How Vegetation Structure Shapes the Soundscape: Acoustic Community Partitioning and Its Implications for Urban Forestry Management
Abstract
1. Introduction
2. Materials and Methods
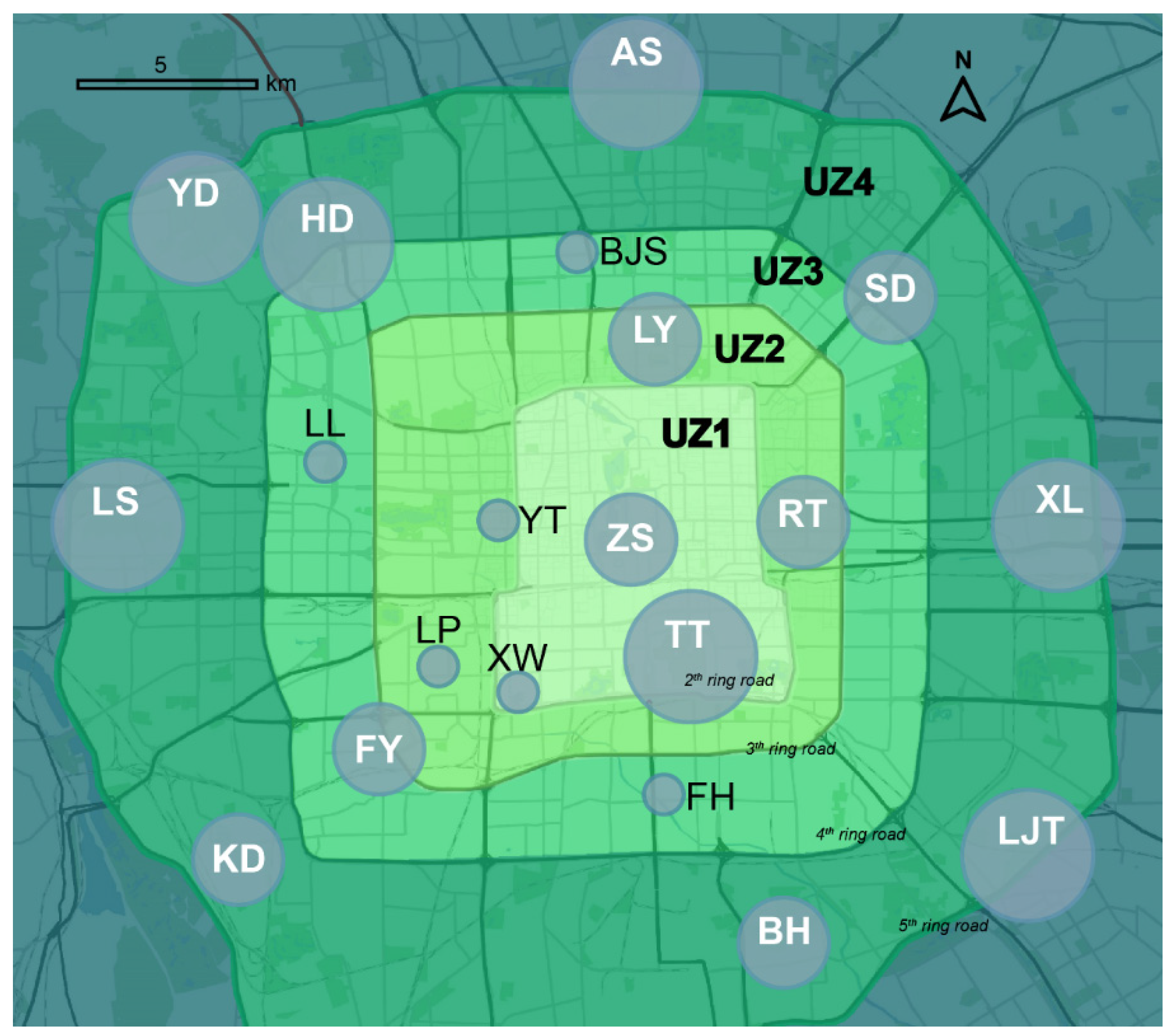
2.1. Field Acoustic Data Collection
2.2. Vegetation Data Collection
2.3. Acoustic Parameters
2.4. Statistical Analyses
2.4.1. Principal Component Analysis
2.4.2. Acoustic Niche Characterization Analysis
2.4.3. Mixed-Effect Modeling
- Yijk denotes the acoustic index value for observation k at plot i in month j;
- Xijk denotes the matrix of 11 standardized vegetation predictors;
- Β denotes the fixed-effect coefficients;
- γi ~ N(0, σplot2) denotes the random intercept for the plot location (60 plots);
- δj ~ N(0, σmonth2) denotes the random intercept for the recording month (2 months);
- εijk ~ N(0, σres2) denotes the residual error.
