Physiological and Flavonoid Metabolic Responses of Black Locust Leaves to Drought Stress in the Loess Plateau of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Throughfall Exclusion Experiment
2.3. Leaf Sample Collection
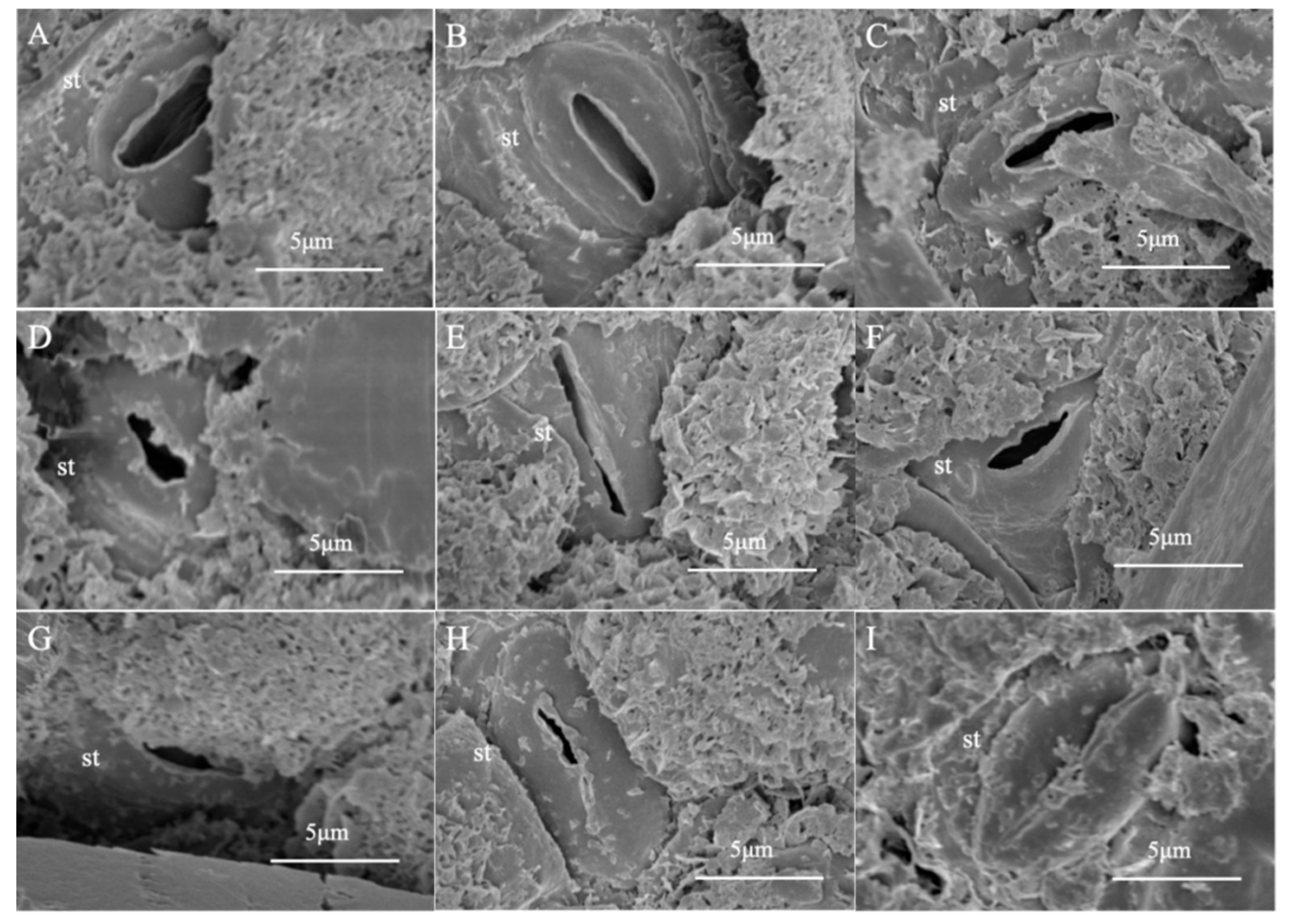
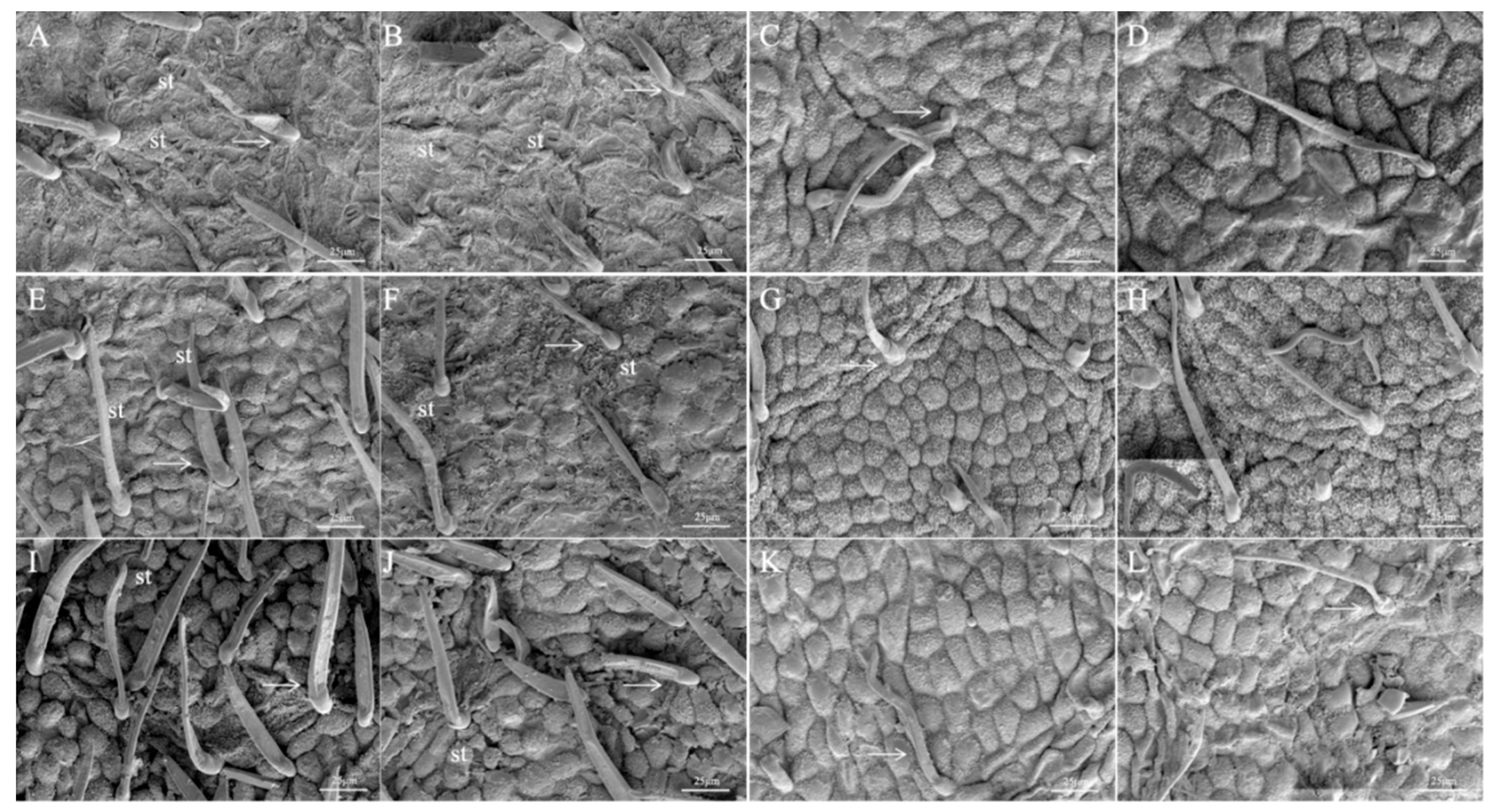
2.4. Leaf Epidermal Micromorphology
2.5. Leaf Physiological Indices
2.6. Analysis and Determination of Hormones in Black Locust Leaves
2.7. Flavonoid Metabolome Analysis
2.8. Data Processing
3. Results
3.1. Effects of Drought Stress on Leaf Structure of Black Locust
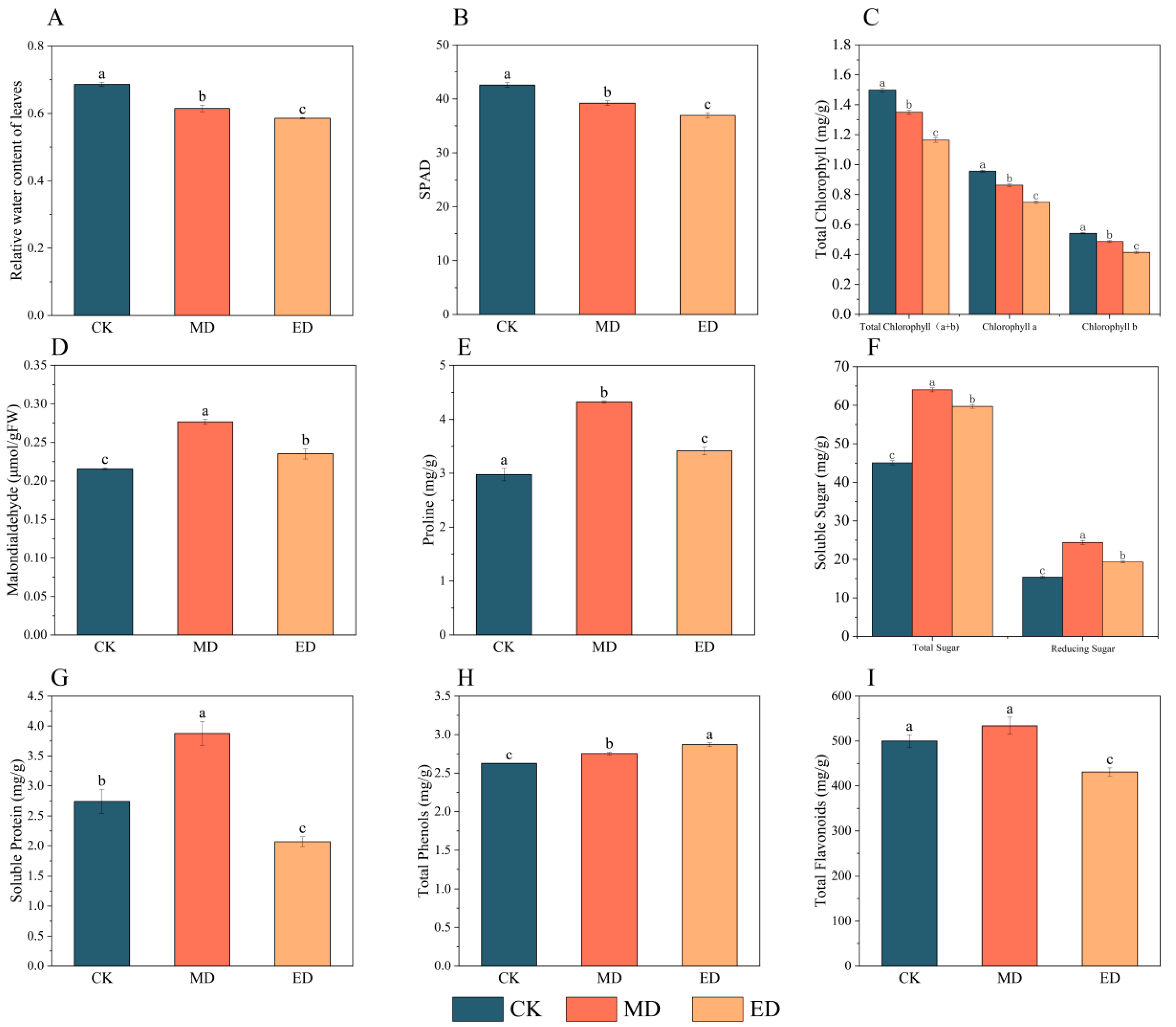
3.2. Effects of Drought Stress on Leaf Physiology of Black Locust
3.2.1. Leaf Relative Water Content
3.2.2. Photosynthetic Pigment Content
3.2.3. Malondialdehyde and Osmoregulatory Substances
3.2.4. Total Phenol and Total Flavone
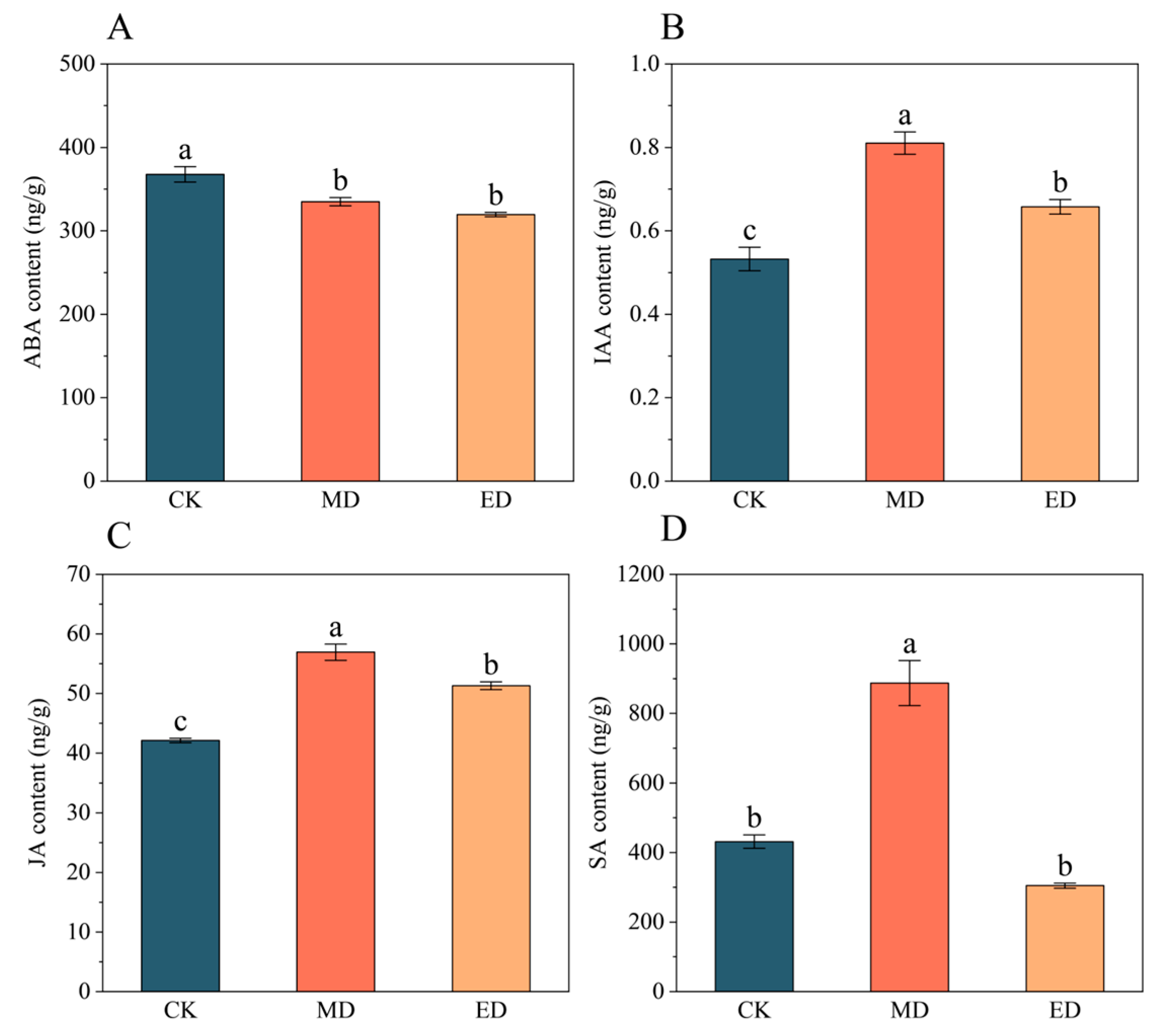
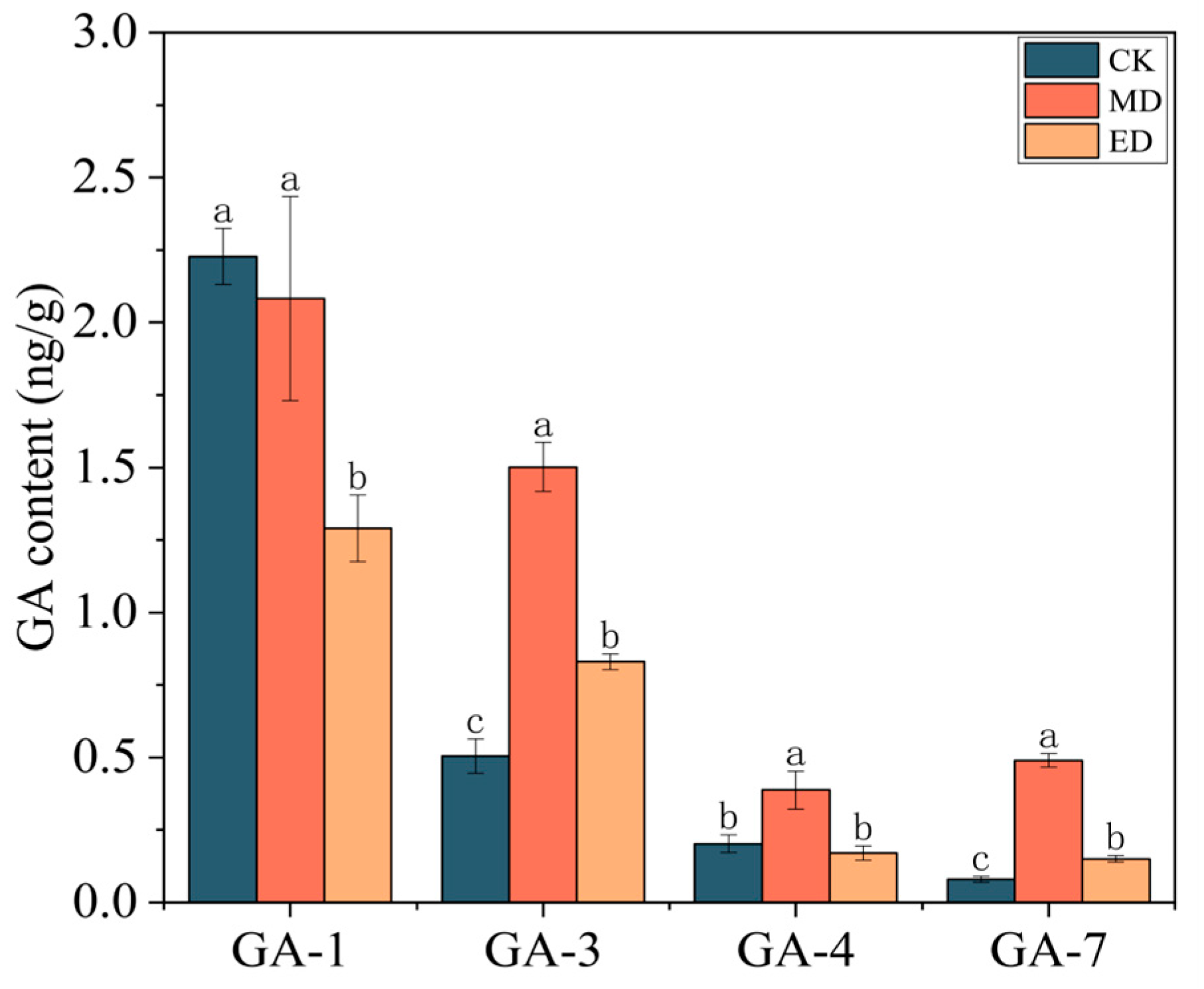
3.3. Hormone Content
3.4. Flavonoids Metabolome
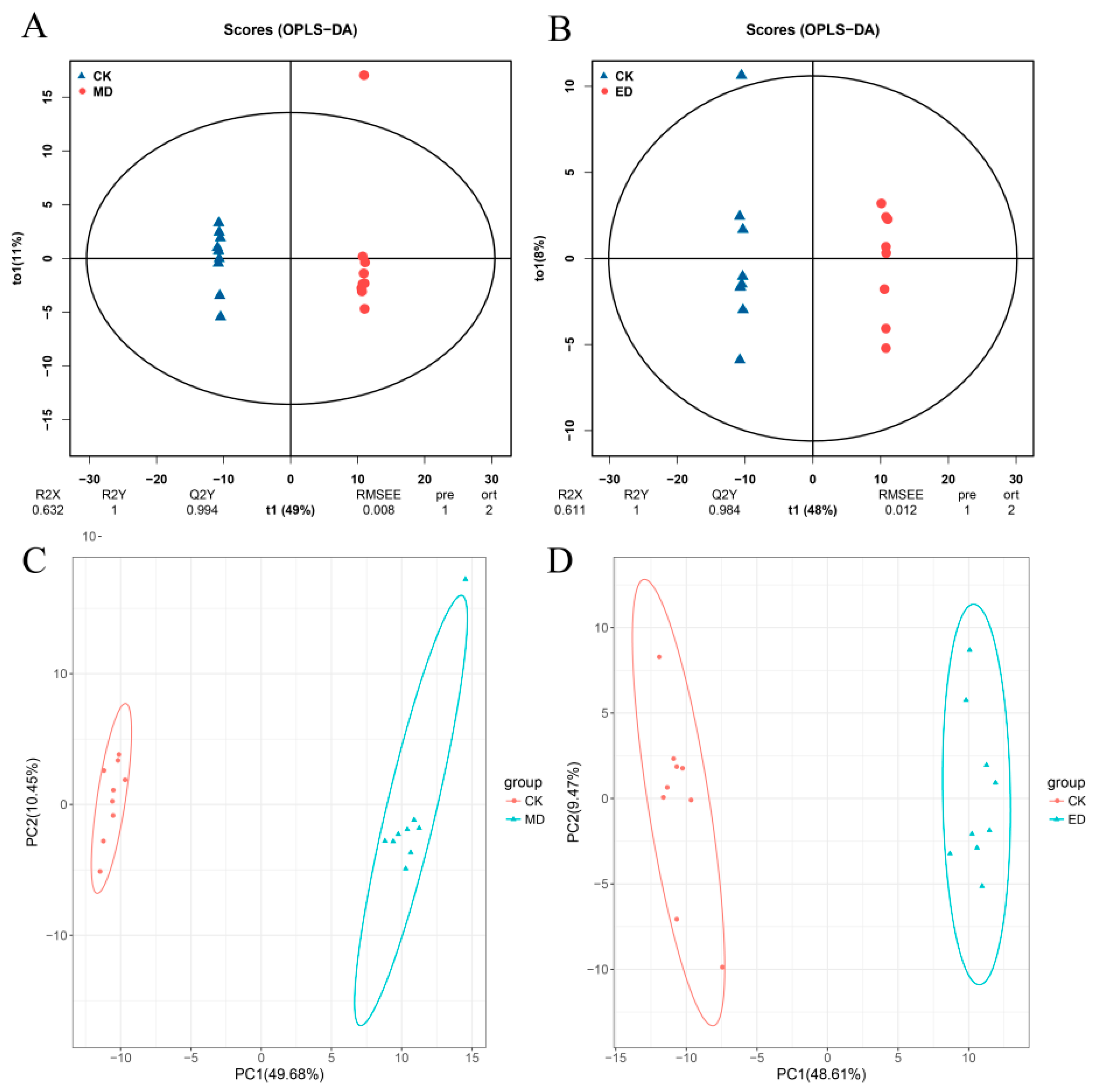
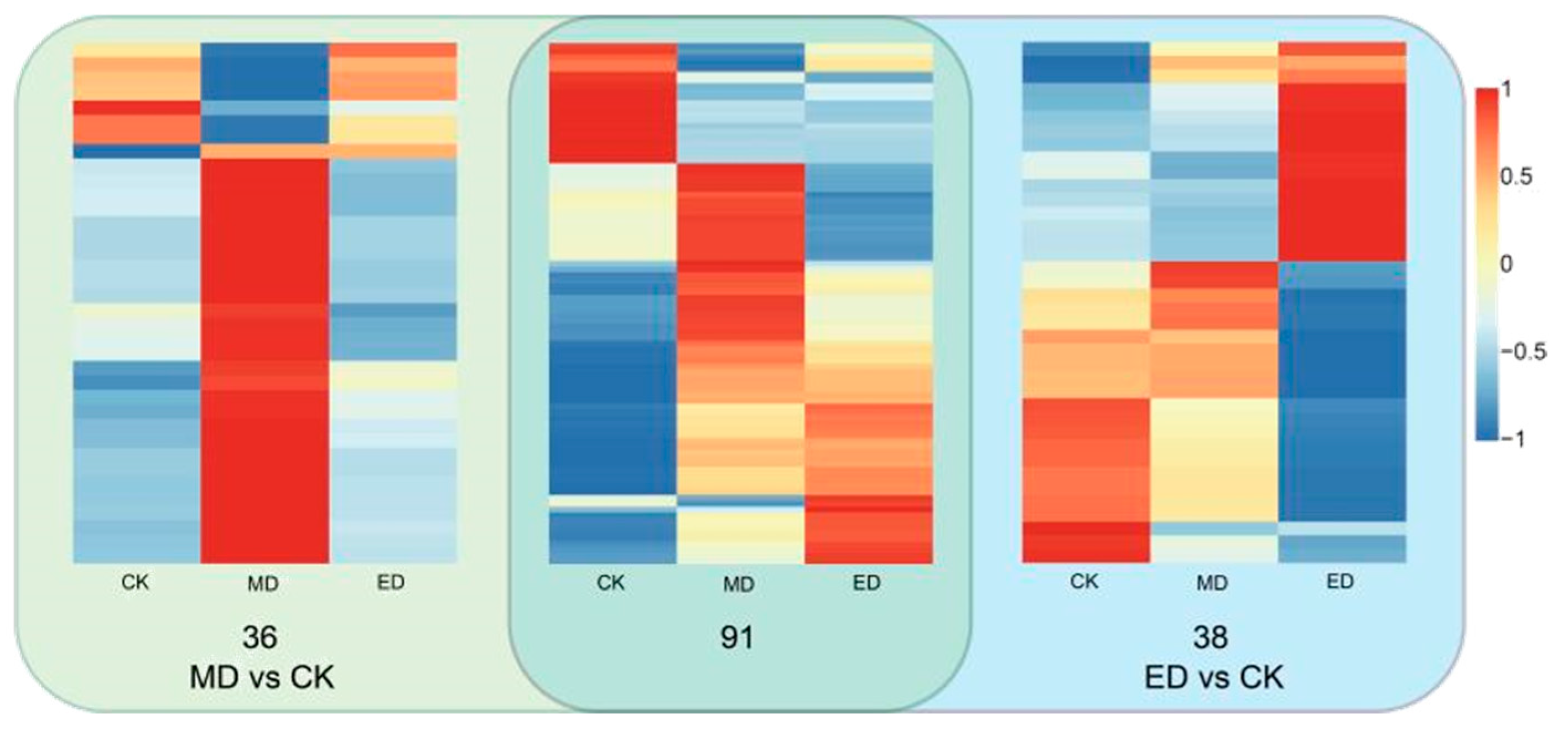
3.4.1. Identification of Differential Metabolites
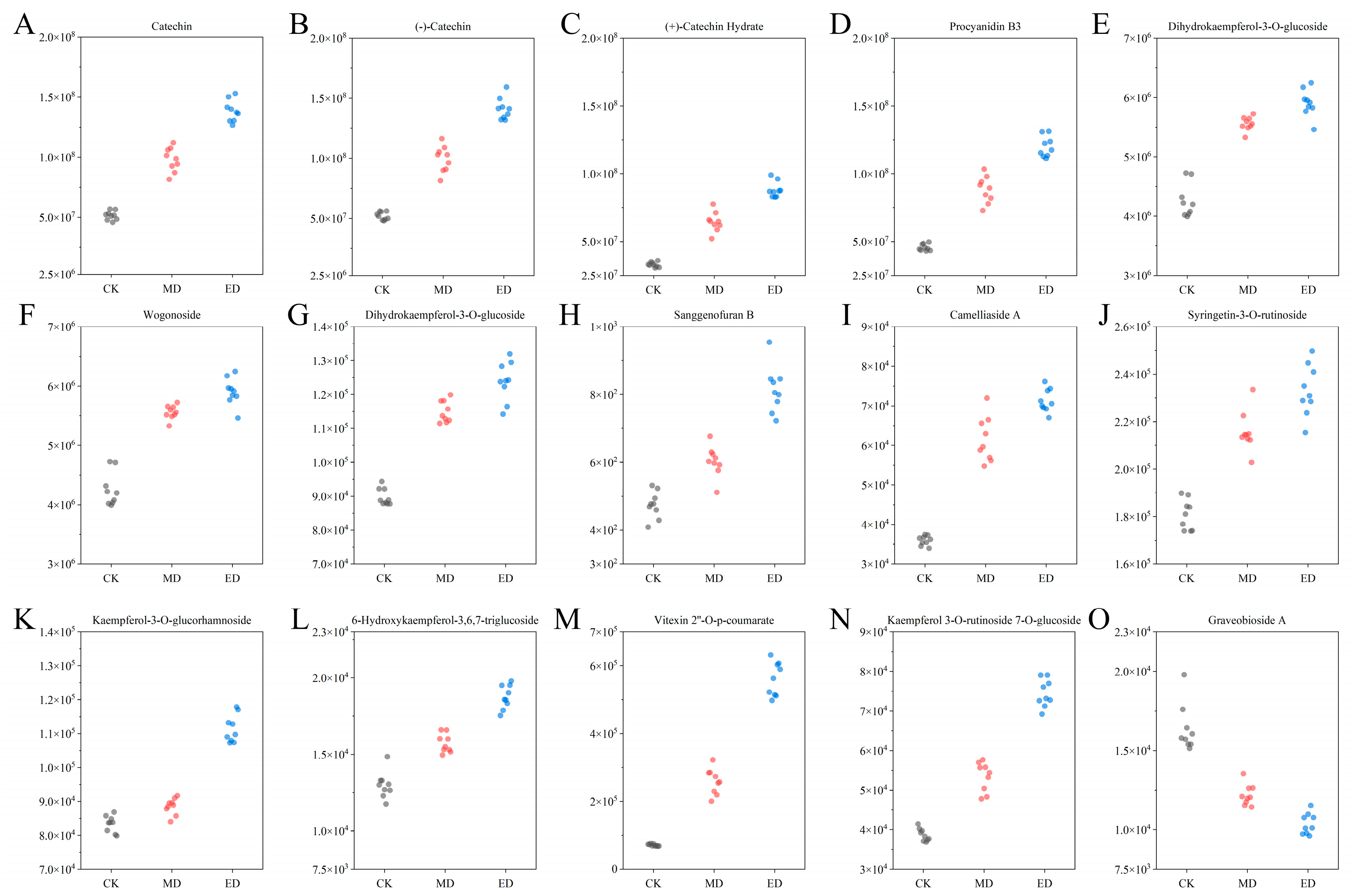
3.4.2. Heat Map Analysis of Flavonoid Differential Metabolites
4. Discussion
4.1. Effects of Drought Stress on Leaf Structure
4.2. Effects of Drought Stress on Leaf Physiology of Black Locust
4.3. Effects of Drought Stress on Hormone Content in Black Locust Leaves
4.4. Effects of Drought Stress on Flavonoid Metabolome in Black Locust Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, X.; Zhang, Z.; Zhao, X.; Huang, M.; Wu, X.; Guo, T. Differentiated Responses of Plant Water Use Regulation to Drought in Robinia pseudoacacia Plantations on the Chinese Loess Plateau. Agric. Water Manag. 2024, 291, 108659. [Google Scholar] [CrossRef]
- Huang, L.; Shao, M. Advances and Perspectives on Soil Water Research in China’s Loess Plateau. Earth-Sci. Rev. 2019, 199, 102962. [Google Scholar] [CrossRef]
- Cao, S.; Chen, L.; Liu, Z.; Wang, G. A New Tree-Planting Technique to Improve Tree Survival and Growth on Steep and Arid Land in the Loess Plateau of China. J. Arid Environ. 2008, 72, 1374–1382. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, S.; Guo, H.; Chen, S.; Li, Z. Climatic Warming-Induced Drought Stress Has Resulted in the Transition of Tree Growth Sensitivity from Temperature to Precipitation in the Loess Plateau of China. Biology 2023, 12, 1275. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Jiao, J.-Y.; Su, Y.; Chen, Y. The Efficiency of Large-Scale Afforestation with Fish-Scale Pits for Revegetation and Soil Erosion Control in the Steppe Zone on the Hill-Gully Loess Plateau. CATENA 2014, 115, 159–167. [Google Scholar] [CrossRef]
- Wu, J.; Miao, C.; Zheng, H.; Duan, Q.; Lei, X.; Li, H. Meteorological and Hydrological Drought on the Loess Plateau, China: Evolutionary Characteristics, Impact, and Propagation. JGR Atmos. 2018, 123, 11569–11584. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Shao, M.; Jia, X.; Li, X. Spatiotemporal Analysis of Multiscalar Drought Characteristics across the Loess Plateau of China. J. Hydrol. 2016, 534, 281–299. [Google Scholar] [CrossRef]
