Hotspots of Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.1.1. Species Distribution Data
2.1.2. Plant Functional Trait Data
2.1.3. Environmental Data
2.1.4. Phylogenetic Tree Construction
2.2. Data Analyses
2.2.1. Species Distribution Modeling
2.2.2. Diversity Index Calculation
2.2.3. Hotspots Identification
3. Results
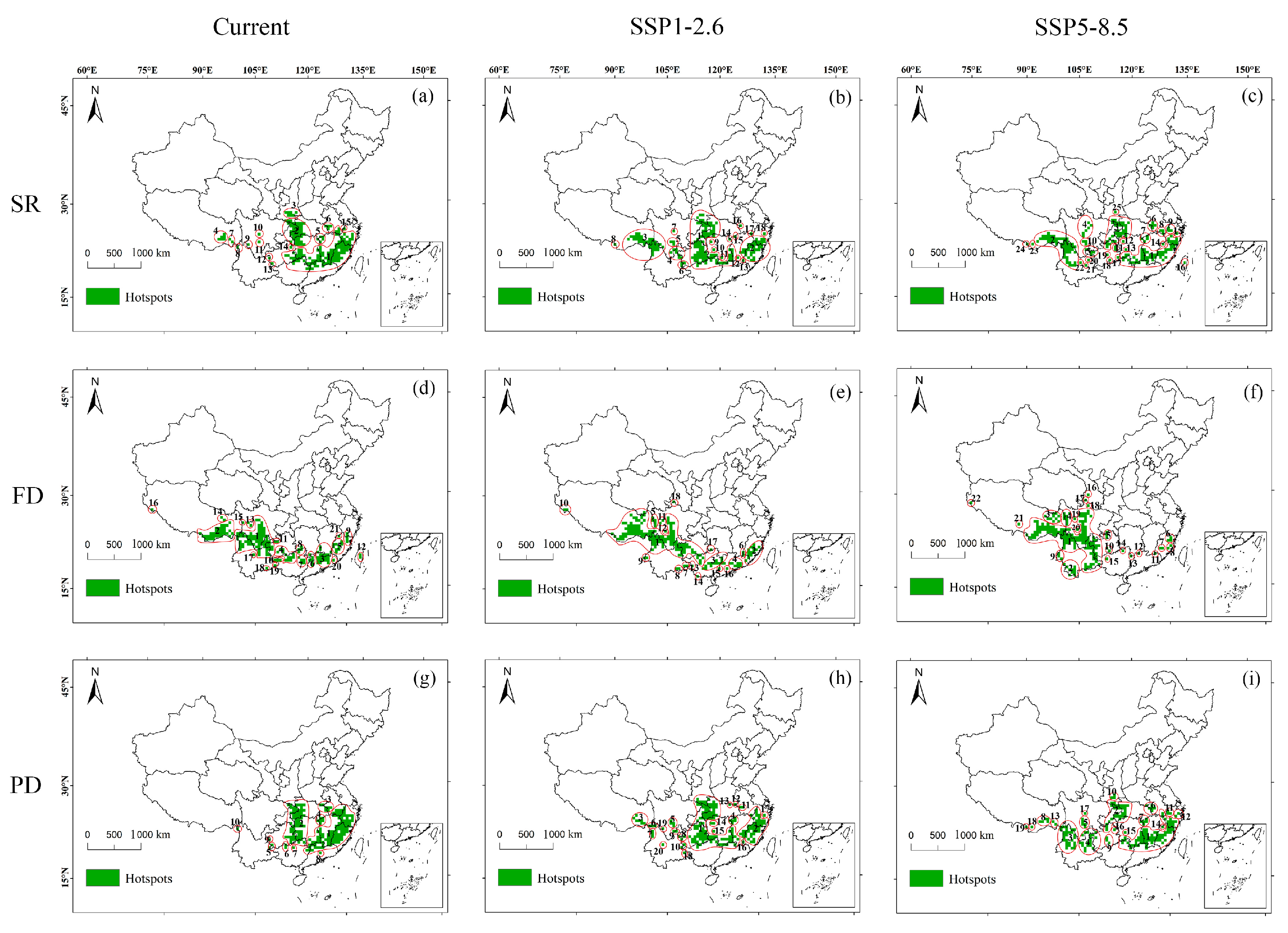
3.1. Spatial Distribution of Potential Hotspots for the Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios
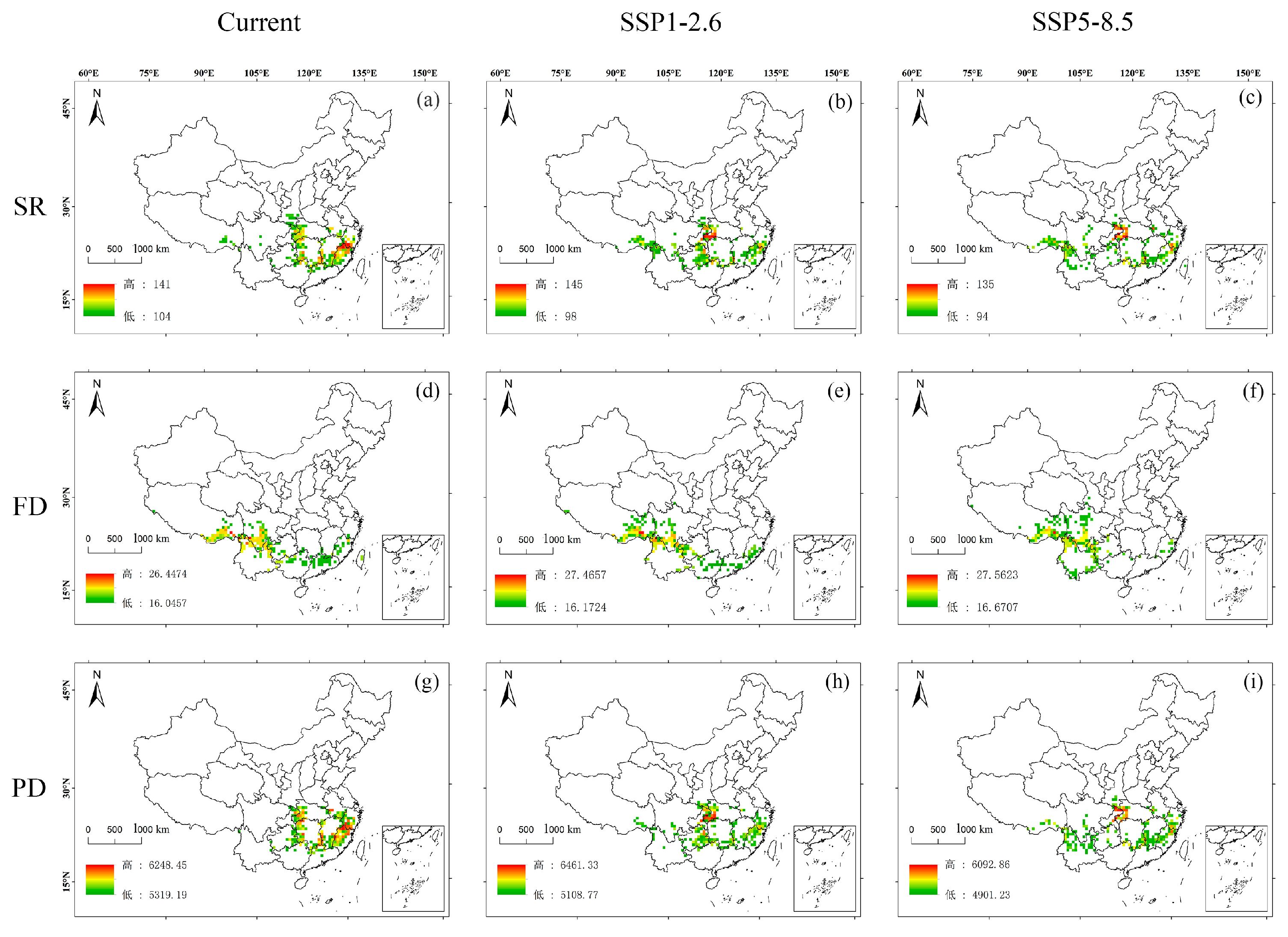
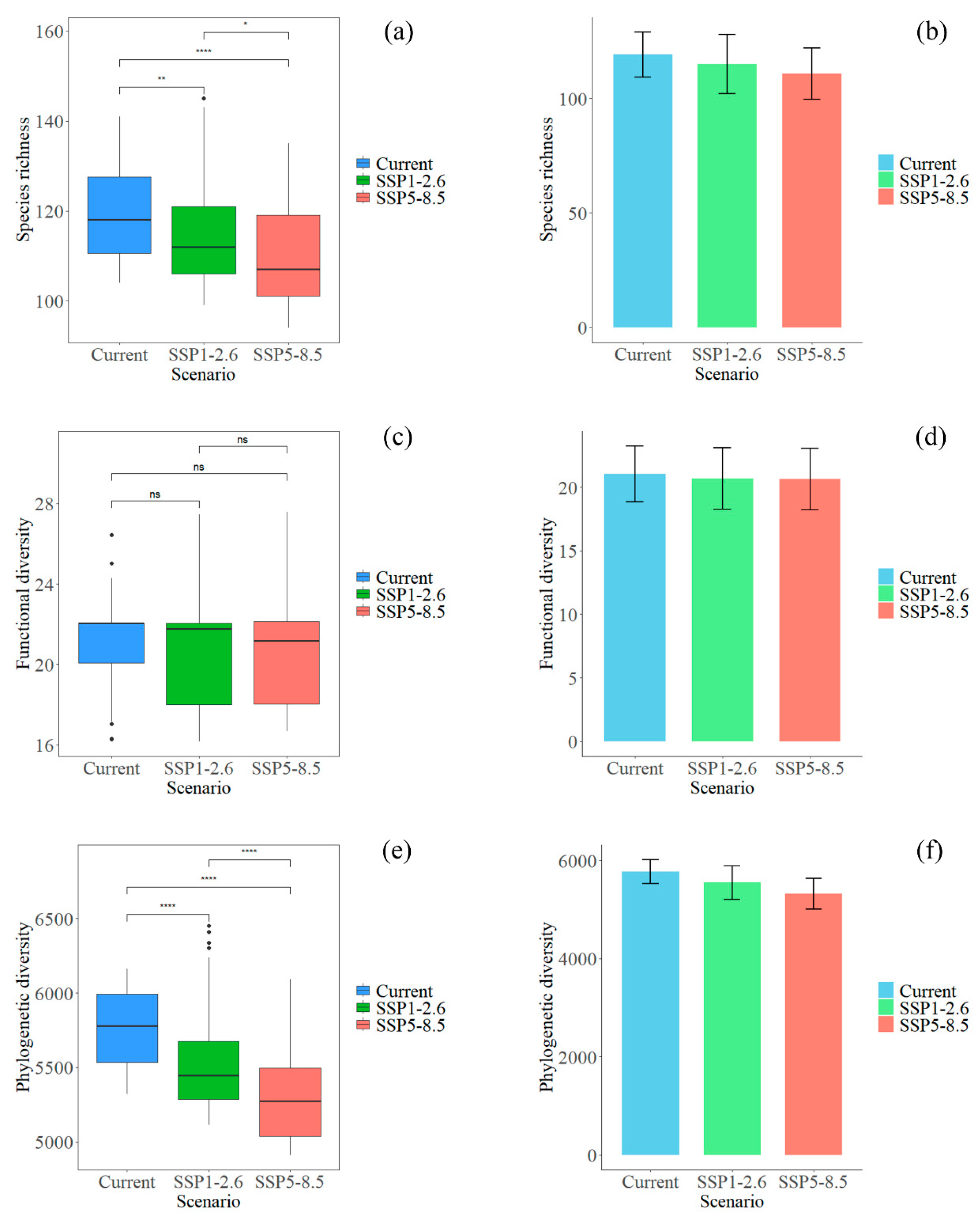
3.2. Spatial Distribution of Plant Diversity in Potential Hotspots for the Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios
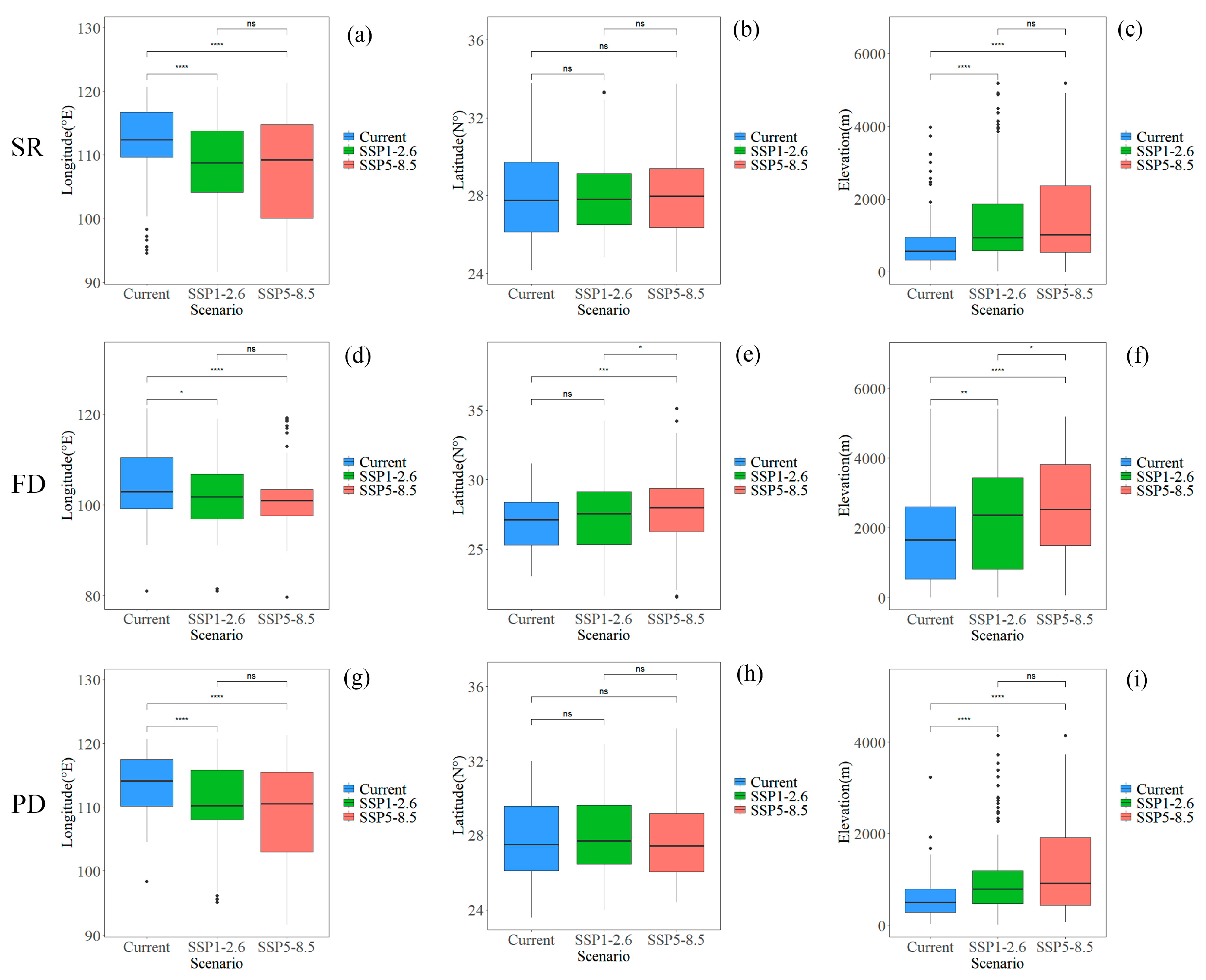
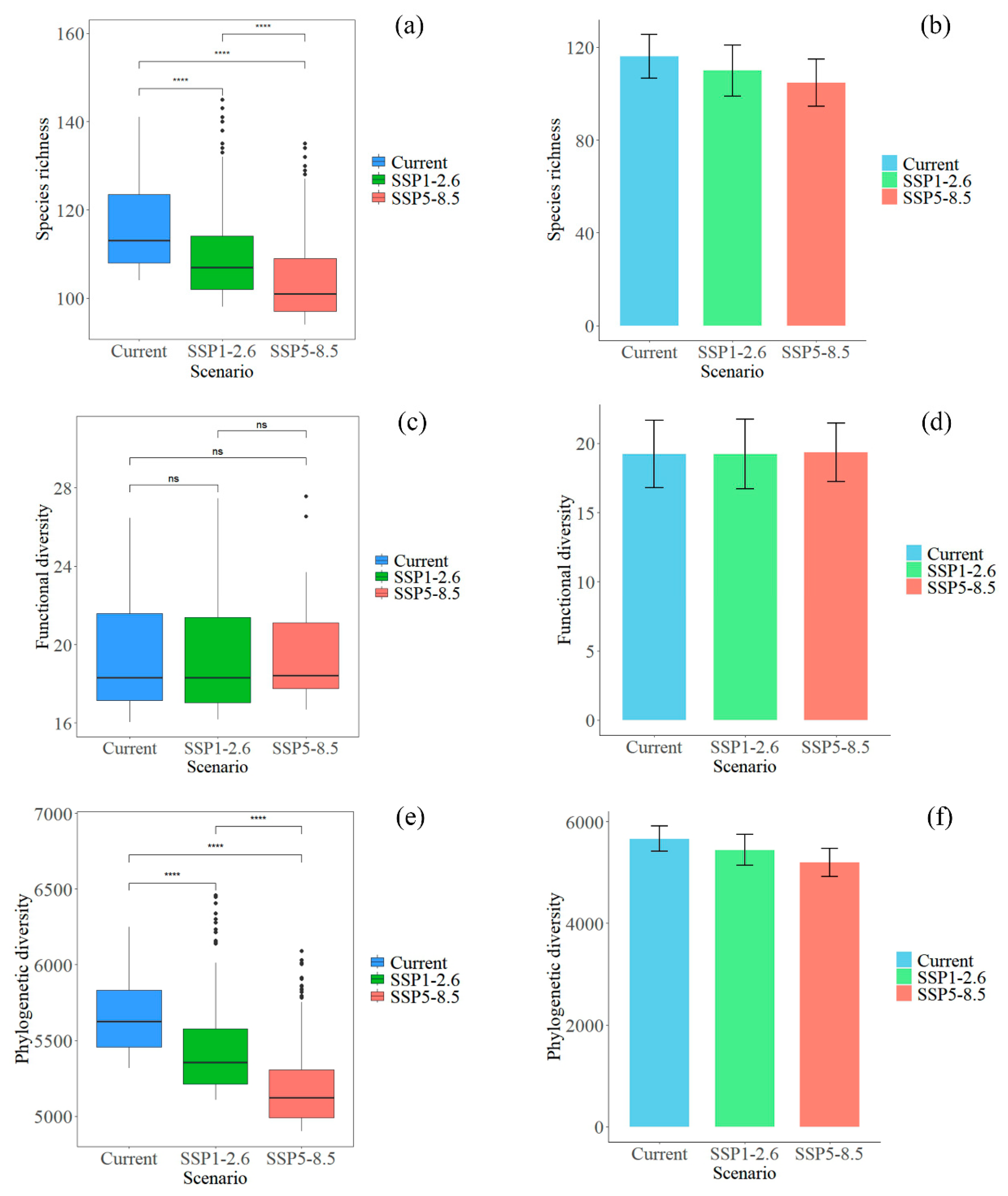
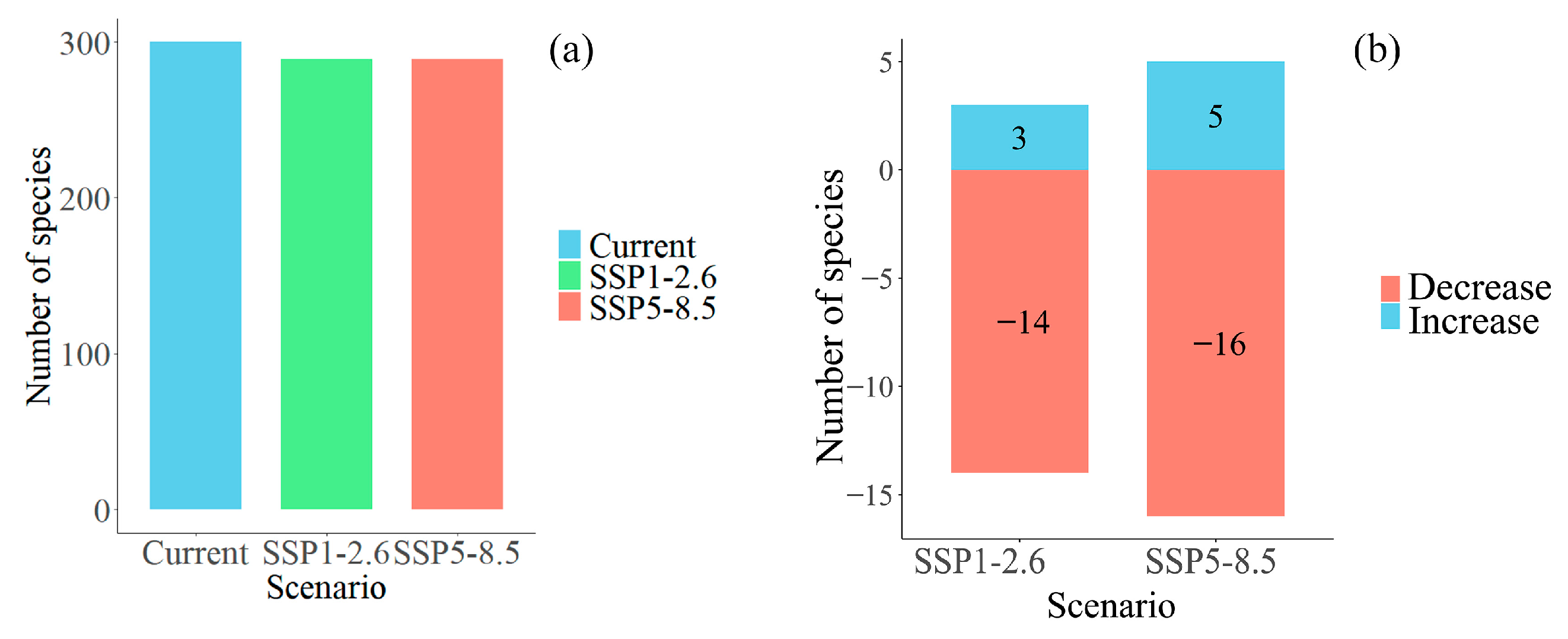
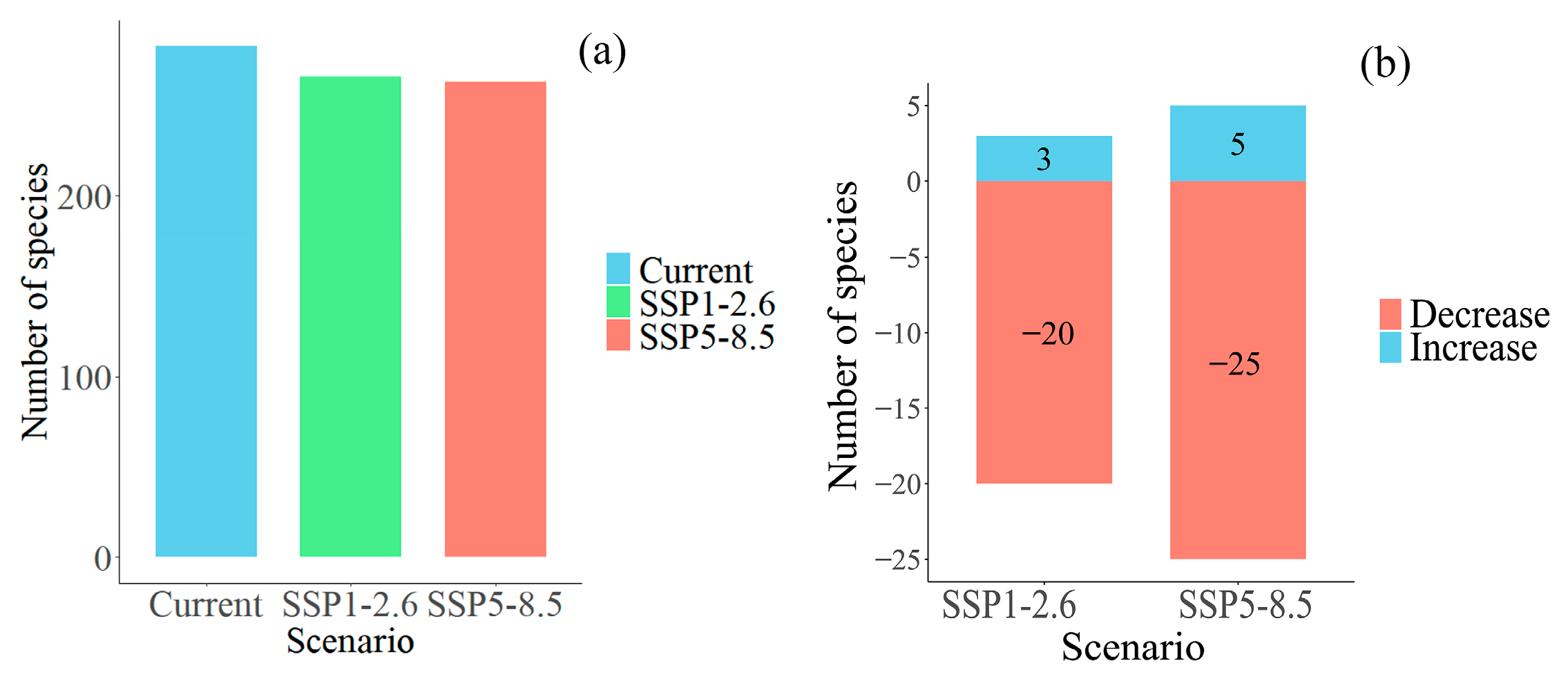
3.3. Changes in Plant Diversity in Potential Shared Hotspots for the Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios
4. Discussion
4.1. Potential Impacts of Climate and Land Use Changes on the Spatial Distribution of Hotspots for the Chinese Endemic Tree Plant Diversity
4.2. Potential Impacts of Climate and Land Use Changes on the Plant Diversity of Hotspots for the Chinese Endemic Tree Plant Diversity
4.3. Implications for Biodiversity Conservation in China
4.4. Uncertainties of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manning, P.; Van Der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Osborn, D.; Newbold, T.; Adams, G.L.; Albaladejo Robles, G.; Boakes, E.H.; Braga Ferreira, G.; Chapman, A.S.; Etard, A.; Gibb, R.; Millard, J.; et al. Climate and land-use change homogenise terrestrial biodiversity, with consequences for ecosystem functioning and human well-being. Emerg. Top. Life Sci. 2019, 3, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.N. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Zhu, F.; Li, X. Climate change may cause distribution area loss for tree species in southern China. For. Ecol. Manag. 2022, 511, 120134. [Google Scholar] [CrossRef]
