Influence of Canopy Environmental Characteristics on Regen-eration of Nine Tree Species in Broadleaved Korean Pine Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sets
2.2. Regeneration Site Canopy Environment Measurement
2.3. Canopy Environmental Effects on Regeneration Spatial Distribution
2.4. Canopy Environmental Effects on Seedling-to-Sapling Transition
3. Results
3.1. Canopy Environmental Factors’ Influence on Regeneration
3.2. Composite Canopy Features’ Influence on Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kane, V.R.; Gersonde, R.F.; Lutz, J.A.; Mcgaughey, R.J.; Bakker, J.D.; Franklin, J.F. Patch dynamics and the development of structural and spatial heterogeneity in Pacific Northwest forests. Can. J. For. Res. 2011, 41, 2276–2291. [Google Scholar] [CrossRef]
- Remmert, H. The Mosaic-Cycle Concept of Ecosystems; Springer: Berlin, Germany, 1991. [Google Scholar]
- Gravel, D.; Canham, C.D.; Beaudet, M.; Messier, C. Shade tolerance, canopy gaps and mechanisms of coexistence of forest trees. Oikos 2010, 119, 475–484. [Google Scholar] [CrossRef]
- Yamamoto, S.I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Král, K.; Daněk, P.; Janík, D.; Krůček, M.; Vrška, T. How cyclical and predictable are Central European temperate forest dynamics in terms of development phases? J. Veg. Sci. 2018, 29, 84–97. [Google Scholar] [CrossRef]
- Whitmore, T. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Negron-Juarez, R.I.; Marra, D.M.; Di Vittorio, A.; Tews, J.; Roberts, D.; Ribeiro, G.H.; Trumbore, S.E.; Higuchi, N. The steady-state mosaic of disturbance and succession across an old-growth Central Amazon forest landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 3949–3954. [Google Scholar] [CrossRef]
- Krüger, K.; Senf, C.; Jucker, T.; Pflugmacher, D.; Seidl, R. Gap expansion is the dominant driver of canopy openings in a temperate mountain forest landscape. J. Ecol. 2024, 112, 1501–1515. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Xu, H.C. Natural Forests of Pinus Koraiensis in China; China Forestry Publishing House: Beijing, China, 2001. [Google Scholar]
- Tian, A.; Halik, Ü.; Fu, W.; Sawirdin, S.; Cheng, S.; Lei, J. Research history of forest gap as small-scale disturbances in forest ecosystems. Forests 2023, 15, 21. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Feldmann, E.; Glatthorn, J.; Ammer, C.; Leuschner, C. Regeneration dynamics following the formation of understory gaps in a Slovakian beech virgin forest. Forests 2020, 11, 585. [Google Scholar] [CrossRef]
- Valerio, M.; Ibáñez, R.; Gazol, A. The role of canopy cover dynamics over a decade of changes in the understory of an Atlantic beech-oak forest. Forests 2021, 12, 938. [Google Scholar] [CrossRef]
- Martini, F.; Xia, S.W.; Zou, C.; Goodale, U.M. Seedling growth and survival responses to multiple soil properties in subtropical forests of south China. For. Ecol. Manag. 2020, 474, 118382. [Google Scholar] [CrossRef]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1988, 75, 81–86. [Google Scholar] [CrossRef]
- Brokaw, N.V. Gap-phase regeneration in a tropical forest. Ecology 1985, 66, 682–687. [Google Scholar] [CrossRef]
- Sharma, L.; Shrestha, K.; Måren, I. Tree regeneration in gap-understory mosaics in a subtropical Shorea robusta (Sal) forest. J. For. Res. 2019, 30, 2061–2068. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadouria, R.; Srivastava, P.; Devi, R.S.; Chaturvedi, R.; Raghubanshi, A. Effects of light availability on leaf attributes and seedling growth of four tree species in tropical dry forest. Ecol. Processes 2020, 9, 2. [Google Scholar] [CrossRef]
