Leaf Plasticity Responses of Four Urban Garden Plants to Low-Light Environments Under Viaducts
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Points and Plant Materials
2.2. Setting of Light Measurement Plots in Shaded Green Areas Under Viaduct
2.3. Leaf Morphological Characteristics
2.4. Observation of Leaf Stomata and Anatomical Structure
2.5. Physiological Index Determination
2.6. Photosynthesis and Chlorophyll Fluorescence Parameters Measurement
2.7. Statistical Analysis
3. Results
3.1. Morphological Characteristics of Plant Leaves Under Different Light Intensities
3.2. Photosynthetic Parameters and Chlorophyll Fluorescence Parameters Under Different Light Intensities
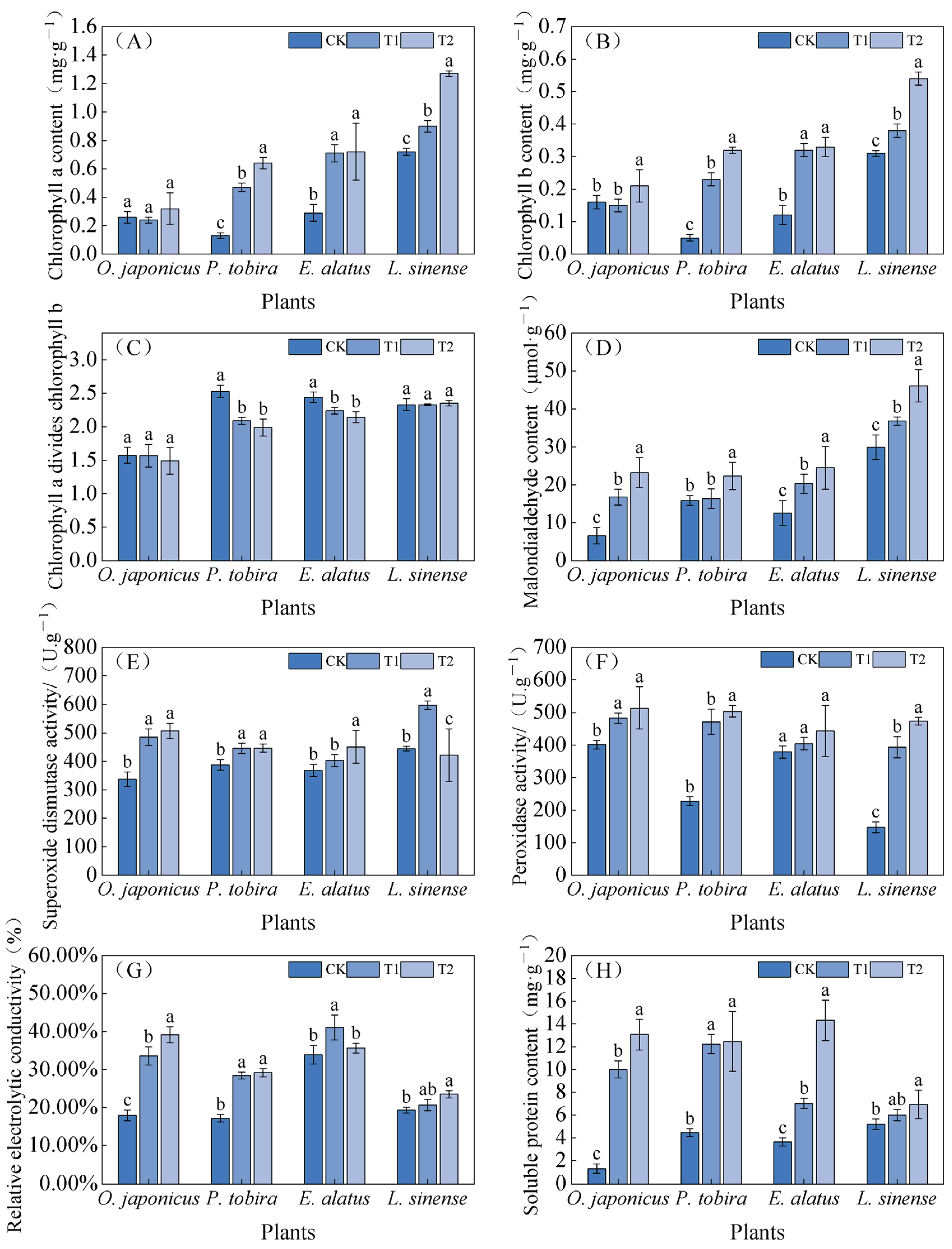
3.3. Physiological Indices Under Different Light Intensities
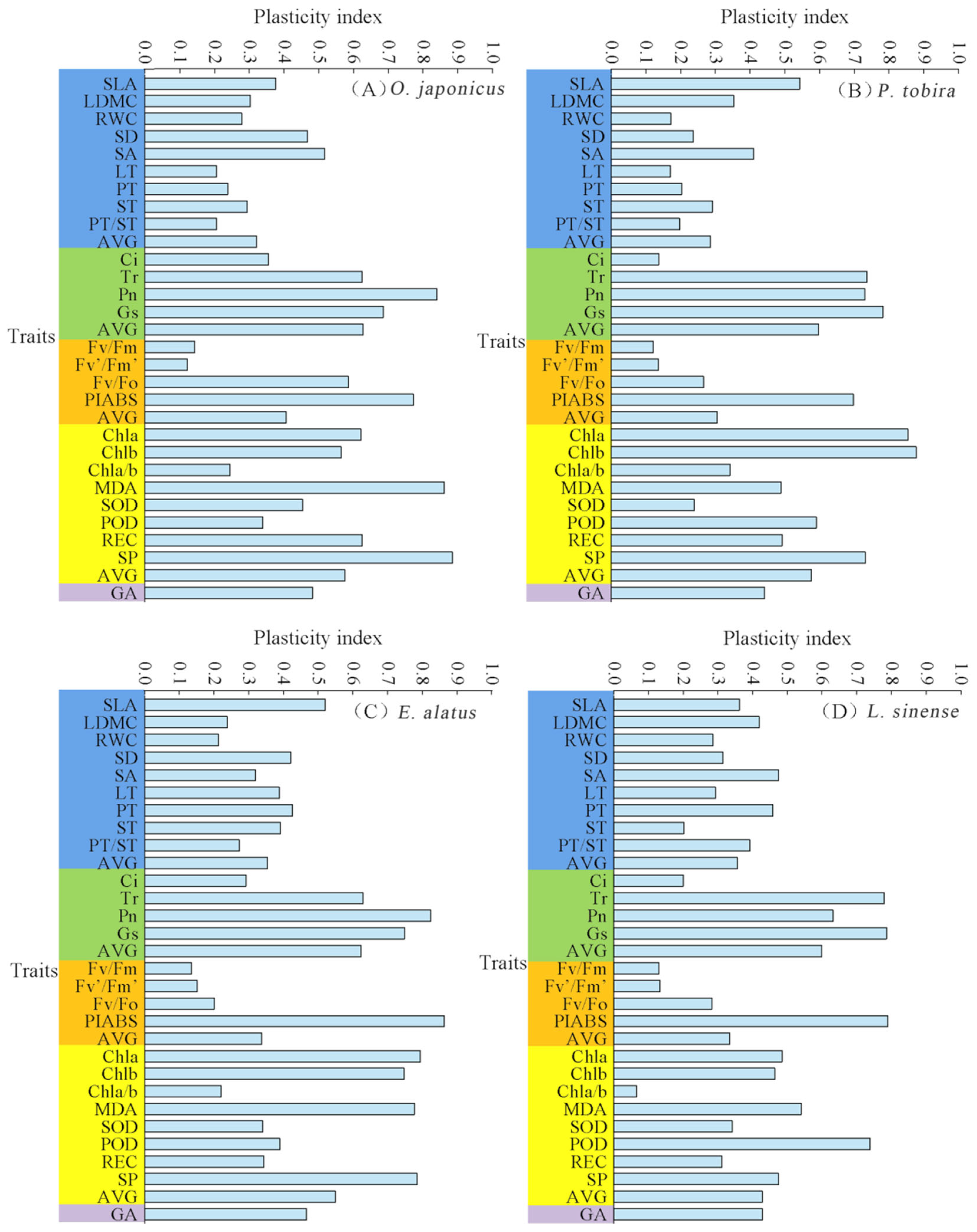
3.4. PPI of Plant Leaves Under Different Light Intensities
3.5. Double Factor Variance Analysis of Species and Light Intensities on Different Indexes
4. Discussion
4.1. Response of Leaf Phenotypic Indicators to Shade
4.2. Response of Photosynthetic Parameters to Shade
4.3. Response of Leaf Physiological Indicators to Shade
4.4. Differences in the Plasticity Index of Morphological Traits Among Four Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Luo, Y.; Miao, S.; Ding, H.; Jin, T.; Chen, J. Pseudo-simultaneous measurements for the spatial-temporal characteristics of accumulation and coarse mode particles near an urban viaduct within street canyons. Atmos. Pollut. Res. 2019, 10, 1643–1654. [Google Scholar] [CrossRef]
- Hao, C.; Xie, X.; Huang, Y.; Huang, Z. Study on influence of viaduct and noise barriers on the particulate matter dispersion in street canyons by CFD modeling. Atmos. Pollut. Res. 2019, 10, 1723–1735. [Google Scholar] [CrossRef]
- Yan, L. Research of Shaded Green Space Landscape Based on Natural Light Environment Under the Urban Viaducts—Case Study on Viaducts in Wuhan City. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Wang, X.; Xin, Y.; Song, K.; Da, L. Characteristics of light condition under viaduct and suitable greening distribution in city. Chin. J. Ecol. 2006, 25, 938–943. [Google Scholar] [CrossRef]