- Li, K.J.; Liu, X.F.; Zhang, J.H.; Zhou, X.L.; Yang, L.; Shen, S.K. Complexity responses of Rhododendron species to climate change in China reveal their urgent need for protection. For. Ecosyst. 2023, 10, 100124. [Google Scholar] [CrossRef]
- Luo, W.; Sun, C.; Yang, S.; Chen, W.; Sun, Y.; Li, Z.; Liu, J.; Tao, W.; Tao, J. Contrasting range changes and drivers of four forest foundation species under future climate change in China. Sci. Total Environ. 2024, 942, 173784. [Google Scholar] [PubMed]
- Aguilar, R.; Cristóbal-Pérez, E.J.; Balvino-Olvera, F.J.; De Jesús Aguilar-Aguilar, M.; Aguirre-Acosta, N.; Ashworth, L.; Lobo, J.A.; Martén-Rodríguez, S.; Fuchs, E.J.; Sanchez-Montoya, G. Habitat fragmentation reduces plant progeny quality: A global synthesis. Ecol. Lett. 2019, 22, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Metzger, R.; Rollo, A.; Myers, R.A. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc. R. Soc. B Biol. Sci. 2007, 274, 1023–1028. [Google Scholar] [CrossRef]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [PubMed]
- Keith, D.A.; Akçakaya, H.R.; Thuiller, W.; Midgley, G.F.; Pearson, R.G.; Phillips, S.J.; Regan, H.M.; Araújo, M.B.; Rebelo, T.G. Predicting extinction risks under climate change: Coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 2008, 4, 560–563. [Google Scholar]
- Habibullah, M.S.; Din, B.H.; Tan, S.H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.S.; Rasmussen, P.C.; Ridgely, R.S. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar]
- Vane-Wright, R.I.; Humphries, C.J.; Williams, P.H. What to protect?—Systematics and the agony of choice. Biol. Conserv. 1991, 55, 235–254. [Google Scholar]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Ying, L.; Wang, Z.; Huang, J.; Zang, R.; Jiang, Y. Hotspot analyses indicate significant conservation gaps for evergreen broadleaved woody plants in China. Sci. Rep. 2017, 7, 1859. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Skidmore, A.K.; Wang, T.; Huang, J.; Ma, K.; Groen, T.A. Rhododendron diversity patterns and priority conservation areas in China. Divers. Distrib. 2017, 23, 1143–1156. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Fu, J.; Chi, X.; Du, W.; Dimitrov, D.; Liu, J.; Xi, Z.; Wu, J.; Xu, X. Diversity patterns and conservation gaps of Magnoliaceae species in China. Sci. Total Environ. 2022, 813, 152665. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, X.; Zhang, Y.; Wang, Y.; Zhou, Z. Species richness, endemism, and conservation of wild Rhododendron in China. Glob. Ecol. Conserv. 2023, 41, e02375. [Google Scholar]
- Oliver, T.H.; Isaac, N.J.; August, T.A.; Woodcock, B.A.; Roy, D.B.; Bullock, J.M. Declining resilience of ecosystem functions under biodiversity loss. Nat. Commun. 2015, 6, 10122. [Google Scholar]
- Barrett, M.A.; Brown, J.L.; Junge, R.E.; Yoder, A.D. Climate change, predictive modeling and lemur health: Assessing impacts of changing climate on health and conservation in Madagascar. Biol. Conserv. 2013, 157, 409–422. [Google Scholar] [CrossRef]
- Tu, Y.; Yao, Z.; Guo, J.; Yang, L.; Zhu, Y.; Yang, X.; Shi, Z.; Indree, T. Predicting the potential risk of Caragana shrub encroachment in the Eurasian steppe under anthropogenic climate change. Sci. Total Environ. 2024, 944, 173925. [Google Scholar]
- Yu, F.; Wu, Z.; Shen, J.; Huang, J.; Groen, T.A.; Skidmore, A.K.; Ma, K.; Wang, T. Low-elevation endemic Rhododendrons in China are highly vulnerable to climate and land use change. Ecol. Indic. 2021, 126, 107699. [Google Scholar] [CrossRef]
- Li, W.B.; Teng, Y.; Zhang, M.Y.; Shen, Y.; Liu, J.W.; Qi, J.W.; Wang, X.C.; Wu, R.F.; Li, J.H.; Garber, P.A. Human activity and climate change accelerate the extinction risk to non-human primates in China. Glob. Change Biol. 2024, 30, e17114. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araujo, M.B.; Martinez-Meyer; Brotons, L.; McClean, C.; Miles, L.S. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar]