- Cornacchia, L.; Van De Koppel, J.; Van Der Wal, D.; Wharton, G.; Puijalon, S.; Bouma, T.J. Landscapes of facilitation: How self-organized patchiness of aquatic macrophytes promotes diversity in streams. Ecology 2018, 99, 832–847. [Google Scholar] [CrossRef]
- Yu, Z.L.; Hao, Z.Q. Canopy gap characteristics and its influence on the regeneration of broad-leaved Korean pine forests in Changbai Mountain. J. For. Res. 1998, 9, 160–165. [Google Scholar] [CrossRef]
- Jin, G.; Liu, Y.; Liu, S.; Kim, J.H. Effect of gaps on species diversity in the naturally regenerated mixed broadleaved-korean pine forest of the Xiaoxing’an Mountains, China. J. Ecol. Environ. 2007, 30, 325–330. [Google Scholar] [CrossRef]
- Jin, G.; Tian, Y.; Zhao, F.; Kim, J.H. The pattern of natural regeneration by canopy gap size in the mixed broadleaved-korean pine forest of Xiaoxing’an Mountains, China. J. Korean For. Soc. 2007, 96, 227–234. [Google Scholar]
- Yy, L.; Gz, J.; Fr, L. Influence of forest gaps on seedling establishment in a mixed broadleaved-Korean pine (Pinus koraiensis) forest in Xiao Hinggan Mountains. Chin. Sci. Bull. 2014, 59, 2396–2406. [Google Scholar]
- Guo, Q. Characteristics of canopy patches related to natural regeneration of broad-leaved Korean pine forest. Chin. J. Appl. Ecol. 2002, 13, 1541–1543. [Google Scholar]
- Zang, R.G. Gap Dynamics and Forest Biodiversity; China Forestry Press: Beijing, China, 1999. [Google Scholar]
- Zhang, Y.; Zhang, J.; Wang, L.; Lu, D.; Cai, D.; Wang, B. Influences of dispersal and local environmental factors on stream macroinvertebrate communities in Qinjiang River, Guangxi, China. Aquat. Biol. 2014, 20, 185–194. [Google Scholar] [CrossRef]
- Leibold, M.A.; Chase, J.M. Metacommunity Ecology; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Dong, X.; Du, X.; Sun, Z.; Gu, H.; Chen, X. Spatial pattern and intraspecific association of natural Korean pine population under the influence of habitat gradient. Acta Ecol. Sin. 2020, 40, 5239–5246. [Google Scholar]
- Pak, U.; Guo, Q.; Liu, Z.; Wang, X.; Liu, Y.; Jin, G. Spatial distribution of pinus koraiensis trees and community-level spatial associations in broad-leaved korean pine mixed forests in Northeastern China. Plants 2023, 12, 2906. [Google Scholar] [CrossRef]
- Du, X.; Dong, X.; Gu, H.Y.; Chen, X.W. A study of the vertical and short-range horizontal spatial distribution of leaf area index in broadleaved-Korean pine forest based on stratified Voronoi diagrams. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2025, 49, 83–94. Available online: https://link.cnki.net/urlid/32.1161.S.20231117.0856.002 (accessed on 24 April 2025).
- Illian, J.B.; Penttinen, A.; Stoyan, H.; Stoyan, D. Statistical Analysis and Modelling of Spatial Point Patterns; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, S.; Wang, J.; Duan, W.; Chen, L.; Wang, L.; Du, S.; Zhao, J. Gap characteristics in the mixed broad-leaved Korean pine forest in Xiaoxing’an Mountains. Acta Ecol. Sin. 2013, 33, 5234–5244. [Google Scholar]
- Brazdil, P.B.; Soares, C. A comparison of ranking methods for classification algorithm selection. In European Conference on Machine Learning; López de Mántaras, R., Plaza, E., Eds.; Springer: Berlin, Germany, 2000; pp. 63–75. [Google Scholar] [CrossRef]
- Scolastri, A.; Bricca, A.; Cancellieri, L.; Cutini, M. Understory functional response to different management strategies in Mediterranean beech forests (central Apennines, Italy). For. Ecol. Manag. 2017, 400, 665–676. [Google Scholar] [CrossRef]
- Mangiafico, S.S. Summary and analysis of extension. In Program Evaluation in R; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2016; Available online: http://rcompanion.org/handbook (accessed on 24 April 2025).
