Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Biomass C Sampling and Analysis
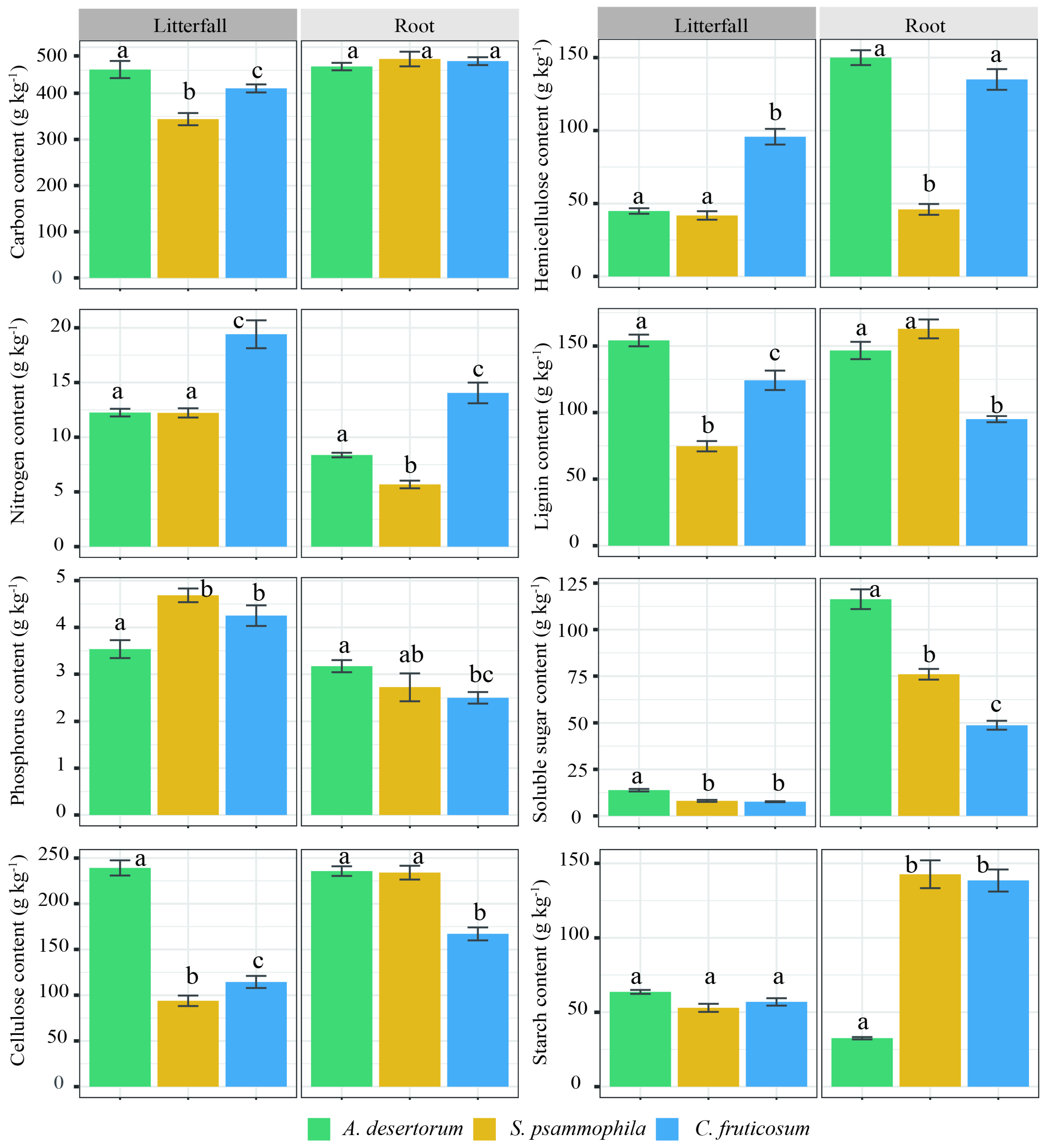
