, P.; Zhang, A.N.; Ma, L.; Xu, G.C.; Zhang, X. Temporal and spatial variation of rainfall erosivity in the Loess Plateau of China and its impact on sediment load. Catena 2022, 210, 105931. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shangguan, Z.P.; Deng, L. Soil aggregate stability and aggregate-associated carbon and nitrogen in natural restoration grassland and Chinese red pine plantation on the Loess Plateau. Catena 2017, 149, 253–260. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.H.; Kim, D.G.; Li, J.W.; Liu, Y.L.; Hai, X.Y.; Huang, C.B.; Shangguan, Z.P.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Wiesmeier, M.; Buness, V.; Berauer, B.J.; Schuchardt, M.A.; Jentsch, A.; Schlingmann, M.; Andrade-Linares, D.; Wolf, B.; Kiese, R.; et al. Rapid loss of organic carbon and soil structure in mountainous grassland topsoils induced by simulated climate change. Geoderma 2024, 442, 116807. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Baum, C.; Eckhardt, K.U.; Kandeler, E. Stability and composition of soil organic matter control respiration and soil enzyme activities. Soil Biol. Biochem. 2008, 40, 1496–1505. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, J.Y.; Wang, S.W.; Fu, Q.; Baoyin, T.; Bao, Z.H.; Li, F.Y. Soil biological nitrogen fixation is closely associated with soil ammonium nitrogen content in a mowing semiarid natural grassland. Appl. Soil Ecol. 2024, 203, 105690. [Google Scholar] [CrossRef]