- Graciano, C.; Guiamét, J.J.; Goya, J.F. Impact of Nitrogen and Phosphorus Fertilization on Drought Responses in Eucalyptus grandis Seedlings. For. Ecol. Manag. 2005, 212, 40–49. [Google Scholar] [CrossRef]
- Jia, G.; Chen, L.; Yu, X.; Liu, Z. Soil Water Stress Overrides the Benefit of Water-use Efficiency from Rising CO2 and Temperature in a Cold Semi-arid Poplar Plantation. Plant Cell Environ. 2022, 45, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, Z.; Zhang, F.; Bourque, C.P.-A.; Jia, X.; Li, X.; Liu, P.; Yu, H.; Tian, Y.; Jin, C.; et al. Sensitivity of Gross Primary Production and Evapotranspiration to Heat and Drought Stress in a Young Temperate Plantation in Northern China. For. Ecosyst. 2025, 12, 100275. [Google Scholar] [CrossRef]
- Wang, B.; Chen, W.; Tian, D.; Li, Z.; Wang, J.; Fu, Z.; Luo, Y.; Piao, S.; Yu, G.; Niu, S. Dryness Limits Vegetation Pace to Cope with Temperature Change in Warm Regions. Glob. Chang. Biol. 2023, 29, 4750–4757. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Du, C.; Ni, X.; Yan, M.; Meng, Q.; He, J. Physiological and Transcriptome Analysis Reveals the Mechanism of Gymnocarpos przewalskii Response to Drought Stress. BMC Plant Biol. 2025, 25, 155. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex Plant Responses to Drought and Heat Stress under Climate Change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Y.; Zhu, W.; Zhou, Y. Morphological, Physiological, and Biochemical Composition of Mulberry (Morus spp.) under Drought Stress. Forests 2023, 14, 949. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, X.; Zi, N.; Ren, W.; Yin, J.; Yuan, T.; Wang, M.; Yuan, F.; Liu, Y. Drought Stress Memory Enhances the Tolerance of Alfalfa Medicago sativa L. in Response to a Subsequent Drought: A Physiological and Omics Perspective. Environ. Exp. Bot. 2025, 230, 106088. [Google Scholar] [CrossRef]
- Lin, C.; Hu, W.; Qin, X.; Fei, Y.; Hu, D. Effects of Serendipita indica on the Morphological and Physiological Characteristics of Agrostis stolonifera L. Under Drought Stress. Agronomy 2025, 15, 234. [Google Scholar] [CrossRef]
- Skelton, R.P.; Midgley, J.J.; Nyaga, J.M.; Johnson, S.D.; Cramer, M.D. Is Leaf Pubescence of Cape Proteaceae a Xeromorphic or Radiation-Protective Trait? Aust. J. Bot. 2012, 60, 104. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Mao, J.; Xu, Y.; Wang, S.; Wang, L.; Qiao, Z.; Liu, S.; Cao, X. Physiological Responses and Metabolic Characteristics of Proso Millet Under Drought Stress During Germination Period. Food Sci. Nutr. 2025, 13, e70001. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Huang, H.; Wu, D.; Yan, J.; Qi, Y.; Song, S.; Xie, D. Arabidopsis DELLA and JAZ Proteins Bind the WD-Repeat/bHLH/MYB Complex to Modulate Gibberellin and Jasmonate Signaling Synergy. Plant Cell 2014, 26, 1118–1133. [Google Scholar] [CrossRef]