- Ghehsareh Ardestani, E.; Rigi, H.; Honarbakhsh, A. Predicting optimal habitats of Haloxylon persicum for ecosystem restoration using ensemble ecological niche modeling under climate change in southeast Iran. Restor. Ecol. 2021, 29, e13492. [Google Scholar]
- Moraitis, M.L.; Valavanis, V.D.; Karakassis, I. Modelling the effects of climate change on the distribution of benthic indicator species in the Eastern Mediterranean Sea. Sci. Total Environ. 2019, 667, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Goettsch Mittermeier, C. Megadiversity: Earth’s Biologically Wealthiest Nations; CEMEX: New York, NY, USA, 1997. [Google Scholar]
- Peng, S.; Zhang, J.; Zhang, X.; Li, Y.; Liu, Y.; Wang, Z. Conservation of woody species in China under future climate and land-cover changes. J. Appl. Ecol. 2022, 59, 141–152. [Google Scholar]
- Pires, M.B.; Kougioumoutzis, K.; Norder, S.; Dimopoulos, P.; Strid, A.; Panitsa, M. The future of plant diversity within a Mediterranean endemism centre: Modelling the synergistic effects of climate and land-use change in Peloponnese, Greece. Sci. Total Environ. 2024, 947, 174622. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Chen, J.H.; Ying, J.S.; Ma, K.P. Features and distribution patterns of Chinese endemic seed plant species. J. Syst. Evol. 2011, 49, 81–94. [Google Scholar]
- Wang, Q.; Huang, J.H.; Zang, R.G.; Li, Z.P.; El, K.; Yousry, A. Centres of neo-and paleo-endemism for Chinese woody flora and their environmental features. Biol. Conserv. 2022, 276, 109817. [Google Scholar]
- Peng, S.J.; Hu, R.C.; Velazco, S.J.E.; Luo, Y.; Lyu, T.; Zhang, X.L.; Zhang, J.; Wang, Z.H. Preserving the woody plant tree of life in China under future climate and land-cover changes. Proc. R. Soc. B 2022, 289, 20221497. [Google Scholar]
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M.R. Global patterns in plant height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Echeverría-Londoño, S.; Enquist, B.J.; Neves, D.M.; Violle, C.; Boyle, B.; Kraft, N.J.B.; Maitner, B.S.; McGill, B.; Peet, R.K.; Sandel, B.; et al. Plant Functional Diversity and the Biogeography of Biomes in North and South America. Front. Ecol. Evol. 2018, 6, 219. [Google Scholar]
