The Effects of Different Moso Bamboo Densities on the Physiological Growth of Indocalamus latifolius Cultivated in Moso Bamboo Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set up
2.2. Harvest and Measurements
2.3. Statistical Analysis
3. Results
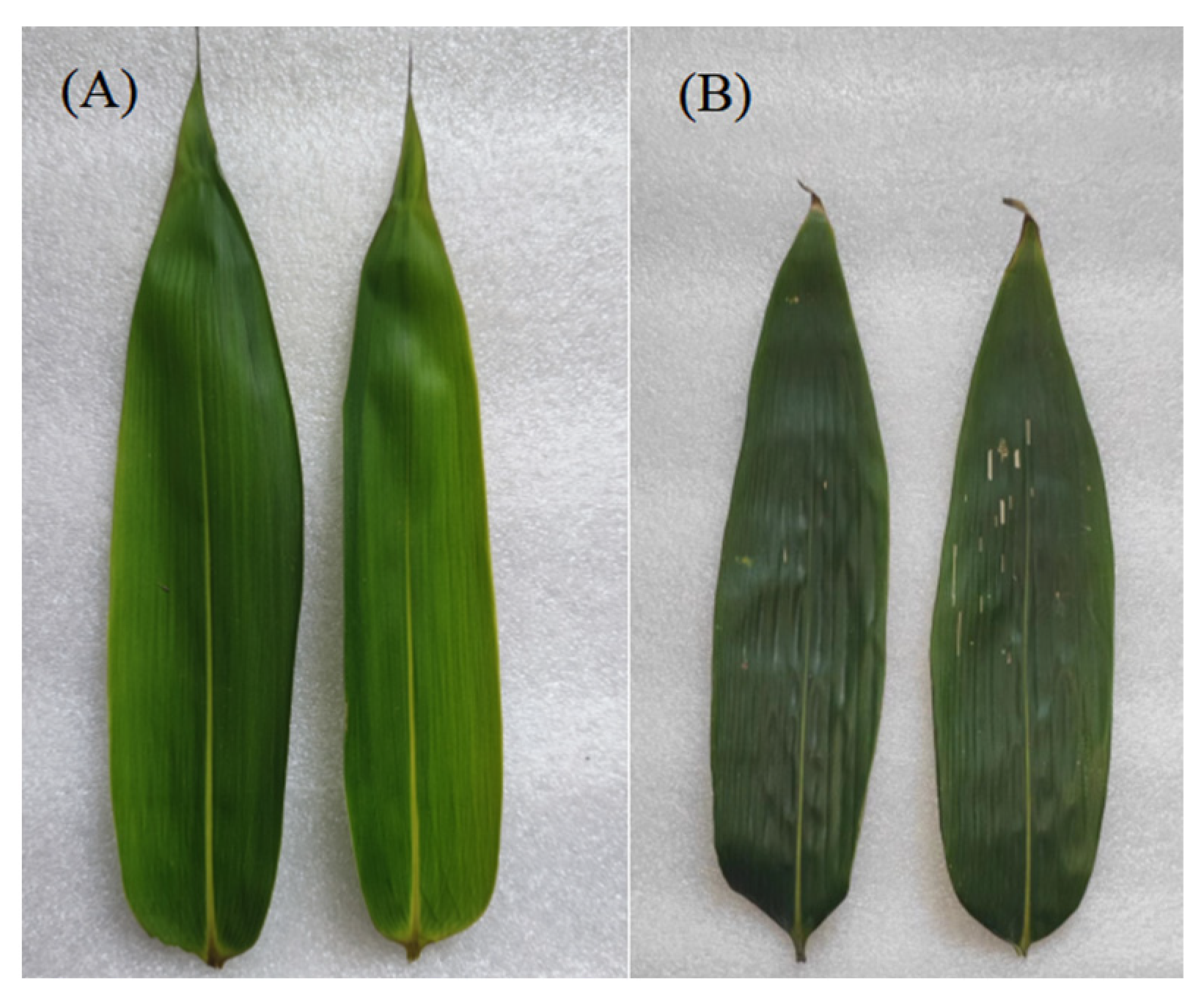
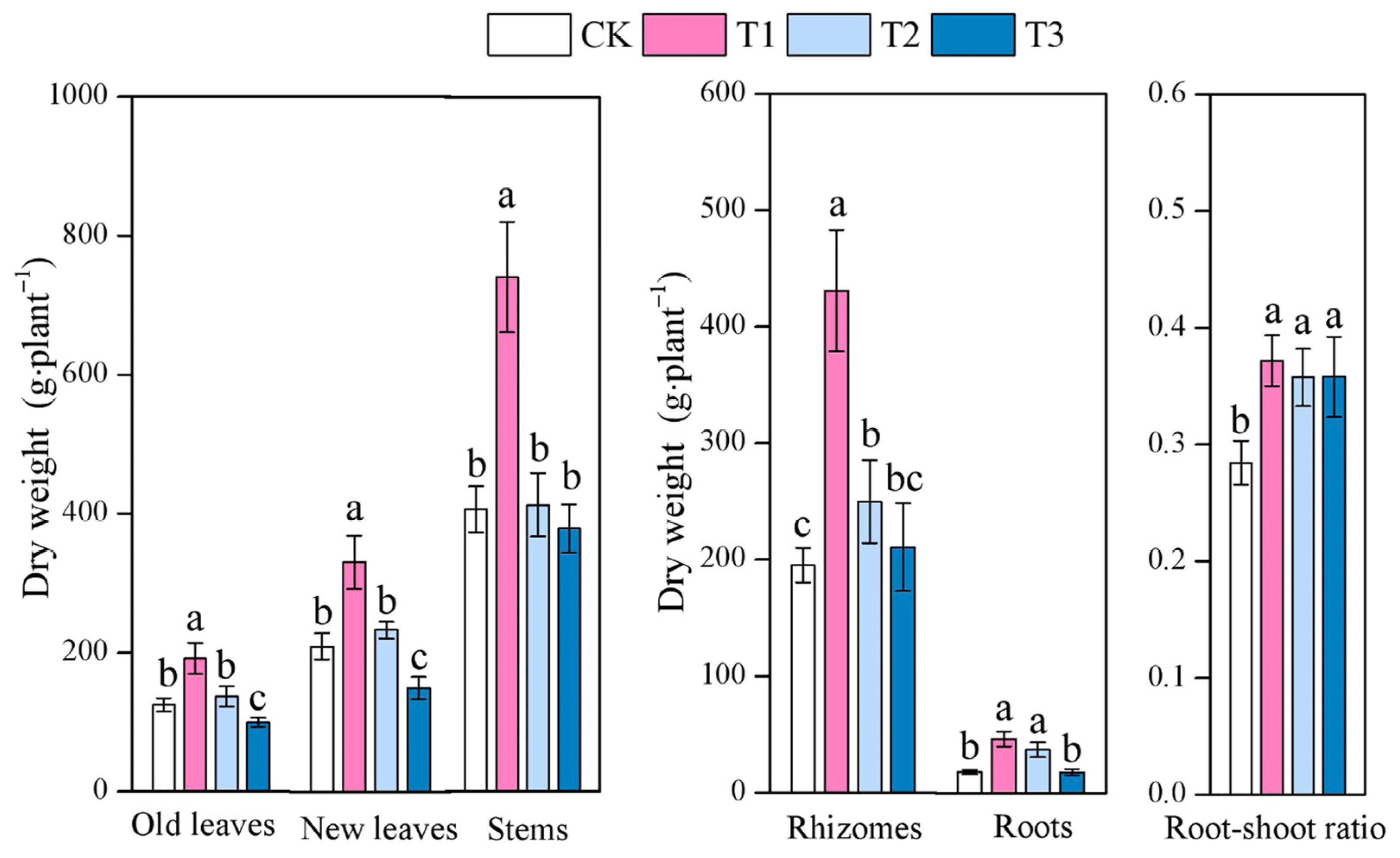
3.1. Effect of Different Moso Bamboo Densities on the Biomass Allocation of I. latifolius
3.2. Effect of Different Moso Bamboo Densities on the Nutrient Elements and Unstructured Carbohydrates Allocation of I. latifolius
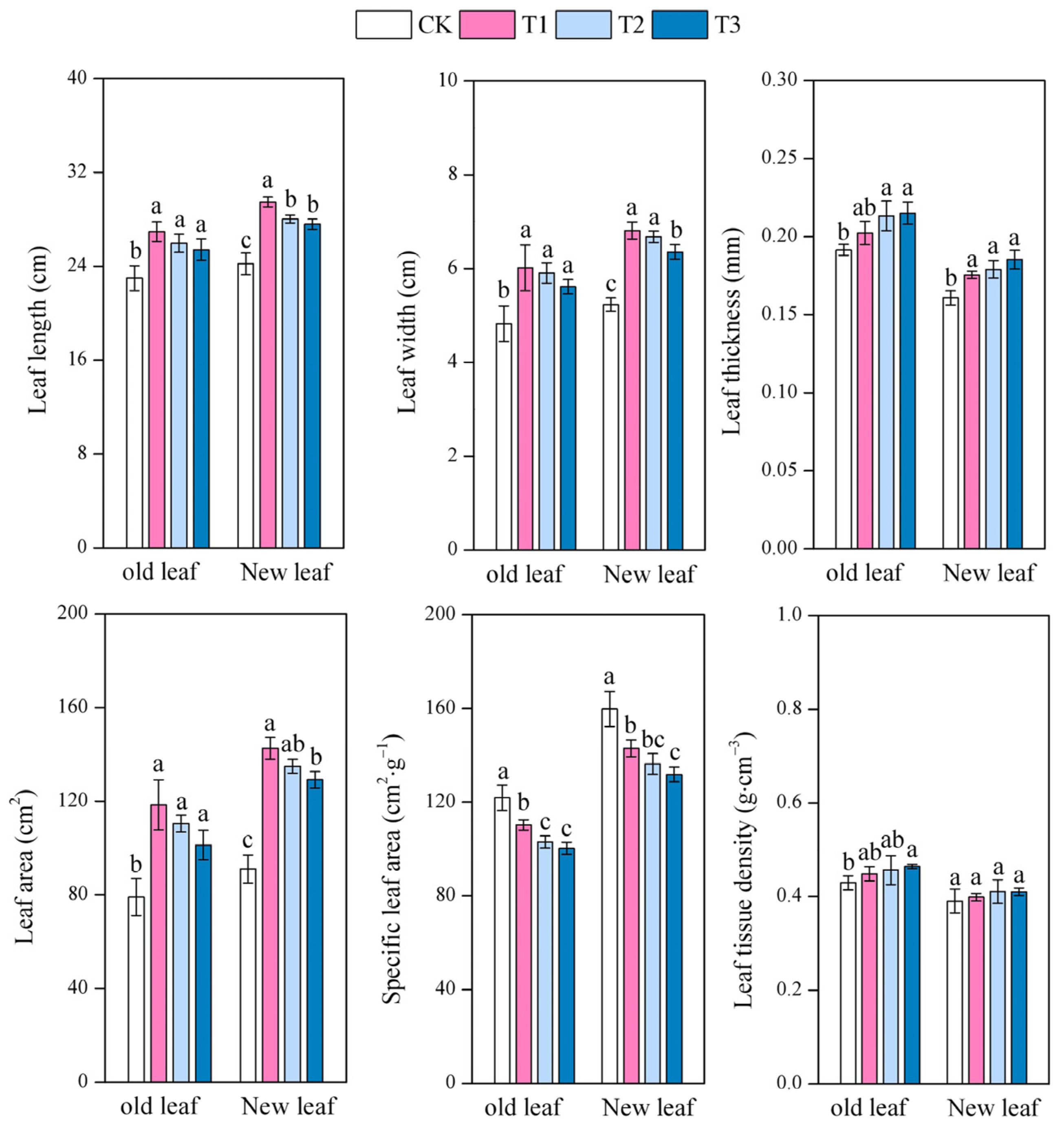
3.3. Effect of Different Moso Bamboo Densities on the Leaf Functional Traits of I. latifolius
3.4. Effect of Different Moso Bamboo Densities on the Root Morphology of I. latifolius
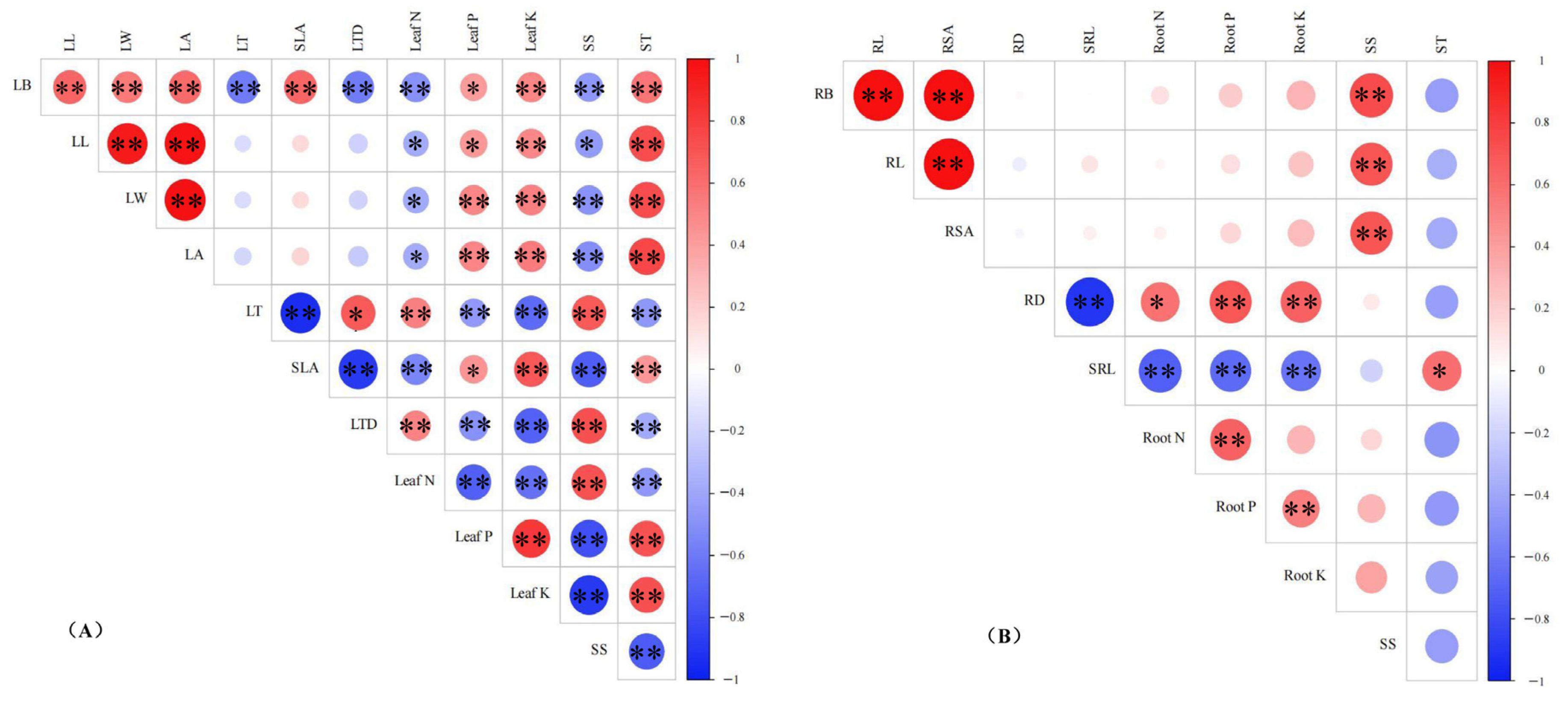
3.5. Correlation Analysis Among the Indicators Parameters
4. Discussion
4.1. Responses of Biomass Allocation to Different Moso Bamboo Densities
4.2. Responses of Leaf Functional Traits to Different Moso Bamboo Densities
4.3. Responses of Root Morphology to Different Moso Bamboo Densities
4.4. Responses of Nutrient Elements and Photosynthate Allocation to Different Moso Bamboo Densities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Z.H.; Lu, Y.; Li, L.B.; Zhao, Q.; Feng, Q.; Gao, Z.M.; Lu, H.Y.; Hu, T.; Yao, N.; Liu, K.Y.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Ge, Z.P.; Deng, X.; Shi, Y.J.; Xu, L.; Zhou, G.M.; Ye, Y.Z.; Zhang, F.G.; Zhou, Y.F.; Li, C.; et al. Abandonment lead to structural degradation and changes in carbon allocation patterns in Moso bamboo forests. For. Ecol. Manag. 2019, 449, 117449. [Google Scholar] [CrossRef]
- Yang, B.; Meng, X.; Singh, A.K.; Wang, P.; Song, L.; Zakari, S.; Liu, W. Intercrops improve surface water availability in rubber-based agroforestry systems. Agric. Ecosyst. Environ. 2020, 298, 106937. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, J.C.; Yang, Z.Y.; Shi, R.; Li, Q.; Wang, Y.B.; He, L.; Chen, Y.J. Analysis of biochemical components and antioxidant capacity of Indocalamus leaves of different varieties. Sci. Technol. Food Ind. 2022, 43, 93–100. [Google Scholar]
