Abstract
Factors such as climate change, fire, and overgrazing have been commonly considered the main causes of the global expansion of shrub invasion in grasslands over the past 160 years. Nevertheless, the influence of soil substrates on the progression of shrub encroachment has been insufficiently examined. This study examines the fundamental characteristics of the shrub-encroached desert steppe communities of Caragana tibetica in the Mongolian Plateau. Combining field surveys (field surveys and drone aerial photography) and laboratory experiments, using Spearman’s rank correlation analysis and structural equation modeling (SEM), this research systematically explores the impact of varying degrees of soil sandification on the survival of shrubs and herbaceous plants within these grassland communities. The findings indicate the following: (1) In the eight shrub-encroached grassland plots, the soil exhibited a significantly higher sand content compared to silt and clay, with the sand content generally exceeding 64%. (2) The coverage of shrub species is predominantly influenced by soil factors, particularly the soil sand content. (The path coefficient is 0.56, with p < 0.01). In contrast, herbaceous plants are more strongly influenced by climatic factors. (The path coefficient is 0.83, with p < 0.001). This study examines the response patterns of Caragana tibetica communities to edaphic and climatic factors, highlighting the pivotal role of soil sandification in the initiation and succession of shrub encroachment. The findings furnish a theoretical framework for forecasting future trends in grassland shrub encroachment and provide empirical evidence for the conservation and sustainable management of shrub-encroached grasslands.
1. Introduction
Shrub encroachment is defined by the gradual rise in shrub density and coverage in grassland ecosystems, resulting in substantial ecological alterations [1]. Over the past 160 years, shrub encroachment has been a major form of transformation in global grasslands [2,3]. It is estimated that between 10% and 20% of global grasslands are significantly affected by shrub encroachment [4]. Shrub encroachment in grasslands has become a focal point for many researchers [5,6]. Studies have proposed that shrub encroachment in grasslands is strongly associated with factors such as fire frequency [6], climate change [7,8], overgrazing [9], and fluctuations in atmospheric CO2 concentrations [10]. Shrub encroachment in grasslands is influenced by internal mechanisms, including vegetation–climate feedback, soil erosion–vegetation feedback, and fire–vegetation feedback. These mechanisms modify the competition and interactions between species, disrupting original coexistence patterns and stable dynamics, which ultimately results in a transition of stable states between herbaceous and shrub communities [11]. However, the conclusions on this topic remain a subject of considerable debate [5,6,11].
Shrub-encroached grasslands constitute a vital part of Inner Mongolia grasslands, with particular prominence in desert grassland areas [12]. Although shrub encroachment is quite common in the grasslands of Inner Mongolia [13], these shrub-encroached grasslands are mostly located on the edges of sandy areas or in regions where the surface is covered with sand. Under the influence of climate change and human activities, grassland ecosystems around the world are being invaded by shrubs, especially in the temperate semi-arid regions of the Northern Hemisphere [14]. Du et al. [15] were among the first to observe the relationship between shrub encroachment in grasslands and soil texture. In Leymus chinensis grasslands, shrubs are predominantly found on sandy chestnut soil but rarely occur on relatively moist black calcium soil. Furthermore, shrub encroachment in grasslands is often accompanied by soil sandification [16]. The sandblasting process alters soil conditions and plant growth conditions, potentially reducing the cover of herbaceous plants while enhancing the growth and survival of shrubs, thereby promoting shrub encroachment and providing advantages to shrubs [17]. Sandy soil, due to its rapid precipitation infiltration capacity and ability to reduce soil moisture evaporation, provides an advantage for shrubs, thereby promoting their expansion in grassland ecosystems [18]. Therefore, the occurrence of shrub encroachment in grasslands (particularly in Inner Mongolia) may be closely linked to soil substrates, with soil sandification potentially influencing the competitive dynamics between shrubs and herbaceous plants, thereby shaping the process of shrub encroachment in desert grasslands.
In the current research on the relationship between shrub encroachment in grasslands and soil sandification, researchers mostly assume that the occurrence of shrub encroachment is a prerequisite for sandification. This suggests that shrub encroachment leads to the deposition and absorption of resources and nutrients beneath the shrubs, concentrating organic matter under the canopy. This process results in the formation of “fertile islands” and increases soil erosion in patches without shrub encroachment [19]. The formation of fertile islands enhances the spatial heterogeneity of the soil and strengthens the ability of shrubs to resist environmental disturbances, creating a positive feedback loop between shrub expansion and soil sandification [1]. However, previous studies have focused mostly on analyzing the correlation between the two without considering soil sandification as a prerequisite for shrub encroachment in grasslands, and an in-depth understanding of the causal relationship between soil sandification and shrub encroachment in grasslands is still lacking. The mechanism by which soil texture affects shrub encroachment in grasslands may be related to the competitive qualities of shrubs and herbaceous plants under specific soil conditions [20]. Whether shrubs can become established successfully in grasslands depends on their ability to gain a competitive advantage over herbaceous plants and reproduce successfully [21]. The difference in competitive ability between shrubs and herbaceous plants in a community is closely related to soil texture [3]. This is because sandy soil facilitates the rapid infiltration of rainfall while preventing soil water from evaporating through capillary action [22]. This ensures the preservation of soil moisture in deeper layers, which may promote shrub growth and provide shrubs with a relative competitive advantage over herbaceous plants [23], as shrubs tend to utilize water from deeper soil layers compared to herbaceous plants [24]. This may play a significant role in promoting the occurrence and development of shrub encroachment in grasslands. However, the role of soil substrates in shrub encroachment has not received sufficient attention. This study focuses on shrub-encroached desert grassland at the northern foothills of Yinshan Mountain. The relationships between shrubs and herbaceous plants in desert grasslands and environmental factors such as soil and climate are analyzed. The aim of this study is to explore the impact of soil desertification at varying degrees on the survival and competitive abilities of shrubs and herbaceous plants in shrub-encroached grasslands. We hypothesize that an increase in the degree of desertification promotes the growth of shrub plants, thereby increasing the extent of shrub encroachment. This study can deepen our understanding of the causes of shrub encroachment and provide theoretical support for the management, utilization, and ecological restoration of shrub-encroached grasslands.
2. Materials and Methods
2.1. Overview of the Study Area
This study focuses on the Caragana tibetica population in the shrub-encroached desert grassland region of Inner Mongolia. This region has a continental monsoon climate, with average annual precipitation ranging from 170.84 mm to 245.62 mm and average annual temperatures between 3.4 °C and 7.2 °C. The average annual wind speed is above level 3, with some areas experiencing more than 100 days of strong winds annually. The topography is characteristic of the Mongolian Plateau, with altitudes ranging from 1024 m to 1557 m. The soil types are primarily Luvic Calcisols and Calcic Kastanozems [25]. The vegetation type is desert steppe [26], which mainly consists of xerophytic and strong xerophytic plants. The utilization of grasslands is primarily based on free grazing by local herders, with a moderate-to-heavy grazing intensity, and grazing pressure has markedly increased in recent decades [27]. The main shrubs include Caragana tibetica and Caragana microphylla. Herbaceous plants include Stipa tianschanica var. klemenzii, Stipa caucasica subsp. glareosa, Allium polyrhizum, and Cleistogenes squarrosa [28].