- Zhang, T.Y.; Cheng, C.X.; Wu, X.D. Mapping the spatial heterogeneity of global land use and land cover from 2020 to 2100 at a 1 km resolution. Sci. Data 2023, 10, 748. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Smith, S.A.; Brown, J.W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 2018, 105, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, W.; Chase, M.W.; Christenhusz, M.J.; Maurin, O.; Forest, F.; Van Wyk, A.E.P. From the frying pan: An unusual dwarf shrub from Namibia turns out to be a new brassicalean family. Phytotaxa 2020, 439, 171–185. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD–a platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Davies, T.J.; Cadotte, M.W. Quantifying biodiversity: Does it matter what we measure? In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. [Google Scholar]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Zhong, X.; Qiu, B.; Liu, X. Functional diversity patterns of macrofauna in the adjacent waters of the Yangtze River Estuary. Mar. Pollut. Bull. 2020, 154, 111032. [Google Scholar] [PubMed]
- Yu, F.; Wang, T.; Groen, T.A.; Skidmore, A.K.; Yang, X.; Ma, K.; Wu, Z. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019, 659, 515–528. [Google Scholar] [PubMed]
- Wu, Y.; Dubay, S.G.; Colwell, R.K.; Ran, J.; Lei, F. Mobile hotspots and refugia of avian diversity in the mountains of south-west China under past and contemporary global climate change. J. Biogeogr. 2017, 44, 615–626. [Google Scholar]
- Ma, Y.; Mao, X.; Wang, J.; Zhang, L.; Jiang, Y.; Geng, Y.; Ma, T.; Cai, L.; Huang, S.; Hollingsworth, P. Pervasive hybridization during evolutionary radiation of Rhododendron subgenus Hymenanthes in mountains of southwest China. Natl. Sci. Rev. 2022, 9, nwac276. [Google Scholar]