- Wang, K. The Research on Site Condition and Greening Strategy of Shaded Green Space Under Viaduct—Take the Main Urban Region of Wuhan as an Example. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2017. [Google Scholar]
- Xing, Q.; Zeng, L.; Hu, Y.; Liu, Z.; Qin, J. Effects of shade environment under viaducts on physiological characteristics of 5 greening plant species. Acta Agric. Shanghai 2021, 37, 78–85. [Google Scholar] [CrossRef]
- Chai, S.; Tang, J.; Mallik, A.; Shi, Y.; Zou, R.; Li, J.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BMC Ecol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Luo, S.; Pan, J.; Yao, S.; Guo, Y.; Wang, G. Light Regimes Regulate Leaf and Twigs Traits of Camellia oleifera (Abel) in Pinus massoniana Plantation Understory. Forests 2022, 13, 918. [Google Scholar] [CrossRef]
- Wang, X.; Su, W.; Bu, Q.; Zhang, P. Light Characteristics and Greening Suggestions under Viaduct in Luoyang City. For. Inventory Plan. 2022, 48, 214–221. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, C.; Ding, S.; Shang, X.; Yang, W.; Fang, S. Effect of light regime and provenance on leaf characteristics, growth and flavonoid accumulation in Cyclocarya paliurus (Batal) Iljinskaja coppices. Bot. Stud. 2017, 28, 57. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, M.; Yue, Q.; Yang, Y.; Luo, Z.; Lv, B.; He, R.; Feng, S.; Yang, M. Effects of Low-Light Environments on the Growth and Physiological and Biochemical Parameters of Indocalamusand Seasonal Variations in Leaf Active Substance Contents. Plants 2023, 12, 3993. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Chen, J.; Zhang, M.; Zhang, J.; Tong, Z.; Yang, Q. Leaf Plasticity of the Subtropical Evergreen Tree Phoebe bournei Increases with Ontogeny in Response to Sun and Shade. Forests 2023, 14, 1683. [Google Scholar] [CrossRef]
- Hallik, L.; Niinemets, U.; Kull, O. Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biol. 2012, 14, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Arrieta, S.; Aranda, I.; Lorenzo, D.; Sanchez-Gomez, D.; Tena, D. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiol. 2005, 25, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zeng, D.; Timothy, J.F.; Yao, C.; Yu, Z. Response of leaf anatomy of Chenopodium acuminatum to soil resource availability in a semi-arid grassland. Plant Ecol. 2010, 209, 375–382. [Google Scholar] [CrossRef]