2.2. Plot Establishment and Survey
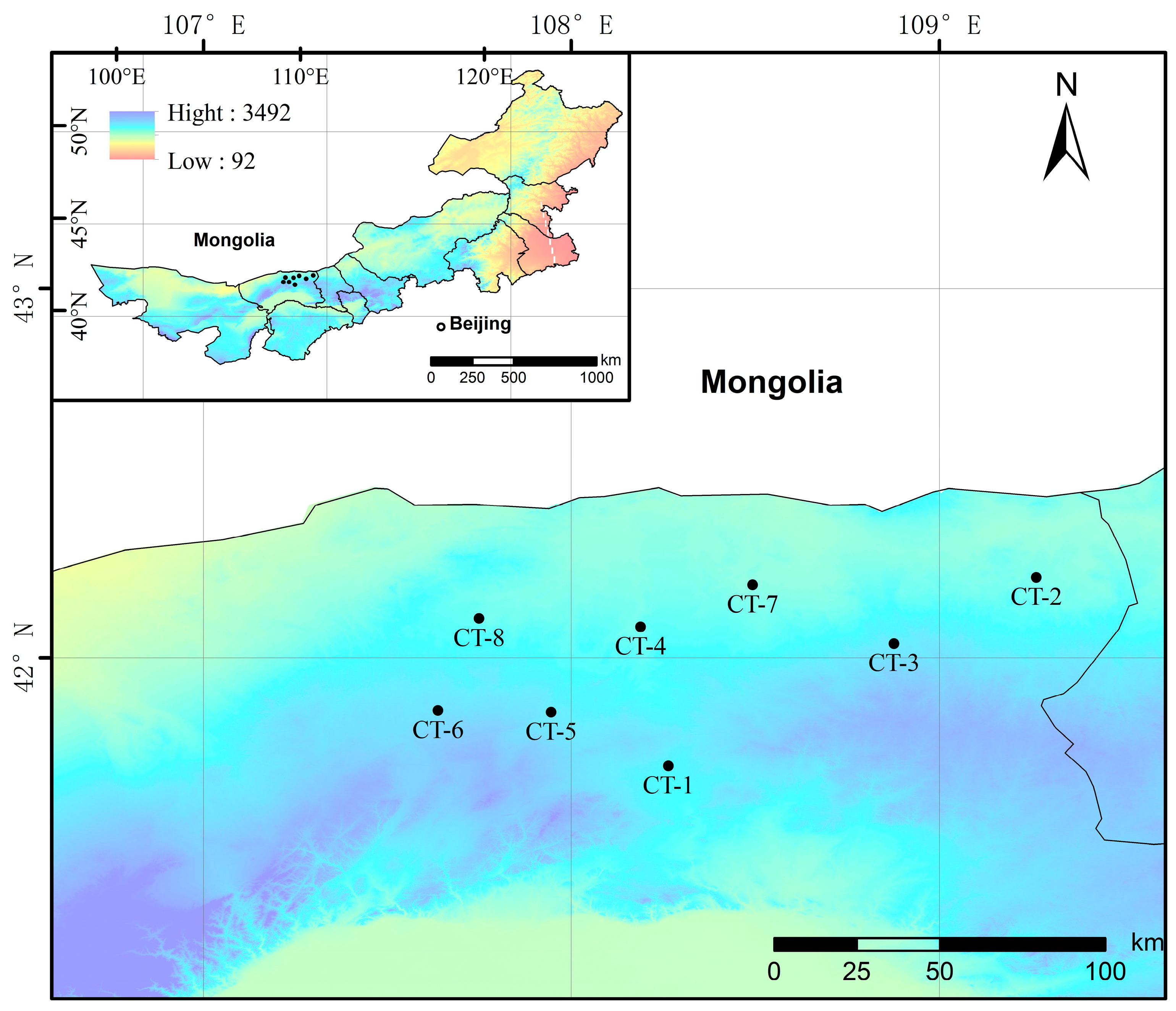
We conducted a survey in areas where the distribution of Caragana tibetica communities is relatively concentrated on the Mongolian Plateau. Sampling plots were established using a uniform distribution method, with plot intervals controlled to approximately 20–30 km, and locations were selected to ensure flat terrain and minimal anthropogenic disturbance [25]. A total of 8 plots were established, which were labeled CT-1–CT-8 (Figure 1). Each 100 m × 100 m plot was assessed for its fundamental characteristics, such as latitude and longitude, elevation, landform classification, vegetation type, and soil data. Visible light images of the plots were captured using drone aerial photography. Within each plot, three 10 m × 10 m shrub quadrats were randomly selected to investigate plant species, height, crown width, and coverage. In each shrub quadrat, one 1 m × 1 m herbaceous quadrat was chosen for investigations of plant species, height, and coverage. The aboveground parts of the herbaceous plants were clipped and taken to the laboratory, where their fresh weights were recorded, and then the aboveground parts were dried to measure their dry weights and calculate aboveground biomass. Soil samples were collected from beneath shrubs from within 15 cm of the plants, sealed in plastic bags, and taken to the laboratory for air-drying and sieving through a 2 mm mesh.
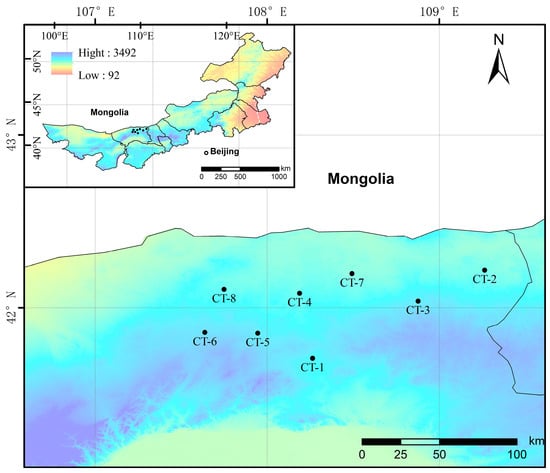
Figure 1.
Schematic map of the study area.
2.3. Soil Analysis
The soil organic matter content was determined using the potassium dichromate volumetric method. Soil-available nitrogen was measured using the alkali diffusion method, whereas soil-available phosphorus was assessed using the sodium bicarbonate method. Soil pH was determined using a potentiometric method [29]. Soil particle size was analyzed with a laser particle size analyzer, and the particles were classified according to the following international standards: sand (0.02–2 mm), silt (0.002–0.02 mm), and clay (<0.002 mm) [30].
2.4. Climate Data Acquisition
Climate data were obtained from CHELSA (Climatologies at high resolution for the Earth’s land surface areas, http://chelsa-climate.org/, accessed on 14 April 2025), which included metrics such as the annual mean temperature, annual mean precipitation, the mean temperature of the coldest season, and evapotranspiration. The aridity index (AI) was calculated using the following formula:
where P is the annual mean precipitation, and PET is the evapotranspiration.