3. Results
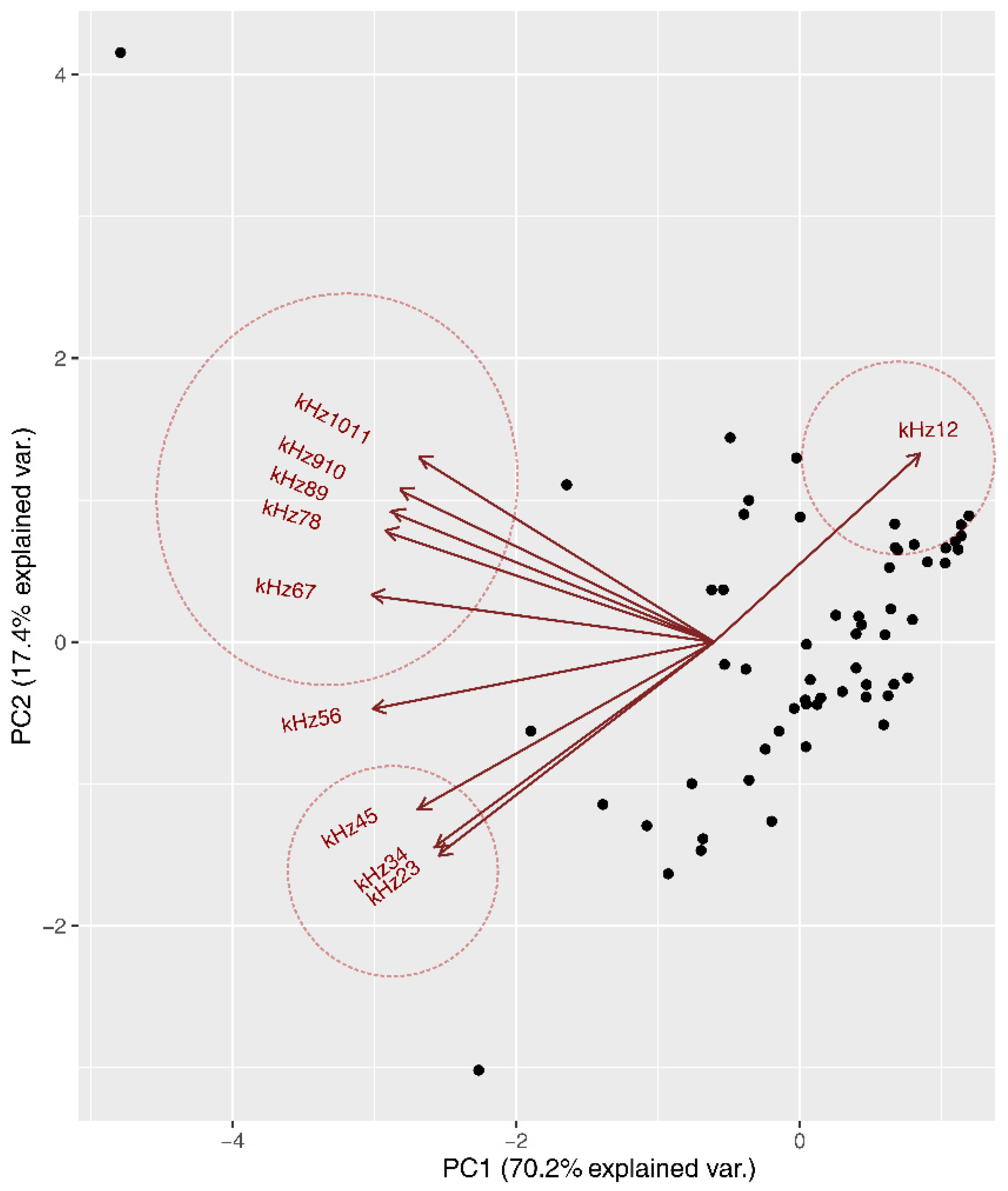
3.1. Acoustic Community Composition
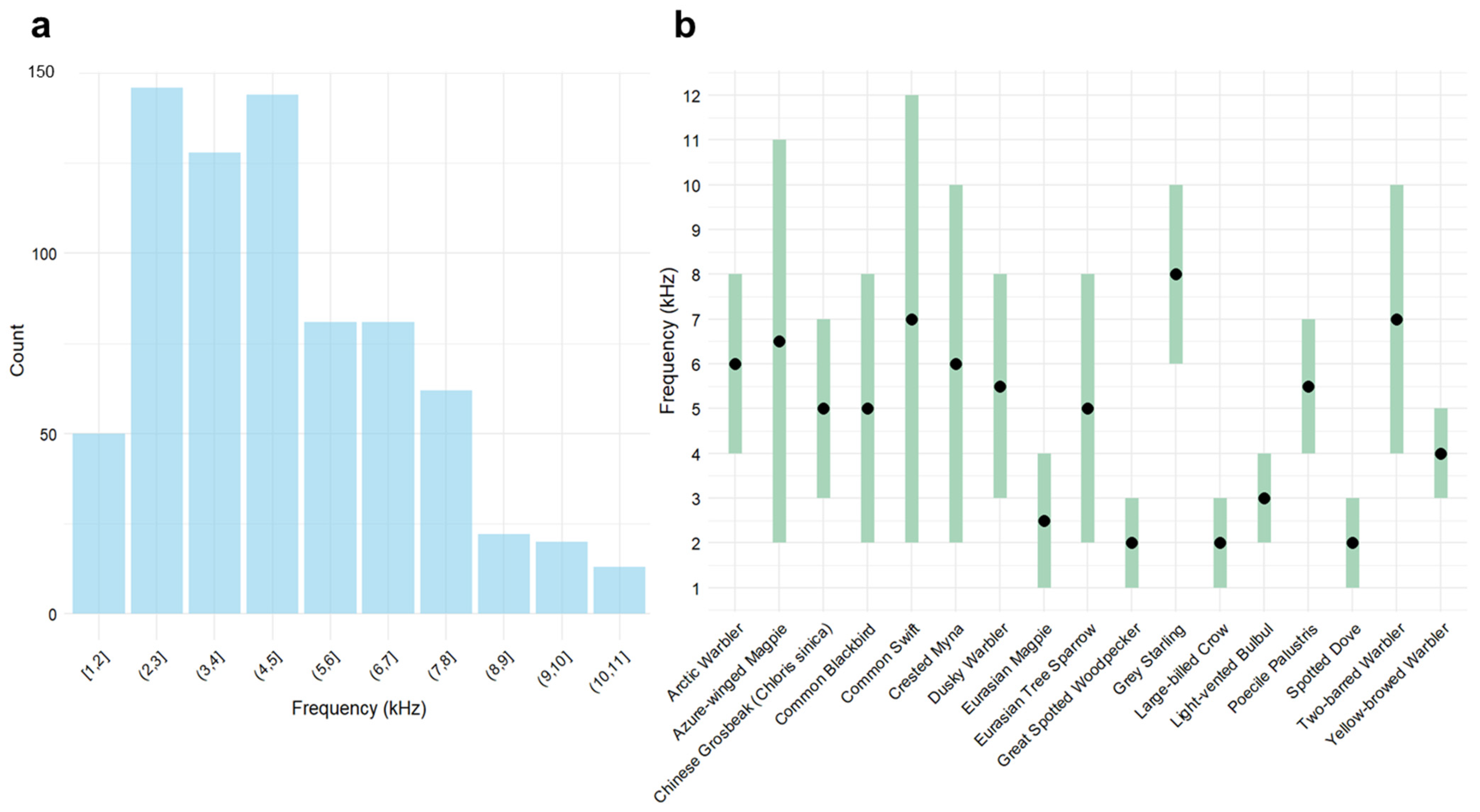
3.1.1. Frequency Partitioning Patterns
3.1.2. Acoustic Niche Characterization
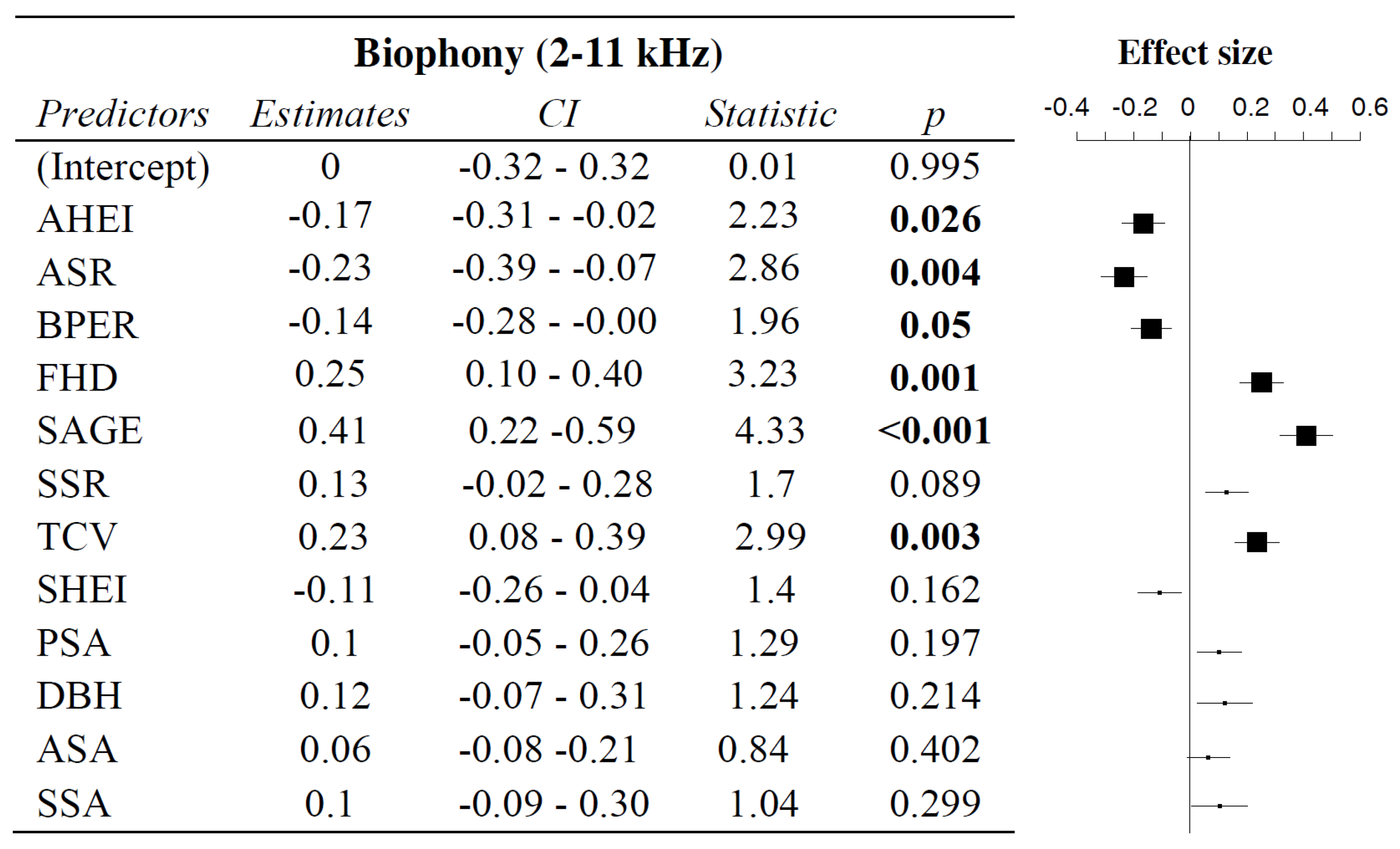
3.2. Influence of Vegetation Characteristics on Biophony
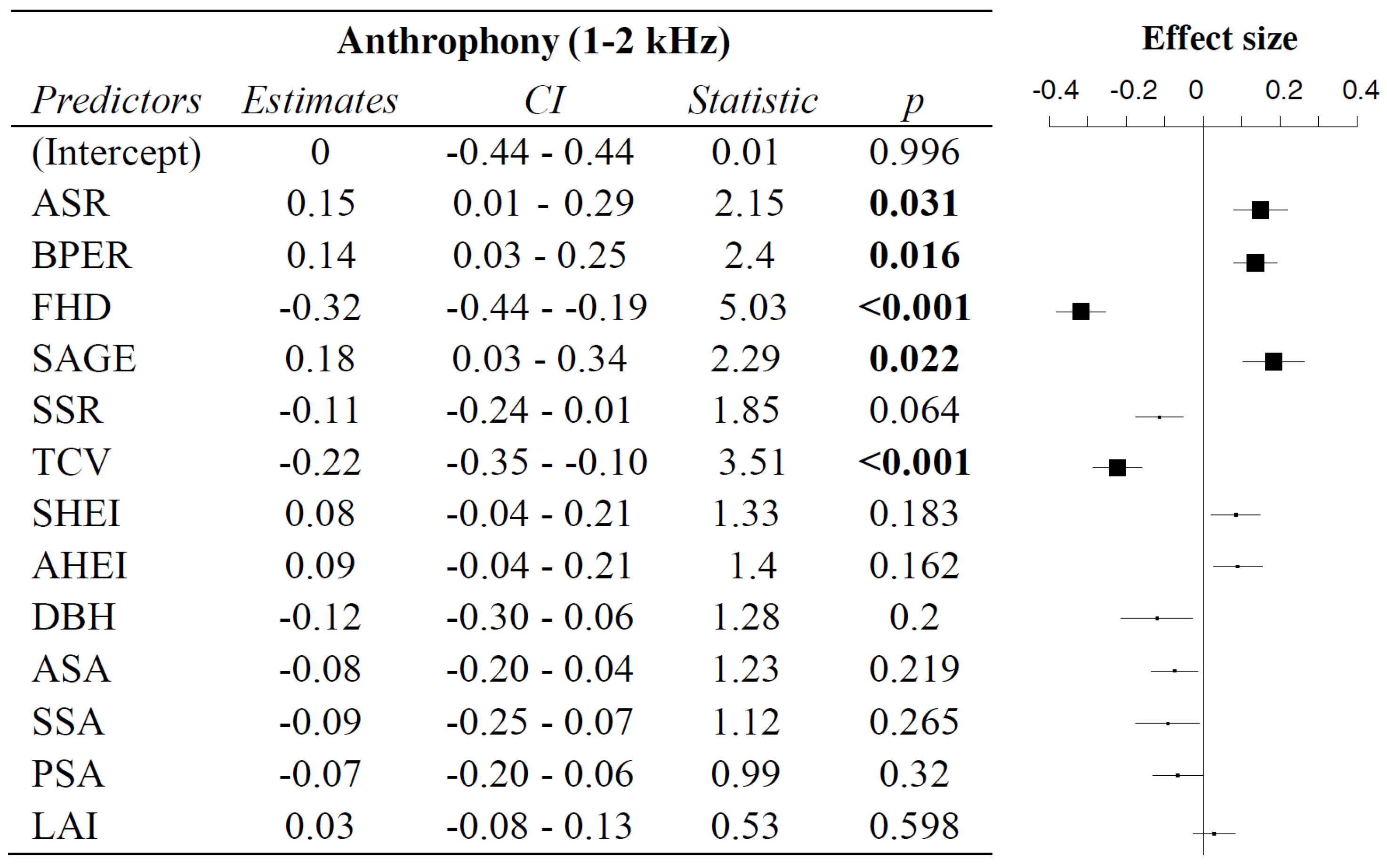
3.3. Influence of Vegetation Characteristics on Anthrophony
3.4. Holistic Effects of Vegetation Characteristics on Soundscape
4. Discussion
4.1. Vertical Vegetation Structure Provides Critical Support for Acoustic Communities
4.2. Potential of Using Acoustic Methods Assessing Urban Forests
4.3. Acoustic Data-Driven Urban Forest Management Framework
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | Abbreviation | Description |
|---|---|---|
| Arbor species richness | ASR | Number of tree species in the sample plot |
| Shrub species richness | SSR | Number of shrub species in the sample plot |
| Herb species richness * | HSR * | Number of herbaceous species in the sample plot |
| Total plant species richness * | PSR * | Total number of plant species in the sample plot |
| Arbor species abundance | ASA | Number of trees in the sample plot |
| Shrub species abundance | SSA | Number of shrubs in the sample plot |
| Total plant species abundance | PSA | Total number of plants in the sample plot |
| Mean diameter at breast height (m) | DBH | Average diameter at breast height of trees in the sample plot |
| Leaf area index | LAI | The total leaf area of plants per unit area of land |
| Arbor height (m) | AHEI | Average height of trees in the sample plot |
| Shrub height (m) | SHEI | Average height of shrubs in the sample plot |
| Bare ground percentage (%) | BPER | Percentage of bare ground in the sample plot |
| Stand age (Year) | SAGE | Average age of trees within each diameter structure |
| Foliage height diversity | FHD | The vertical structural diversity of vegetation at a location |
| Tree height coefficient of variation | TCV | Differences in tree height within a specific stand, reflecting vertical structural complexity |
References
- Burivalova, Z.; Towsey, M.; Boucher, T.; Truskinger, A.; Apelis, C.; Roe, P.; Game, E.T. Using Soundscapes to Detect Variable Degrees of Human Influence on Tropical Forests in Papua New Guinea. Conserv. Biol. 2018, 32, 205–215. [Google Scholar] [CrossRef]
