Global Meta-Analysis of Mangrove Primary Production: Implications for Carbon Cycling in Mangrove and Other Coastal Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Screening
2.2. Data Analysis
3. Gross Primary Production
4. Aboveground Net Primary Production
4.1. Wood Production
4.2. Root Production
5. Total Tree Net Primary Production
6. Long-Term Trends in Mangrove Primary Production
7. Algal Primary Production
7.1. Microalgae and Cyanobacterial Mats
7.2. Macroalgae
8. A Revised Mangrove Carbon Budget
| Component | Inputs | Component | Outputs | ||
|---|---|---|---|---|---|
| Original | Current | Original | Current | ||
| Mangrove GPP | 20.0 | 19.7 | RCANOPY | 8.0 | 14.8 |
| Macroalgal GPP | 6.2 | 8.6 | RMACROALGAE | 2.9 | 4.0 |
| Microalgal GPP | 4.4 | 5.5 | RMICROALGAE | 2.3 | 2.9 |
| Sediment DIC production | 19.8 | 19.8 | RTIDAL WATER | 3.3 | 3.3 |
| Groundwater from terrestrial sources | 2.6 | 12.8 | RSEDIMENT | 6.7 | 6.7 |
| DICEXPORT | 24.8 | 24.8 | |||
| DOCEXPORT | 3.8 | 3.8 | |||
| POCEXPORT | 1.9 | 1.9 | |||
| Burial | 1.6 | 1.6 | |||
| CH4 AIR/SEA EXPORT | 0.06 | 0.06 | |||
| Total Net Flux | 53.0 | 66.4 | 55.4 | 63.9 | |
| Net Balance | −2.4 | 2.5 | |||
9. Ecosystem Contributions to Coastal Blue Carbon Fluxes
| Ecosystem | Area | RE | Global RE | GPP | Global GPP | Mean PGPP/RE | NEP | Global NEP |
|---|---|---|---|---|---|---|---|---|
| Mangroves | 14.5 | 1498 | 217 | 1971 | 286 | 1.3 | 473 | 69 |
| Salt marshes | 0.8 | 192 | 10 | 234 | 16 | 1.5 | 42 | 6 |
| Seagrasses | 18.1 | 2367 | 504 | 2853 | 608 | 1.2 | 486 | 103 |
| Macroalgae | 158.7 | 368 | 583 | 1294 | 2054 | 3.5 | 926 | 1469 |
| Tidal flats | 7.5 | 45 | 6 | 96 | 12 | 2.1 | 51 | 6 |
| Coral reefs | 34.8 | 912 | 546 | 998 | 599 | 1.1 | 86 | 49 |
| Totals | 234.4 | 5383 | 1866 | 7446 | 3575 | - | 2064 | 1702 |
| Mangrove contribution | 6% | 28% | 12% | 11% | 8% | - | 23% | 4% |
10. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Guo, J.; Shen, Y. Spatiotemporal evolution and impact mechanisms of gross primary productivity in the tropics. Forests 2024, 15, 913. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, J.; Han, J.; Zhang, T.; Yang, S.; Wang, J.; Prodhan, F.A.; Yao, F. Projected increases in global terrestrial net primary productivity loss caused by drought under climate change. Earth’s Future 2022, 10, e2022EF002681. [Google Scholar] [CrossRef]
- Twilley, R.R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Rovai, A. Productivity and carbon dynamics in mangrove wetlands. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–162. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon balance in salt marsh and mangrove ecosystems: A global synthesis. J. Mar. Sci. Eng. 2022, 8, 767. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Castañeda, E.; Barr, J.G.; Engel, V.; Fuentes, J.D.; Troxler, T.G.; Twilley, R.R.; Bouillon, S.; Smith, T.J., III; O’Halloran, T.L. Current methods to evaluate net primary production and carbon budgets in mangrove forests. In Methods in Biogeochemistry of Wetlands, Vol. 10; Delaune, R.D., Reddy, K.R., Richardson, C.J., Megonigal, J.P., Eds.; Wiley: Boston, MA, USA, 2013; pp. 253–288. [Google Scholar] [CrossRef]
- Gu, X.; Zhao, H.; Peng, C.; Guo, X.; Lin, Q.; Yang, Q.; Chen, L. The mangrove blue carbon sink potential: Evidence from three net primary production methods. For. Ecol. Manag. 2022, 504, 119848. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Twilley, R.R.; Day, J.W., Jr. Mangrove wetlands. In Estuarine Ecology, 2nd ed.; Day, J.W., Jr., Kemp, W.M., Yanez-Arancibia, A., Crump, B.C., Eds.; Wiley: Boston, MA, USA, 2012; pp. 165–202. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Suwa, R.; Khan, M.N.I.; Hagihara, A. Canopy photosynthesis, canopy respiration and surplus production in a subtropical mangrove Kandelia candel forest, Okinawa Island, Japan. Mar. Ecol. Prog. Ser. 2006, 320, 131–139. [Google Scholar] [CrossRef]
- Jayakody, J.M.A.L.; Amarasinghe, M.D.; Pahalawattaarachchiand, V.P.; De Silva, K.H.W.L. Vegetation structure and potential gross primary productivity of mangroves at Kadolkele in Meegamuwa (Negombo) estuary, Sri Lanka. Sri Lanka J. Aquat. Sci. 2008, 13, 95–108. [Google Scholar] [CrossRef]
- As-syakur, A.R.; Osawa, T.; Adnyana, I.W.S. Estimation of gross primary production using satellite data and GIS in urban area, Denpasar. Int. J. Remote Sens. Earth Sci. 2010, 7, 84–95. [Google Scholar] [CrossRef]
- Barr, J.G.; Engel, V.; Fuentes, J.D.; Fuller, D.O.; Kwon, H. Modelling light use efficiency in a subtropical mangrove forest equipped with CO2 eddy covariance. Biogeosciences 2013, 10, 2145–2158. [Google Scholar] [CrossRef]
- Chanda, A.; Akhand, A.; Manna, S.; Dutta, S.; Hazra, S.; Das, I.; Dadhwal, V.K. Characterizing spatial and seasonal variability of carbon dioxide and water vapour fluxes above a tropical mixed mangrove forest canopy, India. J. Earth Syst. Sci. 2013, 122, 503–513. [Google Scholar] [CrossRef]
- Chen, H.; Lu, W.; Yan, G.; Yang, S.; Lin, G. Typhoons exert significant but differential impacts on net ecosystem exchange of subtropical mangrove forests in China. Biogeosciences 2014, 11, 5323–5333. [Google Scholar] [CrossRef]
- Li, Q.; Lu, W.; Chen, H.; Luo, Y.; Lin, G. Differential responses of net ecosystem exchange of carbon dioxide to light and temperature between spring and neap tides in subtropical mangrove forests. Sci. World J. 2014, 2014, 943697. [Google Scholar] [CrossRef]
- Leopold, A.; Marchand, C.; Renchon, A.; Deborde, J.; Quiniou, T.; Allenbach, M. Net ecosystem carbon dioxide exchange in the ‘Coeur de Voh’ mangrove, New Caledonia: Effects of water stress on mangrove productivity in a semiarid climate. Agric. For. Meteorol. 2016, 223, 217–232. [Google Scholar] [CrossRef]
- Rodda, S.R.; Thumaty, K.C.; Jha, C.S.; Dadhwal, V.K. Seasonal variations of carbon dioxide, water vapor and energy fluxes in tropical Indian mangroves. Forests 2022, 7, 35. [Google Scholar] [CrossRef]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Shrestha, S.; Miranda, I.; Kumar, A.; Pardo, M.L.E.; Dahal, S.; Rashid, T.; Remillard, C.; Mishra, D.R. Identifying and forecasting potential biophysical risk areas within a tropical mangrove ecosystem using multi-sensor data. Int. J. Appl. Earth Obser. Geoinform. 2019, 74, 281–294. [Google Scholar] [CrossRef]
- Kripa, M.K. Modelling the gross primary productivity (GPP) of an Indian mangrove vegetation. Environ. Ecol. 2019, 37, 221–228. [Google Scholar]
- Liu, J.; Lai, D.Y.F. Subtropical mangrove wetland is a stronger carbon dioxide sink in the dry than wet seasons. Agric. For. Meteorol. 2019, 278, 107644. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Selvam, V.; Burman, P.K.D.; Chakraborty, S.; Karipot, A.; Nagarajan, R.; Ramasubramanian, R.; Song, Q.; Zhang, Y.; Grace, J. Seasonal variations of net ecosystem (CO2) exchange in the Indian tropical mangrove forest of Pichavaram. Estuar. Coast. Shelf Sci. 2020, 243, 106828. [Google Scholar] [CrossRef]