2.5. Acquisition of Shrub Cover Data
Aerial drone photography was carried out in ideal conditions, including sunny weather, a light breeze or no wind, and typically around noon. This was to reduce shadow interference caused by the sun’s angle, ensuring better results for subsequent tasks. Aerial imagery was captured using the DJI Phantom 4 drone (DJI, Shenzhen, China), featuring a built-in 1-inch, 20-megapixel CMOS image sensor, which has an effective pixel count of 12 million in the gimbal camera. RGB visible light images of the sample plots were captured through drone aerial photography. During the flight, images were taken at regular time intervals. The area and flight path were preset, with a lateral overlap of 75% and a longitudinal overlap of 70%. The gimbal pitch angle was adjusted to −90 degrees. The drone operated at a speed of 8 m/s and an altitude of 50 m, covering an aerial photography area of 300 m × 300 m.
The visible light images were stitched and orthorectified using Pix4Dmapper 4.8 (PIX4D, Swiss Confederation, Bern, Switzerland). These processed images were then imported into ArcGIS 10.8 (Environmental Systems Research Institute, Redlands, CA, USA) to set the projected coordinate system and perform image registration. Using eCognition Developer 64 (Definiens Imaging, Munich, Germany), vegetation information was extracted with the visible light difference vegetation index (VDVI) for the automatic classification of vegetation and soil, thus identifying shrub coverage. Finally, the classified data were imported into ArcGIS to eliminate interference, resulting in vegetation cover and density outcomes. (Figure 2).

Figure 2.
Drone aerial imagery of 8 sample plots surveyed in this research. (A photo of the Tibetan tibetica is presented in the bottom right corner).
2.6. Other Data Processing
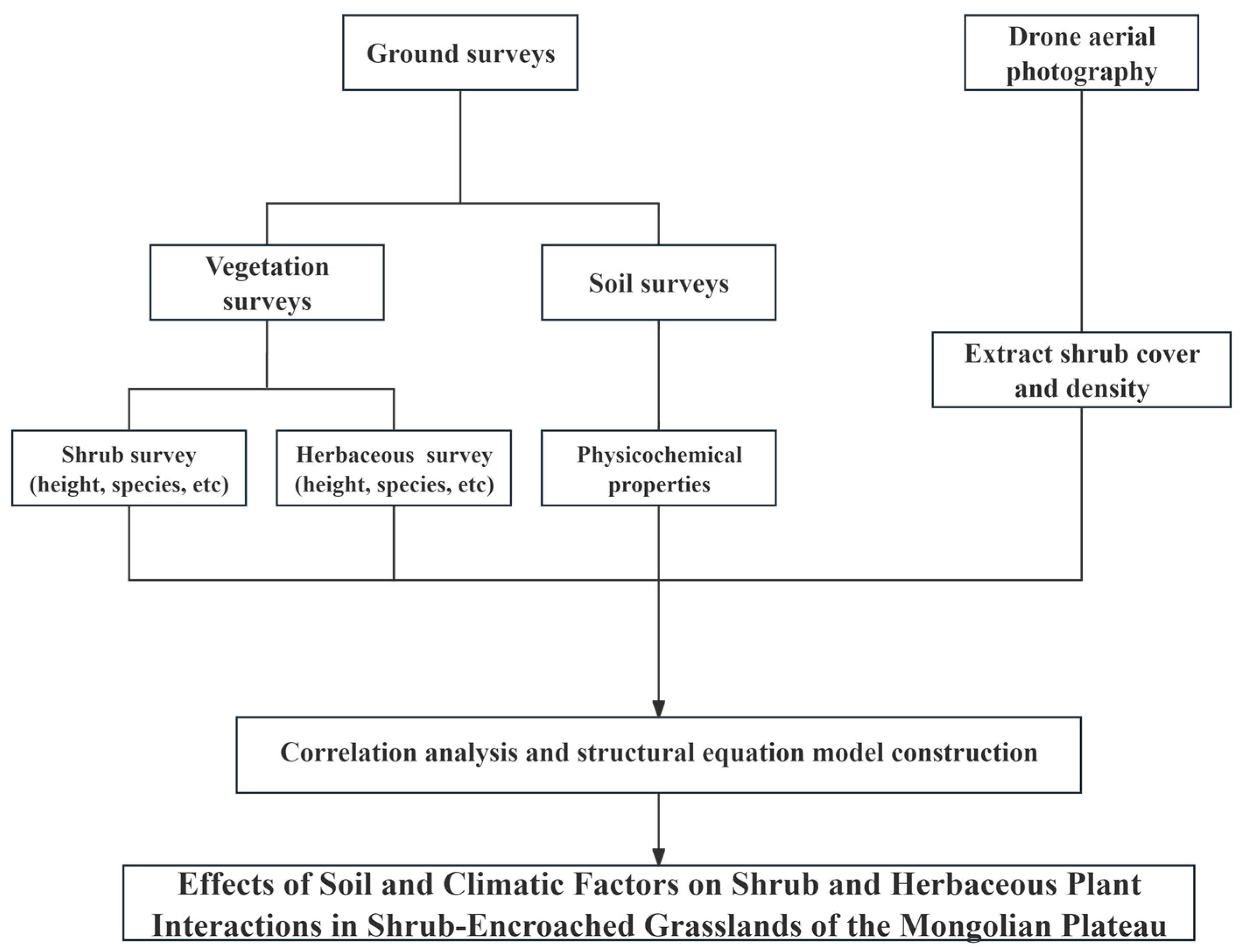
Preliminary statistical analysis and data processing were conducted using Excel 2016 software. Data analysis was performed with R 4.3.2 software using the vegan package [31] to analyze the differences in community characteristics such as shrub cover and height. The correlation analysis between environmental characteristics and community features was conducted using the Hmisc package [32]. The lavaan package [33] was employed for structural equation modeling to explore the relationships among shrub communities (represented by shrub cover), herbaceous plants (represented by biomass), and environmental factors. The structural equation model fit was evaluated using the chi-squared-to-degrees of freedom ratio (x2/df), the comparative fit index (CFI), normed fit index (NFI), standardized root mean square residual (SRMR), and root mean square error of approximation (RMSEA). A smaller chi-squared-to-degrees of freedom ratio is preferable, with a p-value less than 0.05 and an RM value less than 0.05, indicating the ideal model fit. GFI and FI values greater than 0.9 are considered ideal for fitting, whereas an SRMR value less than 0.9 is considered reasonable for fitting the data (Figure 3).
Figure 3.
Flowchart of this study.
3. Results
3.1. Basic Information on Caragana tibetica Communities and Soil Particle Size Characteristics
The overall species richness of the communities in the study area ranged from 8 to 14. The Caragana tibetica community cover ranged from 1.53% to 10.24%, the density varied between 308.00 and 2292.00 plants per hectare, and the height ranged from 8.56 cm to 13.22 cm. On average, there were nine species of herbaceous plants, with an average biomass of 27.11 g/m2. Among them, CT-1 presented the highest cover and density at 10.24% and 2292 plants per hectare, respectively, whereas CT-7 presented the lowest cover and density at 1.53% and 308 plants per hectare, respectively (Table 1).

Table 1.
Table of key characteristics of the C. tibetica.
In plant ecology, life forms are typically represented by the growth habits and survival strategies of plants to adapt to different environmental conditions. One of the common methods for classifying life forms is based on the position of the renewal buds during unfavorable seasons. This classification method was proposed by Danish botanist Christen C. Raunkiær and is known as the Raunkiær life form system [34]. The results of the survey indicate that the surveyed area displays a diverse range of plant species, encompassing 14 families, 27 genera, and 36 species. The Poaceae family is the most diverse family within the surveyed area, containing nine genera and eleven species (Table 2). The detailed statistics for each family are provided below:

Table 2.