- Yang, L.T.; Xie, Y.Y.; Zuo, K.Y.; Xu, S.; Gu, R.; Chen, S.L.; Guo, Z.W. Effects of ramet ratio on photosynthetic physiology of Indocalamus decorus clonal system under heterogeneous light environment. Chin. J. Plant Ecol. 2022, 46, 88–101. [Google Scholar]
- Dai, Y.S.; Yang, T.; Shen, L.; Wang, X.Y.; Zhang, W.L.; Liu, T.T.; Zhang, W. Root growth, distribution, and physiological characteristics of alfalfa in a poplar/alfalfa silvopastoral system compared to sole-cropping in Northwest Xinjiang. China Agrofor. Syst. 2021, 95, 1137–1153. [Google Scholar] [CrossRef]
- Wei, W.W.; Liu, T.T.; Shuai, Z.; Lei, S.; Wang, X.Y.; Li, L.H.; Zhu, Y.; Zhang, W. Root spatial distribution and belowground competition in an apple/ryegrass agroforestry system. Agric. Syst. 2024, 215, 103869. [Google Scholar] [CrossRef]
- Cui, R.; Qi, S.; Wu, B.C.; Zhang, D.; Zhang, L.; Zhou, P.; Ma, N.; Huang, X. The influence of stand structure on understory herbaceous plants species diversity of Platycladus orientalis plantations in Beijing, China. Forests 2022, 13, 1921. [Google Scholar] [CrossRef]
- Yan, N.; Xu, X.F.; Wang, Z.D.; Huang, J.Z.; Guo, D.P. Interactive effects of temperature and light intensity on photosynthesis and antioxidant enzyme activity in Zizania latifolia Turcz. plants. Photosynthetica 2013, 51, 127–138. [Google Scholar] [CrossRef]
- Grunwald, Y.; Yaaran, A.; Moshelion, M. Illuminating plant water dynamics: The role of light in leaf hydraulic regulation. New Phytol. 2024, 241, 1404. [Google Scholar] [CrossRef]
- Feng, L.Y.; Raza, M.A.; Li, Z.C.; Chen, Y.K.; Khalid, M.H.B.; Du, J.B.; Liu, W.G.; Wu, X.L.; Song, C.; Yu, L.; et al. The influence of light lntensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Wegener, F.; Beyschlag, W.; Werner, C. High intraspecific ability to adjust both carbon uptake and allocation under light and nutrient reduction in Halimium halimifolium L. Front. Plant Sci. 2015, 6, 609. [Google Scholar]