- Jan, M.F.; Altaf, M.T.; Liaqat, W.; Liu, C.; Mohamed, H.I.; Li, M. Approaches for the Amelioration of Adverse Effects of Drought Stress on Soybean Plants: From Physiological Responses to Agronomical, Molecular, and Cutting-Edge Technologies. Plant Soil 2025, 1–53. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black Locust (Robinia pseudoacacia L.) Range Contraction and Expansion in Europe under Changing Climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, V.-N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, Growth and Management of Black Locust (Robinia pseudoacacia L.), a Non-Native Species Integrated into European Forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Gong, J.; Fu, B.; Huang, Y. The Effect of Land Cover/Vegetation on Soil Water Dynamic in the Hilly Area of the Loess Plateau, China. CATENA 2007, 70, 200–208. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Wang, X.; Deng, J.; Zhang, Z.; Wang, B. Influence of Habitat on the Phylogenetic Structure of Robinia pseudoacacia Forests in the Eastern Loess Plateau, China. Glob. Ecol. Conserv. 2020, 24, e01199. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, M.; Yang, Y.; Zhao, X. Evaluating Drought-Induced Mortality Risk for Robinia pseudoacacia Plantations along the Precipitation Gradient on the Chinese Loess Plateau. Agric. For. Meteorol. 2020, 284, 107897. [Google Scholar] [CrossRef]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental Controls on Sap Flow in Black Locust Forest in Loess Plateau, China. Sci. Rep. 2017, 7, 13160. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Zhang, C.; Zhao, Y.; Du, G.; Li, M.; Si, B. From Comfort Zone to Mortality: Sequence of Physiological Stress Thresholds in Robinia pseudoacacia Seedlings during Progressive Drought. Front. Plant Sci. 2023, 14, 1149760. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, R.; Qi, L.; Sun, H.; Zhang, P.; Li, Z. Water Consumption Turning Point for Robinia pseudoacacia Occurs at Its Middle Stand Age. Plant Soil 2025, 1–16. [Google Scholar] [CrossRef]
- Meerdink, S.K.; Roberts, D.A.; King, J.Y.; Roth, K.L.; Gader, P.D.; Caylor, K.K. Using Hyperspectral and Thermal Imagery to Monitor Stress of Southern California Plant Species during the 2013–2015 Drought. ISPRS J. Photogramm. Remote Sens. 2025, 220, 580–592. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, L.; Bai, Y.; Li, Z.; Yuan, C.; Li, Z.; Gao, G. Rainfall Partitioning in the Robinia pseudodcacia Plantations with Different Thinning Intensities in the Semiarid Loess Plateau of China. Ecol. Front. 2025, 45, 257–267. [Google Scholar] [CrossRef]