- Hubbell, S.P.; Foster, R.B.; O’brien, S.T.; Harms, K.E.; Condit, R.; Wechsler, B.; Wright, S.J.; De Lao, S.L. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 1999, 283, 554–557. [Google Scholar] [CrossRef]
- Calabrese, G.; Perrino, E.V.; Ladisa, G.; Aly, A.; Tesfmichael Solomon, M.; Mazdaric, S.; Benedetti, A.; Ceglie, F.G. Short-term effects of different soil management practices on biodiversity and soil quality of Mediterranean ancient olive orchards. Org. Agric. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S. Joint control of plant ecological strategy by climate, regeneration mode, and ontogeny in Northeastern Chinese forests. Ecol. Evol. 2021, 11, 6703–6715. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.B.; Farinosi, E.J.; Willis, J.L.; Gottschalk, K.W. Managing for diversity: Harvest gap size drives complex light, vegetation, and deer herbivory impacts on tree seedlings. Ecosphere 2016, 7, e01397. [Google Scholar] [CrossRef]
- Wang, L.X. Effect of Gap Size, Soil Moisture and Light on the Plant in the Pinus-koraiensis Broadleaved Mixed Forest. Master’s Thesis, Northeast Forestry University, Harbin, China, 2013. [Google Scholar]
- Vilhar, U. Water regulation ecosystem services following gap formation in fir-beech forests in the Dinaric Karst. Forests 2021, 12, 224. [Google Scholar] [CrossRef]
- Rada, F.; García-Núñez, C.; Rangel, S. Low temperature resistance in saplings and ramets of Polylepis sericea in the Venezuelan Andes. Acta Oecol. 2009, 35, 610–613. [Google Scholar] [CrossRef]
- Frankel, S.; Berenbaum, M. Effects of light regime on antioxidant content of foliage in a tropical forest community. Biotropica 1999, 31, 422–429. [Google Scholar] [CrossRef]
- Grebe, S.; Trotta, A.; Bajwa, A.A.; Mancini, I.; Bag, P.; Jansson, S.; Tikkanen, M.; Aro, E.M. Specific thylakoid protein phosphorylations are prerequisites for overwintering of Norway spruce (Picea abies) photosynthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 17499–17509. [Google Scholar] [CrossRef]
- Shimizu, M.; Ishida, A.; Tange, T.; Yagi, H. Leaf turnover and growth responses of shade-grown saplings of four Shorea rain forest species to a sudden increase in light. Tree Physiol. 2006, 26, 449–457. [Google Scholar] [CrossRef]