- Bahur, E.; Kara, O.; Fathi, R.A.; Susam, Y.E.; Riza, M.; Arif, M.; Akhtar, K. Wattle fencing improved soil aggregate stability, organic carbon stocks and biochemical quality by restoring highly eroded mountain region soil. J. Environ. Manag. 2021, 288, 112489. [Google Scholar] [CrossRef]
- Adnan, M.; Xu, M.G.; Syed, A.A.S.; Muhammad, M.A.; Sun, N.; Wang, B.R.; Cai, Z.J.; Qudsia, S.; Muhammad, N.; Khalid, M.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef]
- Kou, T.J.; Zhu, P.; Huang, S.; Song, Z.W.; Deng, A.X.; Gao, H.J.; Peng, C.; Zhang, W.J. Effects of long-term cropping regimes on soil carbon sequestration and aggregate composition in rainfed farmland of Northeast China. Soil Till. Res. 2012, 118, 132–138. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The role of soil organic matter in sustaining soil fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Zhou, H.L.; Qu, Q.; Xu, H.W.; Wang, M.G.; Xue, S. Effects of vegetation restoration on soil microbial necromass carbon and organic carbon in grazed and degraded sandy land. J. Environ. Manag. 2025, 382, 125380. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wei, X.R.; Gao, J.L.; Zhang, X.C. Dynamics of soil aggregate-associated organic carbon along an afforestation chronosequence. Plant Soil 2015, 391, 237–251. [Google Scholar] [CrossRef]
- Wang, D.Y.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Qu, Y.P.; Jian, B.H.; Deng, D.Z.; Liu, M.; Yang, J.T.; Chen, D.C.; Huang, Y. Effects of grazing exclusion on vegetation community characteristics over 22 years in the Zoige alpine meadows from China. Acta Oecol. 2023, 118, 103892. [Google Scholar] [CrossRef]