- Yang, B.; Peng, C.; Harrison, S.P.; Wei, H.; Wang, H.; Zhu, Q.; Wang, M. Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests 2018, 9, 754. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-Structural Carbohydrate Dynamics in Robinia pseudoacacia Saplings under Three Levels of Continuous Drought Stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Rosner, S.; Karlsson, B. Hydraulic Efficiency Compromises Compression Strength Perpendicular to the Grain in Norway Spruce Trunkwood. Trees 2011, 25, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Klein, A.; Muller, U.; Karlsson, B. Hydraulic and Mechanical Properties of Young Norway Spruce Clones Related to Growth and Wood Structure. Tree Physiol. 2007, 27, 1165–1178. [Google Scholar] [CrossRef]
- Tiedge, K.; Li, X.; Merrill, A.T.; Davisson, D.; Chen, Y.; Yu, P.; Tantillo, D.J.; Last, R.L.; Zerbe, P. Comparative Transcriptomics and Metabolomics Reveal Specialized Metabolite Drought Stress Responses in Switchgrass (Panicum virgatum). New Phytol. 2022, 236, 1393–1408. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Wang, X.; Song, W.; Wang, Q.; Wang, X.; Li, S.; Fu, B. Exogenous Melatonin Ameliorates Drought Stress in Agropyron mongolicum by Regulating Flavonoid Biosynthesis and Carbohydrate Metabolism. Front. Plant Sci. 2022, 13, 1051165. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Zhou, J.; Fu, W.; Guo, L.; Zhang, X.; Chen, B. Integrated Transcriptomics and Metabolomics Reveal Specific Phenolic and Flavonoid Accumulation in Licorice (Glycyrrhiza uralensis Fisch.) Induced by Arbuscular Mycorrhiza Symbiosis under Drought Stress. Plant Physiol. Biochem. 2023, 205, 108173. [Google Scholar] [CrossRef]
- Feng, X.; Bai, S.; Zhou, L.; Song, Y.; Jia, S.; Guo, Q.; Zhang, C. Integrated Analysis of Transcriptome and Metabolome Provides Insights into Flavonoid Biosynthesis of Blueberry Leaves in Response to Drought Stress. IJMS 2024, 25, 11135. [Google Scholar] [CrossRef]
- Chen, G.; Li, D.; Yao, P.; Chen, F.; Yuan, J.; Ma, B.; Yang, Z.; Ding, B.; He, N. Metabolic and Transcriptional Analysis Reveals Flavonoid Involvement in the Drought Stress Response of Mulberry Leaves. IJMS 2024, 25, 7417. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gichuki, D.K.; Zhou, H.; Hou, Y.; Gituru, R.W.; Wang, Q.; Xin, H. Transcriptional Modification and the Accumulation of Flavonoid in the Leaves of Cissus rotundifolia Lam. in Respond to Drought Stress. Stress Biol. 2025, 5, 19. [Google Scholar] [CrossRef]
- He, C.; Du, W.; Ma, Z.; Jiang, W.; Pang, Y. Identification and Analysis of Flavonoid Pathway Genes in Responsive to Drought and Salinity Stress in Medicago truncatula. J. Plant Physiol. 2024, 302, 154320. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chu, G.; Wang, J.; Luo, H.; Yang, Z.; Sun, L.; Rong, W.; Wang, M. Integrated Metabolomic and Transcriptomic Analysis of Specialized Metabolites and Isoflavonoid Biosynthesis in Sophora alopecuroides L. under Different Degrees of Drought Stress. Ind. Crops Prod. 2023, 197, 116595. [Google Scholar] [CrossRef]