- Mo, Z.Q.; Fu, C.N.; Zhu, M.S.; Milne, R.I.; Yang, J.B.; Cai, J.; Qin, H.T.; Zheng, W.; Hollingsworth, P.M.; Li, D.Z. Resolution, conflict and rate shifts: Insights from a densely sampled plastome phylogeny for Rhododendron (Ericaceae). Ann. Bot. 2022, 130, 687–701. [Google Scholar] [CrossRef]
- Brighenti, S.; Hotaling, S.; Finn, D.S.; Fountain, A.G.; Hayashi, M.; Herbst, D.; Saros, J.E.; Tronstad, L.M.; Millar, C.I. Rock glaciers and related cold rocky landforms: Overlooked climate refugia for mountain biodiversity. Glob. Change Biol. 2021, 27, 1504–1517. [Google Scholar]
- He, X.; Burgess, K.S.; Yang, X.F.; Ahrends, A.; Gao, L.M.; Li, D.Z. Upward elevation and northwest range shifts for alpine Meconopsis species in the Himalaya–Hengduan Mountains region. Ecol. Evol. 2019, 9, 4055–4064. [Google Scholar]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Xiang, W.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar]
- Moradi, H.; Noroozi, J.; Fourcade, Y. Plant endemic diversity in the Irano-Anatolian global biodiversity hotspot is dramatically threatened by future climate change. Biol. Conserv. 2025, 302, 110963. [Google Scholar]
- Rodrigues, A.S.; Gaston, K.J. Maximising phylogenetic diversity in the selection of networks of conservation areas. Biol. Conserv. 2002, 105, 103–111. [Google Scholar] [CrossRef]
- Cai, H.; Lyu, L.; Shrestha, N.; Tang, Z.; Su, X.; Xu, X.; Dimitrov, D.; Wang, Z. Geographical patterns in phylogenetic diversity of Chinese woody plants and its application for conservation planning. Divers. Distrib. 2021, 27, 179–194. [Google Scholar] [CrossRef]
- Mishler, B.D.; Knerr, N.; González-Orozco, C.E.; Thornhill, A.H.; Laffan, S.W.; Miller, J.T. Phylogenetic measures of biodiversity and neo-and paleo-endemism in Australian Acacia. Nat. Commun. 2014, 5, 4473. [Google Scholar] [CrossRef]
- Luo, A.; Li, Y.; Shrestha, N.; Xu, X.; Su, X.; Li, Y.; Lyu, T.; Waris, K.; Tang, Z.; Liu, X. Global multifaceted biodiversity patterns, centers, and conservation needs in angiosperms. Sci. China Life Sci. 2024, 67, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Zang, R.; Ding, Y. Functional diversity increases with species diversity along successional gradient in a secondary tropical lowland rainforest. Trop. Ecol. 2014, 55, 393–401. [Google Scholar]