- World Bank. World Bank Forests for People, the Planet and Climate; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Pei, N.; Wang, C.; Sun, R.; Xu, X.; He, Q.; Shi, X.; Gu, L.; Jin, J.; Liao, J.; Li, J.; et al. Towards an Integrated Research Approach for Urban Forestry: The Case of China. Urban For. Urban Green. 2019, 46, 126472. [Google Scholar] [CrossRef]
- Xu, F.; Wang, X.; Li, L. NPP and Vegetation Carbon Sink Capacity Estimation of Urban Green Space Using the Optimized CASA Model: A Case Study of Five Chinese Cities. Atmosphere 2023, 14, 1161. [Google Scholar] [CrossRef]
- Queiroz, H.L.; Sobanski, M.B.; Magurran, A.E. Reproductive Strategies of Red-Bellied Piranha (Pygocentrus Nattereri Kner, 1858) in the White Waters of the Mamirauá Flooded Forest, Central Brazilian Amazon. Environ. Biol. Fishes 2010, 89, 11–19. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; White, J.C.; Wulder, M.A.; Næsset, E. Remote Sensing in Forestry: Current Challenges, Considerations and Directions. For. Int. J. For. Res. 2024, 97, 11–37. [Google Scholar] [CrossRef]
- Sueur, J.; Farina, A. Ecoacoustics: The Ecological Investigation and Interpretation of Environmental Sound. Biosemiotics 2015, 8, 493–502. [Google Scholar] [CrossRef]
- Pijanowski, B.C.; Villanueva-Rivera, L.J.; Dumyahn, S.L.; Farina, A.; Krause, B.L.; Napoletano, B.M.; Gage, S.H.; Pieretti, N. Soundscape Ecology: The Science of Sound in the Landscape. BioScience 2011, 61, 203–216. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Dong, A.; Lin, L. The Relationship between Acoustic Indices, Vegetation, and Topographic Characteristics Is Spatially Dependent in a Tropical Forest in Southwestern China. Ecol. Indic. 2022, 142, 109229. [Google Scholar] [CrossRef]
- Skowno, A.L.; Bond, W.J. Bird Community Composition in an Actively Managed Savanna Reserve, Importance of Vegetation Structure and Vegetation Composition. Biodivers. Conserv. 2003, 12, 2279–2294. [Google Scholar] [CrossRef]
- Davison, C.W.; Assmann, J.J.; Normand, S.; Rahbek, C.; Morueta-Holme, N. Vegetation Structure from LiDAR Explains the Local Richness of Birds across Denmark. J. Anim. Ecol. 2023, 92, 1332–1344. [Google Scholar] [CrossRef]
- Do Nascimento, L.A.; Campos-Cerqueira, M.; Beard, K.H. Acoustic Metrics Predict Habitat Type and Vegetation Structure in the Amazon. Ecol. Indic. 2020, 117, 106679. [Google Scholar] [CrossRef]
- Farina, A.; James, P. The Acoustic Communities: Definition, Description and Ecological Role. Biosystems 2016, 147, 11–20. [Google Scholar] [CrossRef]
- Tucker, D.; Gage, S.H.; Williamson, I.; Fuller, S. Linking Ecological Condition and the Soundscape in Fragmented Australian Forests. Landsc. Ecol. 2014, 29, 745–758. [Google Scholar] [CrossRef]