- Su, B.Y.; Song, Y.X.; Song, C.; Cui, L.; Yong, T.W.; Yang, W.Y. Growth and photosynthetic responses of soybean seedlings to maize shading in relay intercropping system in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Bahn, M.; Lattanzi, F.A.; Hasibeder, R.; Wild, B.; Koranda, M.; Danese, V.; Brüggemann, N.; Schmitt, M.; Siegwolf, R.; Richter, A. Responses of belowground carbon allocation dynamics to extended shading in mountain grassland. New Phytol. 2013, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Wang, G.; Liu, R.; Huang, D.; Song, M.; Zhang, Y.; Wang, H.; Wang, Y.; Shao, R. Maize yield increased by matching canopy light and nitrogen distribution via controlled-release urea/urea adjustment. Field Crops Res. 2024, 308, 109284. [Google Scholar] [CrossRef]
- Xie, H.T.; Yu, M.K.; Cheng, X.R. Effects of light intensity variation on nitrogen and phosphorus contents, allocation and limitation in five shade-enduring plants. Chin. J. Plant Ecol. 2017, 41, 559–569. [Google Scholar]
- Tang, J.C.; Sun, B.; Cheng, R.M.; Shi, Z.M.; Luo, D.; Liu, S.R.; Centritto, M. The Effect of Low Irradiance on Leaf Nitrogen Allocation and Mesophyll Conductance to CO2 in Seedlings of Four Tree Species in Subtropical China. Plants 2021, 10, 2213. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Di, Y.; Wang, H.M. Differences in Fine-Root Biomass of Trees and Understory Vegetation among Stand Types in Subtropical Forests. PLoS ONE 2015, 10, e0128894. [Google Scholar] [CrossRef] [PubMed]
- Cardinael, R.; Mao, Z.; Prieto, I.; Stokes, A.; Dupraz, C.; Kim, J.H.; Jourdan, C. Competition with winter crops induces deeper rooting of walnut trees in a Mediterranean alley cropping agroforestry system. Plant Soil 2015, 391, 219–235. [Google Scholar] [CrossRef]
- Natalie, C.; Bever, J.D. Carbon allocation and competition maintain variation in plant root mutualisms. Ecol. Evol. 2018, 8, 5792–5800. [Google Scholar]