- Qin, H.; Wang, Y.; Zhang, F.; Chen, J.; Zhang, G.; Dong, G. Application of species, phylogenetic and functional diversity to the evaluation on the effects of ecological restoration on biodiversity. Ecol. Inform. 2016, 32, 53–62. [Google Scholar]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; Van Der Bank, M. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar]
- Ricotta, C. A note on functional diversity measures. Basic Appl. Ecol. 2005, 6, 479–486. [Google Scholar]
- Mantyka-Pringle, C.S.; Visconti, P.; Di Marco, M.; Martin, T.G.; Rondinini, C.; Rhodes, J.R. Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 2015, 187, 103–111. [Google Scholar] [CrossRef]
- Hu, J.L.; Ci, X.Q.; Zhang, X.Y.; Zhou, R.; Xiao, J.H.; Liu, Z.F.; Zhang, C.Y.; Jin, X.; Li, J. Assessment of multidimensional diversity and conservation of threatened timber trees in China under climate change. Biol. Conserv. 2024, 300, 110871. [Google Scholar] [CrossRef]
- Mazel, F.; Guilhaumon, F.; Mouquet, N.; Devictor, V.; Gravel, D.; Renaud, J.; Cianciaruso, M.V.; Loyola, R.; Diniz-Filho, J.A.F.; Mouillot, D. Multifaceted diversity–area relationships reveal global hotspots of mammalian species, trait and lineage diversity. Glob. Ecol. Biogeogr. 2014, 23, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Pillet, M.; Goettsch, B.; Merow, C.; Maitner, B.; Feng, X.; Roehrdanz, P.R.; Enquist, B. Elevated extinction risk of cacti under climate change. Nat. Plants 2022, 8, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Moat, J.; Gole, T.W.; Davis, A.P. Least concern to endangered: Applying climate change projections profoundly influences the extinction risk assessment for wild Arabica coffee. Glob. Change Biol. 2019, 25, 390–403. [Google Scholar] [CrossRef]