- Bradfer-Lawrence, T.; Gardner, N.; Bunnefeld, L.; Bunnefeld, N.; Willis, S.G.; Dent, D.H. Guidelines for the Use of Acoustic Indices in Environmental Research. Methods Ecol. Evol. 2019, 10, 1796–1807. [Google Scholar] [CrossRef]
- Zhao, Y.; Sheppard, S.; Sun, Z.; Hao, Z.; Jin, J.; Bai, Z.; Bian, Q.; Wang, C. Soundscapes of Urban Parks: An Innovative Approach for Ecosystem Monitoring and Adaptive Management. Urban For. Urban Green. 2022, 71, 127555. [Google Scholar] [CrossRef]
- Krause, B. Bioacoustics, Habitat Ambience in Ecological Balance. Whole Earth Rev. 1987, 57, 14–18. [Google Scholar]
- Zhou, D.; Chu, L.M. How Would Size, Age, Human Disturbance, and Vegetation Structure Affect Bird Communities of Urban Parks in Different Seasons? J. Ornithol. 2012, 153, 1101–1112. [Google Scholar] [CrossRef]
- Karaconstantis, C.; Desjonquères, C.; Gifford, T.; Linke, S. Spatio-temporal Heterogeneity in River Sounds: Disentangling Micro-and Macro-Variation in a Chain of Waterholes. Freshw. Biol. 2020, 65, 96–106. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-64144-5. [Google Scholar]
- Farina, A.; Gage, S.H. Ecoacoustics: The Ecological Role of Sounds; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 1-119-23069-1. [Google Scholar]
- Pijanowski, B.C.; Farina, A.; Gage, S.H.; Dumyahn, S.L.; Krause, B.L. What Is Soundscape Ecology? An Introduction and Overview of an Emerging New Science. Landsc. Ecol. 2011, 26, 1213–1232. [Google Scholar] [CrossRef]
- Lawrence, B.T.; Hornberg, J.; Haselhoff, T.; Sutcliffe, R.; Ahmed, S.; Moebus, S.; Gruehn, D. A Widened Array of Metrics (WAM) Approach to Characterize the Urban Acoustic Environment; a Case Comparison of Urban Mixed-Use and Forest. Appl. Acoust. 2022, 185, 108387. [Google Scholar] [CrossRef]
- Gewers, F.L.; Ferreira, G.R.; Arruda, H.F.D.; Silva, F.N.; Comin, C.H.; Amancio, D.R.; Costa, L.D.F. Principal Component Analysis: A Natural Approach to Data Exploration. ACM Comput. Surv. 2021, 54, 70:1–70:34. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, Statistics for Biology and Health; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The Coefficient of Determination R2 and Intra-Class Correlation Coefficient from Generalized Linear Mixed-Effects Models Revisited and Expanded. J. R. Soc. Interface 2017, 13, 20170213. [Google Scholar] [CrossRef]