- Ma, J.M.; Liu, D.; Zhao, P.Y.; Dou, M.; Yang, X.H.; Liu, S.L.; Nian, F.Z.; Tong, W.J.; Li, J.Y.; Xu, Z.L.; et al. Intercropping of tobacco and maize at seedling stage promotes crop growth through manipulating rhizosphere microenvironment. Front. Plant Sci. 2024, 15, 1470229. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhuo, G.Y.; Zhang, D.Q.; Zhuo, G.Y. Soil organic carbon accumulation modes between pioneer and old-growth forest ecosystems. J. Appl. Ecol. 2020, 57, 2419–2428. [Google Scholar] [CrossRef]
- Zhao, J.C.; Cai, C.J. Physiological integration improves nitrogen use efficiency of moso bamboo: An isotopic (15N) assessment. For. Ecol. Manag. 2023, 542, 121073. [Google Scholar] [CrossRef]
- Zhuang, M.H.; Li, Y.C.; Chen, S.L. Advances in the researches of bamboo physiological integration and its ecological significance. J. Bamboo Res. 2011, 30, 5–9. [Google Scholar]
- Tong, R.; Zhou, B.Z.; Jiang, L.N.; Ge, X.G.; Cao, Y.H. The growth of Chinese fir is limited by nitrogen: Evidences from N:P ratio, N or P variability and NuRE based on a regional investigation. For. Ecol. Manag. 2020, 460, 117905. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H. Mobile carbohydrates in Himalayan treeline trees I. evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar]
- Xie, H.T.; Yu, M.K.; Cheng, X.R. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physio. Bioc. 2018, 124, 146–154. [Google Scholar] [CrossRef]
- Zhang, M.; Ming, Y.; Wang, B.H.; Jin, H.L. Strategies for adaptation to high light in plants. Abiotech 2024, 5, 381–393. [Google Scholar] [PubMed]
- Jing, X.; Cai, C.J.; Fan, S.H.; Liu, G.L.; Li, X.J. Research advances in ecological adaptability of bamboo under environmental stresses. World For. Res. 2018, 31, 36–41. [Google Scholar]
- Zhang, Y.P.; Onda, Y.C.; Kato, H.; Feng, B.; Gomi, T. Understory biomass measurement in a dense plantation forest based on drone-SfM data by a manual low-flying drone under the canopy. J. Environ. Manag. 2022, 312, 114862. [Google Scholar] [CrossRef] [PubMed]