- Wu, X.W.; Wang, Y.C.; Sun, S.C. Long-term fencing decreases plant diversity and soil organic carbon concentration of the Zoige alpine meadows on the eastern Tibetan Plateau. Plant Soil 2021, 458, 191–200. [Google Scholar] [CrossRef]
- Li, Q.; Shen, X.; Huang, Q.; Sun, F.; Zhou, J.; Ma, X.; Ran, Z.; Chen, Y.; Li, Z.; Yan, Y.; et al. Resource islands of Salix cupularis facilitating seedling emergence of the companion herbs in the restoration process of desertified alpine meadow, the Tibetan Plateau. J. Environ. Manag. 2021, 289, 112434. [Google Scholar] [CrossRef] [PubMed]
- Du, C.J.; Jing, J.; Shen, Y.; Liu, H.X.; Gao, Y.H. Short-term grazing exclusion improved topsoil conditions and plant characteristics in degraded alpine grasslands. Ecol. Indic. 2020, 108, 105680. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.L.; Ji, B.M.; Struik, P.C.; Jin, K.; Tang, S.M. Coupling between the responses of plants, soil, and microorganisms following grazing exclusion in an overgrazed grassland. Front. Plant Sci. 2021, 12, 640789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qu, Y.; Zeng, H.; Yang, J.; Liu, L.; Deng, D.; Ma, Y.; Chen, D.; Jian, B.; Guan, L.; et al. Long-term ecological restoration increased plant diversity and soil total phosphorus content of the alpine flowing sand land in northwest Sichuan, China. Heliyon 2024, 10, e24035. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Lu, R.K. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Elliott, E. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; He, H. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Wu, Y.C.; Li, Z.C.; Cheng, C.F.; Liu, R.J.; Wang, B.; Ge, R.L.T. Effects of understory removal on soil labile organic carbon pool in a Cinnamomum camphora plantation. Chin. J. Appl. Ecol. 2013, 24, 3341–3346. [Google Scholar] [CrossRef]