- Bernal, P.L.; Defossé, G.E.; Quinteros, C.P.; Bava, J.O. Sapling growth and crown expansion in canopy gaps of Nothofagus pumilio (lenga) forests in Chubut, Patagonia, Argentina. Forest Syst. 2012, 21, 489–497. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesiński, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2021, 482, 118866. [Google Scholar] [CrossRef]
- Kursar, T.A. Relating tree physiology to past and future changes in tropical rainforest tree communities. Clim. Change 1998, 39, 363–379. [Google Scholar] [CrossRef]
- Piovesan, G.; Lüttge, U. Tree growth dynamics during early ontogenetic stages in closed forests. Trees 2018, 32, 671–673. [Google Scholar] [CrossRef]
- Zhou, G. Survival Strategy of Korean Pine Under Forest and Driving Mechanism of Seasonal Light Environment. Doctoral Dissertation, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Man, R.; Lieffers, V.J. Seasonal photosynthetic responses to light and temperature in white spruce (Picea glauca) seedlings planted under an aspen (Populus tremuloides) canopy and in the open. Tree Physiol. 1997, 17, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Landhäusser, S.M.; Stadt, K.J.; Lieffers, V.J. Photosynthetic strategies of summergreen and evergreen understory herbs of the boreal mixedwood forest. Oecologia 1997, 112, 173–178. [Google Scholar] [CrossRef]
- Liu, X.; Liang, M.; Etienne, R.S.; Wang, Y.; Staehelin, C.; Yu, S. Experimental evidence for a phylogenetic Janzen–Connell effect in a subtropical forest. Ecol. Lett. 2012, 15, 111–118. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, J.; Wang, X.; Hao, G.; Wang, G.G. A systematic evaluation of gap size and within-gap position effects on seedling regeneration in a temperate secondary forest, Northeast China. For. Ecol. Manag. 2021, 490, 119140. [Google Scholar] [CrossRef]
- Du, X.; Dong, X.; Gu, H.; Chen, X. Diffuse radiation environment of regeneration seedlings and saplings under a broadleaved-Korean pine forest. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 145–156. [Google Scholar] [CrossRef]
- Zang, R.; Guo, Z.; Gao, W. Gap regeneration in a broadleaved-Korean pine forest in Changbai Mountain Natural Reserve. Chin. J. Appl. Ecol. 1998, 9, 349–353. [Google Scholar]