- Chen, L.; Luo, W.; Huang, J.; Peng, S.; Xiong, D. Leaf photosynthetic plasticity does not predict biomass responses to growth irradiance in rice. Physiol. Plant. 2021, 173, 2155–2165. [Google Scholar] [CrossRef]
- Centofante, R.A.; Neto, R.A.; Filho, V.C.S.; Oliveira, A.E.; Assis, S.E.; Rosa, M.; Silva, G.F. Corrigendum to “Light quality on the morphoanatomy and physiology of Campomanesia pubescens (DC.) O. Berg. Seedlings”. Sci. Hortic. 2020, 267, 109083. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, P.; Gong, W.; Gul, H.; Zhu, J.; Yang, F.; Wang, X.; Tai, W.; Liu, J.; Tian, P.; et al. Morphological and physiological variation of soybean seedlings in response to shade. Front. Plant Sci. 2022, 13, 1015414. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Zhao, B. Physiological and Microstructural Responses of Two Rhododendron Cultivars to High Temperature and Low Light. Hortic. Environ. Biotechnol. 2020, 61, 1–14. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Chen, J.; Li, L.; Cheng, J.; Wang, S.; Liu, J. Plasticity of leaf traits of Juglans regia L. f. luodianense liu et xu seedlings under different light conditions in karst habitats. Forests 2021, 12, 81. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, C. Intraspecific differences in plant functional traits are related to urban atmospheric particulate matter. BMC Plant Biol. 2021, 21, 430. [Google Scholar] [CrossRef]
- An, J.; Wei, X.; Yao, Y.; Jin, N.; Wu, G.; Wang, X. Plasticity of Phoebe bournei seedlings adapting to low light environment. Guihaia 2024, 44, 501–509. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Li, M.; Zhu, X.; Shi, C.; Ding, G. Morphological and Physiological Responses of Pinus massoniana Seedlings to different light gradients. Forests 2021, 12, 523. [Google Scholar] [CrossRef]