- Han, Z.G.; Zhou, Y.C.; Ren, J.J. Effects of organic matter-bound multivalent cations on the internal and external formation of soil aggregates under Pinus massoniana forest. J. Soil Water Conserv. 2021, 76, 568–576. [Google Scholar] [CrossRef]
- Su, Z.X.; Zhu, S.; Wei, Z.H.; He, Y.X.; Su, B.Q.; Zhang, K.; Ma, X.; Shangguan, Z.P. Vegetation restoration changed the soil aggregate stability and aggregate carbon stabilization pathway according to δ13C signatures. Agric. Ecosyst. Environ. 2025, 378, 109317. [Google Scholar] [CrossRef]
- Shu, X.Y.; Hu, Y.H.; Liu, W.J.; Xia, L.; Zhang, Y.Y.; Zhou, W.; Liu, W.L.; Zhang, Y.L. Linking between soil properties, bacterial communities, enzyme activities, and soil organic carbon mineralization under ecological restoration in an alpine degraded grassland. Front. Microbiol. 2023, 14, 1131836. [Google Scholar] [CrossRef]
- Bing, H.J.; Wu, Y.H.; Zhou, J.; Sun, H.Y.; Luo, J.; Wang, J.P.; Yu, D. Stoichiometric variation of carbon, nitrogen, and phosphorus in soils and its implication for nutrient limitation in alpine ecosystem of Eastern Tibetan Plateau. J. Soil Sediment 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Shi, J.W.; Deng, L.; Yang, L.; Dong, Y.J.; Liao, Y.; Li, J.W.; Liu, Y.R.; Ren, C.J.; Yang, F.; Shangguan, Z.P.; et al. Deciphering microbial divers of soil organic matter mineralization in surface and subsurface soil during long-term vegetation succession. Agric. Ecosyst. Environ. 2024, 374, 109186. [Google Scholar] [CrossRef]