The species list recorded in this study.
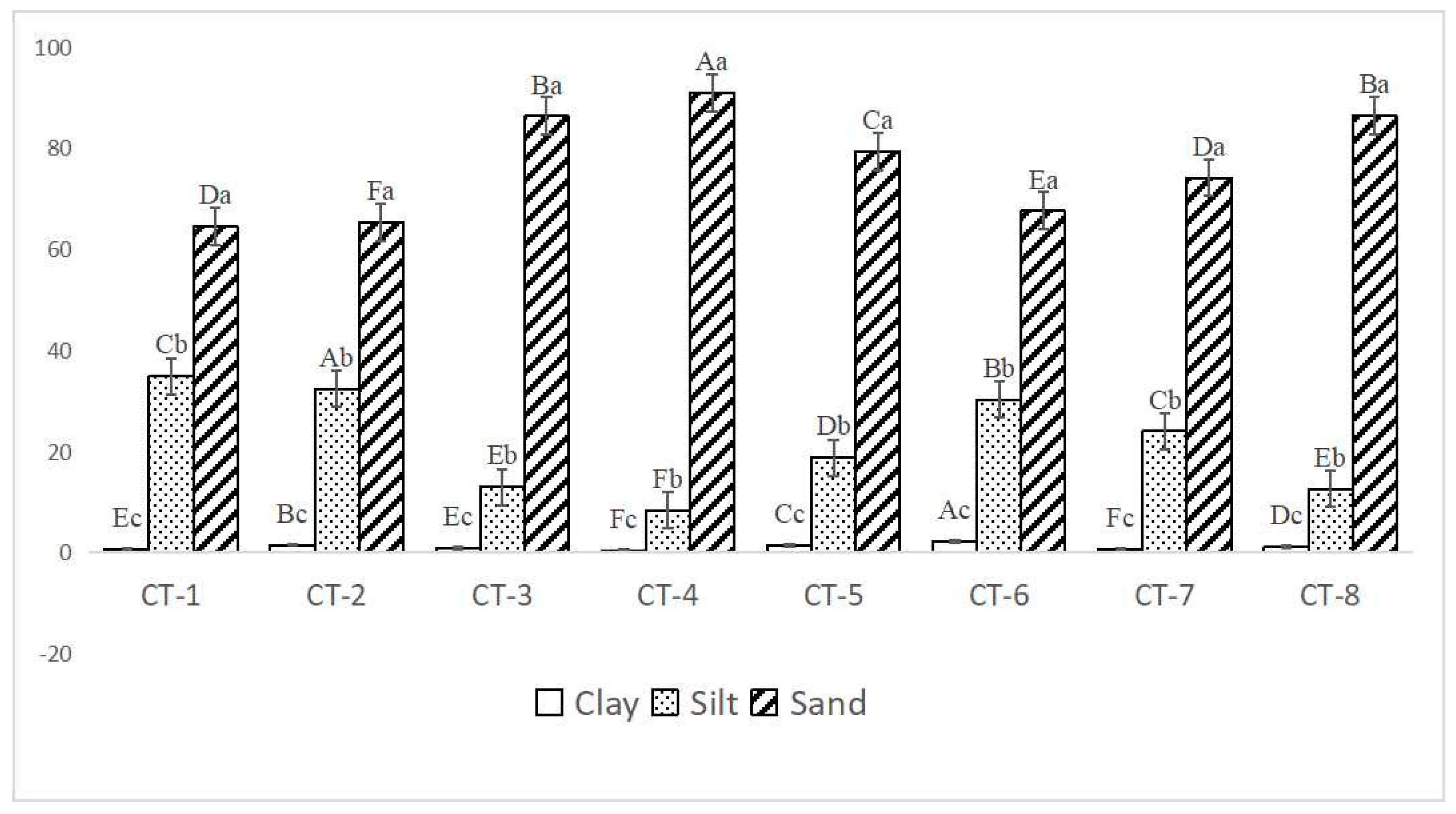
As shown in Figure 4, the soil in the Caragana tibetica community was predominantly composed of sand particles, accounting for 64.42% to 90.88%. The proportion of clay particles was relatively small, ranging from 0.12% to 2.16%. In all the sample plots, the sand content was significantly greater than the silt and clay contents.
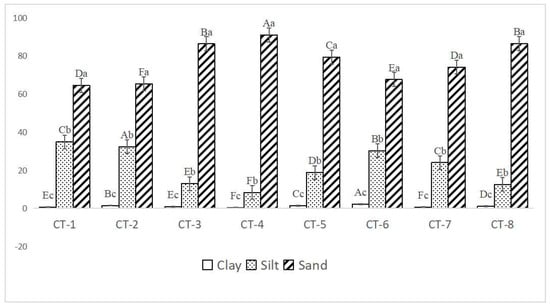
Figure 4.
The soil particle composition of Caragana tibetica shrubs in different sample plots. Different uppercase letters indicate the differences between different sample plots, while different lowercase letters indicate the differences in soil particle composition within the same sample plot. Clay—clay content in soil; silt—silt content in soil; Sand—sand content in soil. The Y axis is measured in percentages.