- Peng, S.; Shrestha, N.; Luo, Y.; Li, Y.; Cai, H.; Qin, H.; Ma, K.; Wang, Z. Incorporating global change reveals extinction risk beyond the curfent Red List. Curr. Biol. 2023, 33, 3669–3678.e4. [Google Scholar] [CrossRef]
- Asamoah, E.F.; Beaumont, L.J.; Maina, J.M. Climate and land-use changes reduce the benefits of terrestrial protected areas. Nat. Clim. Change 2021, 11, 1105–1110. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Li, B.V. How to protect half of Earth to ensure it protects sufficient biodiversity. Sci. Adv. 2018, 4, eaat2616. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Acevedo, M.A.; Beaudrot, L.; Meléndez-Ackerman, E.J.; Tremblay, R.L. Local extinction risk under climate change in a neotropical asymmetrically dispersed epiphyte. J. Ecol. 2020, 108, 1553–1564. [Google Scholar] [CrossRef]
| Index | Current | SSP1-2.6 | SSP5-8.5 | |||
|---|---|---|---|---|---|---|
| Number | Fragmentation Index | Number | Fragmentation Index | Number | Fragmentation Index | |
| SR | 15 | 0.032 | 18 | 0.036 | 25 | 0.042 |
| FD | 21 | 0.037 | 18 | 0.038 | 22 | 0.038 |
| PD | 10 | 0.028 | 20 | 0.038 | 19 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Xu, S.; Dong, S.; Yu, F.; Huang, J.; Xu, Y.; Yao, J.; Ding, Y.; Zang, R. Hotspots of Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios. Forests 2025, 16, 599. https://doi.org/10.3390/f16040599
Cao Z, Xu S, Dong S, Yu F, Huang J, Xu Y, Yao J, Ding Y, Zang R. Hotspots of Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios. Forests. 2025; 16(4):599. https://doi.org/10.3390/f16040599
Chicago/Turabian StyleCao, Zhe, Shuyi Xu, Shuixing Dong, Fangyuan Yu, Jihong Huang, Yue Xu, Jie Yao, Yi Ding, and Runguo Zang. 2025. "Hotspots of Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios" Forests 16, no. 4: 599. https://doi.org/10.3390/f16040599
APA StyleCao, Z., Xu, S., Dong, S., Yu, F., Huang, J., Xu, Y., Yao, J., Ding, Y., & Zang, R. (2025). Hotspots of Chinese Endemic Tree Plant Diversity Under Different Climate and Land Use Scenarios. Forests, 16(4), 599. https://doi.org/10.3390/f16040599