- Rasquinha, D.N.; Mishra, D.R. Tropical cyclones shape mangrove productivity gradients in the Indian subcontinent. Sci. Rep. 2021, 11, 17355. [Google Scholar] [CrossRef]
- Alvarado-Berrientos, M.S.; López-Adame, H.; Lazcano-Hernández, H.E.; Arellano-Verdejo, J.; Hernández-Arana, H.A. Ecosystem-atmosphere exchange of CO2, water, energy in a basin mangrove of the northeastern coast of the Yucatan Peninsula. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005811. [Google Scholar] [CrossRef]
- Kanniah, K.D.; Kang, C.S.; Sharma, S.; Amir, A.A. Remote sensing to study mangrove fragmentation and its impacts on leaf area index and gross primary productivity in the south of Peninsular Malaysia. Remote Sens. 2021, 13, 1427. [Google Scholar] [CrossRef]
- Lele, N.; Kripa, M.K.; Panda, M.; Das, S.K.; Nivas, A.H.; Divakaran, N.; Naik-Gaonkar, S.; Sawant, A.; Pattnaik, A.K.; Samal, R.N.; et al. Seasonal variation in photosynthetic rates and satellite-based GPP estimation over mangrove forest. Environ. Monit. Assess. 2021, 193, 61. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X. Response of mangrove carbon fluxes to drought stress detected by photochemical reflectance index. Remote Sens. 2021, 13, 4053. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, Y.; Zhang, Y.; Lu, X.; Liu, Z.; Weng, Q. Potential sun-induced chlorophyll fluorescence for indicating mangrove canopy photosynthesis. J. Geophys. Res Biogeosci. 2021, 126, e2020JG006159. [Google Scholar] [CrossRef]
- Rodda, S.R.; Thumaty, K.C.; Faraoda, R.; Jha, C.S.; Dadhwal, V.K. Unique characteristics of carbon dioxide exchange in Sundarbans mangrove forests and their relationship with environmental factors. Estuar. Coast. Shelf Sci. 2022, 267, 107764. [Google Scholar] [CrossRef]
- Romadhoni, L.S.R.; As-Syakur, A.R.; Hidayah, Z.; Wiyanto, D.B.; Safitri, R.; Utama, R.Y.S.; Wijana, I.M.S.; Anugrah, A.P.; Antara, I.M.O.G. Annual characteristics of gross primary productivity (GPP) in mangrove forest during 2016–2022 as revealed by Sentinel-s remote sensing imagery. IOP Conf. Ser. Earth Environ. Sci. 2022, 1016, 012051. [Google Scholar] [CrossRef]
- Burman, P.K.D.; Chakraborty, S.; El-Madany, T.S.; Ramasubramanian, R.; Gogoi, N.; Gnanamoorthy, P.; Murkute, C.; Nagarajan, R.; Karipot, A. A comparative study of ecohydrologies of a tropical mangrove and a broadleaf deciduous forest using eddy covariance measurement. Meteorol. Atmos. Phys. 2022, 134, 4. [Google Scholar] [CrossRef]
- Zhao, X.; Chunlin Wang, C.; Li, T.; Zhang, C.; Fan, X.; Zhang, Q.; Zhang, Q.; Chen, X.; Zou, X.; Shen, C.; et al. Net carbon dioxide and methane emissions from restored mangrove wetland: New insights based on a case study in estuary of the Pearl River, China. Sci. Total Environ. 2022, 811, 151619. [Google Scholar] [CrossRef]
- Zheng, Y.; Takeuchi, W. Estimating mangrove forest gross primary production by quantifying environmental stressors in the coastal area. Sci. Rep. 2022, 12, 2238. [Google Scholar] [CrossRef]
- Harahap, M.; Basyuni, M.; Sulistyono, N.; Sasmito, S.D.; Latifah, S.; Delvian Amelia, R.; Bimantara, Y.; Harahap, S.S.H.; Larekeng, S.H.; Sumarga, E.; et al. Carbon stock and carbon dioxide fluxes in various land covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia. Sustainability 2023, 15, 15196. [Google Scholar] [CrossRef]
- Kumar, T.; Das, P.K. Estimation of gross primary productivity of Indian Sundarbans mangrove forests using field measurements and Landsat 8 Operational Land Imager data. Trop. Ecol. 2023, 64, 167–179. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Chen, X.; Xiao, C.; Fan, X.; Shen, C.; Sun, M.; Shen, Z.; Zhang, Q. Variations in carbon dioxide and methane exchange in response to multiple biophysical factors from a mangrove wetland park in Southeastern China. Atmos 2024, 14, 805. [Google Scholar] [CrossRef]
- Wu, L.; Guo, E.; An, Y.; Xiong, Q.; Shi, X.; Zhang, X.; Sun, Z. Evaluating the losses and recovery of GPP in the subtropical mangrove forest directly attacked by tropical cyclone: Case study of Hainan Island. Forests 2023, 15, 2094. [Google Scholar] [CrossRef]
- Vargas-Terminel, M.L.; Rodríguez, J.C.; Yépez, E.A.; Robles-Zazueta, C.A.; Watts, C.; Garatuza-Payán, J.; Vargas, R.; Sanchez-Mejia, Z.M. Ecosystem-atmosphere carbon dioxide exchange from semiarid mangroves in the Gulf of California. J. Arid. Environ. 2024, 208, 104872. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Wang, J.; Zhen, J.; Shen, Z.; Xiang, K.; Xiang, H.; Wang, Y.; Wu, G. Spatiotemporal dynamics and geo-environmental factors influencing mangrove gross primary productivity during 2000-2020 in Gaoqiao Mangrove Reserve, China. For. Ecosyst. 2023, 10, 100137. [Google Scholar] [CrossRef]
- Gou, R.; Buchmann, N.; Chi, J.; Luo, Y.; Mo, L.; Shekhar, A.; Feigenwinter, I.; Hörtnagl, L.; Lu, W.; Cui, X.; et al. Temporal variations of carbon and water fluxes in a subtropical mangrove forest: Insights from a decade-long eddy covariance measurement. Agric. For. Meteorol. 2022, 343, 109764. [Google Scholar] [CrossRef]
- Burman, P.K.D.; Das Das, P. A data-driven approach to assess the impact of climate change on a tropical mangrove in India. J. Geophys. Res Biogeosci. 2024, 129, e2023JG007911. [Google Scholar] [CrossRef]
- Manne, M.; Rajitha, K.; Chakraborty, S.; Gnanamoorthy, P. A path analysis approach to model the gross primary productivity of mangroves using climate data and optimal indices. Model. Earth Syst. Environ. 2024, 10, 509–522. [Google Scholar] [CrossRef]
- Munoz-Albiter, M.E.; Velazquez-Rodriguez, A.S.; Sanchez-Mejia, Z.M. Seasonal gross primary production in response to environmental factors from arid mangroves in the Gulf of California. Remote Sens. Appl. Soc. Environ. 2024, 33, 101096. [Google Scholar] [CrossRef]
- Sun, Z.; An, Y.; Kong, J.; Zhao, J.; Cui, W.; Nie, T.; Zhang, T.; Liu, W.; Wu, L. Exploring the spatiotemporal patterns of global mangrove gross primary production and quantifying the factors affecting its estimation, 1996–2020. Sci. Total Environ. 2024, 908, 168262. [Google Scholar] [CrossRef]
- Wang, J.; Yu, G.; Han, L.; Yao, Y.; Sun, M.; Yan, Z. Ecosystem carbon exchange across China’s coastal wetlands: Spatial patterns, mechanisms, and magnitudes. Agric. For. Meteorol. 2024, 345, 109859. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, S.; Zhou, G.; Zhou, G.; Tieszen, L.L.; Baldocchi, D.; Bernhofer, C.; Gholz, H.; Goldstein, A.H.; Goulden, M.L.; et al. Deriving a light use efficiency model from eddy covariance flux data for predicting daily gross primary production across biomes. Agric. For. Meteorol. 2007, 143, 189–207. [Google Scholar] [CrossRef]