3.2. Correlation Analysis of Caragana Tibetica Plant Communities and Their Relationships with Environmental Factors
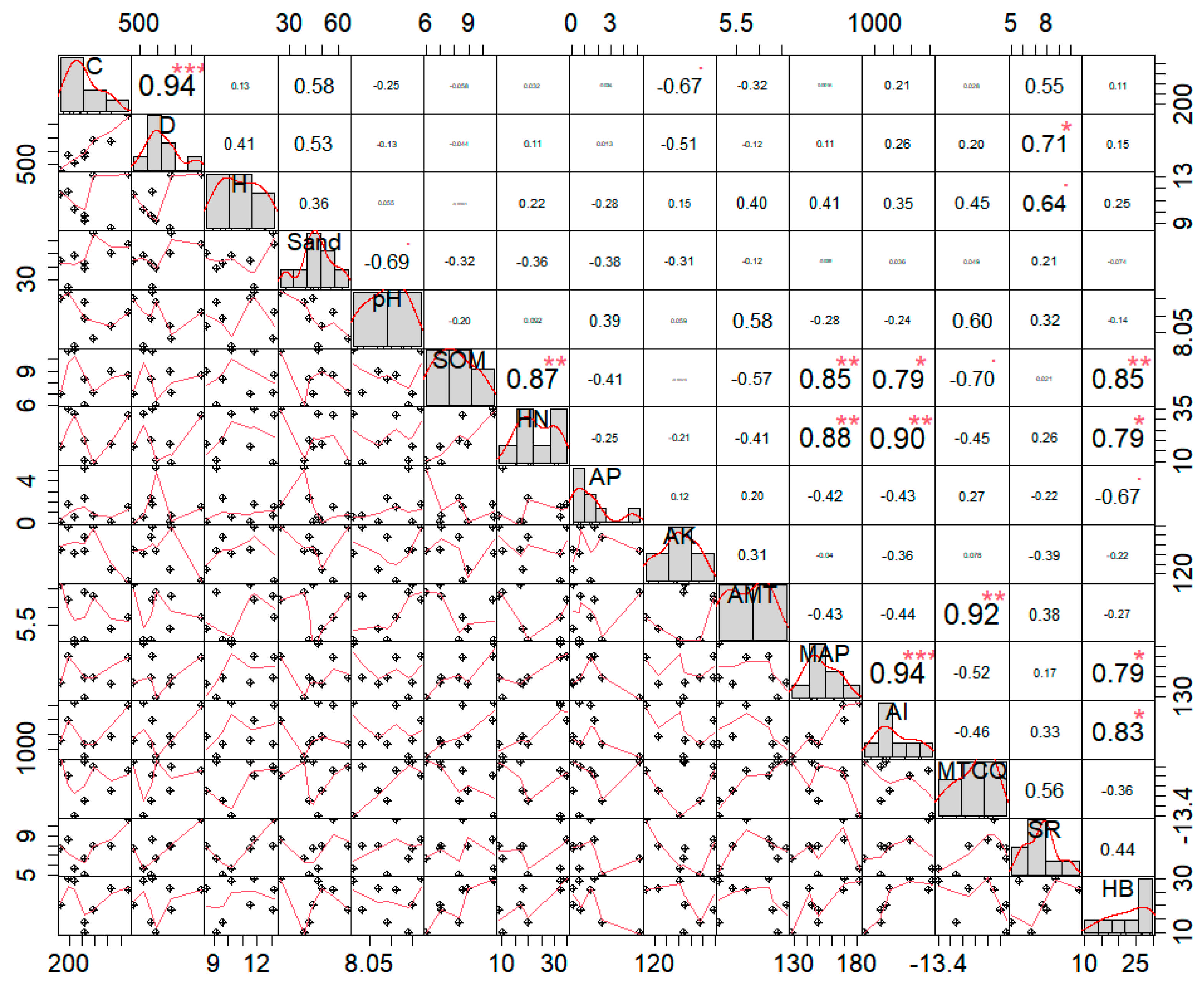
Figure 5 clearly shows that the coverage and density of the Caragana tibetica population were highly significantly positively correlated (p < 0.001), with a correlation coefficient of 0.94. The sand content was positively correlated with the coverage, density, and height of Caragana tibetica, with correlation coefficients of 0.58, 0.53, and 0.36, respectively, but these correlations were not significant (p > 0.05). Additionally, the number of herbaceous plant species in the community was significantly and positively correlated with the density of the Caragana tibetica population (p < 0.01), with a correlation coefficient of 0.71. The biomass of herbaceous plants was positively correlated with the soil organic matter content, with a correlation coefficient of 0.85 (p < 0.05).
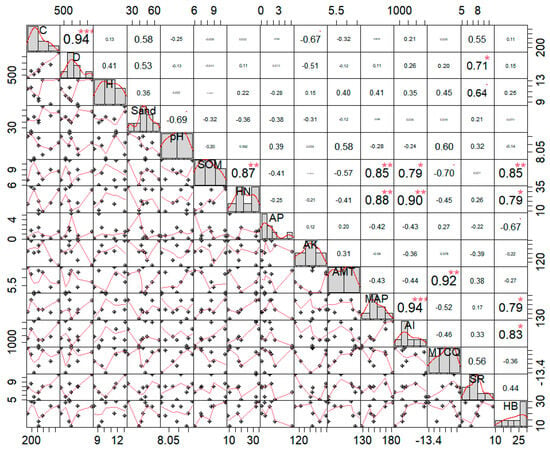
Figure 5.
Correlation analysis between coverage, density, and height and their relationship with environmental factors. “*”—p < 0.05; “**”—p < 0.01; “***”—p < 0.001. C—shrub cover in the plot; D—shrub density in the plot; H—average height of the thicket; Sand—sand content in the plot; pH—soil pH in the plot; SOM—soil organic matter in the plot; HN—soil-available nitrogen in the plot; AP—soil-available phosphorus in the plot; AK—soil-available potassium in the plot; AMT—average annual temperature in the plot; MAP—average annual precipitation in the plot; AI—drought index in the plot; MTCQ—mean temperature of the coldest season in the plot. SR—species richness; HB—herbal biomass.
3.3. Analysis of Factors Influencing Caragana tibetica Communities
The fit indices for the structural equation model indicated a good fit between the model and the actual data (Table 3), and the model results were highly reliable.

Table 3.
The model fit indices and their evaluation criteria.
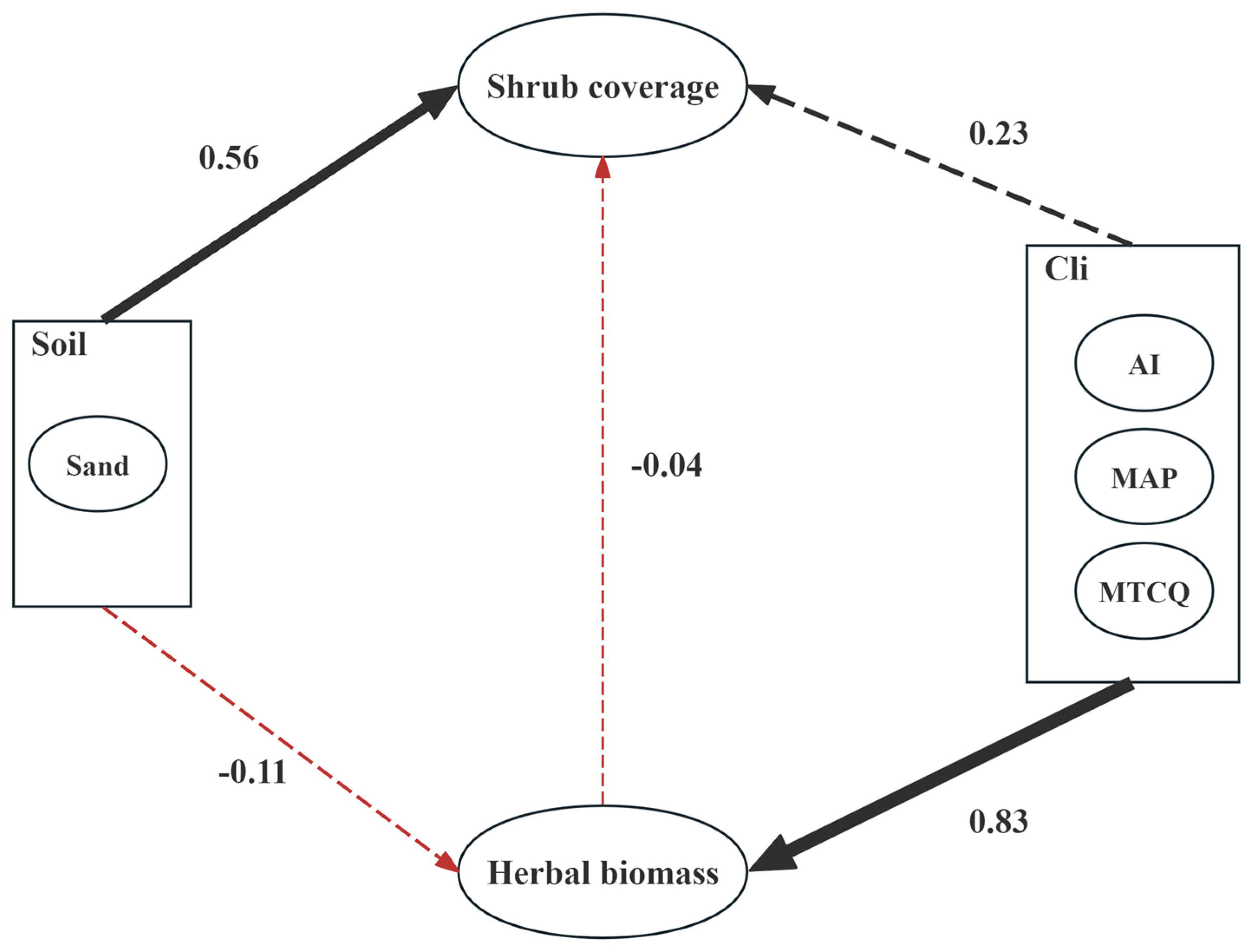
The pathways of the influence of soil and climatic factors on community shrubs and herbaceous plants are shown in Figure 4. The factors affecting shrub coverage included three main factors: soil factors, climatic factors, and herbaceous vegetation factors. Among the soil factors, sand content was the main indicator; among the climate factors, the average temperature in the coldest season, annual precipitation, and drought index were the main indicators; among the herbaceous vegetation factors, herbaceous vegetation biomass was the main indicator. Through the analysis, the path coefficients of these three variables on Caragana population coverage could be obtained.