- Chen, J.Y. The Coarse Woody Debris Effect to Regeneration of Seedling in a Typical Mixed Broadleaved-Korean Pine Forest in Xiaoxing’an Mountains, China. Doctoral Dissertation, Northeast Forestry University, Harbin, China, 2016. [Google Scholar]
- Galasso, G.; Domina, G.; Adorni, M.; Ardenghi, N.M.G.; Banfi, E.; Bedini, G.; Bertolli, A.; Brundu, G.; Calbi, M.; Cecchi, L.; et al. Notulae to the Italian alien vascular flora: 1. Ital. Bot. 2016, 43, 39–64. [Google Scholar] [CrossRef][Green Version]
- Wright, J.S. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]

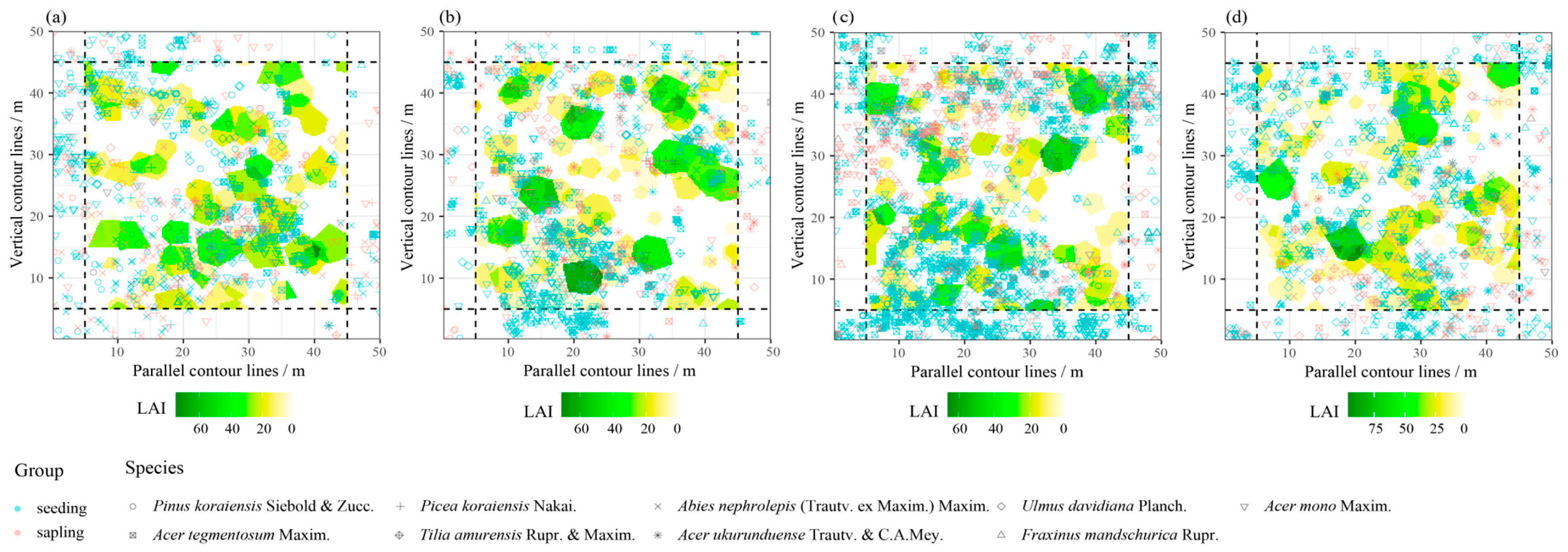

| Species | Average Local LAI | Local LAI Standard Deviation | Canopy Percent | |||
|---|---|---|---|---|---|---|
| Seeding | Sapling | Seeding | Sapling | Seeding | Sapling | |
| Pinus koraiensis | 9.991 ± 7.333 | 8.464 ± 4.868 | 9.634 ± 4.226 | 9.600 ± 4.115 | 0.541 ± 0.247 | 0.492 ± 0.208 |
| Picea koraiensis | 10.897 ± 7.668 | 11.869 ± 8.832 | 10.740 ± 4.590 | 10.489 ± 4.26 | 0.570 ± 0.208 | 0.585 ± 0.250 |
| Abies nephrolepis | 10.344 ± 7.432 | 9.018 ± 7.933 | 10.396 ± 4.821 | 9.192 ± 4.361 | 0.505 ± 0.260 | 0.476 ± 0.255 |
| Ulmus davidiana | 10.300 ± 8.825 | 7.175 ± 6.040 | 9.266 ± 5.17 6 | 7.840 ± 4.530 | 0.572 ± 0.243 | 0.442 ± 0.255 |
| Acer mono | 13.574 ± 10.604 | 11.501 ± 9.965 | 11.900 ± 6.674 | 10.728 ± 6.537 | 0.605 ± 0.239 | 0.520 ± 0.265 |
| Acer tegmentosum | 12.598 ± 10.011 | 8.572 ± 7.823 | 11.367 ± 6.092 | 9.267 ± 4.852 | 0.558 ± 0.259 | 0.432 ± 0.254 |
| Acer ukurunduense | 9.153 ± 7.517 | 8.737 ± 7.039 | 9.240 ± 6.398 | 10.086 ± 5.599 | 0.556 ± 0.269 | 0.46 ± 0.256 |
| Tilia amurensis | 15.857 ± 11.677 | 11.067 ± 9.214 | 12.839 ± 7.166 | 9.733 ± 4.531 | 0.636 ± 0.261 | 0.544 ± 0.265 |
| Fraxinus mandshurica | 12.999 ± 11.296 | 4.150 ± 2.426 | 11.285 ± 6.259 | 6.665 ± 2.218 | 0.561 ± 0.243 | 0.319 ± 0.172 |
| Species | Vertical Distribution Tendency Degree | Local Coniferous LAI | Local Broadleaved LAI | |||
| Seeding | Sapling | Seeding | Sapling | Seeding | Sapling | |
| Pinus koraiensis | 1.011 ± 0.302 | 0.882 ± 0.254 | 3.253 ± 5.827 | 0.904 ± 1.617 | 6.739 ± 5.540 | 7.560 ± 4.807 |