- Huang, M.; Piao, S.; Ciais, P.; Peñuelas, J.; Wang, X.; Keenan, T.F.; Peng, S.; Berry, J.A.; Wang, K.; Mao, J.; et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 2019, 3, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, T.; Yang, X.Q.; Chao, Q.; Wang, C.; Lai, D.Y.; Liu, J.; Zhu, X.; Zhao, X.; Fan, X.; et al. Mango-GPP: A process-based model for simulating gross primary productivity of mangrove ecosystems. J. Adv. Model. Earth Syst. 2023, 15, e2023MS003714. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Friess, D.A.; Wang, S.; Li, Y. Stronger increases but greater variability in global mangrove productivity compared to that of adjacent terrestrial forests. Nat. Ecol. Evol. 2024, 8, 239–250. [Google Scholar] [CrossRef]
- Chen, Y.; Kirwan, M.L. Rapid greening in mangroves. Nat. Ecol. Evol. 2024, 8, 186–187. [Google Scholar] [CrossRef]
- Golley, F.; Odum, H.T.; Wilson, R.F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef]
- Christensen, B. Biomass and primary production of Rhizophora apiculata Bl. in a mangrove forest in southern Thailand. Aquat. Bot. 1978, 4, 43–52. [Google Scholar] [CrossRef]
- Sukardjo, S.; Yamada, I. Biomass and productivity of a Rhizophora mucronata Lamarck plantation in Tritih, central Java, Indonesia. For. Ecol. Manag. 1992, 49, 195–209. [Google Scholar] [CrossRef]
- Amarasinghe, M.D.; Balasubramanium, S. Net primary productivity of two mangrove forest stands on the northwestern coast of Sri Lanka. Hydrobiologia 1992, 247, 37–47. [Google Scholar] [CrossRef]
- Ong, J.E. Mangroves—A carbon source and sink. Chemosphere 1993, 27, 1097–1107. [Google Scholar] [CrossRef]
- Ong, J.E.; Gong, W.K.; Clough, B.F. Structure and productivity of a 20-year-old stand of Rhizophora apiculata Bl. mangrove forest. J. Biogeogr. 1995, 22, 417–424. [Google Scholar] [CrossRef]
- Hossain, M.; Othman, S.; Bujang, J.S.; Kusnan, M. Net primary productivity of Bruguiera parviflora (Wright & Arn.) dominated mangrove forest at Kuala Selangor, Malaysia. For. Ecol. Manag. 2008, 255, 179–182. [Google Scholar] [CrossRef]
- Khan Md, N.I.; Suwa, R.; Hagihara, A. Biomass and aboveground net primary production in a subtropical mangrove stand of Kandelia obovata (S.,L.) Yong at Manko Wetland, Japan. Wetl. Ecol. Manag. 2009, 17, 585–599. [Google Scholar] [CrossRef]
- Poungparn, S.; Poungparn, S.; Komiyama, A.; Patanaponpaipoon, P.; Jintana, V.; Sangtiean, T.; Tanapermpool, P.; Piriyatota, S.; Maknual, C.; Kato, S. Site-independent allometric relationships for estimating above-ground weights of mangroves. Tropics 2002, 12, 147–158. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Kamara, M.; Sharma, S.; Hagihara, A. Stand structure, phenology and litterfall dynamics of a subtropical mangrove Bruguiera gymnorrhiza. J. For. Res. 2016, 27, 513–523. [Google Scholar] [CrossRef]
- Peng, C.J.; Qian, J.W.; Guo, X.D.; Zhao, H.W.; Hu, N.X.; Yang, Q.; Chen, C.P.; Chen, L.Z. Vegetation carbon stocks and net primary productivity of the mangrove forests in Shenzhen, China. J. Appl. Ecol. 2016, 27, 2059–2065. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Ahmed, S.; Osawa, A. Biomass and net primary productivity of mangrove communities along the oligohaline zone of the Sundarbans, Bangladesh. For. Ecosyst. 2017, 4, 16. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Mouctar, K.; Sharma, S.; Osawa, A. Comparison of biomass and net primary productivity among three species in a subtropical mangrove forest at Manko Wetland, Okinawa, Japan. Reg. Stud. Mar. Sci. 2019, 25, 100475. [Google Scholar] [CrossRef]
- Njana, M.A.; Zahabu, E.; Malimbwi, R.E. Carbon stocks and productivity of mangrove forests in Tanzania. South For. 2018, 80, 217–232. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Taylor, P.; Chadwick, K.D.; Dahlin, K.; Doughty, C.E.; Malhi, Y.; Smith, W.K.; Sullivan, B.W.; Wieder, W.R.; Townsend, A.R. A comparison of plot-based satellite and Earth system model estimates of tropical forest net primary production. Glob. Biogeochem. Cycles 2015, 29, 626–644. [Google Scholar] [CrossRef]
- Okimoto, Y.; Nose, A.; Ikeda, K.; Agarie, S.; Oshima, K.; Tateda, Y.; Ishii, T.; Nhan, D.D. An estimation of CO2 fixation capacity in mangrove forest using two methods of CO2 exchange and growth curve analysis. Wetl. Ecol. Manag. 2008, 16, 155–171. [Google Scholar] [CrossRef]
- Okimoto, Y.; Nose, A.; Oshima, K.; Tateda, Y.; Ishii, T. A case study for an estimation of carbon fixation capacity in the mangrove plantation of Rhizophora apiculata trees in Trat, Thailand. For. Ecol. Manag. 2013, 310, 1016–1026. [Google Scholar] [CrossRef]
- Analuddin, K.; La Ode, K.; La Ode, M.Y.H.; Andi, S.; Idin, S.; La, S.; Rahim, S.; La Ode, A.F.; Kazuo, N. Aboveground biomass, productivity and carbon sequestration in Rhizophora stylosa forest of southeast Sulawesi, Indonesia. Biodiversitas 2020, 21, 1316–1325. [Google Scholar] [CrossRef]
- Aksornkoae, S. Structure, Regeneration and Productivity of Mangroves in Thailand. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 1975. [Google Scholar]
- Robertson, A.I.; Daniel, P.A.; Dixon, P. Mangrove forest structure and productivity in the Fly River estuary, Papua New Guinea. Mar. Biol. 1991, 111, 147–155. [Google Scholar] [CrossRef]
- Cox, E.F.; Allen, J. Stand Structure and Productivity of the Rhizophora mangle in Hawaii; USDA Forest Service/UNL Faculty Publications; USDA: Washington, DC, USA, 1999; Volume 43, pp. 1–64. [Google Scholar]
- Alongi, D.M.; Dixon, P. Mangrove primary production and above and below-ground biomass in Sawi Bay, southern Thailand. Phuket Mar. Biol. Cent. Spec. Publ. 2000, 22, 31–38. [Google Scholar]
- Alongi, D.M.; Sasekumar, A.; Chong, V.C.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Brunskill, G.J. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in Peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Kairo, J.G.; Lang’at, J.K.; Dahdouh-Guebas, F.; Bosire, J.; Karachi, M. Structural development and productivity of replanted mangrove plantations in Kenya. For. Ecol. Manag. 2008, 255, 2670–2677. [Google Scholar] [CrossRef]
- Selvam, P.P.; Geevarghese, G.A.; Ramachandran, P.; Ramachandran, R. Spatial assessment of net canopy photosynthetic rates and species diversity in Pichavaram mangrove forest, Tamil Nadu. Indian J. Ecol. 2018, 45, 717–723. [Google Scholar]
- Sahu, S.K.; Kathiresan, K. The age and species composition of mangrove forest directly influence the net primary productivity and carbon sequestration potential. Biocatal. Agric. Biotechnol. 2019, 20, 101235. [Google Scholar] [CrossRef]
- Sasekumar, A.; Loi, J.J. Litter production in three mangrove forest zones in the Malay Peninsula. Aquat. Bot. 1983, 17, 283–290. [Google Scholar] [CrossRef]