The results of the structural equation model indicated that the main factor affecting the coverage of Caragana plant populations was soil factors, with a path coefficient of 0.56 (p < 0.01, z = 1.972). The path coefficient for climatic factors was 0.23, but it was not significant (p = 0.65). The path coefficient for the influence of herbaceous plants on shrubs was −0.4, but it was not significant (p = 0.93). Additionally, the coefficient for the influence of climatic factors on herbaceous plant biomass was 0.83 (p < 0.001, z = 5.474). The coefficient for the influence of soil factors on herbaceous plant biomass and species number was −0.11, which was not significant (p = 0.57). The coefficient for the influence of climate factors on shrub coverage via herbaceous biomass was −0.03 (p = 0.93, Table 4).

Table 4.
Direct and indirect effects of soil and climate factors on shrubs and herbaceous plants.
Table 4 shows that among the factors affecting the coverage of Caragana shrublands, soil factors had the greatest impact, with a total effect of 0.556, and both direct and indirect effects were positive. The herbaceous plant and climatic factors followed, with total effects of −0.04 and 0.2, respectively. Among them, climatic factors had a dual effect on the coverage of Caragana shrublands.
4. Discussion
The explanation for the appearance of shrubs in desert grasslands is not singular; there could be multiple reasons ranging from desertification to ecosystem enhancement [35]. The Inner Mongolia grasslands are located in the transition zone between arid and semiarid climates [36]. In this region, the habitat conditions are harsh, and the degree of wind erosion is severe. Soil desertification and grassland shrub encroachment coexist in this area [37]. This study revealed that the soil sand content in all plots exceeded 60%, with a maximum of 90.88%, demonstrating a clear trend of desertification (Figure 4). Moreover, as the sand content increased, the shrub cover tended to increase (Figure 5). This positive correlation suggests that soil desertification may have facilitated the growth and expansion of shrub areas to some extent. Current research on the relationship between grassland shrub encroachment and soil desertification is mostly based on the premise that shrub encroachment occurs first. It is believed that once shrub encroachment forms, the deposition, and absorption of resources and nutrients beneath the shrubs lead to the formation of “fertile islands” and increase soil erosion in non-shrubland patches [19]. The creation of fertile islands enhances soil spatial heterogeneity, thus establishing a positive feedback loop between “shrub increase and soil desertification” [1]. However, some researchers have also observed that sandy environments can promote shrub encroachment, as sandy soil conditions may be more favorable for shrub growth [38,39]. Consequently, these conditions could serve as both a cause and an initial stage of shrub encroachment. For example, Xie et al. [18] conducted an analysis of the characteristics of sandy habitats with a focus on how the physical and chemical properties of the soil influence shrub growth. The results suggest that sandy soil can increase the survival ability of shrubs, thus exacerbating the phenomenon of shrub encroachment.
In addition, the results from SEM indicate that soil desertification positively influences shrub cover, with the path coefficient of soil factors to the shrub cover reaching 0.53 and being statistically significant (p < 0.05, Figure 6). This finding is partly supported by the research of Niu et al. [17], who demonstrated that sand habitats alter the physical and chemical properties of grassland soil, thereby providing more favorable growth conditions for shrubs. Furthermore, sand can increase soil water retention and alter soil structure, thereby enhancing the competitiveness of shrubs and promoting their expansion in grasslands. The process through which desertified soil facilitates shrub proliferation may pertain to the impact of soil substrates on the competitive benefits of shrubs versus herbaceous plants within the ecosystem [22]. This is because the interactions between shrubs and herbaceous plants are governed primarily by underground competition, particularly for groundwater, which is mediated by soil properties [24,40]. Compared with herbaceous plants, shrubs generally have deeper taproots, which allow them to access moisture from deeper soil layers [41]. Sandy soil aids in the swift infiltration of rainfall and prevents the evaporation of soil moisture through capillary action [23]. This ensures the retention of soil moisture in deeper layers [23,42], which may provide a more favorable environment for shrub growth, as studies have shown that shrubs tend to utilize deeper soil moisture [24,37]. Therefore, as desertification progresses and soil moisture shifts to deeper layers, this may potentially benefit shrub growth, promoting the expansion of shrub encroachment. In contrast, herbaceous plants, which rely on shallow moisture, exhibit greater sensitivity to climatic factors such as precipitation [43] (Figure 6). Zhang et al. [44] reported a strong positive correlation between shrub encroachment and the sand content of surface soil and showed that shrubs exhibit increased competitiveness in coarse-textured soil. Thereby, desertified soil may play a positive role in promoting shrub encroachment in grasslands.
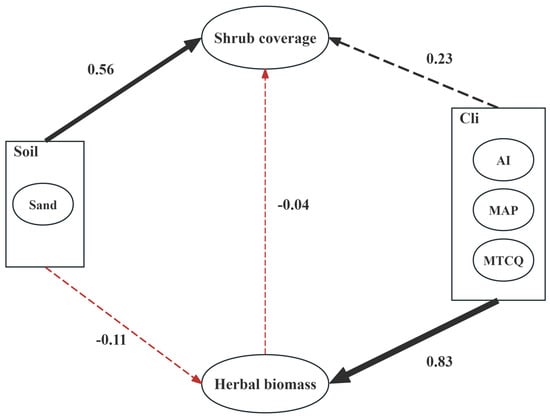
Figure 6.
The structural equation models pathways of soil and climate factors on shrubs and herbaceous plants. Dashed lines represent non-significant p-values; solid lines represent significant p-values; black represents positive path coefficients; red represents negative path coefficients; and thicker arrows indicate greater influence.
This study also revealed that the annual precipitation, average temperature of the coldest season, and drought index had minimal direct impacts on the shrubs (path coefficient = 0.23, p > 0.05). This may be due to the relatively small spatial scale of the study area and the minimal climatic variation between different plots. However, interestingly, herbaceous plants exhibited a higher sensitivity to climatic factors.