- Nemeth, E.; Pieretti, N.; Zollinger, S.A.; Geberzahn, N.; Partecke, J.; Miranda, A.C.; Brumm, H. Bird Song and Anthropogenic Noise: Vocal Constraints May Explain Why Birds Sing Higher-Frequency Songs in Cities. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122798. [Google Scholar] [CrossRef]
- Gage, S.H.; Axel, A.C. Visualization of Temporal Change in Soundscape Power of a Michigan Lake Habitat over a 4-Year Period. Ecol. Inform. 2014, 21, 100–109. [Google Scholar] [CrossRef]
- Basile, M.; Storch, I.; Mikusiński, G. Abundance, Species Richness and Diversity of Forest Bird Assemblages—The Relative Importance of Habitat Structures and Landscape Context. Ecol. Indic. 2021, 133, 108402. [Google Scholar] [CrossRef]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.-Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity Conservation in Agriculture Requires a Multi-Scale Approach. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef]
- MacArthur, R.H. Environmental Factors Affecting Bird Species Diversity. Am. Nat. 1964, 98, 387–397. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal Species Diversity Driven by Habitat Heterogeneity/Diversity: The Importance of Keystone Structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Nikolov, S.C. Effect of Stand Age on Bird Communities in Late-Successional Macedonian Pine Forests in Bulgaria. For. Ecol. Manag. 2009, 257, 580–587. [Google Scholar] [CrossRef]
- Reise, J.; Kukulka, F.; Flade, M.; Winter, S. Characterising the Richness and Diversity of Forest Bird Species Using National Forest Inventory Data in Germany. For. Ecol. Manag. 2019, 432, 799–811. [Google Scholar] [CrossRef]
- Kebrle, D.; Hofmeister, J.; Kodet, V.; Hošek, J. Rare Birds Benefit from the Maturation of Central European Production Forests Unless These Are Limited by Spruce Dominance. SSRN 2023. [Google Scholar] [CrossRef]
- Szarvas, F.; Hanzelka, J.; Reif, J.; Voříšek, P.; Vermouzek, Z.; Michálek, J. Ecological Traits Predict Habitat Associations in Central European Forest Birds: Implications for Forest Management. SSRN 2025. [Google Scholar] [CrossRef]
- Muñoz-Pedreros, A.; González-Urrutia, M.; Encina-Montoya, F.; Norambuena, H.V. Effects of Vegetation Strata and Human Disturbance on Bird Diversity in Green Areas in a City in Southern Chile. Avian Res. 2018, 9, 38. [Google Scholar] [CrossRef]
- Catfolis, B.; Vanroy, T.; Verheyen, K.; Baeten, L.; Martel, A.; Pasmans, F.; Strubbe, D.; Lens, L. Avian Nutritional Condition Increases with Forest Structural Complexity. Ecol. Indic. 2023, 154, 110536. [Google Scholar] [CrossRef]