- Barbosa, M.A.M.; Chitwood, H.D.; Azevedo, A.A.; Araújo, L.W.; Ribeiro, M.D.; Peres, P.E.L.; Martins, V.C.S.; Zsögön, A. Bundle sheath extensions affect leaf structural and physiological plasticity in response to irradiance. Plant Cell Environ. 2019, 42, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Niu, X. Landscape Optimization Design Along the Viaduct in Zhengzhou. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2021. [Google Scholar]
- Wei, C.; Luo, G.; Jin, Z.; Li, J.; Li, Y. Physiological and structural changes in leaves of Platycrater arguta seedlings exposed to increasing light intensities. Plants 2024, 13, 1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Jin, Z.; Yang, Z.; Li, Y. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of Heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 258, 151418. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Deng, Y.; Shao, Q.; Li, C.; Ye, X.; Tang, R. Differential responses of double petal and multi petal jasmine to shading: II. Morphology, anatomy and physiology. Sci. Hortic. 2012, 144, 19–28. [Google Scholar] [CrossRef]
- Favaretto, V.F.; Martinez, C.A.; Soriani, H.H.; Furriel, R.P. Differential responses of antioxidant enzymes in pioneer and latesuccessional tropical tree species grown under sun and shade conditions. Environ. Exp. Bot. 2011, 70, 20–28. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.; Mu, H.; Li, X.; Zhang, X.; Wen, Y.; Zhang, X. Effects of brassinolide on growth, photosynthetic rate and antioxidant enzyme activity of ornamental gourd under salt stress. Russ. J. Plant Physiol. 2023, 70, 137. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Ni, Y.; Lin, G.; Chen, K.; Wu, C.; Zhang, Y. Flavonoid compounds and photosynthesis in Passiflora plant leaves under varying light intensities. Plants 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.M.Z.; Almeida, L.P.; Giacobbo, C.L.; Galon, L.; Perboni, A.T.; Bermeo, P.; Danner, M.A. Campomanesia xanthocarpa (Myrtaceae: Myrtoideae) seedlings reveal morpho-physiological plasticity under shade conditions. Braz. J. Biol. 2023, 83, 276695. [Google Scholar] [CrossRef]
- Wang, S.; Song, S.; Li, J.; Yang, T.; He, Y.; Wei, X.; Jiang, M. Photosynthetic characteristics of Sinojackia huangmeiensis, a plant species with extremely small population. Chin. J. Ecol. 2023, 43, 701–708. [Google Scholar] [CrossRef]