| Picea koraiensis | 0.954 ± 0.372 | 1.033 ± 0.376 | 5.717 ± 7.657 | 6.940 ± 7.479 | 5.180 ± 4.279 | 4.929 ± 4.127 |
| Abies nephrolepis | 1.071 ± 0.341 | 1.002 ± 0.341 | 4.967 ± 6.883 | 4.069 ± 7.113 | 5.377 ± 5.229 | 4.949 ± 5.017 |
| Ulmus davidiana | 1.040 ± 0.343 | 0.885 ± 0.348 | 5.676 ± 8.651 | 3.648 ± 5.654 | 4.624 ± 4.140 | 3.527 ± 2.735 |
| Acer mono | 1.194 ± 0.345 | 1.086 ± 0.401 | 10.031 ± 10.463 | 8.370 ± 9.919 | 3.543 ± 4.236 | 3.131 ± 3.449 |
| Acer tegmentosum | 1.099 ± 0.353 | 0.992 ± 0.372 | 7.631 ± 9.796 | 4.171 ± 7.023 | 4.967 ± 4.687 | 4.401 ± 4.568 |
| Acer ukurunduense | 1.017 ± 0.346 | 0.956 ± 0.348 | 6.362 ± 7.441 | 4.943 ± 6.302 | 2.791 ± 3.240 | 3.793 ± 4.065 |
| Tilia amurensis | 1.187 ± 0.311 | 0.953 ± 0.344 | 11.664 ± 10.084 | 6.426 ± 8.873 | 4.194 ± 4.666 | 4.641 ± 3.828 |
| Fraxinus mandshurica | 1.090 ± 0.370 | 0.893 ± 0.364 | 7.488 ± 9.122 | 1.247 ± 1.882 | 5.511 ± 6.555 | 2.903 ± 1.872 |
| Species | Average Local LAI | Local LAI Standard Deviation | ||||||
|---|---|---|---|---|---|---|---|---|
| Seeding | Sapling | Seeding | Sapling | |||||
| Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | |
| Pinus koraiensis | 1.077 | 0.075 | 1.003 | 0.486 | 1.050 | 0.174 | 1.064 | 0.214 |
| Picea koraiensis | 1.123 | 0.103 | 1.161 | 0.031 * | 1.166 | 0.044 * | 1.155 | 0.037 * |
| Abies nephrolepis | 1.082 | 0.003 ** | 0.958 | 0.247 | 1.128 | <0.001 *** | 1.005 | 0.469 |
| Ulmus davidiana | 1.046 | 0.192 | 0.828 | 0.012 * | 0.969 | 0.272 | 0.811 | 0.006 ** |
| Acer mono | 1.258 | <0.001 *** | 1.077 | 0.011 * | 1.215 | <0.001 *** | 1.085 | 0.006 ** |
| Acer tegmentosum | 1.183 | <0.001 *** | 0.909 | 0.047 * | 1.185 | <0.001 *** | 0.990 | 0.425 |
| Acer ukurunduense | 0.975 | 0.341 | 0.942 | 0.196 | 0.915 | 0.080 | 1.053 | 0.220 |
| Tilia amurensis | 1.321 | <0.001 *** | 1.080 | 0.156 | 1.264 | <0.001 *** | 1.046 | 0.281 |
| Fraxinus mandshurica | 1.182 | <0.001 *** | 0.574 | 0.013 * | 1.167 | <0.001 *** | 0.699 | 0.059 |
| Species | Canopy Percent | Vertical Distribution Tendency Degree | ||||||
| Seeding | Sapling | Seeding | Sapling | |||||
| Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | |
| Pinus koraiensis | 1.104 | 0.027 * | 1.004 | 0.480 | 0.970 | 0.284 | 0.772 | 0.002 ** |