- Liu, B.; Tang, X.; Wang, L.; Zhang, P.; He, J.; Yue, C. Physiological responses of a black locust plantation to drought stress based on a throughfall exclusion experiment in semi-arid northwestern China. For. Int. J. For. Res. 2025, 98, 220–232. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample Preparation for Scanning Electron Microscopy of Plant Surfaces—Horses for Courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Zhang, X.; Zhao, D.; Tao, J. Effects of Fulvic Acid on the Photosynthetic and Physiological Characteristics of Paeonia ostii under Drought Stress. Plant Signal. Behav. 2020, 15, 1774714. [Google Scholar] [CrossRef]
- De Oliveira Maia Júnior, S.; De Andrade, J.R.; De Oliveira Sousa, V.F.; Silva, P.C.; Aguiar, D.L.; De Sousa Torres, A.M.; Diniz, D.C.; De Assis Figueiredo, F.A.M.M.; De Oliveira Reis, F.; Ferraz, T.M. Seed Priming with Brassinosteroids Mitigates Pre-Flowering Drought Stress in Soybean Varieties. J. Soil Sci. Plant Nutr. 2025, 1–6. [Google Scholar] [CrossRef]
- Wei, S.; Bian, Y.; Zhao, Q.; Chen, S.; Mao, J.; Song, C.; Cheng, K.; Xiao, Z.; Zhang, C.; Ma, W.; et al. Salinity-Induced Palmella Formation Mechanism in Halotolerant Algae Dunaliella salina Revealed by Quantitative Proteomics and Phosphoproteomics. Front. Plant Sci. 2017, 8, 810. [Google Scholar] [CrossRef]
- Ahsan, M.; Younis, A.; Jamal, A.; Alshaharni, M.O.; Algopishi, U.B.; Al-Andal, A.; Sajid, M.; Naeem, M.; Khan, J.A.; Radicetti, E.; et al. Melatonin Induces Drought Stress Tolerance by Regulating the Physiological Mechanisms, Antioxidant Enzymes, and Leaf Structural Modifications in Rosa centifolia L. Heliyon 2025, 11, e41236. [Google Scholar] [CrossRef]
- Bakhtiari, E.S.; Mousavi, A.; Yadegari, M.; Haghighati, B.; Martínez-García, P.J. Physiological and Biochemical Responses of Almond (Prunus dulcis) Cultivars to Drought Stress in Semi-Arid Conditions in Iran. Plants 2025, 14, 734. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Chai, X.; Graciano, C. Involvement of Soluble Proteins in Growth and Metabolic Adjustments of Drought-stressed Calligonum mongolicum Seedlings under Nitrogen Addition. Plant Biol. J. 2021, 23, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Bing, Y.; Jing, G.; Yan, W.; Ben, H.; Liang, P. Effects of Drought Stress on Secondary Metabolite Contents and Antioxidant Enzyme Activities in Callus of Polygala tenuifolia Willd. North. Hortic. 2020, 3, 109–116. [Google Scholar]
- Zhi, X.; Bian, X.; Yu, J.; Xiao, X.; Duan, B.; Huang, F.; Jiang, Z.; Zhou, G.; Ma, N. Comparative Metabolomics Analysis of Tolerant and Sensitive Genotypes of Rapeseed (Brassica napus L.) Seedlings under Drought Stress. Agric. Water Manag. 2024, 296, 108797. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The Mechanical Diversity of Stomata and Its Significance in Gas-Exchange Control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, Y.; Zhao, B.; Zhao, Y.; Chen, Y.; Zhou, Q.; Wang, H. Photosynthetic Performance and Carbon Metabolism in the Ear Organs of Oats under Drought Stress. Front. Plant Sci. 2025, 15, 1463284. [Google Scholar] [CrossRef]