- Alam, R.; Mahmood, H.; Biswas, T.; Rahman, M. Physiologically adaptive plasticity in nutrient resorption efficiency of Avicennia officinalis L. under fluctuating saline environments in the Sundarbans of Bangladesh. Hydrobiologia 2019, 828, 41–56. [Google Scholar] [CrossRef]
- Saenger, P.; Snedaker, S.C. Pantropical trends in mangrove above-ground biomass and annual litterfall. Oecologia 1993, 96, 293–299. [Google Scholar] [CrossRef]
- May, J.D. Spatial variation in litter production by the mangrove Avicennia marina var. australasica in Rangaunu harbour, New Zealand. N. Z. J. Mar. Freshw. Res. 1999, 33, 162–173. [Google Scholar] [CrossRef]
- Utrera-Lopez, M.; Moreno-Casasola, P. Mangrove litter dynamics in La Mancha Lagoon, Veracruz, Mexico. Wetl. Ecol. Mang. 2008, 16, 11–22. [Google Scholar] [CrossRef]
- Bernini, E.; Rezende, C.E. Litterfall in a mangrove in Southeast Brazil. Pan-American J. Aquat. Sci. 2010, 5, 508–519. [Google Scholar]
- Sanchez-Andres, R.; Sánchez-Carrillo, S.; Alatorre, L.C.; Cirujano, S.; Álvarez-Cobelas, M. Litterfall dynamics and nutrient decomposition of arid mangrove in the Gulf of California: Their role in sustaining ecosystem heterotrophy. Estuar. Coast. Shelf Sci. 2010, 89, 191–199. [Google Scholar] [CrossRef]
- Agraz Hernández, C.M.; García Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno Casasola, P. Forest structure, productivity and species phenology of mangrove in the La Mancha lagoon in the Atlantic coast of Mexico. Wetl. Ecol. Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Metcalfe, K.N.; Franklin, D.C.; McGuinness, K.A. Mangrove litter fall: Extrapolation from traps to a large tropical macrotidal harbour. Estuar. Coast. Shelf Sci. 2011, 95, 245–252. [Google Scholar] [CrossRef]
- Coronado-Molina, C.; Alvarez-Guillen, H.; Day, J.W.; Reyes, E.; Perez, B.C.; Vera-Herrera, F.; Twilley, R. Litterfall dynamics in carbonate and deltaic mangrove ecosystems in the Gulf of Mexico. Wetl. Ecol. Manag. 2012, 20, 1230136. [Google Scholar] [CrossRef]
- Farooqui, Z.A.F.A.R.; Shafique, S.; Khan, K.L.; Ali, A.; Iqbal, P.; Siddiqui, P.J. Assessment of litter production in semi-arid mangrove forests near active Indus River mouth (Hajambro Creek) and Karachi Backwaters, Pakistan. Pakistan J. Bot. 2012, 44, 1763–1768. [Google Scholar]
- Lopen-Medellin, X.; Ezcurra, E. The productivity of mangrove in northwestern Mexico: A meta-analysis of current data. J. Coast. Conserv. 2012, 16, 399–403. [Google Scholar] [CrossRef]
- Poungparn, S.; Komiyama, A.; Sangteian, T.; Maknual, C.; Patanaponpaiboon, P.; Suchewaboripont, V. High primary productivity under submerged soil raises the net ecosystem productivity of a secondary mangrove forest in eastern Thailand. J. Trop. Ecol. 2012, 28, 303–306. [Google Scholar] [CrossRef]
- Sharma, S.; Hoque, A.R.; Analuddin, K.; Hagihara, A. Litterfall dynamics in an overcrowded mangrove Kandelia obovata (S.L.) Young stand over five years. Estuar. Coast. Shelf Sci. 2012, 98, 31–41. [Google Scholar] [CrossRef]
- Adame, M.F.; Zaldívar-Jimenez, A.; Teutli, C.; Caamal, J.P.; Andueza, M.T.; López-Adame, H.; Cano, R.; Hernández-Arana, H.A.; Torres-Lara, R.; Herrera-Silveira, J.A. Drivers of mangrove litterfall within a karstic region affected by frequent hurricanes. Biotropica 2013, 45, 147–154. [Google Scholar] [CrossRef]
- Sakardjo, S.; Alongi, D.M.; Kusmana, C. Rapid litter production and accumulation in Bornean mangrove forests. Ecosphere 2013, 4, 79. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, Y.P.; Chen, G.C. Litter production and litter elemental composition in two rehabilitated Kandelia obovata mangrove forests in Jiulongjiang estuary, China. Mar. Environ. Res. 2013, 83, 63–72. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Yang, Q.; Tam, N.F.; Liao, W.; Zan, Q. Long-term differences in annual litter production between alien (Sonneratia apetala) and native (Kandelia obovata) mangrove species in Futian, Shenzhen, China. Mar. Pollut. Bull. 2014, 85, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Ntyam, S.C.O.; Armah, A.K.; Ajonina, G.N.; George, W.; Adomako, J.K.; Elvis, N.; Obiang, B.O. Importance of mangrove litter production in the protection of Atlantic coastal forest of Cameroon and Ghana. In The Land/Ocean Interactions in the Coastal Zone of West and Central Africa; Diop, S., Barusseau, J.-P., Descamps, C., Eds.; Springer: Cham, Switzerland, 2014; pp. 123–137. [Google Scholar] [CrossRef]
- Wang’ondu, V.W.; Bosire, J.O.; Kairo, J.G.; Kinyamario, J.I.; Mwaura, F.B.; Dahdouh-Guebas, F.; Koedam, N. Litterfall dynamics of restored mangroves (Rhiophora mucronata Lamk. and Sonneratia alba) in Kenya. Restor. Ecol. 2014, 22, 824–831. [Google Scholar] [CrossRef]
- Hoque, M.M.; Mustafa Kamal, A.H.; Idris, M.H.; Haruna Ahmed, O.; Rafiqul Hoque, A.T.M.; Masum Billah, M. Litterfall production in a tropical mangrove of Sarawak, Malaysia. Zool. Ecol. 2015, 25, 157–165. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Paul, S.K.; Ahmed, S.; Azad, M.S.; Osawa, A. Phenology and litterfall production of Bruguiera sexangula (Lour.) Pour. In the Sundarbans mangrove forests, Bangladesh. For. Sci. Technol. 2019, 15, 165–172. [Google Scholar] [CrossRef]
- Rani, V.; Sreelekshmi, S.; Preethy, C.M.; Bijoy Nandan, S. Phenology and litterfall dynamics structuring ecosystem productivity in a tropical mangrove stand on southwest coast of India. Reg. Stud. Mar. Sci. 2016, 8, 400–407. [Google Scholar] [CrossRef]
- Mulya, M.B.; Arlen, H.J. Production of litter and detritus related to the density of mangrove. IOP Conf. Ser. Earth Environ. Sci. 2018, 130, 012033. [Google Scholar] [CrossRef]
- Torres, J.R.; Barba, E.; Choix, F.J. Mangrove productivity and phenology in relation to hydroperiod and physical chemistry properties of water and sediment in Biosphere Reserve, Centla Wetland, Mexico. Trop. Conserv. Sci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Dewiyanti, I.; Nurfadillah, N.; Setiawati, T.; Yanti, F.; Ebrahimi, S.A. Litter production and decomposition of mangrove in the northern coast of Aceh Besar district, Aceh province. IOP Conf. Ser. Mat. Sci. Eng. 2019, 567, 012025. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomotsune, M.; Suchewaboripont, V.; Iimura, Y.; Kida, M.; Yoshitake, S.; Kondo, M.; Kinjo, K. Stand dynamics and aboveground net primary productivity of a mature subtropical mangrove forest on Ishigaki Island, southwestern Japan. Reg. Stud. Mar. Sci. 2019, 27, 100516. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.G.; Lluch-Cota, S.E.; Rivera-Monroy, V.H.; Lluch-Cota, D.B.; Troyo-Diéguez, E.; Oechel, W.; Serviere-Zaragoza, E. Mangrove wetland productivity and carbon stocks in an arid zone of the Gulf of California (La Paz Bay, Mexico). For. Ecol. Manag. 2019, 442, 135–147. [Google Scholar] [CrossRef]