- Zellweger, F.; Braunisch, V.; Baltensweiler, A.; Bollmann, K. Remotely Sensed Forest Structural Complexity Predicts Multi Species Occurrence at the Landscape Scale. For. Ecol. Manag. 2013, 307, 303–312. [Google Scholar] [CrossRef]
- Jin, C.; Jiao, J.; Wu, C.; Mu, Y.; Zheng, S.; You, L.; Wu, W.; Liu, J.; Jiang, B. Sparse Large Trees in Secondary and Planted Forests Highlight the Need to Improve Forest Conservation and Management. Sci. Total Environ. 2024, 954, 176363. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F. The Ecology, Distribution, Conservation and Management of Large Old Trees. Biol. Rev. 2017, 92, 1434–1458. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, C.; Li, L.; Gao, B.; Wu, R.; Pei, N.; Liu, Y. Anthropogenic Noise and Habitat Structure Shaping Dominant Frequency of Bird Sounds along Urban Gradients. iScience 2024, 27, 109056. [Google Scholar] [CrossRef]
- Slabbekoorn, H. Songs of the City: Noise-Dependent Spectral Plasticity in the Acoustic Phenotype of Urban Birds. Anim. Behav. 2013, 85, 1089–1099. [Google Scholar] [CrossRef]
- Turner, A.; Fischer, M.; Tzanopoulos, J. Sound-mapping a coniferous forest—Perspectives for biodiversity monitoring and noise mitigation. PLoS ONE 2018, 13, e0189843. [Google Scholar] [CrossRef]
- Camacho-Cervantes, M.; Schondube, J.E.; Castillo, A.; MacGregor-Fors, I. How Do People Perceive Urban Trees? Assessing Likes and Dislikes in Relation to the Trees of a City. Urban Ecosyst. 2014, 17, 761–773. [Google Scholar] [CrossRef]
- Lacasta, A.M.; Peñaranda, A.; Cantalapiedra, I.R. Chapter 3.9—Green Streets for Noise Reduction. In Nature Based Strategies for Urban and Building Sustainability; Pérez, G., Perini, K., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 181–190. ISBN 978-0-12-812150-4. [Google Scholar]
- Dzhambov, A.M.; Dimitrova, D.D. Green Spaces and Environmental Noise Perception. Urban For. Urban Green. 2015, 14, 1000–1008. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, C.; Li, L.; Sun, B.; Luo, S.; Liao, J.; Wang, Q.; Wu, R.; Xu, X.; Lepczyk, C.A.; et al. Can Urban Forests Provide Acoustic Refuges for Birds? Investigating the Influence of Vegetation Structure and Anthropogenic Noise on Bird Sound Diversity. J. For. Res. 2024, 35, 33. [Google Scholar] [CrossRef]
- Cardozo, R.; Machado, R.B. Bird Communities in the Dry Chaco of South America: Vegetation Structure and Climate Effects. Perspect. Ecol. Conserv. 2024, 22, 374–383. [Google Scholar] [CrossRef]