- Licaj, I.; Fiorillo, A.; Di Meo, M.C.; Varricchio, E.; Rocco, M. Effect of Polyethylene Glycol-Simulated Drought Stress on Stomatal Opening in “Modern” and “Ancient” Wheat Varieties. Plants 2024, 13, 1575. [Google Scholar] [CrossRef]
- Tian, J.; Pang, Y.; Zhao, Z. Drought, Salinity, and Low Nitrogen Differentially Affect the Growth and Nitrogen Metabolism of Sophora japonica (L.) in a Semi-Hydroponic Phenotyping Platform. Front. Plant Sci. 2021, 12, 715456. [Google Scholar] [CrossRef]
- Hegebarth, D.; Buschhaus, C.; Wu, M.; Bird, D.; Jetter, R. The Composition of Surface Wax on Trichomes of Arabidopsis thaliana Differs from Wax on Other Epidermal Cells. Plant J. 2016, 88, 762–774. [Google Scholar] [CrossRef]
- Zekri, M.A.; Lang, I. Lack of Trichomes and Variation in Stomata Properties Influence the Quantum Efficiency of Photosynthesis in Arabidopsis. Environ. Exp. Bot. 2024, 227, 105948. [Google Scholar] [CrossRef]
- Kang, J.-H.; Liu, G.; Shi, F.; Jones, A.D.; Beaudry, R.M.; Howe, G.A. The Tomato odorless-2 Mutant Is Defective in Trichome-Based Production of Diverse Specialized Metabolites and Broad-Spectrum Resistance to Insect Herbivores. Plant Physiol. 2010, 154, 262–272. [Google Scholar] [CrossRef]
- Ning, P.; Wang, J.; Zhou, Y.; Gao, L.; Wang, J.; Gong, C. Adaptional Evolution of Trichome in Caragana korshinskii to Natural Drought Stress on the Loess Plateau, China. Ecol. Evol. 2016, 6, 3786–3795. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.J.; Nie, J.; Yang, Z.Y.; Zhao, X.L. Leaf Morphological Responses of Indigofera bungeana to Drought Stress. Acta Bot. Boreali-Occident. Sin. 2020, 40, 613–623. [Google Scholar]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Jin, S.; Peng, Z.; Zhang, S. The Impact of Varying Degrees of Drought Stress and Rehydration Treatment on the Physiological Indicators of Robinia pseudoacacia Seedlings. J. Northeast. For. Univ. 2024, 52, 27–39. [Google Scholar]
- Siyu, J.; Zuodeng, P. Changes in response of carbon and water physiological parameters of Robinia pseudoacacia seedlings to long-term drought and rehydration. J. Beijing For. Univ. 2023, 45, 43–56. [Google Scholar]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Lo Gullo, M.A.; Nardini, A.; Casolo, V. Hydraulic Recovery from Xylem Embolism in Excised Branches of Twelve Woody Species: Relationships with Parenchyma Cells and Non-Structural Carbohydrates. Plant Physiol. Biochem. 2019, 139, 513–520. [Google Scholar] [CrossRef]
- Song, S.; Qu, Z.; Zhou, X.; Wang, X.; Dong, S. Effects of Weak and Strong Drought Conditions on Physiological Stability of Flowering Soybean. Plants 2022, 11, 2708. [Google Scholar] [CrossRef]