- Dou, X.; Xu, X.; Shu, X.; Zhang, Q.; Cheng, X. Shifts in soil organic carbon and nitrogen dynamics for afforestation in central China. Ecol. Eng. 2016, 87, 263–270. [Google Scholar] [CrossRef]
- Lu, L.X.; Song, T.Q.; Peng, W.X.; Zeng, F.P.; Wang, K.L.; Xu, Y.L.; Yu, Z.; Liu, Y. Profile distribution of soil aggregates organic carbon in primary forests in Karst cluster-peak depression region. Chin. J. Appl. Ecol. 2012, 23, 1167–1174. [Google Scholar] [CrossRef]
- Zheng, J.J.; Fang, H.J.; Cheng, S.L.; Yu, G.R.; Zhang, P.L.; Xu, M.J.; Li, Y.N. Effects of N addition on soil organic carbon components in an alpine meadow on the eastern Qinghai-Tibetan Plateau. Acta Ecol. Sin. 2012, 32, 5363–5372. [Google Scholar] [CrossRef]
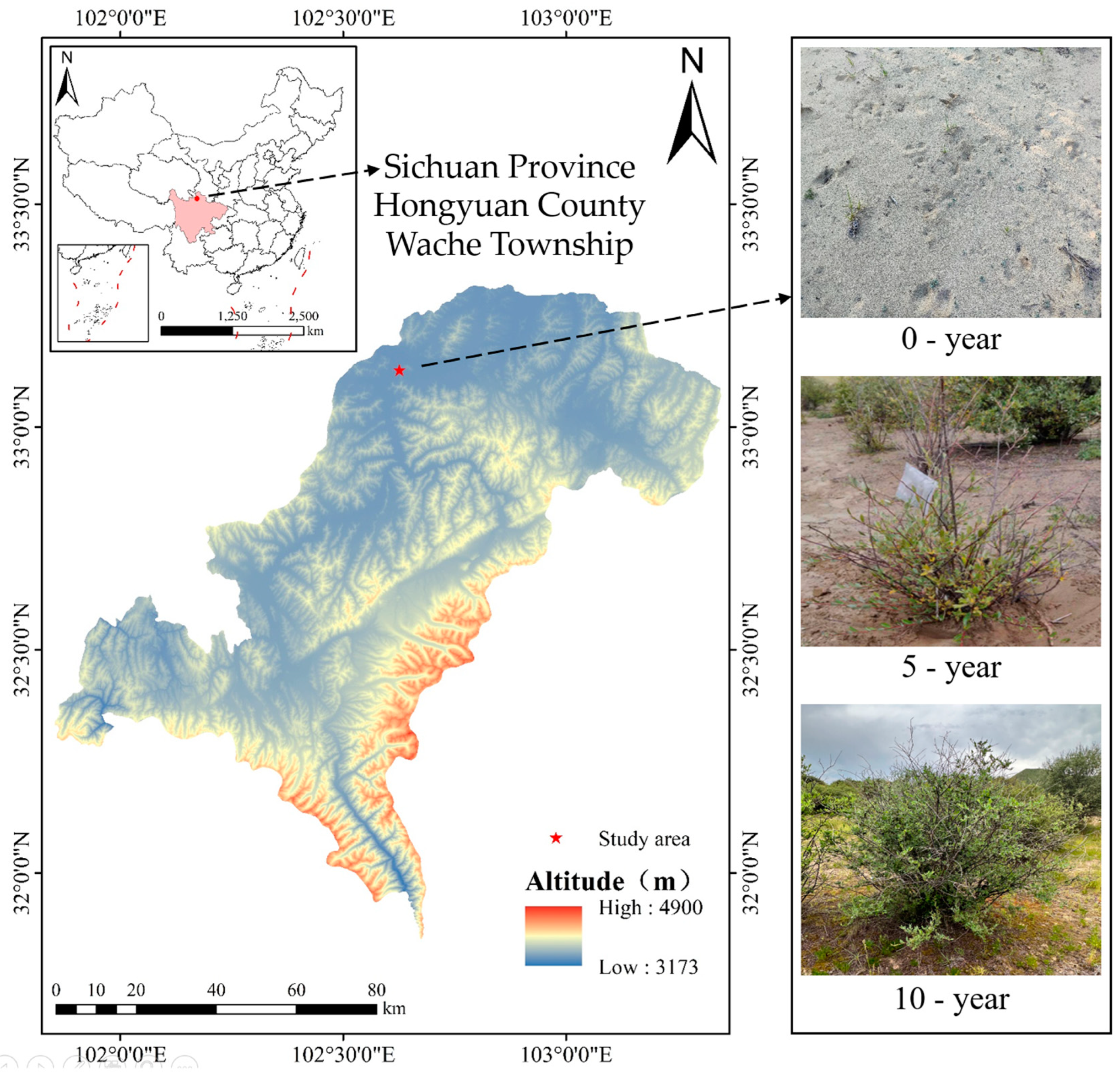
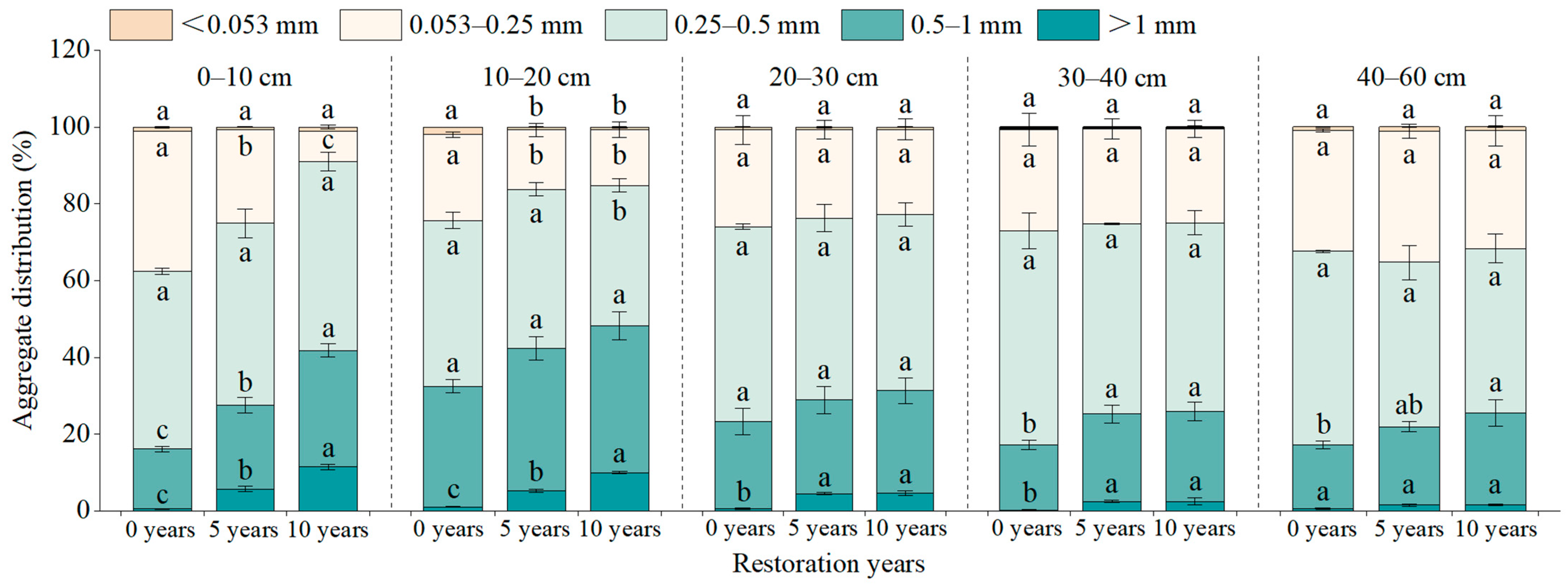
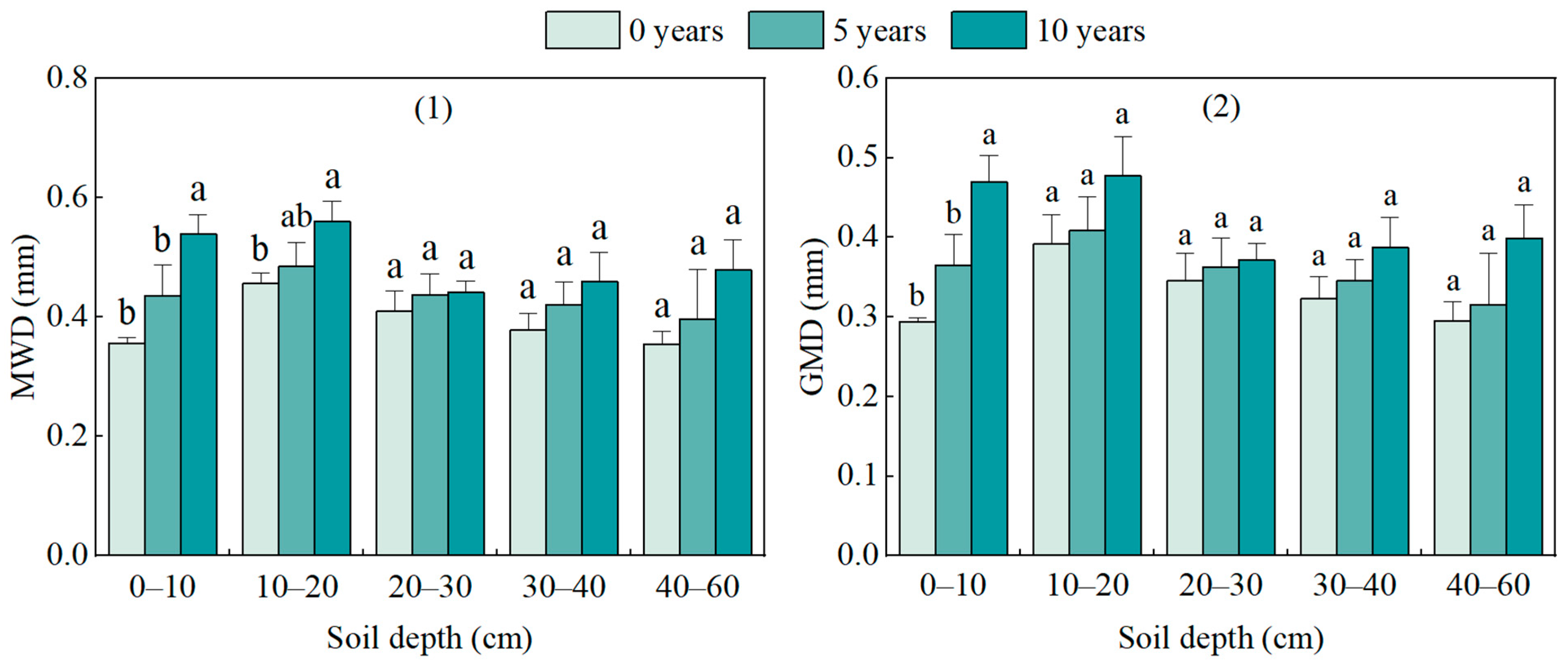
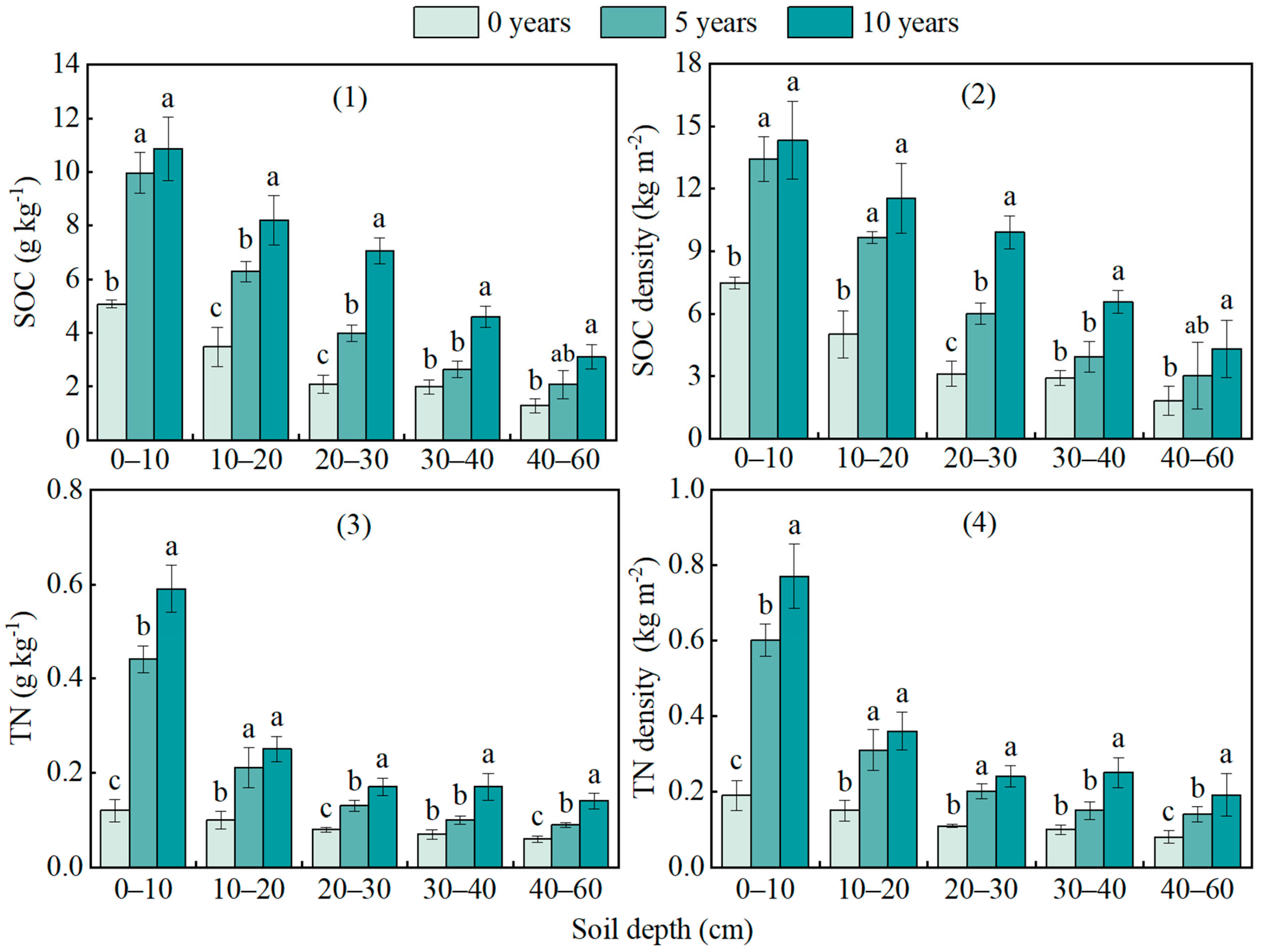
| Restoration Years | Coordinates | Altitude (m) | Slope (°) | Shrub Height (cm) | Vegetation Coverage (%) |
|---|---|---|---|---|---|
| 0–1 | 102°37′14.62″ E, 33°10′62.63″ N | 3450.2 | 9 | 0 | 0 |
| 0–2 | 102°37′14.61″ E, 33°10′62.73″ N | 3450.3 | 9 | 0 | 0 |
| 0–3 | 102°37′14.61″ E, 33°10′62.87″ N | 3450.5 | 8 | 0 | 0 |
| 0–4 | 102°37′14.71″ E, 33°10′62.74″ N | 3450.2 | 9 | 0 | 0 |
| 5–1 | 102°37′27.12″ E, 33°10′51.38″ N | 3451.1 | 8 | 62 | 22 |
| 5–2 | 102°37′27.21″ E, 33°10′51.37″ N | 3451.3 | 8 | 64 | 25 |
| 5–3 | 102°37′27.11″ E, 33°10′51.48″ N | 3451.3 | 9 | 67 | 23 |
| 5–4 | 102°37′27.22″ E, 33°10′51.41″ N | 3451.2 | 9 | 68 | 27 |