- Riaño, K.; Briones, O. Leaf physiological response to light environment of three tree fern species in a Mexican cloud forest. J. Trop. Ecol. 2013, 29, 217–228. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Zhang, X.; Zhou, J.; Guy, R.D. Photosynthetic performance and growth responses of Liriope muscari (Decne.) L.H. Bailey (Asparagaceae) to different levels of irradiance in three seasons. Flora 2021, 278, 151798. [Google Scholar] [CrossRef]
- Leng, H.; Su, M.; Zhang, C. Responses of photosynthetic traits and adaplive mechanisms of Rhododendron pulchrum Sweetto different light environments. Chin. J. Ecol. 2023, 42, 1793–1801. [Google Scholar] [CrossRef]
- Büssis, D.; Groll, V.U.; Fisahn, J.; Altmann, T. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol. 2006, 33, 1037–1043. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2012, 64, 495–505. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, K.; Pan, Y.; Ren, T.; Lu, J.; Wang, M.; Shen, Q.; Guo, S. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 2019, 42, 2231–2244. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Wang, Z.; Tan, T.; Li, S.; Li, J.; Wang, B.; Zhang, J.; Cheng, Y.; Wu, X.; et al. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant Biol. 2019, 19, 34. [Google Scholar] [CrossRef]
- Tsunoda, Y.; Ohno, Y.; Ishizuka, W. Photoinhibition sensitivity of Abies sachalinensis saplings under different leaf mass per area conditions: Insights into acclimation to current and future light environments. Forests 2024, 15, 1916. [Google Scholar] [CrossRef]
- Hong, M.; Ma, Z.; Wang, X.; Shen, Y.; Mo, Z.; Wu, M.; Chen, B.; Zhang, T. Effects of light intensity and ammonium stress on photosynthesis in Sargassum fusiforme seedlings. Chemosphere 2020, 273, 128605. [Google Scholar] [CrossRef] [PubMed]