- Shokat, S.; Liu, F.; Großkinsky, D.K. Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes. Plants 2024, 13, 2942. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Molero, G.; Erice, G.; Avice, J.C.; Nogués, S. Plant Physiology and Proteomics Reveals the Leaf Response to Drought in Alfalfa (Medicago sativa L.). J. Exp. Bot. 2011, 62, 111–123. [Google Scholar] [CrossRef]
- Landi, M. Commentary to: “Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds” by Hodges et al., Planta (1999) 207:604–611. Planta 2017, 245, 1067. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Saleem, A.; Tahir, M.; Khilji, S.A.; Sajid, Z.A.; Landry, K.B.; El-Sheikh, M.A.; Ahmad, P. Modulation of the Polyamines, Osmolytes and Antioxidant Defense System to Ameliorate Drought Stress Tolerance in Hordeum vulgare L. Using Ascorbic Acid. S. Afr. J. Bot. 2024, 171, 726–736. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, H.; Cai, S.; Guo, Q.; Dai, Y.; Wang, H.; Wan, S.; Yuan, Y. Rice Growth and Leaf Physiology in Response to Four Levels of Continuous Drought Stress in Southern China. Agronomy 2024, 14, 1579. [Google Scholar] [CrossRef]
- Sattar, A.; El-Yazied, A.A.; Alharbi, B.M.; El-Gawad, H.G.A.; Abbas, Z.K.; El-Absy, K.M.; Mahmoud, S.F.; Althaqafi, M.M.; Darwish, D.B.E.; Al-Harbi, N.A.; et al. Application of Biostimulants Alleviated Drought Stress in Sugar Beet (Beta vulgaris L.) by Improving Oxidative Defense System, Osmolytes Accumulation and Root Yield. J. Soil Sci. Plant Nutr. 2024, 24, 7167–7183. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Shekari, F.; Maleki, H.H. Influence of Exogenous Polyamines on Antioxidant Defence and Essential Oil Production in Valerian (Valeriana officinalis L.) Plants under Drought Stress. Acta Agric. Slov. 2016, 107, 81–91. [Google Scholar] [CrossRef]
- Mbinda, W.; Ombori, O.; Dixelius, C.; Oduor, R. Xerophyta viscosa Aldose Reductase, XvAld1, Enhances Drought Tolerance in Transgenic Sweetpotato. Mol. Biotechnol. 2018, 60, 203–214. [Google Scholar] [CrossRef]
- Akram, N.A.; Waseem, M.; Ameen, R.; Ashraf, M. Trehalose Pretreatment Induces Drought Tolerance in Radish (Raphanus sativus L.) Plants: Some Key Physio-Biochemical Traits. Acta Physiol. Plant 2016, 38, 3. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Jing, M.; Zhang, N.; Liu, C.; Yan, J. Variation of Root Soluble Sugar and Starch Response to Drought Stress in Foxtail Millet. Agronomy 2023, 13, 359. [Google Scholar] [CrossRef]
- Jimenez, S.; Dridi, J.; Gutierrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, Biochemical and Molecular Responses in Four Prunus Rootstocks Submitted to Drought Stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Nahar, S.; Vemireddy, L.R.; Sahoo, L.; Tanti, B. Antioxidant Protection Mechanisms Reveal Significant Response in Drought-Induced Oxidative Stress in Some Traditional Rice of Assam, India. Rice Sci. 2018, 25, 185–196. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Yan, C.; Chen, Z.; Li, X.; Zeng, B.; Hu, B. Comparative Physiological, Biochemical, Metabolomic, and Transcriptomic Analyses Reveal the Formation Mechanism of Heartwood for Acacia Melanoxylon. BMC Plant Biol. 2024, 24, 308. [Google Scholar] [CrossRef]