- Uebel, K.; Bonn, A.; Marselle, M.; Dean, A.J.; Rhodes, J.R. Understory Vegetation Can Promote Bird Sounds and Reduce Traffic Noise in Urban Park Soundscapes. Urban Ecosyst. 2025, 28, 71. [Google Scholar] [CrossRef]
- Maisey, A.C.; Haslem, A.; Leonard, S.W.J.; Bennett, A.F. Foraging by an Avian Ecosystem Engineer Extensively Modifies the Litter and Soil Layer in Forest Ecosystems. Ecol. Appl. 2021, 31, e02219. [Google Scholar] [CrossRef]
- Vergara, P.M.; Fierro, A.; Alaniz, A.J.; Carvajal, M.A.; Lizama, M.; Llanos, J.L. Landscape-Scale Effects of Forest Degradation on Insectivorous Birds and Invertebrates in Austral Temperate Forests. Landsc. Ecol. 2021, 36, 191–208. [Google Scholar] [CrossRef]
- Sueur, J.; Gasc, A.; Grandcolas, P.; Pavoine, S. Global Estimation of Animal Diversity Using Automatic Acoustic Sensors. In Sensors for Ecology: Towards Integrated Knowledge of Ecosystems; CNRS: Paris, France, 2012; pp. 99–117. [Google Scholar]
- Henry, K.S.; Lucas, J.R. Auditory Sensitivity and the Frequency Selectivity of Auditory Filters in the Carolina Chickadee, Poecile Carolinensis. Anim. Behav. 2010, 80, 497–507. [Google Scholar] [CrossRef]
- Būhning-Gaese, K. Determinants of Avian Species Richness at Different Spatial Scales. J. Biogeogr. 1997, 24, 49–60. [Google Scholar] [CrossRef]
- Panda, B.P.; Prusty, B.A.K.; Panda, B.; Pradhan, A.; Parida, S.P. Habitat Heterogeneity Influences Avian Feeding Guild Composition in Urban Landscapes: Evidence from Bhubaneswar, India. Ecol. Process 2021, 10, 31. [Google Scholar] [CrossRef]
- Lu, X.; Jia, Y.; Wang, Y. The Effect of Landscape Composition, Complexity, and Heterogeneity on Bird Richness: A Systematic Review and Meta-Analysis on a Global Scale. Landsc. Ecol. 2024, 39, 132. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Yao, Z.; Cui, S. Soundscape for Urban Ecological Security Evaluation. Basic Appl. Ecol. 2024, 76, 50–57. [Google Scholar] [CrossRef]
- Raihan, A. A Review on the Role of Green Vegetation in Improving Urban Environmental Quality. Eco Cities 2024, 4, 2387. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Biondi, D.; dos Reis, A.R.N.; de Oliveira, J.D.; Biondi, D.; dos Reis, A.R.N. The Role of Urban Green Areas in Noise Pollution Attenuation. DYNA 2022, 89, 210–215. [Google Scholar] [CrossRef]
- Ali Khan, S.; Fatima, K.; Hussain, S.; Minhal Ali, M.; Mannan, A.; Iqbal Butt, N. Mitigation of noise pollution in urban areas by strategically planting trees and shrubs. J. Hcare. Dvp. Ctries 2023, 7, 41–47. [Google Scholar] [CrossRef]