Compared with shrubs, herbaceous plants are more significantly affected by climatic factors, which have a positive influence (p < 0.01). In comparison, soil factors had a minimal effect (path coefficient = −0.11, p > 0.05), suggesting that the herbaceous plant community is shaped predominantly by climatic rather than soil factors. Research by Zhu et al. indicated that as rainfall increased, the biomass and coverage of herbaceous plants significantly increased, whereas the changes in shrubs were not distinct [42]. Moreover, the number of herbaceous plant species was significantly and positively correlated with shrub cover (Figure 5). The above results indicate a complex trade-off–synergy relationship between shrubs and herbaceous plants. The reason for this is that, in arid and semiarid regions, there is intense competition for resources, particularly water, between shrubs and herbaceous plants [24]. On the other hand, shrub encroachment enhances spatial heterogeneity within communities [1]. This facilitates the colonization of different species, and shrubs provide shelter for herbaceous plants, thereby increasing the species diversity of herbaceous plants. In conclusion, although we did not find a direct effect of climatic factors on shrubs, future climate change may affect herbaceous plants in shrub-encroached grasslands, thereby indirectly influencing the shrub encroachment process [45,46]. Furthermore, at larger spatial scales, climate change (especially the effect of low-temperature limitations) may significantly impact shrub encroachment [47], which is an important area for future research to address.
In summary, shrubs and herbaceous plants in shrub-encroached grasslands may be influenced by different environmental factors, while the sandy soil environment may positively affect shrub growth, creating a positive feedback loop with shrub encroachment. Therefore, future research on the mechanisms of shrub encroachment should enhance the focus on the role of soil substrates. Practices for restoring and reducing shrub-encroached grasslands should also consider the implementation of soil property improvements to achieve optimal restoration outcomes. In addition, the impact of soil degradation on the competition for resources between shrubs and herbaceous plants is another aspect that needs further investigation in the future.
5. Conclusions
This study examines the key factors influencing plant communities in shrub-encroached desert grasslands of the Mongolian Plateau, focusing on the relationships between vegetation characteristics and soil and climatic conditions. Eight Caragana tibetica communities were surveyed and analyzed to assess the influence of soil and climate factors on shrubs and herbaceous plants in these ecosystems. We found that the soil in all the plots surveyed demonstrated a marked trend toward desertification (The sand content is consistently above 64%). Shrub coverage is predominantly influenced by soil factors, and soil desertification is a key driver of the extent of shrub encroachment (The path coefficient is 0.56, with p < 0.01). In contrast, herbaceous plants are more significantly influenced by climatic factors than by soil factors (The path coefficient is 0.83, with p < 0.001). This study offers a novel perspective with which to understand the occurrence and succession of shrub encroachment in grasslands, providing a scientific foundation for ecological restoration and conservation efforts.
Author Contributions
Y.L., L.D., J.L. and J.W. designed the experiments. Y.L., L.D., L.Y., H.L. and S.C. performed the experiments. Y.L., L.D. and S.L. analyzed the data. Y.L. and L.D. wrote the manuscript. Y.L., L.D., J.L. and J.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32401670), the Inner Mongolia Natural Science Foundation (2022QN03010), the Special Project of Basic Scientific Research Business Expenses of China Institute of Water Resources and Hydropower Research (MK0199A122021), and the Science and Technology Program of Inner Mongolia Autonomous Region (2023YFHH0065, 2023YFHH0067).
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; Shen, H.; Zhou, L.; Zhu, Y.; Chen, L.; Hu, H.; Zhang, P.; Fang, J. Shrub encroachment increases soil carbon and nitrogen stocks in temperate grasslands in China. Land Degrad. Dev. 2019, 30, 756–767. [Google Scholar] [CrossRef]
- D’Odorico, P.; Okin, G.S.; Bestelmeyer, B.T. A synthetic review of feedbacks and drivers of shrub encroachment in arid grasslands. Ecohydrology 2012, 5, 520–530. [Google Scholar] [CrossRef]
- Sala Osvaldo, E.; Maestre Fernando, T. Grass–woodland transitions: Determinants and consequences for ecosystem functioning and provisioning of services. J. Ecol. 2014, 102, 1357–1362. [Google Scholar] [CrossRef]
- Van Auken, O.W. Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 2000, 31, 197–215. [Google Scholar] [CrossRef]
- Peng, H.Y.; Li, X.Y.; Li, G.Y.; Zhang, Z.H.; Zhang, S.Y.; Li, L.; Zhao, G.Q.; Jiang, Z.Y.; Ma, Y.J. Shrub encroachment with increasing anthropogenic disturbance in the semiarid Inner Mongolian grasslands of China. Catena 2013, 109, 39–48. [Google Scholar] [CrossRef]
- Urbina, I.; Grau, O.; Sardans, J.; Ninot, J.M.; Peñuelas, J. Encroachment of shrubs into subalpine grasslands in the Pyrenees changes the plant-soil stoichiometry spectrum. Plant Soil 2020, 448, 37–53. [Google Scholar] [CrossRef]
- Knapp, A.K.; Briggs, J.M.; Collins, S.L.; Archer, S.R.; BRET-HARTE, M.S.; Ewers, B.E.; Peters, D.P.; Young, D.R.; Shaver, G.R.; Pendall, E.; et al. Shrub encroachment in North American grasslands: Shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob. Change Biol. 2008, 14, 615–623. [Google Scholar] [CrossRef]
- Shackleton, C.M.; Scholes, R.J. Above ground woody community attributes, biomass and carbon stocks along a rainfall gradient in the savannas of the central lowveld, South Africa. S. Afr. J. Bot. 2010, 77, 184–192. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Y.; Xu, D.; Xu, X.; Wang, C.; Wang, X.; Chen, J.; Xin, X.; Eldridge, D.J. The fertile island effect collapses under extreme overgrazing: Evidence from a shrub-encroached grassland. Plant Soil 2020, 448, 201–212. [Google Scholar] [CrossRef]
- Bond William, J.; Midgley Jeremy, J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Lou, S.; Hu, A.; Ren, J.; Hu, J.; Zhang, J.; Hou, F. Shrub Encroachment in Grasslands: Processes, Mechanisms, and Effects. Acta Prataculturae Sin. 2018, 27, 219–227. [Google Scholar]