- Azad, M.d.S.; Kamruzzaman, M.; Paul, S.K.; Kanzaki, M. Litterfall release, vegetative, and reproductive phenology investigation of Heritiera fomes Buch-Ham in the Sundarbans mangrove forests, Bangladesh: Relationship with environmental variables. For. Sci. Technol. 2020, 16, 105–115. [Google Scholar] [CrossRef]
- Robertson, A.I.; Dixon, P.; Zagorskis, I. Phenology and litter production in the mangrove genus Xylocarpus along rainfall and temperature gradients in tropical Australia. Mar. Freshw. Res. 2020, 72, 551–562. [Google Scholar] [CrossRef]
- Robertson, A.I.; Dixon, P.; Daniel, P.A.; Zagorskis, I. Primary production in forests of the mangrove palm Nypa fruticans. Aquat. Bot. 2020, 16, 103288. [Google Scholar] [CrossRef]
- Dangan-Galon, F.; Dolorosa, R.G.; Seguerra, R.O. Mangrove leaf litter production in the Iwahig River estuary ecosystem of Princess Princesa Bay, Palawan, The Philippines. Palawan Sci. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Dewiyani, I.; El Rahimi, S.A.; Damora, A.; Ulfah, M. Mangrove litter production in correlation to environmental properties of water in Pusong Cium, Seruway, Aceh Tamiang. IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 012030. [Google Scholar] [CrossRef]
- de Oliveira Gomes, I.E.; Vescovi, L.C.; Bernardino, A.F. The collapse of mangrove litterfall production following a climate-related forest loss in Brazil. Mar. Pollut. Bull. 2021, 453, 110910. [Google Scholar] [CrossRef]
- Torres, J.R.; Sánchez-Mejía, Z.M.; Arreola-Lizárraga, J.A.; Galindo-Félix, J.I.; Mascareño-Grijalva, J.J.; Rodríguez-Pérez, G. Environmental factors controlling structure, litter productivity, and phenology of mangroves in arid region of the Gulf of California. Acta Oecol. 2022, 117, 103861. [Google Scholar] [CrossRef]
- Abohasassan, R.A. Litterfall production and nutrient dynamics in mangrove systems on the Red Sea coast of Saudi Arabia. JKAU Meterol. Environ. Arid. Land Agric. Sci. 2023, 32, 15–35. [Google Scholar] [CrossRef]
- Cintron-Molero, G.; Coelho, C., Jr.; Paiva, L.M.; Schaeffer-Novelli, Y. Latitudinal variations in Brazilian mangroves’ annual litterfall as evidence of maximum power and geo-ecological manifestation. In Brazilian Mangroves and Salt Marshes; Schaeffer-Novelli, Y., Abuchahla, G.M.d.O., Cintron-Molero, G., Eds.; Springer: Cham, Switzerland, 2023; pp. 99–111. [Google Scholar] [CrossRef]
- Dali, G.L.A. Litter production in two mangrove forests along the coast of Ghana. Heliyon 2023, 9, e17004. [Google Scholar] [CrossRef]
- Purnobasuki, H.; Amaliyah, S.; Kobayashi, K. Production and decomposition of mangrove species Rhizophora apiculata Blume in Surabaya east coast, Indonesia. Borneo J. Resour. Sci. Technol. 2023, 13, 53–66. [Google Scholar] [CrossRef]
- Rumondang, A.L.; Kusmana, C.E.C.E.P.; Wilarso, S. Mangrove litterfall productivity in the all-high tides area of Angke Kapuk protected mangrove forest, Jakarta. Seybold Rep. 2023, 18, 1742–1754. [Google Scholar] [CrossRef]
- Nyamsi-Moussian, L.; Gordon, A.N.; Essome-Koum, G.L.; Kotte-Mapoko, E.F.; Boubakary Konango-Same, A.; Ngo-Massou, V.M.; Shangguan, Z.; Wang, M.Y. Assessment of standing biomass and litterfall production in reforested mangrove stands within Doula-Edea National Park (Cameroon). J. Mar. Biol. Environ. Sci. 2024, 5, 117–129. [Google Scholar]
- Shij, K.; Suresh, S.; Jilesh, V.; Nayar, T.S. Leaf litter production and decomposition in a riverine mangrove forest in India. Wetl. Ecol. Manag. 2024, 32, 59–77. [Google Scholar] [CrossRef]
- Kresnasari, D.; Zainuri, M.; Muskanafola, M.R.; Pribadi, R.; Wiarta, R. Litter productivity dynamics in Segara Anakan mangrove forest, Cilacap, Indonesia. Indonesia J. Mar. Sci. 2025, 30, 152–162. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castaneda, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycle 2008, 22, GB2013. [Google Scholar] [CrossRef]
- Adame, M.F.; Cormier, N.; Taillardat, P.; Iram, N.; Rovai, A.; Sloey, T.M.; Yando, E.S.; Blanco-Libreros, J.F.; Arnaud, M.; Jennerjahn, T.; et al. Deconstructing the mangrove carbon cycle: Gains, transformation, and losses. Ecosphere 2024, 15, e4806. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Ribeiro, R.D.A.; Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E. Spatial variability of mangrove primary productivity in the neotropics. Ecosphere 2019, 10, e02841. [Google Scholar] [CrossRef]
- Ouyang, X.; Guo, F. Patterns of mangrove productivity and support for marine fauna. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1783–1802. [Google Scholar] [CrossRef]
- Chatting, M.; Al-Maslamani, I.; Walton, M.; Skov, M.W.; Kennedy, H.; Husrevoglu, S.; Le Vay, L. Past, present and future global mangrove primary productivity. Sci. Total Environ. 2024, 957, 177446. [Google Scholar] [CrossRef]
- Velázquez-Pérez, C.; Romero-Berny, E.I.; Miceli-Méndez, C.L.; Moreno-Casasola, P.; López, S. Geoforms and biogeography defining mangrove primary productivity: A meta-analysis for the American Pacific. Forests 2024, 15, 1215. [Google Scholar] [CrossRef]
- Walcker, R.; Gandois, L.; Proisy, C.; Corenblit, D.; Mougin, E.; Laplanche, C.; Ray, R.; Fromard, F. Control of “blue carbon” storage by mangrove aging: Evidence from a 66-year chronosequence in French Guiana. Glob. Change Biol. 2018, 11, 93. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, W.; Dong, W.; Liu, S. Seasonal patterns of litterfall in forest ecosystems worldwide. Ecol. Complex. 2014, 20, 240–247. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Midway, S.R.; Friess, D.A.; Trettin, C.C.; Bukoski, J.J.; Stovall, A.E.; Pagliosa, P.R.; Fonseca, A.L.; et al. Macroecological patterns of forest structure and allometric scaling in mangrove forests. Glob. Ecol. Biogeogr. 2021, 30, 1000–1013. [Google Scholar] [CrossRef]
- Xiong, Y.; Cakir, R.; Phan, S.M.; Ola, A.; Krauss, K.W.; Lovelock, C.E. Global patterns of tree stem growth and stand aboveground wood production in mangrove forests. For. Ecol. Manag. 2019, 444, 382–392. [Google Scholar] [CrossRef]
- Banin, L.; Lewis, S.L.; Lopez-Gonzalez, G.; Baker, T.R.; Quesada, C.A.; Chao, K.J.; Burslem, D.F.; Nilus, R.; Abu Salim, K.; Keeling, H.C.; et al. Tropical forest wood production: A cross-continental comparison. J. Ecol. 2014, 102, 1025–1037. [Google Scholar] [CrossRef]
- Day, J.W., Jr.; Conner, W.H.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The productivity and composition of mangrove forests, Laguna de Terminos, Mexico. Aquat. Bot. 1987, 27, 267–284. [Google Scholar] [CrossRef]
- Imbert, D.; Rollet, B. Phytomasse aeinne et production primaire dans la mangrove du Grande Cul-de-Sac Marin (Guateloupe, Antilles, Francaise). Bull. Ecol. 1989, 20, 27–39. [Google Scholar]