- Semchenko, M.; Lepik, M.; Götzenberger, L.; Zobel, K. Positive effect of shade on plant growth: Ameliorationof stress or active regulation of growth rate? J. Ecol. 2012, 100, 459–466. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.Y.; Wo, Y.; Yang, N.; Lin, Z.L.; Zhao, L.X.; Li, T.Q.; Gao, K. Effects of Shading on Biomass and Material Distribution Rule of Jerusalem artichoke. J. Inner Mongolia Univ. Natl. (Nat. Sci.) 2021, 3, 225–230. [Google Scholar]
- Smith, T.M.; Shackleton, S.E. The effects of shading on the establishment and growth of Acacia tortilis seedlings. S. Afr. J. Bot. 1988, 54, 375–379. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wu, X.; Zhu, C.; Huang, S.; Yang, F.; Hou, X. Adaptational responses of leaf functional traits of Dicranopteris dichotoma to environmental factors in different vegetational restoration stages. Glob. Ecol. Conserv. 2023, 44, e02484. [Google Scholar] [CrossRef]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, M.G.; Lu, J.Y.; Yang, H.M. Effects of shading on the growth and leaf photosynthetic characteristics of three forages in an apple orchard on the Loess Plateau of eastern Gansu, China. PeerJ 2019, 7, e7594. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Yu, M.; Wang, Q.; Wang, L.; Yang, H.P.; Zhao, Y.J.; Dong, H.Z. Leaf structure and seed histochemistry analyses provided structural insights into the improved yield and quality of tree peony seed under light shading conditions. Sci. Rep. 2020, 10, 4328. [Google Scholar] [CrossRef] [PubMed]
- Moura, B.B.; Alves, E.S. Climatic factors influence leaf structure and thereby affect the ozone sensitivity of Ipomoea nil ‘Scarlet O’Hara’. Environ. Pollut. 2014, 194, 11–16. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.M.; Zhang, J. Preliminary Studies on the Influence of Light Intensity on Growth and Saponin Contents of DIoscorea zingiberensis. Acta Bot. Boreali Occident. Sin. 2011, 31, 536–542. [Google Scholar]
- Wang, W.Y.; Zhao, D.D.; Zhang, G.X.; Su, T.X. Comparison of anatomical structure and shade tolerance of three species of Ranunculaceae under shading. J. Southern Agric. 2024, 6, 1713–1723. [Google Scholar]