| 10–1 | 102°37′09.35″ E, 33°10′58.41″ N | 3450.8 | 10 | 116 | 51 |
| 10–2 | 102°37′09.45″ E, 33°10′58.42″ N | 3450.6 | 9 | 119 | 52 |
| 10–3 | 102°37′09.36″ E, 33°10′58.50″ N | 3451.1 | 9 | 124 | 55 |
| 10–4 | 102°37′09.34″ E, 33°10′58.35″ N | 3450.7 | 10 | 126 | 56 |
| Depth (cm) | Years | SWC (%) | pH | TP (g kg−1) | AP (mg kg−1) | Sand (%) | Slit (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|
| 0–10 | 0 | 7.68 ± 0.87 b | 7.31 ± 0.07 b | 0.15 ± 0.00 b | 7.94 ± 0.24 a | 95.96 ± 1.31 a | 2.09 ± 0.13 c | 2.04 ± 0.08 c |
| 5 | 11.06 ± 0.93 a | 7.28 ± 0.08 a | 0.18 ± 0.01 a | 8.08 ± 0.65 a | 93.70 ± 0.63 b | 3.07 ± 0.15 b | 3.23 ± 0.50 b | |
| 10 | 11.14 ± 1.44 a | 6.59 ± 0.13 a | 0.18 ± 0.02 a | 8.40 ± 1.17 a | 91.13 ± 0.90 c | 4.40 ± 0.61 a | 4.47 ± 0.40 a | |
| 10–20 | 0 | 7.41 ± 0.95 a | 7.23 ± 0.05 a | 0.14 ± 0.00 a | 7.78 ± 0.23 b | 96.60 ± 0.69 a | 1.79 ± 0.10 b | 1.94 ± 0.14 a |
| 5 | 9.81 ± 2.80 a | 7.31 ± 0.09 a | 0.16 ± 0.01 a | 9.12 ± 0.33 a | 95.37 ± 1.26 ab | 2.45 ± 0.16 ab | 2.60 ± 0.51 a | |
| 10 | 10.48 ± 3.91 a | 6.71 ± 0.04 a | 0.17 ± 0.03 a | 9.36 ± 1.93 a | 93.75 ± 1.67 b | 3.19 ± 0.95 a | 3.06 ± 0.89 a | |
| 20–30 | 0 | 6.46 ± 0.21 a | 7.20 ± 0.08 a | 0.14 ± 0.01 b | 7.79 ± 0.77 a | 96.37 ± 0.15 a | 1.71 ± 0.08 b | 1.92 ± 0.08 b |
| 5 | 8.07 ± 1.32 a | 7.14 ± 0.15 a | 0.17 ± 0.01 a | 8.72 ± 1.11 a | 95.72 ± 1.00 a | 2.04 ± 0.17 ab | 2.23 ± 0.83 ab | |
| 10 | 7.71 ± 1.47 a | 6.64 ± 0.09 b | 0.17 ± 0.00 a | 9.05 ± 1.10 a | 93.93 ± 0.91 b | 3.01 ± 0.85 a | 3.07 ± 0.29 a | |