- Marçal, D.M.S.; Avila, R.T.; Quiroga-Rojas, L.F.; de Souza, R.P.B.; Gomes Junior, C.C.; Ponte, L.R.; Barbosa, M.L.; Oliveira, L.A.; Martins, S.C.V.; Ramalho, J.D.C.; et al. Elevated [CO2] benefits coffee growth and photosynthetic performance regardless of light availability. Plant Physiol. Biochem. 2021, 158, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, Y.; Zhang, M.; Hong, A.; Yang, H.; Liu, Y. Shade effects on growth, photosynthesis and chlorophyll fluorescence parameters of three Paeonia species. PeerJ 2020, 8, e9316. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Dayananda, B.; Li, J. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Song, X.; Jiang, M.; Zhao, X.; Cao, X. The inhibitory effects of simulated light sources on the activity of algae cannot be ignored in photocatalytic inhibition. Chemosphere 2022, 309, 136611. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arenadoi, C. The interplay between light quality and biostimulant application affects the antioxidant capacity and photosynthetic traits of soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, L.; Zhou, J.; Li, Q.; Yi, S.; Sun, Q.; Zheng, Y. Changes in levels of enzymes and osmotic adjustment compounds in key species and their relevance to vegetation succession in abandoned croplands of a semiarid sandy region. Ecol. Evol. 2020, 10, 2269–2280. [Google Scholar] [CrossRef]
- Oh, H.E.; Yoon, A.; Park, Y.G. Red light enhances the antioxidant properties and growth of Rubus hongnoensis. Plants 2021, 10, 2589. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global trends in phenotypic plasticity of plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Ntawuhiganayo, E.B.; Uwizeye, F.K.; Zibera, E.; Dusenge, M.E.; Ziegler, C.; Ntirugulirwa, B.; Nsabimana, D.; Wallin, G.; Uddling, J. Traits controlling shade tolerance in tropical montane trees. Tree Physiol. 2020, 40, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, F.; Ma, J. Flora of Zhengzhou; Science and Technology Press of China: Beijing, China, 2008. [Google Scholar]
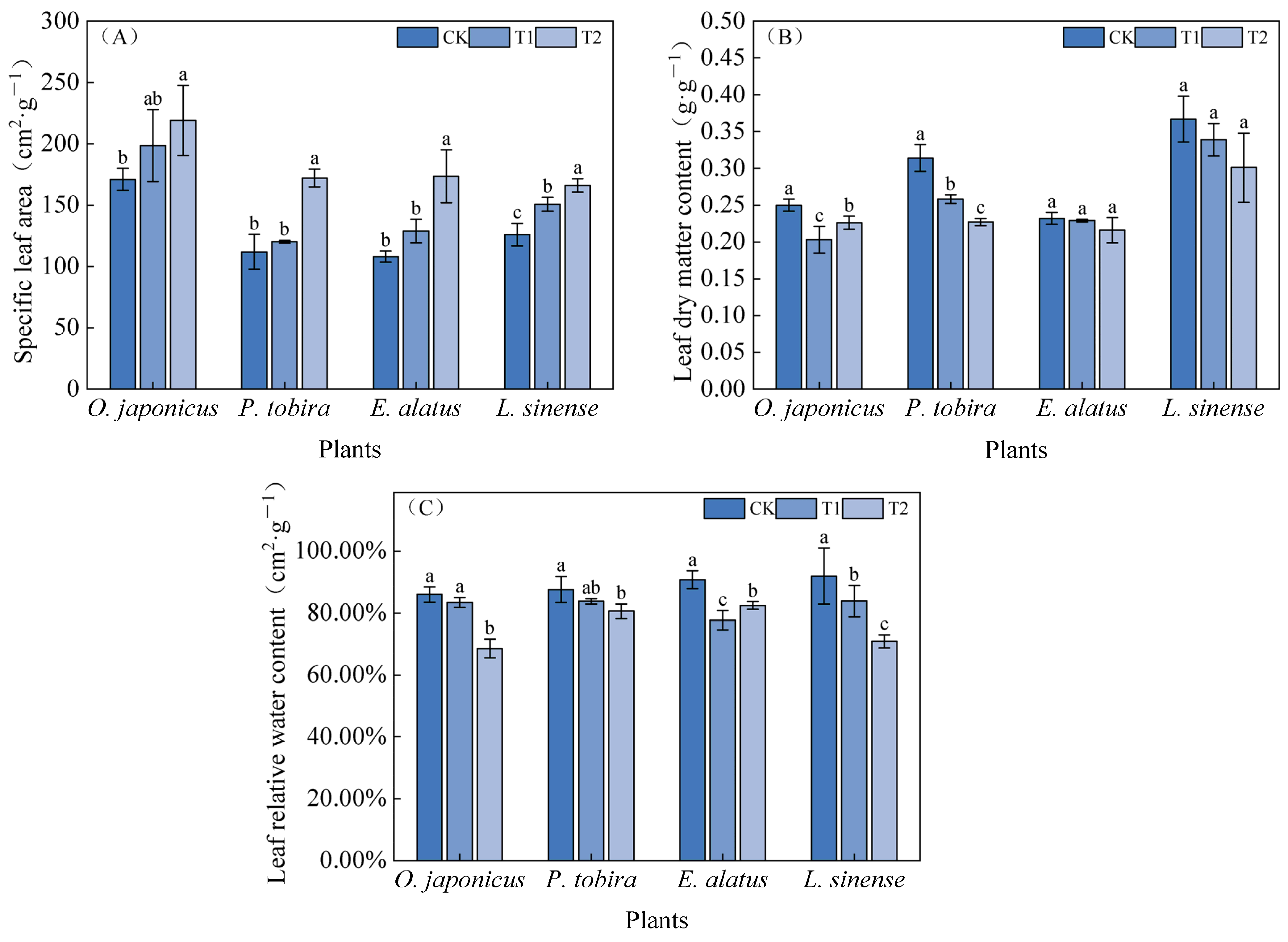
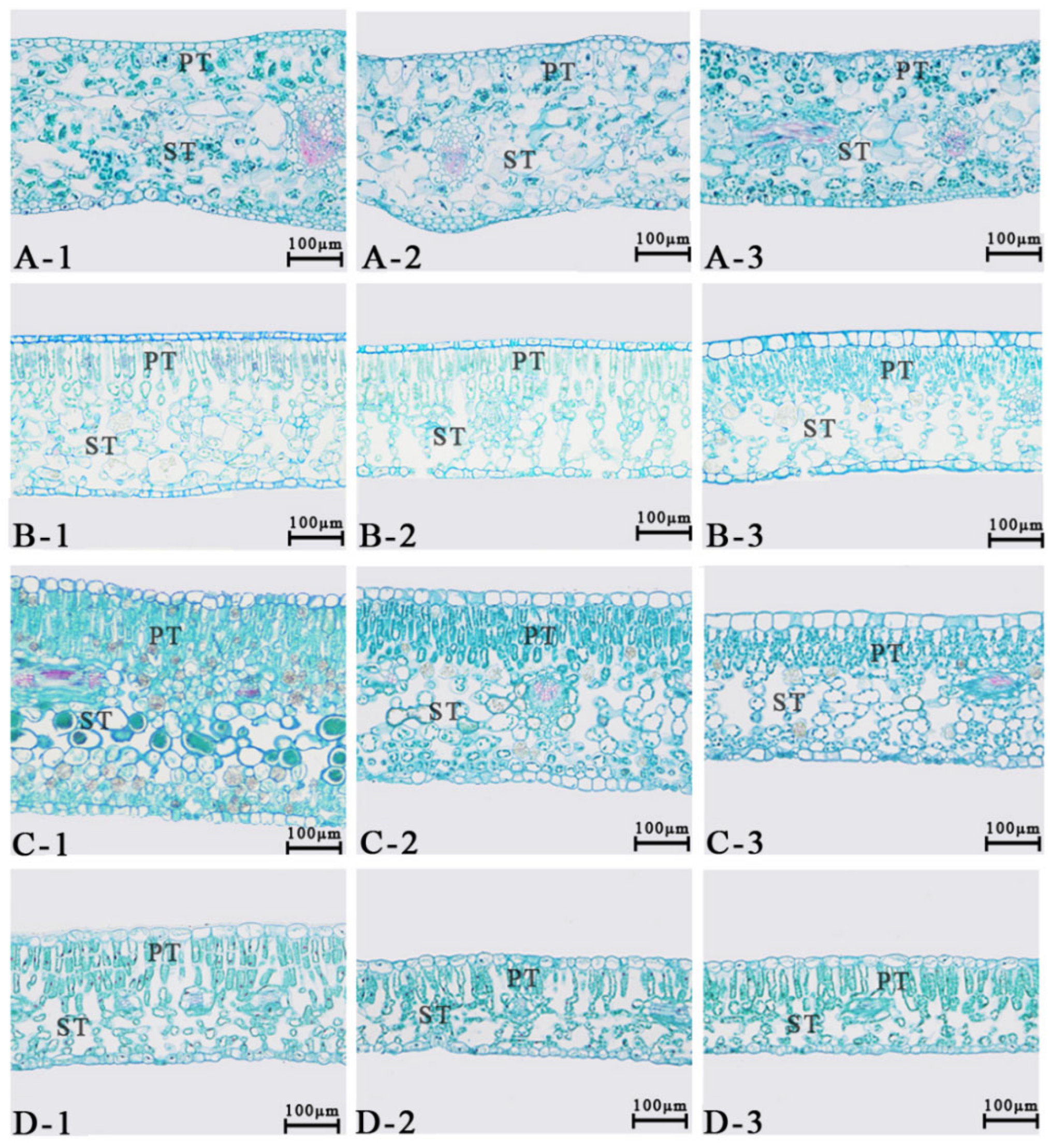
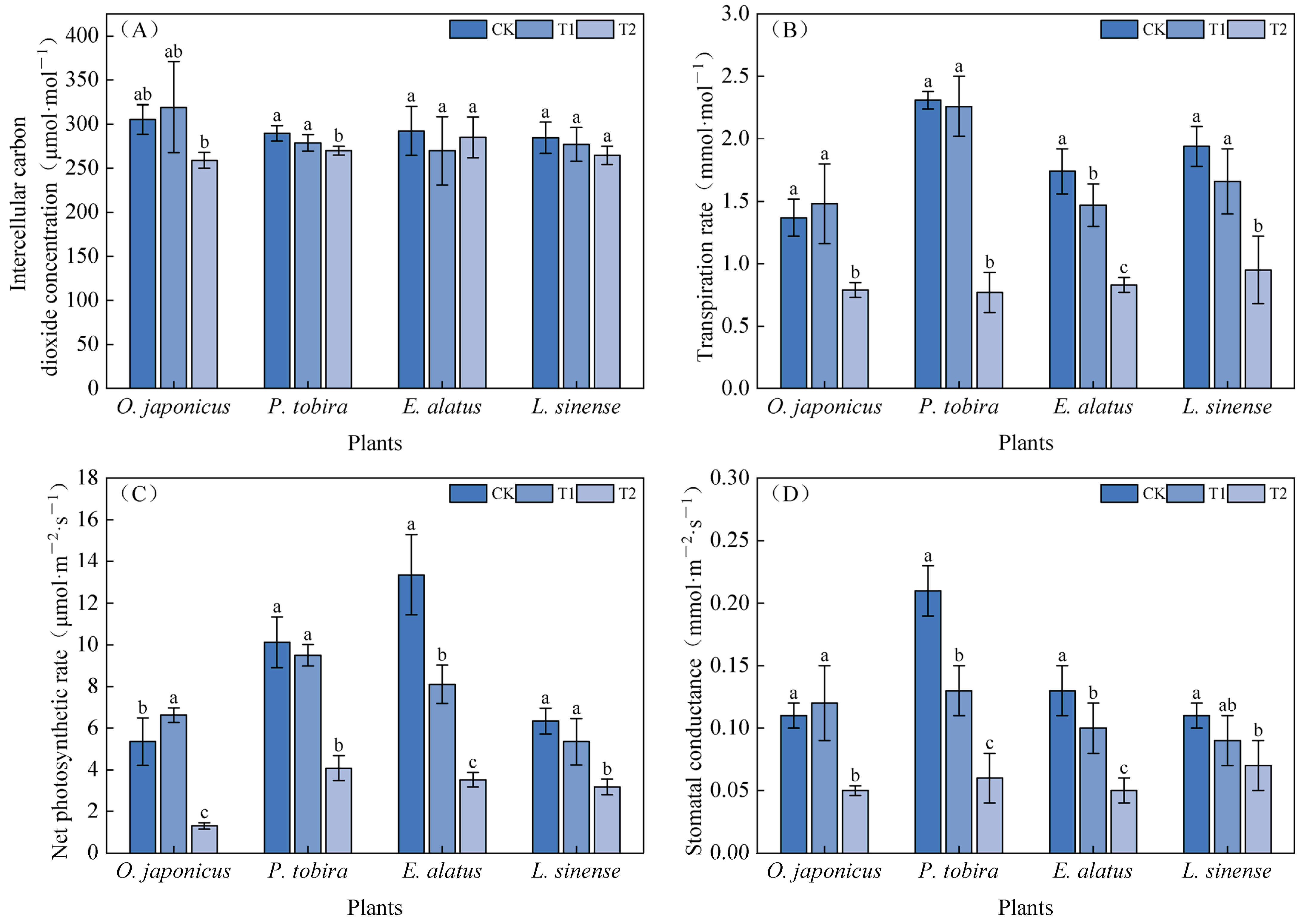
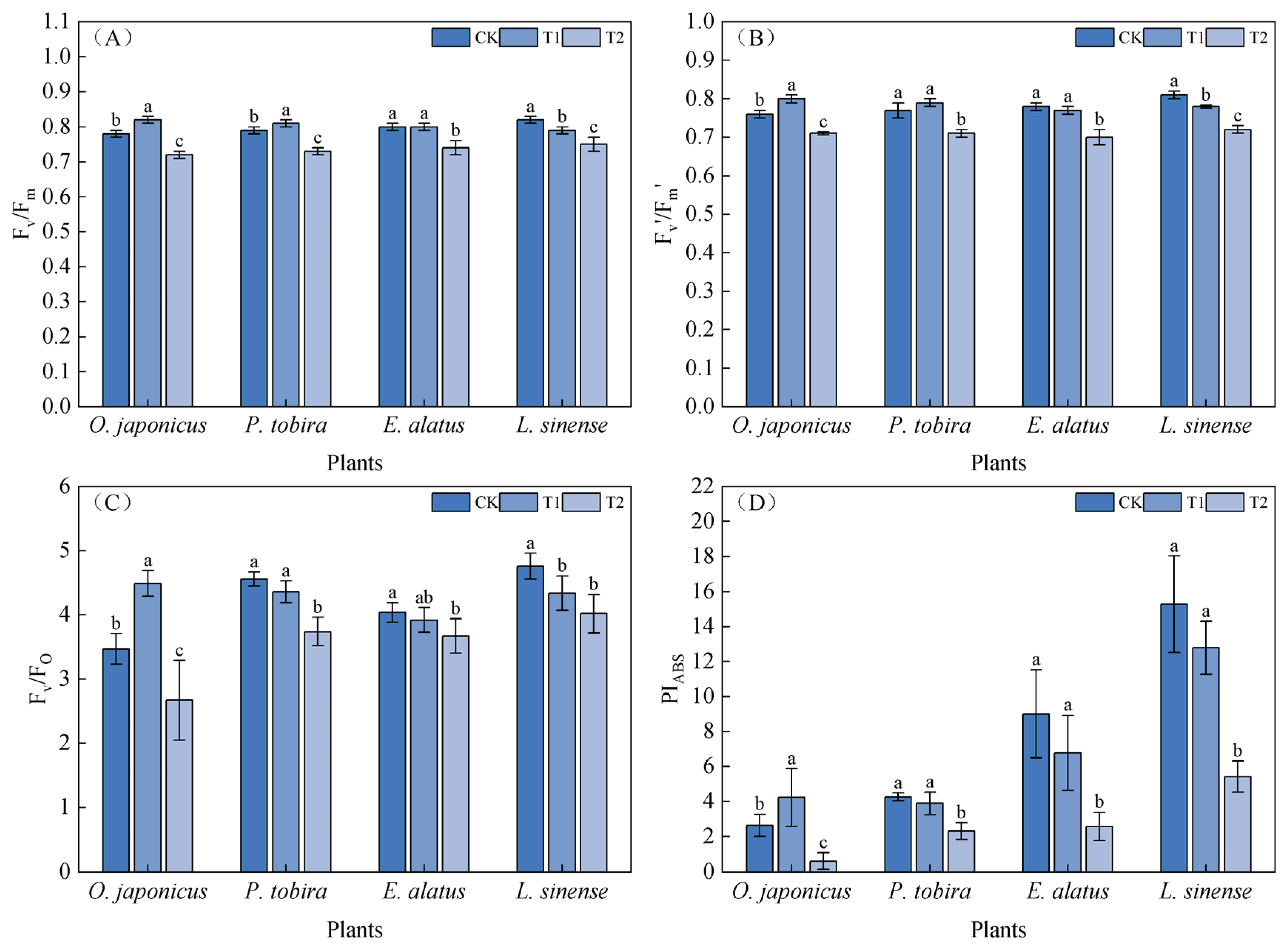
| Trait | Treatments | Species | |||
|---|---|---|---|---|---|
| Ophiopogon Japonicus | Pittosporum Tobira | Euonymus Alatus | Ligustrum Sinense | ||
| SD/(N/mm2) | CK | 272.35 ± 28.34 a | 261.66 ± 6.68 a | 275.36 ± 19.9 a | 404.79 ± 26.62 a |