- Aguiar, A.C.; Robinson, S.A.; French, K. Friends with benefits: The effects of vegetative shading on plant survival in a green roof environment. PLoS ONE 2019, 14, e0225078. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.J.; Wang, Y.J.; Wang, N.; Yang, J.L.; Xu, S.; He, X.Y.; Chen, W. Effects of light intensity on physiological and growth characteristics of paeonia suffruticosa var. papaveracea. Chin. J. Ecol. 2020, 39, 2963–2973. [Google Scholar]
- Liu, S.Q.; Liu, Y.Z.; Liu, S.Q.; Ma, Y.; Dai, M.M.; Liu, J.R.; Liu, C. Effects of Shade Stress on the Synthesis of Cellulose and Lignin in Maize Nodal Roots. J. Agron. Crop Sci. 2025, 211, e70015. [Google Scholar]
- Gaucher, C.; Gougeon, S.; Mauffette, Y.; Christian, M. Seasonal variation in biomass and carbohydrate partitioning of understory sugar maple (Acer saccharum) and yellow birch (Betula alleghaniensis) seedlings. Tree Physiol. 2005, 25, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Zhao, G.J.; Shen, Y.X.; Zhu, H.; Li, Z.J.; Tan, B.L. Shading and Water Addition Alleviate the Elemental Limitations of the Early Restoration Community in a Stressful Environment. Plants 2024, 13, 2626. [Google Scholar] [CrossRef]
- Nan, H.; Mao, Q.G.; Zhong, X.Z.; Sun, X.B.; Xu, Q.H.; Li, M.M.; Chen, H. Factors driving the positive resorption of aluminum and iron from old leaves. Plant Soil 2023, 488, 443–450. [Google Scholar]
- Wu, J.W.; Su, Y.; Wang, J.H.; He, Q.; Qiu, Q.; Ma, J.W.; Li, J.Y. Morphological and physiological acclimation of Catalpa bungei plantlets to different light conditions. Photosynthetica 2018, 56, 537–548. [Google Scholar] [CrossRef]
- Hu, S.L.; Liu, D.; Guo, B.; Li, H.Y.; Zhu, Q.D.; Zhang, Z.H. OsSTP1 mediates sucrose allocation to regulate rice responses to different nitrogen supply levels. Chin. J. Biotechnol. 2024, 40, 3500–3514. [Google Scholar]


| Treatments | Bamboo Density (Plants·ha−1) | The Proportion of Bamboo at Different Ages | Canopy Density |
|---|---|---|---|
| CK | 0 | — | 0 |
| T1 | 1050 | 1:1:1 | 0.30~0.35 |
| T2 | 2100 | 1:1:1 | 0.55~0.60 |
| T3 | 3150 | 1:1:1 | 0.75~0.80 |
| Abbreviations | Full Form |