- Lee, S.Y. Primary productivity and particulate organic matter flow in an estuarine mangrove-wetland in Hong Kong. Mar. Biol. 1990, 106, 453–463. [Google Scholar] [CrossRef]
- Day, J.W., Jr.; Coronado-Molina, C.; Vera-Herrera, F.R.; Twilley, R.; Rivera-Monroy, V.H.; Alvarez-Guillen, H.; Day, R.; Conner, W. A 7-year record of above-ground net primary production in a southeastern Mexican mangrove forest. Aquat. Bot. 1996, 55, 39–60. [Google Scholar] [CrossRef]
- Li, M.S. Nutrient dynamics of a Futian mangrove forest in Shenzhen, south China. Estuar. Coast. Shelf Sci. 1997, 45, 463–472. [Google Scholar] [CrossRef]
- Ross, M.S.; Ruiz, P.L.; Telesnicki, G.J.; Meeder, J.F. Estimating above-ground biomass and production in mangrove communities of Biscayne National Park, Florida (U.S.A.). Wetl. Ecol. Manag. 2001, 9, 27–37. [Google Scholar] [CrossRef]
- Sherman, R.E.; Fahey, T.J.; Martinez, P. Spatial patterns of biomass and aboveground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosyst 2003, 6, 384–398. [Google Scholar] [CrossRef]
- Ong, J.E.; Gong, W.K.; Wong, C.H. Allometry and partitioning of the mangrove, Rhizophora apiculata. For. Ecol. Manag. 2004, 188, 395–408. [Google Scholar] [CrossRef]
- Ewe, S.M.; Gaiser, E.E.; Childers, D.L.; Iwaniec, D.; Rivera-Monroy, V.H.; Twilley, R.R. Spatial and temporal patterns of aboveground net primary productivity (ANPP) along two freshwater-estuarine transects in the Florida Coastal Everglades. Hydrobiologia 2006, 569, 459–474. [Google Scholar] [CrossRef]
- Alongi, D.M. Patterns of mangrove wood and litter production within a beach ridge-fringing reef embayment, Northern Great Barrier Reef coast. Estuar. Coast. 2011, 34, 32–44. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H. Allocation of biomass and net primary productivity of mangrove forests along environment gradients in the Florida Coastal Everglades, USA. For. Ecol. Manag. 2013, 307, 226–241. [Google Scholar] [CrossRef]
- Enoki, T.; Yasuda, K.; Kusumoto, B. Aboveground net primary production and stand dynamics of mangroves along a river on Iriomote Island, southwestern Japan. Tropics 2014, 23, 91–98. [Google Scholar] [CrossRef]
- Njana, M.A.; Bollandsås, O.M.; Eid, T.; Zahabu, E.; Malimbwi, R.E. Above- and belowground tree biomass models for three mangrove species in Tanzania: A nonlinear mixed effects modelling approach. Ann. For. Sci. 2016, 73, 353–369. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Osawa, A.; Deshar, R.; Sharma, S.; Mouctar, K. Species composition, biomass, and net primary productivity of mangrove forest in Okukubi River, Okinawa Island, Japan. Reg. Stud. Mar. Sci. 2017, 12, 19–27. [Google Scholar] [CrossRef]
- Hassan, M.K. Management practices and aboveground biomass production patterns of Rhizophora apiculata plantation: Study from a mangrove area in Samut Songkram Province, Thailand. BioResources 2018, 13, 7826–7850. [Google Scholar] [CrossRef]
- Phan, S.M.; Nguyen, H.T.T.; Nguyen, T.K.; Lovelock, C. Modelling above ground biomass accumulation of mangrove plantations in Vietnam. For. Ecol. Manag. 2019, 432, 376–386. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Danielson, T.M.; Castañeda-Moya, E.; Marx, B.D.; Travieso, R.; Zhao, X.; Gaiser, E.E.; Farfan, L.M. Long-term demography and stem productivity of Everglades mangrove forests (Florida, USA): Resistance to hurricane disturbance. For. Ecol. Manag. 2019, 440, 79–91. [Google Scholar] [CrossRef]
- Cuc, N.T.K.; Hien, H.T. Stand structure and above ground biomass of Kandelia obovata Sheue, H.Y. Liu & J. Yong mangrove plantations in Northern Viet Nam. For. Ecol. Manag. 2021, 483, 118720. [Google Scholar] [CrossRef]
- Mugi, L.M.; Kiss, D.; Kairo, J.G.; Huxham, M.R. Stocks and productivity of dead wood in mangrove forests: A systematic literature review. Front. For. Glob. Change 2022, 5, 767337. [Google Scholar] [CrossRef]
- Robertson, A.I.; Zagorskis, I.; Logan, M. Environmental predictors of forest structure, tree growth and wood production for Xylocarpus granatum in mixed-species mangrove forests. Mar. Freshw. Res. 2024, 75, MF23246. [Google Scholar] [CrossRef]
- Sillanpää, M.; Vantellingen, J.; Friess, D.A. Vegetation regeneration in a sustainably harvested mangrove forest in West Papua, Indonesia. For. Ecol. Manag. 2017, 390, 137–146. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Change Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Poungparn, S.; Komiyama, A.; Umnouysin, S.; Rodtassana, C.; Sangtiean, T.; Maknual, C.; Pravinvongvuthi, T.; Suchewaboripont, V.; Kato, S. Ten-year estimation of net primary productivity in a mangrove forest under a tropical monsoon climate in eastern Thailand: Significance of the temperature environment in the dry season. Forests 2020, 11, 987. [Google Scholar] [CrossRef]
- Krauss, K.W.; Keeland, B.D.; Allen, J.A.; Ewel, K.C.; Johnson, D.J. Effects of season, rainfall, and hydrogeomorphic setting on mangrove tree growth in Micronesia. Biotropica 2007, 39, 161–170. [Google Scholar] [CrossRef]
- Arnaud, M.; Krause, S.; Norby, R.J.; Dang, T.H.; Acil, N.; Kettridge, N.; Gauci, V.; Ullah, S. Global mangrove root production, its controls and roles in the blue carbon budget of mangroves. Glob. Change Biol. 2023, 29, 3256–3270. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ono, K.; Watanabe, S.; Taniguchi, S.; Inoue, T.; Kanayama, K.; Ogawa, T. Estimation of probable annual fine root production and missing dead roots associated with the ingrowth core methods: Attempt with major mangrove species on Iriomote Island, southwestern Japan. Mangrove Sci. 2021, 12, 11–14. [Google Scholar]
- Noguchi, K.; Poungparn, S.; Umnouysin, S.; Patanaponpaiboon, P.; Duangnamol, D.; Yoneda, R.; Utsugi, H.; Sato, T.; Tabuchi, R. Biomass and production rates of fine roots in two mangrove stands in southern Thailand. JARQ 2020, 54, 349–360. [Google Scholar] [CrossRef]
- Ono, K.; Fujimoto, K.; Hirata, Y.; Tabuchi, R.; Taniguchi, S.; Furukawa, K.; Watanabe, S.; Suwa, R.; Lihpai, S. Estimation of total fine root production using continuous inflow methods in tropical mangrove forest on Pohnpei Island, Micronesia: Fine root necromass accumulation is a substantial contributor to blue carbon stocks. Ecol. Res. 2022, 37, 33–52. [Google Scholar] [CrossRef]
- Arnaud, M.; Morris, P.J.; Baird, A.J.; Dang, H.; Nguyen, T.T. Fine root production in a chronosequence of mature reforested mangroves. New Phytol. 2021, 232, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystem in relation to stand and environmental characteristics. For. Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Huaraca, H.W.; Riutta, T.; Girardin, C.A.; Hancco Pacha, F.; Puma Vilca, B.L.; Moore, S.; Rifai, S.W.; del Aguila-Pasquel, J.; Araujo Murakami, A.; Freitag, R.; et al. Fine root dynamics across pantropical rainforest ecosystems. Glob. Change Biol. 2021, 27, 3657–3680. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc. Royal. Soc. B 2012, 279, 3796–3802. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Phil. Trans. Roy Soc. B Biol. Sci. 2011, 366, 3225–3245. [Google Scholar] [CrossRef]