| T1 | 283.17 ± 13.14 a | 236.43 ± 23.48 ab | 287.68 ± 10.23 a | 395.39 ± 57.76 a | |
| T2 | 195.56 ± 30.91 b | 215.55 ± 7.01 b | 180.20 ± 5.90 b | 380.21 ± 42.19 b | |
| SA/(µm2) | CK | 629.97 ± 31.09 b | 1009.28 ± 68.92 b | 1051.91 ± 30.35 b | 186.95 ± 8.00 c |
| T1 | 408.27 ± 25.95 c | 1412.83 ± 17.91 a | 898.60 ± 26.20 c | 241.10 ± 15.56 b | |
| T2 | 757.31 ± 15.57 a | 1523.46 ± 46.08 a | 1214.74 ± 38.68 a | 318.36 ± 13.79 a | |
| LT /(µm) | CK | 294.86 ± 9.16 a | 270.73 ± 2.19 a | 441.32 ± 13.10 a | 233.90 ± 2.14 a |
| T1 | 273.80 ± 5.43 b | 234.50 ± 5.60 b | 344.95 ± 13.01 b | 172.64 ± 6.33 b | |
| T2 | 232.3 ± 10.52 c | 231.71 ± 3.83 b | 293.21 ± 6.37 c | 171.10 ± 3.14 b | |
| PT /(µm) | CK | 78.35 ± 2.57 a | 95.88 ± 3.13 a | 129.70 ± 3.32 a | 91.74 ± 0.51 a |
| T1 | 68.91 ± 2.14 b | 85.37 ± 1.58 b | 93.69 ± 4.72 b | 54.20 ± 2.27 b | |
| T2 | 63.37 ± 1.13 b | 79.16 ± 1.18 c | 76.82 ± 1.33 c | 51.49 ± 1.10 b | |
| ST /(µm) | CK | 176.89 ± 9.81 a | 145.91 ± 3.58 a | 256.01 ± 10.18 a | 102.06 ± 4.49 a |
| T1 | 174.50 ± 1.51 b | 124.54 ± 7.85 b | 199.33 ± 15.58 b | 86.87 ± 2.27 b | |
| T2 | 146.13 ± 13.32 b | 113.23 ± 5.83 b | 167.55 ± 4.99 c | 51.49 ± 1.10 b | |
| PT /ST | CK | 0.45 ± 0.03 a | 0.66 ± 0.04 a | 0.51 ± 0.01 a | 0.90 ± 0.05 a |
| T1 | 0.40 ± 0.01 b | 0.69 ± 0.05 a | 0.47 ± 0.05 b | 0.62 ± 0.02 b | |
| T2 | 0.44 ± 0.04 a | 0.70 ± 0.05 a | 0.46 ± 0.04 b | 0.60 ± 0.01 b | |
| Indexes | Species | Light Intensity | Species × Light Intensity | |||
|---|---|---|---|---|---|---|
| p | F | p | F | p | F | |
| SLA | 0.000 ** | 43.266 | 0.000 ** | 50.669 | 0.191 NS | 1.523 |
| LDMC | 0.000 ** | 95.813 | 0.000 ** | 28.829 | 0.001 ** | 4.812 |
| RWC | 0.001 ** | 6.478 | 0.000 ** | 88.826 | 0.000 ** | 12.786 |
| SD | 0.000 ** | 827.434 | 0.000 ** | 127.004 | 0.000 ** | 42.094 |
| SA | 0.000 ** | 44.432 | 0.000 ** | 12.581 | 0.202 NS | 1.560 |
| LT | 0.000 ** | 500.377 | 0.000 ** | 204.521 | 0.000 ** | 33.760 |
| PT | 0.000 ** | 303.315 | 0.000 ** | 429.775 | 0.000 ** | 42.115 |
| ST | 0.000 ** | 226.844 | 0.000 ** | 54.017 | 0.000 ** | 8.955 |
| PT/ST | 0.000 ** | 95.229 | 0.000 ** | 13.451 | 0.000 ** | 10.840 |