- Fang, Y. Study on The Category and Content of Gibberellin in Different Parts During Dormancy Release in Peach. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Hamed, H.A.; Mahmoud, G.A.-E.; Abeed, A.H.A. Unraveling Growth and Metabolic Dynamics in Drought-Stressed Spinach Plants: Exploring the Contribution of Biological Gibberellin. Sci. Hortic. 2025, 340, 113924. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Kumar, R.; Altaf, M.M.; Kumar, A.; Khan, L.U.; Saqib, M.; Nawaz, M.A.; Saddiq, B.; Bahadur, S.; et al. Phytohormones Mediated Modulation of Abiotic Stress Tolerance and Potential Crosstalk in Horticultural Crops. J. Plant Growth Regul. 2023, 42, 4724–4750. [Google Scholar] [CrossRef]
- Long, J.; Liu, D.; Qiao, W.; Wang, Y.; Miao, Y.; Baosai, H. Response of Elymus nutans Griseb. Seedling Physiology and Endogenous Hormones to Drought and Salt Stress. Sci. Rep. 2024, 14, 17810. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA Perception and Signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Qing, Y.; Yan, Q. Response of endogenous hormones in Camellia reticulata to drought stress and rehydration based on transcriptome analysis. Southwest China J. Agric. Sci. 2024, 37, 913–924. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, Y.; Wei, W.; Yang, J.; Zhong, Q.; Li, Z. Metabolite Analysis of Jerusalem Artichoke (Helianthus tuberosus L.) Seedlings in Response to Polyethylene Glycol-Simulated Drought Stress. Int. J. Mol. Sci. 2021, 22, 3294. [Google Scholar] [CrossRef]
- Sima, N.A.K.; Jabbari, H.; Ebadi, A.; Ghaffari, M.R.; Koobaz, P. Comparative Analysis of Exogenous Hormone Application on Contrasting Canola (Brassica napus L.) Genotypes Under Drought Stress Conditions. J. Soil Sci. Plant Nutr. 2024, 24, 308–317. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Peng, N.; Liu, B.; Yang, Y.; Yue, C.; Hao, W.; He, J. Physiological and Flavonoid Metabolic Responses of Black Locust Leaves to Drought Stress in the Loess Plateau of China. Forests 2025, 16, 695. https://doi.org/10.3390/f16040695
Wang Y, Peng N, Liu B, Yang Y, Yue C, Hao W, He J. Physiological and Flavonoid Metabolic Responses of Black Locust Leaves to Drought Stress in the Loess Plateau of China. Forests. 2025; 16(4):695. https://doi.org/10.3390/f16040695
Chicago/Turabian StyleWang, Yan, Ning Peng, Binbin Liu, Yingbin Yang, Chao Yue, Wenfang Hao, and Junhao He. 2025. "Physiological and Flavonoid Metabolic Responses of Black Locust Leaves to Drought Stress in the Loess Plateau of China" Forests 16, no. 4: 695. https://doi.org/10.3390/f16040695
APA StyleWang, Y., Peng, N., Liu, B., Yang, Y., Yue, C., Hao, W., & He, J. (2025). Physiological and Flavonoid Metabolic Responses of Black Locust Leaves to Drought Stress in the Loess Plateau of China. Forests, 16(4), 695. https://doi.org/10.3390/f16040695