- Twilley, R.R.; Chen, R.H.; Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical carbon ecosystems. Water Air Soil Pollut. 1992, 64, 265–288. [Google Scholar] [CrossRef]
- Lugo, A.E.; Brown, S.; Brinson, M.M. Forested wetlands in freshwater and saltwater environments. Limnol. Oceanogr. 1988, 33, 894–909. [Google Scholar] [CrossRef]
- Alongi, D.M. Impacts of climate change on blue carbon stocks and fluxes in mangrove forests. Forests 2022, 13, 149. [Google Scholar] [CrossRef]
- Alongi, D.M.; Mukhopadhyay, S.K. Contributions of mangroves to coastal carbon cycling in low latitude seas. Agric. For. Meteorol. 2015, 213, 266–272. [Google Scholar] [CrossRef]
- Alongi, D.M. Zonation and seasonality of benthic primary production and community respiration in tropical mangrove forests. Oecologia 1994, 98, 320–327. [Google Scholar] [CrossRef]
- Kwon, B.-O.; Kim, H.; Noh, J.; Lee, S.Y.; Nam, J.; Khim, J.S. Spatiotemporal variability in microphytobenthic across bare intertidal flat, saltmarsh, and mangrove forest of Asia and Australia. Mar. Pollut. Bull. 2020, 151, 110707. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.P.; Ouyang, X.; Lai, D.Y.; Lee, S.Y. The microphytobenthos are abundant and mediate key carbon fluxes in tropical mangroves. Estuar. Coast. 2024, 47, 963–980. [Google Scholar] [CrossRef]
- Joye, S.B.; Lee, R.Y. Benthic microbial mats: Important sources of fixed nitrogen and carbon to the Twin Cays, Belize ecosystem. Atoll Res. Bull. 2004, 528, 1–24. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Grinham, A.; Adame, M.F.; Penrose, H.M. Elemental composition and productivity of cyanobacterial mats in an arid zone estuary in northwestern Australia. Wetl. Ecol. Manag. 2010, 18, 37–47. [Google Scholar] [CrossRef]
- Lyimo, T.J. The microalgae and cyanobacteria of Chwaka Bay. In People, Nature and Research in Chwaka Bay, Tanzania; WIOMSA: Zanzibar, Tanzanian, 2012; pp. 125–141. [Google Scholar]
- Lovelock, C.E.; Simpson, L.T.; Duckett, L.J.; Feller, I.C. Carbon budgets for Caribbean mangrove forests of varying structure and with phosphorus enrichment. Forests 2015, 6, 3528–3546. [Google Scholar] [CrossRef]
- Steinke, T.D.; Naidoo, Y. Biomass of algae epiphytic on pneumatophores of the mangrove, Avicennia marina, in the St. Lucia estuary. S. Afr. J. Bot. 1990, 56, 226–232. [Google Scholar] [CrossRef]
- Schories, D.; Mehlig, U. CO2 gas exchange of benthic microalgae during exposure to air: A technique for the rapid assessment of primary production. Wetl. Ecol. Manag. 2000, 8, 273–280. [Google Scholar] [CrossRef]
- Koch, M.S.; Madden, C.J. Patterns of primary production and nutrient availability in a Bahamas lagoon with fringing mangrove. Mar. Ecol. Prog. Ser. 2001, 219, 109–119. [Google Scholar] [CrossRef]
- Rajesh, K.M.; Gowda, G.; Mendon, M.R.; Gupta, T.R.C. Primary production of benthic microalgae in the tropical semi-enclosed brackish water pond, southwest coast of India. Asian Fish Sci. 2001, 14, 357–366. [Google Scholar]
- Borje, A. Microphytobenthic Productivity in Mangrove Areas of Different Replanting Regimes, Gazi Bay, Kenya. Master’s Thesis, Stockholm University, Stockholm, Sweden, 2004; pp. 1–22. [Google Scholar]
- Kristensen, E.; Flindt, M.R.; Ulomi, S.; Borges, A.V.; Abril, G.; Bouillon, S. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 2008, 370, 53–67. [Google Scholar] [CrossRef]
- Leopold, A.; Marchand, C.; Deborde, J.; Chaduteau, C.; Allenbach, M. Influence of mangrove zonation on CO2 fluxes at the sediment-air interface (New Caledonia). Geoderma 2013, 202–203, 62–70. [Google Scholar] [CrossRef]
- Castellantos-Galindo, G.A.; Cantera, J.; Valencia, N.; Giraldo, S.; Peña, E.; Kluger, L.C.; Wolff, M. Modelling trophic flows in the wettest mangroves in the world: The case of Bahia Malaga in the Colombian Pacific coast. Hydrobiologia 2017, 803, 13–27. [Google Scholar] [CrossRef]
- Bento, L.; Masuda, L.S.M.; Peixoto, R.B.; Enrich-Prast, A. Regulation in the metabolism and community structure of a tropical salt flat after rainfall. J. Coast. Res. 2017, 33, 304–308. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y.; Connolly, R.M. Structural equation modelling reveals factors regulating surface sediment organic carbon content and CO2 efflux in a subtropical mangrove. Sci. Total Environ. 2017, 578, 513–522. [Google Scholar] [CrossRef]
- Chen, S.; Chmura, G.L.; Wang, Y.; Yu, D.; Ou, D.; Chen, B.; Ye, Y.; Chen, G. Benthic microalgae offset the sediment carbon dioxide emission in subtropical mangrove in cold seasons. Limnol. Oceanogr. 2019, 64, 1297–1308. [Google Scholar] [CrossRef]
- Breavington, J.; Steckbauer, A.; Fu, C.; Ennasri, M.; Duarte, C.M. Dynamics of CO2 and CH4 fluxes in Red Sea mangrove soils. Biogeosciences 2025, 22, 117–134. [Google Scholar] [CrossRef]
- Corzo, A.; Haro, S.; Gómez-Ramírez, E.; González, C.J.; Papaspyrou, S.; Garcia-Robledo, E. Intertidal microphytobenthic primary production and net metabolism of a tropical estuary. Mar. Environ. Res. 2024, 202, 106741. [Google Scholar] [CrossRef]
- Karsten, U.; West, J.A. Ecophysiological studies on six species of the mangrove red algal genus Calogrossa. Funct. Plant. Biol. 1993, 20, 729–739. [Google Scholar] [CrossRef]
- Borburema, H.D.; Graiff, A.; Marinho-Soriano, E.; Karsten, U. Photosynthetic performance, growth, pigment content, and photoprotective compounds of the mangrove macroalgae Bostrychia calliptera and Bostrychia montagnei (Rhodophyta) under light stress. Front. Mar. Sci. 2022, 9, 989454. [Google Scholar] [CrossRef]
- Borburema, H.D.; Yokoya, N.S.; de Souza, J.M.C.; Nauer, F.; Barbosa-Silva, M.S.; Marinho-Soriano, E. Ocean warming and increased salinity threaten Bostrychia (Rhodophyta) species from genetically divergent populations. Mar. Environ. Res. 2022, 178, 105662. [Google Scholar] [CrossRef]
- Borburema, H.D.; Yokoya, N.S.; Soares, L.P.; de Souza, J.M.C.; Nauer, F.; Fujii, M.T.; Pasqualetti, C.B.; de Miranda, G.E.C.; Marinho-Soriano, E. Mangrove macroalgae increase their growth under ocean acidification: A study with Bostrychia (Rhodophyla) haplotypes from different biogeographic provinces. J. Exp. Mar. Biol. Ecol. 2022, 552, 151740. [Google Scholar] [CrossRef]
- Cordeiro-Marino, M.; Braga, M.R.A.; Eston, V.R.; Fujii, M.T.; Yokoya, N.S. Mangrove macroalgal communities of Latin America: The state of art and perspectives. In Coastal Plant Communities of Latin. America; Seeliger, U., Ed.; Elsevier: San Diego, CA, USA, 1992; pp. 51–64. [Google Scholar] [CrossRef]