| Ci | 0.427 NS | 1.426 | 0.024 * | 4.040 | 0.130 * | 1.748 |
| Tr | 0.000 ** | 19.308 | 0.000 ** | 130.210 | 0.000 ** | 6.392 |
| Pn | 0.000 ** | 57.277 | 0.000 ** | 174.028 | 0.000 ** | 14.948 |
| GS | 0.000 ** | 15.117 | 0.000 ** | 77.786 | 0.000 ** | 7.592 |
| Fv/Fm | 0.021 * | 3.564 | 0.000 ** | 160.857 | 0.000 ** | 8.329 |
| Fv′/Fm′ | 0.012 * | 4.065 | 0.000 ** | 182.774 | 0.000 ** | 8.138 |
| Fv/Fo | 0.000 ** | 21.493 | 0.000 ** | 36.093 | 0.000 ** | 8.616 |
| PIABS | 0.000 ** | 82.234 | 0.000 ** | 53.423 | 0.000 ** | 7.260 |
| Chl a | 0.000 ** | 73.243 | 0.000 ** | 114.415 | 0.000 ** | 11.973 |
| Chl b | 0.000 ** | 40.293 | 0.000 ** | 122.599 | 0.000 ** | 7.147 |
| Chla/b | 0.000 ** | 42.360 | 0.000 ** | 32.089 | 0.000 ** | 3.558 |
| MDA | 0.000 ** | 67.216 | 0.000 ** | 47.055 | 0.000 ** | 6.831 |
| SOD | 0.000 ** | 21.990 | 0.000 ** | 108.195 | 0.000 ** | 27.002 |
| POD | 0.003 ** | 5.402 | 0.000 ** | 96.929 | 0.107 * | 1.861 |
| SP | 0.000 ** | 21.568 | 0.000 ** | 148.411 | 0.000 ** | 15.640 |
| REC | 0.000 ** | 56.908 | 0.000 ** | 147.234 | 0.000 ** | 14.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Li, H.; Zhou, P.; Guo, J.; Yuan, J.; Wang, J.; Liu, Y.; Zhang, Z.; Lei, Y. Leaf Plasticity Responses of Four Urban Garden Plants to Low-Light Environments Under Viaducts. Forests 2025, 16, 651. https://doi.org/10.3390/f16040651
He D, Li H, Zhou P, Guo J, Yuan J, Wang J, Liu Y, Zhang Z, Lei Y. Leaf Plasticity Responses of Four Urban Garden Plants to Low-Light Environments Under Viaducts. Forests. 2025; 16(4):651. https://doi.org/10.3390/f16040651
Chicago/Turabian StyleHe, Dan, Haitao Li, Pingxi Zhou, Jinlin Guo, Jiangqin Yuan, Jingkun Wang, Yiping Liu, Zhiqiang Zhang, and Yakai Lei. 2025. "Leaf Plasticity Responses of Four Urban Garden Plants to Low-Light Environments Under Viaducts" Forests 16, no. 4: 651. https://doi.org/10.3390/f16040651
APA StyleHe, D., Li, H., Zhou, P., Guo, J., Yuan, J., Wang, J., Liu, Y., Zhang, Z., & Lei, Y. (2025). Leaf Plasticity Responses of Four Urban Garden Plants to Low-Light Environments Under Viaducts. Forests, 16(4), 651. https://doi.org/10.3390/f16040651