| 30–40 | 0 | 6.25 ± 0.30 b | 7.18 ± 0.04 a | 0.14 ± 0.01 a | 7.63 ± 0.76 a | 96.54 ± 0.45 a | 1.67 ± 0.12 b | 1.79 ± 0.35 a |
| 5 | 7.68 ± 1.06 a | 7.30 ± 0.18 a | 0.15 ± 0.01 a | 8.08 ± 0.60 a | 96.00 ± 0.60 a | 2.02 ± 0.12 ab | 1.97 ± 0.49 a | |
| 10 | 6.78 ± 0.45 ab | 6.72 ± 0.08 b | 0.15 ± 0.02 a | 8.40 ± 0.61 a | 95.30 ± 0.80 a | 2.37 ± 0.33 a | 2.34 ± 0.49 a | |
| 40–60 | 0 | 5.60 ± 0.97 a | 7.13 ± 0.09 a | 0.14 ± 0.01 a | 7.84 ± 0.65 a | 96.87 ± 0.50 a | 1.54 ± 0.12 b | 1.59 ± 0.41 a |
| 5 | 6.92 ± 0.50 a | 7.33 ± 0.18 a | 0.15 ± 0.00 a | 8.08 ± 0.65 a | 96.45 ± 1.10 a | 1.71 ± 0.21 b | 1.84 ± 0.94 a | |
| 10 | 6.82 ± 0.77 a | 6.67 ± 0.11 b | 0.15 ± 0.00 a | 8.40 ± 0.84 a | 95.78 ± 0.86 a | 2.21 ± 0.30 a | 2.00 ± 0.57 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Qian, H.; Jiang, H.; Gao, X.; Hu, Y. Effects of Afforestation on Soil Aggregate Stability, Carbon, and Nitrogen in Alpine Sandy Lands. Forests 2025, 16, 782. https://doi.org/10.3390/f16050782
He W, Qian H, Jiang H, Gao X, Hu Y. Effects of Afforestation on Soil Aggregate Stability, Carbon, and Nitrogen in Alpine Sandy Lands. Forests. 2025; 16(5):782. https://doi.org/10.3390/f16050782
Chicago/Turabian StyleHe, Wangyi, Hongyu Qian, Haodong Jiang, Xuan Gao, and Yufu Hu. 2025. "Effects of Afforestation on Soil Aggregate Stability, Carbon, and Nitrogen in Alpine Sandy Lands" Forests 16, no. 5: 782. https://doi.org/10.3390/f16050782
APA StyleHe, W., Qian, H., Jiang, H., Gao, X., & Hu, Y. (2025). Effects of Afforestation on Soil Aggregate Stability, Carbon, and Nitrogen in Alpine Sandy Lands. Forests, 16(5), 782. https://doi.org/10.3390/f16050782