- Miao, L.; Jiang, C.; Xue, B.; Liu, Q.; He, B.; Nath, R.; Cui, X. Vegetation dynamics and factor analysis in arid and semi-arid Inner Mongolia. Environ. Earth Sci. 2015, 73, 2343–2352. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, X.Y.; Yang, X.; Shi, Y.; Zhang, S.Y.; Jiang, Z.Y. Changes in soil properties following shrub encroachment in the semiarid Inner Mongolian grasslands of China. Soil Sci. Plant Nutr. 2020, 66, 369–378. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Liu, B.; Zhang, J.; Wang, L.; Lu, X.; Jiang, M. Effect of shrub encroachment on land surface temperature in semi-arid areas of temperate regions of the Northern Hemisphere. Agric. For. Meteorol. 2022, 320, 108943. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, H.; Penuelas, J.; Sardans, J.; Deng, D.; Cai, X.; Gao, D.; Nie, S.; He, Y.; Lü, X.; et al. Shrub encroachment leads to accumulation of C, N, and P in grassland soils and alters C: N: P stoichiometry: A meta-analysis. Sci. Total Environ. 2024, 951, 175534. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Zhu, J.; Wang, H.; Zhang, M.D.; Lu, S.B.; Zheng, S.X. Population characteristics of Caragana microphylla and the influencing soil factors in shrub-encroached grassland of Inner Mongolia, China. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2024, 35, 1525–1533. [Google Scholar]
- Niu, F.; Pierce, N.A.; Okin, G.S.; Archer, S.R.; Fischella, M.R.; Nadoum, S. Sandblasting promotes shrub encroachment in arid grasslands. New Phytol. 2023, 240, 1817–1829. [Google Scholar] [CrossRef]
- Xie, L.; Li, Y.; Guo, H.; Wang, C.; Chen, Q.; He, P.; Ma, C. Sandy habitats play an important role in shrub encroachment in grasslands. Agronomy 2022, 12, 2858. [Google Scholar] [CrossRef]
- Allington, G.R.; Valone, T.J. Islands of fertility: A byproduct of grazing. Ecosystems 2014, 17, 127–141. [Google Scholar] [CrossRef]
- Good, S.P.; Caylor, K.K. Climatological determinants of woody cover in Africa. Proc. Natl. Acad. Sci. USA 2011, 108, 4902–4907. [Google Scholar] [CrossRef]
- Weber-Grullon, L.; Gherardi, L.; Rutherford, W.A.; Archer, S.R.; Sala, O.E. Woody-plant encroachment: Precipitation, herbivory and grass-competition interact to affect shrub recruitment. Ecol. Appl. 2022, 32, e2536. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, L.; Shao, M.; Li, R.; Zhang, Y. Characteristics and Influencing Factors of Soil Water Recharge Under Different Groundwater Table Depths in the Mu Us Sandy Land. Trans. Chin. Soc. Agric. Eng. 2021, 37, 108–116. [Google Scholar]
- Zhang, Z.Y.; Gao, G.Q.; Xu, X.L.; Yu, M.; Qiang, T.Y. Shrubs proliferated within a six-year exclosure in a temperate grassland-spatiotemporal relationships between vegetation and soil variables. Sci. Cold Arid Reg. 2014, 6, 139–149. [Google Scholar]
- Liu, X.; Zhuang, Q.; Lai, L.; Zhou, J.; Sun, Q.; Yi, S.; Liu, B.; Zheng, Y. Soil water use sources and patterns in shrub encroachment in semiarid grasslands of Inner Mongolia. Agric. For. Meteorol. 2021, 308, 108579. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. World Reference Base for Soil Resources 2022: International Soil Classification and Correlation System; FAO: Rome, Italy, 2022. [Google Scholar]
- Song, Y.; Guo, Z.; Lu, Y.; Yan, D.; Liao, Z.; Liu, H.; Cui, Y. Pixel-level spatiotemporal analyses of vegetation fractional coverage variation and its influential factors in a desert steppe: A case study in Inner Mongolia, China. Water 2017, 9, 478. [Google Scholar] [CrossRef]
- Li, C.; Hao, X.; Zhao, M.; Han, G.; Willms, W.D. Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agric. Ecosyst. Environ. 2008, 128, 109–116. [Google Scholar] [CrossRef]
- Guo, K.; Liu, C.C.; Xie, Z.Q.; Li, Y.; Lu, Z.J.; Ma, K.P. China Vegetation Classification: Concept, approach and applications. Phytocoenologia 2018, 48, 113–120. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, C.L.; Dai, H.L. Soil Agrochemical Analysis and Environmental Monitoring; Chine Land Press: Beijing, China, 2008. [Google Scholar]
- Huang, C. Soil Science; China Agriculture Press: Beijing, China, 2000; pp. 71–75. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Package ‘hmisc’. CRAN 2019, 2019, 235–236. [Google Scholar]
- Rosseel, Y.; Oberski, D.; Byrnes, J.; Vanbrabant, L.; Savalei, V.; Merkle, E.; Hallquist, M.; Rhemtulla, M.; Katsikatsou, M.; Barendse, M.; et al. Package ‘lavaan’. CRAN 2017, 17, 2017. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef]
- Meng, B.; Jin, Z.; Chen, J.; Kuang, Y.; Yi, S.; Lv, Y.; Li, J. Critical signals for grassland desertification prediction in the transition zone between desert and typical steppe in InnerMongolia, China. Ecol. Indic. 2025, 170, 113065. [Google Scholar] [CrossRef]
- Yu, L.; Guo, T.; Sun, Z.; Ma, Y.; Li, Z.; Zhao, Y.; Wang, H. Seed Germination and Threshold Characteristics of Two Dominant Plant Species During the Transition from Desert Steppe to Shrubland. Acta Ecol. Sin. 2021, 41, 4160–4169. [Google Scholar]
- Shi, L.; Zhang, Z.J.; Zhang, C.Y.; Zhang, J.Z. Effects of sand burial on survival, growth, gas exchange and biomass allocation of Ulmus pumila seedlings in the Hunshandak Sandland, China. Ann. Bot. 2004, 94, 553–560. [Google Scholar] [CrossRef]
- Chengyi, Z.; Feihai, Y.; Ming, D. Effects of sand burial on the survival, growth, and biomass allocation in semi-shrub Hedysarum laeve seedlings. Acta Bot. Sin. 2002, 44, 337–343. [Google Scholar]
- Campanella, M.; Bisigato, A.J. Conspecific leaf litter and root competition inhibits shrub emergence in the Patagonian steppe. Plant Ecol. 2019, 220, 985–993. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting Depths, Lateral Root Spreads and Below-Ground/Above-Ground Allometries of Plants in Water-Limited Ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, H.; Akinyemi, D.S.; Zhang, P.; Feng, Y.; Zhao, M.; Kang, J.; Zhao, X.; Hu, H.; Fang, J. Increased precipitation attenuates shrub encroachment by facilitating herbaceous growth in a Mongolian grassland. Funct. Ecol. 2022, 36, 2356–2366. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Li, X.-Y.; Wang, Y.; He, B.; Jiang, Z.; Zhang, S.; Sun, W. Identifying water sources used by alpine riparian plants in a restoration zone on the Qinghai-Tibet Plateau: Evidence from stable isotopes. Sci. Total Environ. 2019, 697, 134092. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, X.Y.; Jiang, Z.Y.; Wang, Y.M. Study on the Relationship Between Shrub Encroachment and Soil Properties in Typical Grasslands of Inner Mongolia. Acta Prataculturae Sin. 2017, 26, 224–230. [Google Scholar]
- Hou, H.; Yan, H.; Bai, X.; Zhang, Y.; Guo, Y.; Zhou, J.; Gao, S. A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment. Plants 2025, 14, 605. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Elorza, M.; Dana, E.D.; González, A.; Sobrino, E. Changes in the high-mountain vegetation of the central Iberian Peninsula as a probable sign of global warming. Ann. Bot. 2003, 92, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, L.; Li, Z.; Zhang, J.; Li, Z.; Miao, B.; Jia, C.; Liang, C.; Wang, L.; Li, F.Y. Phylogenetic structure and formation mechanism of shrub communities in arid and semiarid areas of the Mongolian Plateau. Ecol. Evol. 2019, 9, 13320–13331. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).