| Picea koraiensis | 1.174 | 0.036 * | 1.200 | 0.010 * | 0.886 | 0.118 | 1.018 | 0.417 |
| Abies nephrolepis | 1.028 | 0.167 | 0.960 | 0.259 | 1.077 | 0.004 ** | 0.970 | 0.313 |
| Ulmus davidiana | 1.170 | 0.001 ** | 0.889 | 0.073 | 1.015 | 0.388 | 0.771 | 0.001 ** |
| Acer mono | 1.276 | <0.001 *** | 1.059 | 0.041 * | 1.312 | <0.001 *** | 1.103 | 0.001 ** |
| Acer tegmentosum | 1.150 | <0.001 *** | 0.866 | 0.006 ** | 1.125 | <0.001 *** | 0.941 | 0.139 |
| Acer ukurunduense | 1.138 | 0.013 * | 0.930 | 0.150 | 0.982 | 0.381 | 0.895 | 0.060 |
| Tilia amurensis | 1.316 | <0.001 *** | 1.104 | 0.095 | 1.271 | <0.001 *** | 0.879 | 0.062 |
| Fraxinus mandshurica | 1.154 | <0.001 *** | 0.620 | 0.024 * | 1.110 | <0.001 *** | 0.815 | 0.169 |
| Species | Local Coniferous LAI | Local Broadleaved LAI | ||||||
| Seeding | Sapling | Seeding | Sapling | |||||
| Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | |
| Pinus koraiensis | 0.830 | <0.001 *** | 0.660 | <0.001 *** | 1.301 | <0.001 *** | 1.442 | <0.001 *** |
| Picea koraiensis | 1.090 | 0.173 | 1.160 | 0.030 * | 1.210 | 0.015 * | 1.162 | 0.030 * |
| Abies nephrolepis | 0.992 | 0.390 | 0.912 | 0.071 | 1.111 | <0.001 *** | 1.071 | 0.124 |
| Ulmus davidiana | 1.035 | 0.251 | 0.826 | 0.010 * | 1.099 | 0.030 * | 1.000 | 0.500 |
| Acer mono | 1.364 | <0.001 *** | 1.158 | <0.001 *** | 0.880 | <0.001 *** | 0.865 | <0.001 *** |
| Acer tegmentosum | 1.129 | <0.001 *** | 0.912 | 0.050 | 1.100 | <0.001 *** | 1.001 | 0.490 |
| Acer ukurunduense | 1.106 | 0.039 * | 1.000 | 0.499 | 0.780 | <0.001 *** | 0.960 | 0.277 |
| Tilia amurensis | 1.406 | <0.001 *** | 1.015 | 0.423 | 1.001 | 0.493 | 1.121 | 0.063 |
| Fraxinus mandshurica | 1.141 | <0.001 *** | 0.688 | 0.051 | 1.095 | 0.002 ** | 0.937 | 0.372 |
| Species | PC1 Score | PC2 Score | ||
|---|---|---|---|---|
| p-Value | Cliff’s Delta | p-Value | Cliff’s Delta | |
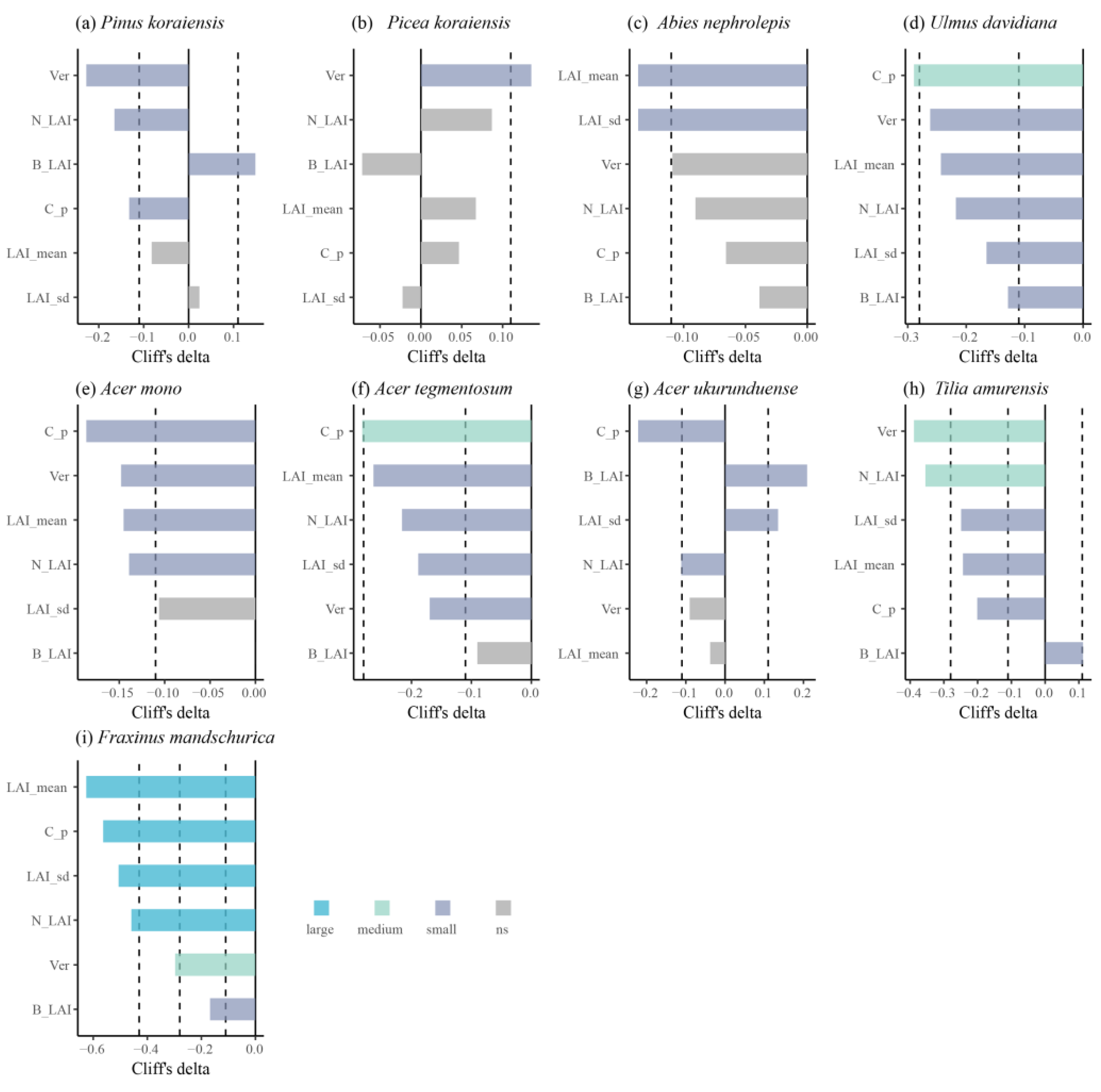
| Pinus koraiensis | 0.096 | −0.157 | 0.068 | −0.172 |
| Picea koraiensis | 0.570 | 0.073 | 0.564 | 0.074 |
| Abies nephrolepis | 0.027 * | −0.140 | 0.914 | 0.007 |
| Ulmus davidiana | 0.001 ** | −0.298 | 0.492 | 0.062 |
| Acer mono | <0.001 *** | −0.160 | 0.689 | −0.013 |