- Bakker, J.J. Improving the Acoustic Absorption of Vertical Greening Systems. Master Thesis, Delft University of Technology, Delft, The Netherlands, 2021. [Google Scholar]
- Zhao, N.; Prieur, J.-F.; Liu, Y.; Kneeshaw, D.; Lapointe, E.M.; Paquette, A.; Zinszer, K.; Dupras, J.; Villeneuve, P.J.; Rainham, D.G.; et al. Tree Characteristics and Environmental Noise in Complex Urban Settings—A Case Study from Montreal, Canada. Environ. Res. 2021, 202, 111887. [Google Scholar] [CrossRef]
- Ow, L.F.; Ghosh, S. Urban Cities and Road Traffic Noise: Reduction through Vegetation. Appl. Acoust. 2017, 120, 15–20. [Google Scholar] [CrossRef]
- Ren, X.; Li, Q.; Yuan, M.; Shao, S. How Visible Street Greenery Moderates Traffic Noise to Improve Acoustic Comfort in Pedestrian Environments. Landsc. Urban Plan. 2023, 238, 104839. [Google Scholar] [CrossRef]



| NDSI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model parameters | Rank | model1 | model2 | model3 | model4 | model5 | Average model | |
| AICc | 695.81 | 697.19 | 697.19 | 697.62 | 697.80 | |||
| delta | 0.00 | 1.39 | 1.39 | 1.81 | 1.99 | |||
| weight | 0.36 | 0.18 | 0.18 | 0.15 | 0.13 | |||
| Vegetation composition | ASR | 8.0% | 26% | |||||
| SSR | 5.5% | |||||||
| ASA | 8.0% | |||||||
| SSA | 4.5% | |||||||
| Vegetation structure | DBH | 12.1% | 74% | |||||
| LAI | 1.5% | |||||||
| AHEI | 10.1% | |||||||
| BPER | 10.1% | |||||||
| SAGE | 11.1% | |||||||
| FHD | 16.6% | |||||||
| TCV | 12.6% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sun, Z.; Bai, Z.; Jin, J.; Wang, C. How Vegetation Structure Shapes the Soundscape: Acoustic Community Partitioning and Its Implications for Urban Forestry Management. Forests 2025, 16, 669. https://doi.org/10.3390/f16040669
Zhao Y, Sun Z, Bai Z, Jin J, Wang C. How Vegetation Structure Shapes the Soundscape: Acoustic Community Partitioning and Its Implications for Urban Forestry Management. Forests. 2025; 16(4):669. https://doi.org/10.3390/f16040669
Chicago/Turabian StyleZhao, Yilin, Zhenkai Sun, Zitong Bai, Jiali Jin, and Cheng Wang. 2025. "How Vegetation Structure Shapes the Soundscape: Acoustic Community Partitioning and Its Implications for Urban Forestry Management" Forests 16, no. 4: 669. https://doi.org/10.3390/f16040669
APA StyleZhao, Y., Sun, Z., Bai, Z., Jin, J., & Wang, C. (2025). How Vegetation Structure Shapes the Soundscape: Acoustic Community Partitioning and Its Implications for Urban Forestry Management. Forests, 16(4), 669. https://doi.org/10.3390/f16040669