- Hoffman, W.E.; Dawes, C.J. Photosynthetic rates and primary production by two Florida benthic red algal species from a salt marsh and a mangrove community. Bull. Mar. Sci. 1980, 30, 358–364. [Google Scholar]
- Burkholder, P.R.; Almodovar, L.R. Studies on mangrove algal communities in Puerto Rico. Florida Sci. 1973, 36, 50–66. [Google Scholar]
- Dawes, C.J.; Moon, R.E.; Davis, M.A. The photosynthetic and respiratory rates and tolerances of benthic algae from a mangrove and salt marsh estuary: A comparative study. Estuar. Coast. Mar. Sci. 1978, 6, 175–185. [Google Scholar] [CrossRef]
- Littler, M.M.; Taylor, P.R.; Littler, D.S.; Sims, R.H.; Norris, J.N. The distribution, abundance and primary productivity of submerged macrophytes in a Belize Barrier Reef mangrove system. Atoll Res. Bull. 1985, 280, 1–20. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Littler, M.M.; Littler, D.S. A comparison of nutrient-limited productivity in macroalgae from a Caribbean Barrier Reef and from a mangrove ecosystem. Aquat. Bot. 1987, 28, 243–255. [Google Scholar] [CrossRef]
- Mann, F.D.; Steinke, T.D. Photosynthetic and respiratory responses of the mangrove-associated red algae, Bostrychia radicans and Caloglossa leprieurii. S. Afr. J. Bot. 1988, 54, 203–207. [Google Scholar] [CrossRef]
- Dawes, C.J. Mangrove structure, litter and macroalgal productivity in a northernmost forest of Florida. Mangr. Salt Marsh 1999, 3, 259–267. [Google Scholar] [CrossRef]
- Pena, E.J.; Zingmark, R.; Nietch, C. Comparative photosynthesis of two species of intertidal epiphytic macroalgae on mangrove roots during submersion and emersion. J. Phycol. 2002, 35, 1206–1214. [Google Scholar] [CrossRef]
- Karsten, U.; Koch, S.; West, J.A.; Kirst, G.O. The intertidal red alga Bostrychia simpliciuscula Harvey ex J. Agardh from a mangrove swamp in Singapore: Acclimation to light and salinity. Aquat. Bot. 1994, 48, 313–323. [Google Scholar] [CrossRef]
- Borburema, H.D.D.S.; Lima, R.P.D.; Miranda, G.E.C.D. Effects of ocean warming, eutrophication and salinity variations on the growth of habitat-forming macroalgae in estuarine environments. Acta Bot. Bras. 2020, 34, 662–672. [Google Scholar] [CrossRef]
- Alongi, D.M. Lateral export and sources of subsurface dissolved carbon and alkalinity in mangroves: Revising the blue carbon budget. J. Mar. Sci. Eng. 2022, 10, 1916. [Google Scholar] [CrossRef]
- James, K.; Macreadie, P.I.; Burdett, H.L.; Davies, I.; Kamenos, N.A. It’s time to broaden what we consider a ‘blue carbon ecosystem’. Glob. Change Biol. 2024, 30, e17261. [Google Scholar] [CrossRef]
- McKenzie, L.J.; Nordlund, L.M.; Jones, B.L.; Cullen-Unsworth, L.C.; Roelfsema, C.; Unsworth, R.K.F. The global distribution of seagrass meadows. Environ. Res. Lett. 2020, 15, 074041. [Google Scholar] [CrossRef]
- Duarte, C.M.; Gattuso, J.-P.; Hancke, K.; Gundersen, H.; Filbee-Dexter, K.; Pedersen, M.F.; Middelburg, J.J.; Burrows, M.T.; Krumhansi, K.A.; Wernberg, T.; et al. Global estimates of the extent and production of macroalgal forests. Glob. Ecol. Biogeogr. 2022, 31, 1422–1439. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Mao, D.; Ren, C.; Song, K.; Zhao, C.; Wang, C.; Xiao, X.; Wang, Y. Mapping global distribution of mangrove forests at 10-m resolution. Sci. Bull. 2023, 68, 1306–1316. [Google Scholar] [CrossRef]
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.B.; Clinton, N.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef]
- Worthington, T.A.; Spalding, M.; Landis, E.; Maxwell, T.L.; Navarro, A.; Smart, L.S.; Murray, N.J. The distribution of global tidal marshes from Earth observation data. Glob. Ecol. Biogeogr. 2024, 33, e13852. [Google Scholar] [CrossRef]
- Lyons, M.B.; Murray, N.J.; Kennedy, E.V.; Kovacs, E.M.; Castro-Sanguino, C.; Phinn, S.R.; Acevedo, R.B.; Alvarez, A.O.; Say, C.; Tudman, P.; et al. New global area estimates for coral reefs from high-resolution mapping. Cell Rep. Sustain. 2024, 1, 100015. [Google Scholar] [CrossRef]
- Pessarrodona, A.; Franco-Santos, R.M.; Wright, L.S.; Vanderlift, M.A.; Howard, J.; Pidgeon, E.; Wernberg, T.; Filbee-Dexter, K. Carbon sequestration and climate change mitigation using macroalgae: A state of knowledge review. Biol. Rev. 2023, 98, 1945–1971. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Endo, T. CO2 flux in tidal flats and salt marshes. In Blue Carbon in Shallow Coastal Ecosystems; Kuwae, T., Hori, M., Eds.; Springer: Singapore, 2019; pp. 223–250. [Google Scholar]
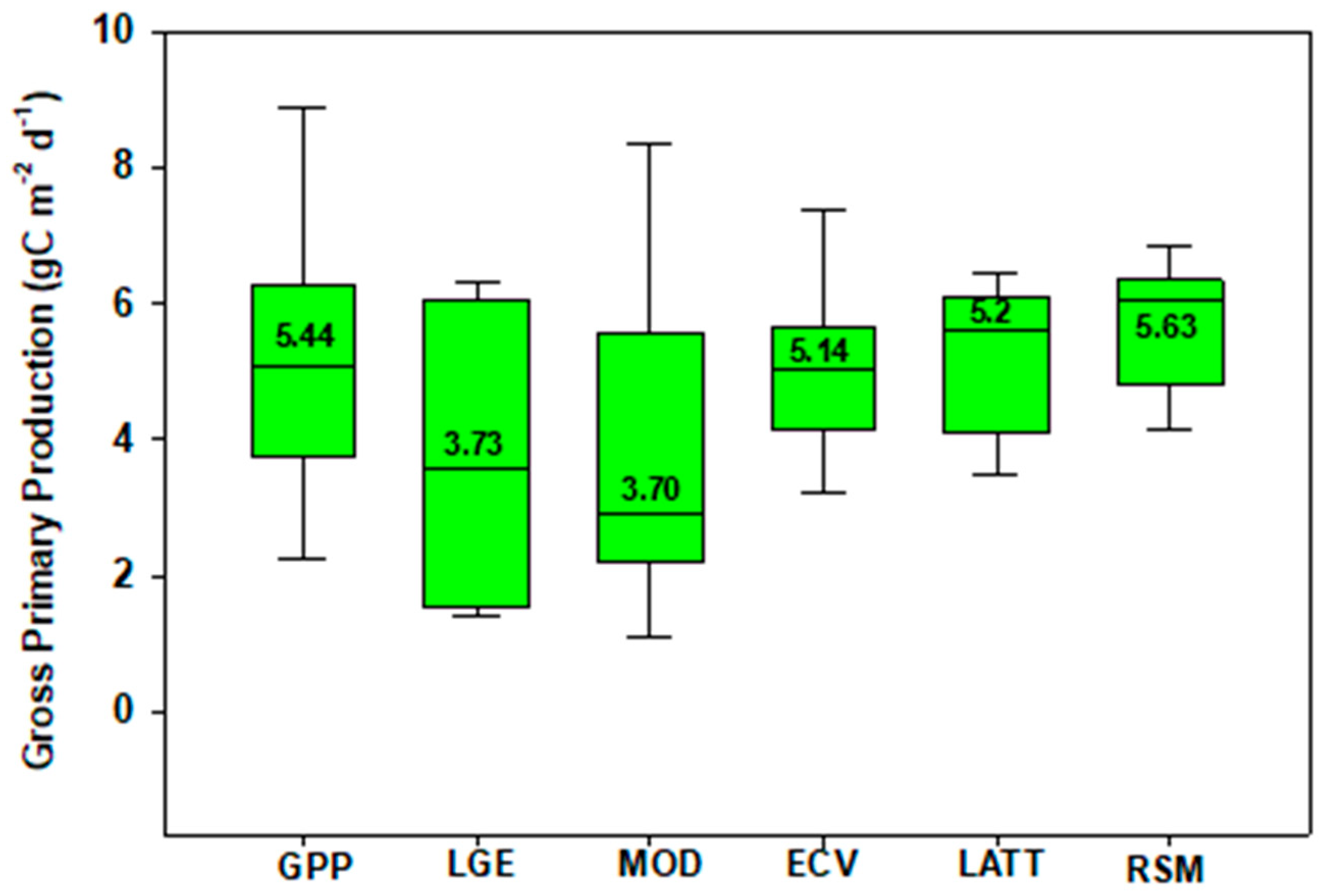

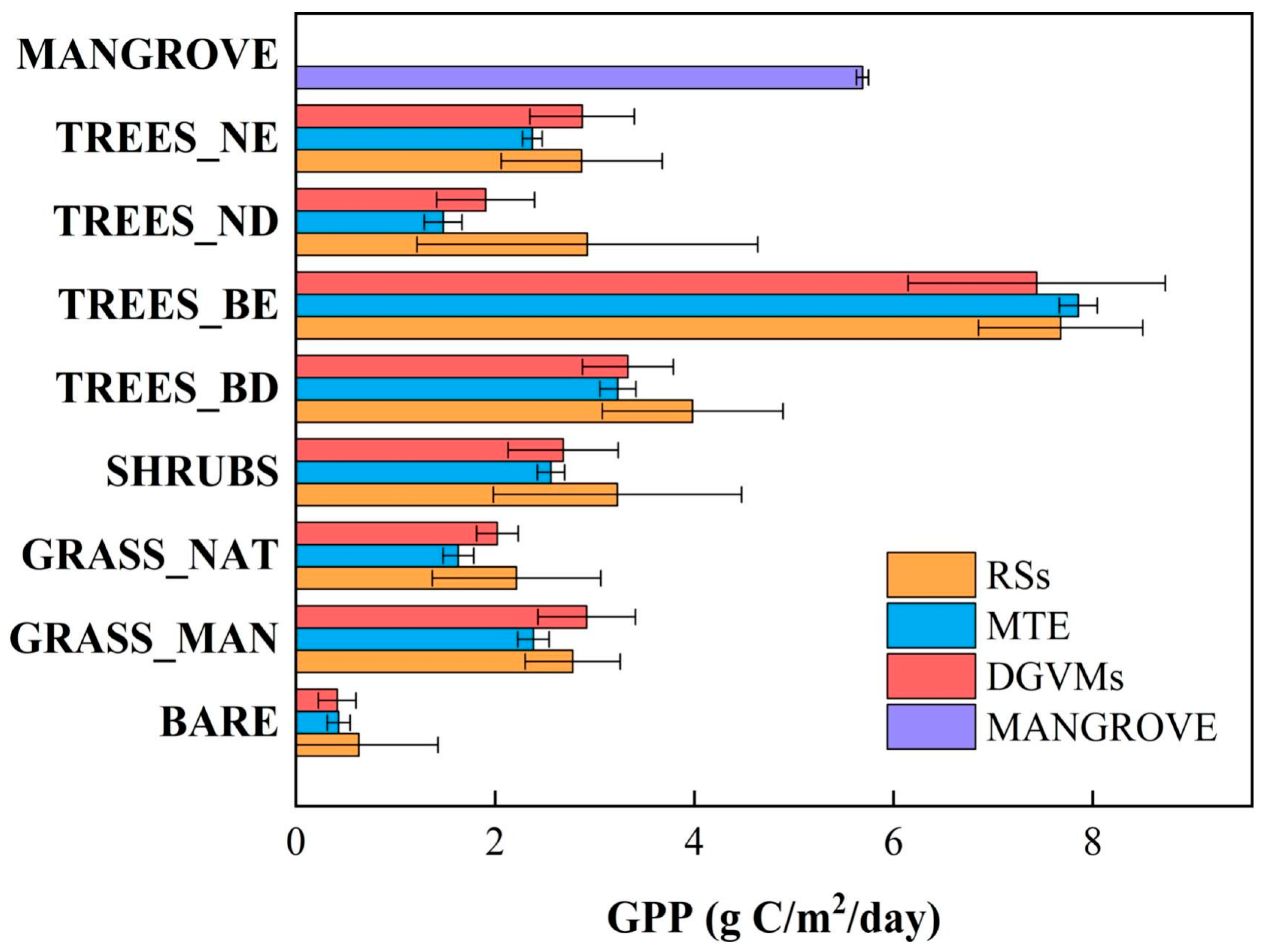


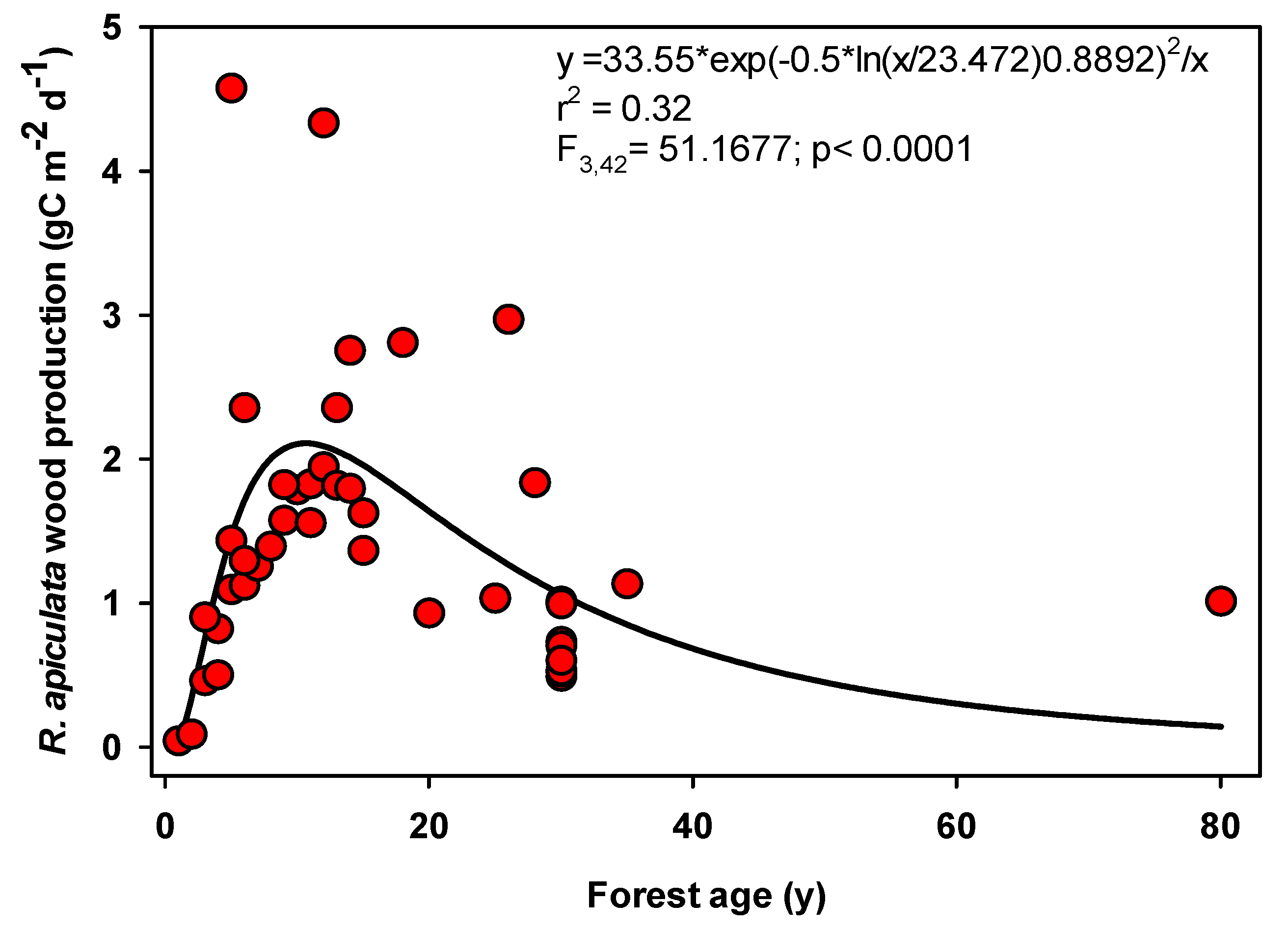
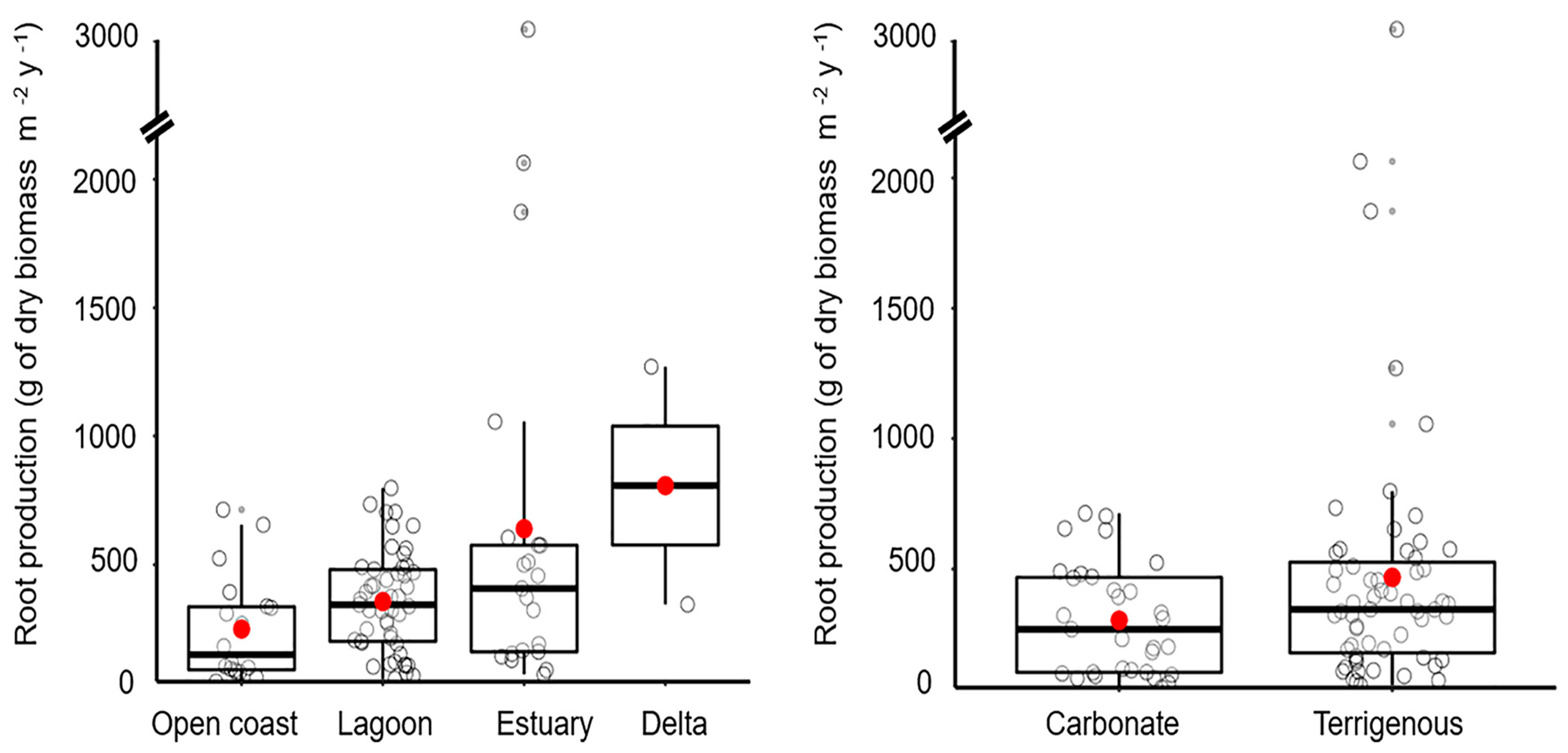
| Method | Size | Mean | ±1 SD | Max | Min | Median |
|---|---|---|---|---|---|---|
| Biomass increment + litterfall b,c | 70 | 3.9 | 2.6 | 11.8 | 0.0 | 3.2 |
| CO2 exchange + litterfall d | 4 | 1.4 | 2.1 | 4.4 | 0.0 | 0.7 |
| Field-based, radiation-based model c,d | 3 | 3.1 | 0.3 | 3.6 | 3.0 | 3.0 |
| Gas exchange a,b,c | 8 | 8.9 | 5.4 | 16.6 | 2.3 | 6.7 |
| Growth curve/growth increment b,c | 14 | 3.6 | 2.8 | 9.3 | 0.7 | 2.4 |
| Light attenuation a | 36 | 12.8 | 9.2 | 30.7 | 1.9 | 9.0 |
| Light interception a,b | 18 | 10.0 | 8.7 | 35.0 | 3.7 | 6.9 |
| Litterfall d | 338 | 2.2 | 1.4 | 8.1 | 0.0 | 1.9 |
| Litter Production | Wood Production | Root Production | Total Mangrove NPP | Global Mangrove Area | Global Mangrove NPP | Reference |
|---|---|---|---|---|---|---|
| 9.0 | 12.1 | NA | 21.1 | 181,900 | 383.4 | [168] |
| 12.8 | NA | NA | 16.3 | 160,000 | 260.5 | [169] |
| 4.2 | 4.2 | 5.2 | 13.6 | 160,000 | 218.0 | [123] |
| 4.3 | 4.2 | 10.8 | 19.3 | 160,000 | 308.7 | [8] |
| 10.0 | 9.0 | 2.0 | 21.0 | 170,000 | 356.9 | [9] |
| 4.3 | 4.2 | 4.7 | 13.1 | 138,000 | 210.0 | [125] |
| 4.4 | 4.9 | 5.9 | 15.2 | 160,000 * | 228.0 | [170] |
| 4.5 | 3.6 | 3.0 | 11.0 | 137,600 | 143.0 | [159] |
| 4.0 | 4.7 | 3.0 | 11.7 | 147,359 | 182.7 | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, D.M. Global Meta-Analysis of Mangrove Primary Production: Implications for Carbon Cycling in Mangrove and Other Coastal Ecosystems. Forests 2025, 16, 747. https://doi.org/10.3390/f16050747
Alongi DM. Global Meta-Analysis of Mangrove Primary Production: Implications for Carbon Cycling in Mangrove and Other Coastal Ecosystems. Forests. 2025; 16(5):747. https://doi.org/10.3390/f16050747
Chicago/Turabian StyleAlongi, Daniel M. 2025. "Global Meta-Analysis of Mangrove Primary Production: Implications for Carbon Cycling in Mangrove and Other Coastal Ecosystems" Forests 16, no. 5: 747. https://doi.org/10.3390/f16050747
APA StyleAlongi, D. M. (2025). Global Meta-Analysis of Mangrove Primary Production: Implications for Carbon Cycling in Mangrove and Other Coastal Ecosystems. Forests, 16(5), 747. https://doi.org/10.3390/f16050747