| Acer tegmentosum | <0.001 *** | −0.263 | 0.683 | 0.023 |
| Acer ukurunduense | 0.440 | −0.068 | 0.147 | −0.128 |
| Tilia amurensis | <0.001 *** | −0.329 | 0.021 * | −0.227 |
| Fraxinus mandshurica | 0.004 ** | −0.556 | 0.640 | 0.091 |
| Species | PC1 Score | PC2 Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Seeding | Sapling | Seeding | Sapling | |||||
| Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | Ratio of Average Rank | p-Value | |
| Pinus koraiensis | 1.036 | 0.251 | 0.909 | 0.129 | 0.721 | <0.001 *** | 0.532 | <0.001 *** |
| Picea koraiensis | 1.060 | 0.269 | 1.133 | 0.063 | 0.791 | 0.014 * | 0.861 | 0.051 |
| Abies nephrolepis | 1.084 | 0.002 ** | 0.960 | 0.258 | 0.924 | 0.003 ** | 0.933 | 0.138 |
| Ulmus davidiana | 1.046 | 0.190 | 0.789 | 0.003 ** | 0.912 | 0.044 * | 0.94 | 0.215 |
| Acer mono | 1.326 | <0.001 *** | 1.104 | 0.001 ** | 1.184 | <0.001 *** | 1.174 | <0.001 *** |
| Acer tegmentosum | 1.179 | <0.001 *** | 0.908 | 0.044 * | 0.965 | 0.099 | 0.988 | 0.413 |
| Acer ukurunduense | 1.024 | 0.348 | 0.947 | 0.218 | 1.112 | 0.034 * | 1.002 | 0.487 |
| Tilia amurensis | 1.344 | <0.001 *** | 1.014 | 0.430 | 1.119 | 0.030 * | 0.877 | 0.059 |
| Fraxinus mandshurica | 1.180 | <0.001 *** | 0.611 | 0.022 * | 0.949 | 0.051 | 1.031 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhang, Y.; Jiang, H.; Dong, X. Influence of Canopy Environmental Characteristics on Regen-eration of Nine Tree Species in Broadleaved Korean Pine Forests. Forests 2025, 16, 757. https://doi.org/10.3390/f16050757
Du X, Zhang Y, Jiang H, Dong X. Influence of Canopy Environmental Characteristics on Regen-eration of Nine Tree Species in Broadleaved Korean Pine Forests. Forests. 2025; 16(5):757. https://doi.org/10.3390/f16050757
Chicago/Turabian StyleDu, Xin, Yelin Zhang, Huiwu Jiang, and Xue Dong. 2025. "Influence of Canopy Environmental Characteristics on Regen-eration of Nine Tree Species in Broadleaved Korean Pine Forests" Forests 16, no. 5: 757. https://doi.org/10.3390/f16050757
APA StyleDu, X., Zhang, Y., Jiang, H., & Dong, X. (2025). Influence of Canopy Environmental Characteristics on Regen-eration of Nine Tree Species in Broadleaved Korean Pine Forests. Forests, 16(5), 757. https://doi.org/10.3390/f16050757