|---|---|
| RL | Root length |
| RSA | Root surface area |
| RD | Root average diameter |
| RLR | Root length ratio |
| rl | Root length of each diameter grade |
| SRL | Specific root length |
| LL | Leaf length |
| LW | Leaf width |
| LT | Leaf thickness |
| LA | Leaf area |
| SLA | Specific leaf area |
| LDM | Leaf dry mass |
| LTD | Leaf tissue density |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| NSCs | Non-structural carbohydrates |
| SS | Soluble sugar |
| ST | Starch |
| Nutrient Element Contents | Treatments | Old Leaves | New Leaves | Stems | Rhizomes | Roots |
|---|---|---|---|---|---|---|
| Nitrogen (mg·g−1) | CK | 17.08 ± 0.33 b | 16.97 ± 0.47 a | 6.35 ± 0.19 a | 4.33 ± 0.09 a | 6.21 ± 0.09 c |
| T1 | 18.35 ± 0.39 a | 15.90 ± 0.68 b | 5.56 ± 0.32 b | 4.14 ± 0.07 b | 6.52 ± 0.17 b | |
| T2 | 17.53 ± 0.47 b | 16.13 ± 0.69 ab | 5.94 ± 0.17 b | 4.04 ± 0.14 b | 6.75 ± 0.16 a | |
| T3 | 17.35 ± 0.33 b | 17.05 ± 0.40 a | 5.92 ± 0.17 b | 4.10 ± 0.11 b | 6.86 ± 0.29 a | |
| Phosphorus (mg·g−1) | CK | 1.59 ± 0.05 a | 1.49 ± 0.15 c | 1.40 ± 0.04 c | 0.93 ± 0.08 b | 0.88 ± 0.04 b |
| T1 | 1.39 ± 0.02 b | 1.73 ± 0.04 b | 1.52 ± 0.07 b | 1.26 ± 0.11 a | 1.02 ± 0.08 a | |
| T2 | 1.42 ± 0.03 b | 1.92 ± 0.08 a | 1.54 ± 0.04 b | 1.21 ± 0.03 a | 1.07 ± 0.08 a | |
| T3 | 1.45 ± 0.04 b | 1.75 ± 0.08 b | 1.68 ± 0.05 a | 1.12 ± 0.09 a | 1.10 ± 0.06 a | |
| Potassium (mg·g−1) | CK | 7.25 ± 0.41 a | 7.58 ± 0.52 b | 6.63 ± 0.50 b | 5.35 ± 0.13 b | 6.41 ± 0.12 b |
| T1 | 6.31 ± 0.32 b | 8.39 ± 0.24 a | 7.62 ± 0.34 a | 6.11 ± 0.33 a | 7.28 ± 0.55 a | |
| T2 | 6.21 ± 0.22 b | 9.09 ± 0.45 a | 7.56 ± 0.41 a | 6.18 ± 0.59 a | 7.26 ± 0.51 a | |
| T3 | 6.33 ± 0.28 b | 8.76 ± 0.75 a | 7.46 ± 0.35 a | 6.01 ± 0.30 a | 7.15 ± 0.35 a |
| Non-Structural Carbohydrates Contents | Treatments | Old Leaves | New Leaves | Stems | Rhizomes | Roots |
|---|---|---|---|---|---|---|
| soluble sugar (mg·g−1) | CK | 21.10 ± 2.49 b | 16.44 ± 0.38 a | 51.91 ± 4.31 a | 61.93 ± 0.96 b | 20.97 ± 1.29 b |
| T1 | 25.82 ± 1.19 a | 13.93 ± 0.88 b | 39.21 ± 2.16 c | 66.97 ± 2.63 a | 25.06 ± 1.88 a | |
| T2 | 23.04 ± 1.55 b | 13.10 ± 1.13 b | 43.50 ± 1.91 b | 64.82 ± 2.24 ab | 23.08 ± 2.22 ab | |
| T3 | 23.47 ± 0.65 ab | 10.68 ± 1.05 c | 45.11 ± 2.00 b | 60.05 ± 2.46 b | 21.33 ± 1.11 b | |
| Starch (mg·g−1) | CK | 207.85 ± 4.02 b | 224.95 ± 6.25 b | 241.63 ± 7.08 a | 330.86 ± 10.72 a | 222.42 ± 10.60 a |
| T1 | 220.77 ± 7.88 a | 240.37 ± 4.62 a | 221.54 ± 5.59 b | 320.30 ± 10.47 ab | 202.73 ± 6.83 b | |
| T2 | 220.22 ± 5.30 a | 246.81 ± 4.22 a | 242.90 ± 6.56 a | 305.22 ± 7.03 b | 210.67 ± 5.63 ab | |
| T3 | 218.82 ± 7.43 a | 241.4 ± 5.19 a | 240.11 ± 4.29 a | 281.91 ± 11.49 c | 209.77 ± 7.83 ab | |
| Sugar/starch | CK | 0.101 ± 0.010 b | 0.073 ± 0.001 a | 0.215 ± 0.012 a | 0.187 ± 0.008 b | 0.094 ± 0.007 b |
| T1 | 0.117 ± 0.004 a | 0.058 ± 0.004 b | 0.177 ± 0.009 b | 0.209 ± 0.005 a | 0.124 ± 0.012 a | |
| T2 | 0.105 ± 0.007 ab | 0.053 ± 0.005 b | 0.179 ± 0.009 b | 0.212 ± 0.009 a | 0.109 ± 0.009 ab | |
| T3 | 0.107 ± 0.005 ab | 0.044 ± 0.004 c | 0.188 ± 0.007 b | 0.213 ± 0.011 a | 0.102 ± 0.008 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Zhao, J.; Yang, Z. The Effects of Different Moso Bamboo Densities on the Physiological Growth of Indocalamus latifolius Cultivated in Moso Bamboo Forests. Forests 2025, 16, 636. https://doi.org/10.3390/f16040636
Ni H, Zhao J, Yang Z. The Effects of Different Moso Bamboo Densities on the Physiological Growth of Indocalamus latifolius Cultivated in Moso Bamboo Forests. Forests. 2025; 16(4):636. https://doi.org/10.3390/f16040636
Chicago/Turabian StyleNi, Huijing, Jiancheng Zhao, and Zhenya Yang. 2025. "The Effects of Different Moso Bamboo Densities on the Physiological Growth of Indocalamus latifolius Cultivated in Moso Bamboo Forests" Forests 16, no. 4: 636. https://doi.org/10.3390/f16040636
APA StyleNi, H., Zhao, J., & Yang, Z. (2025). The Effects of Different Moso Bamboo Densities on the Physiological Growth of Indocalamus latifolius Cultivated in Moso Bamboo Forests. Forests, 16(4), 636. https://doi.org/10.3390/f16040636