2.3. Chemical Composition of Litterfall and Fine Root
2.4. Soil Sampling and Analysis
2.5. Statistical Analysis
3. Results
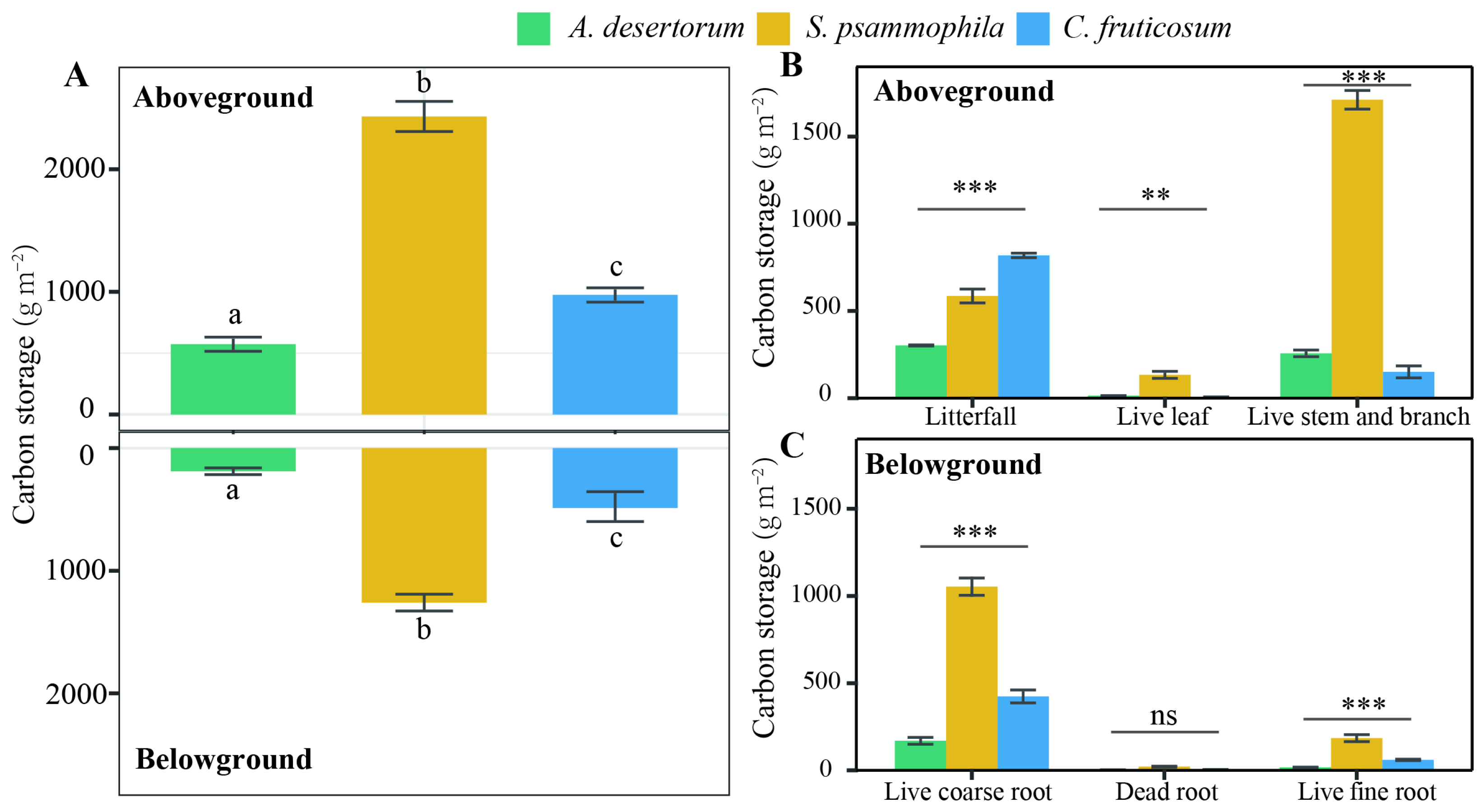
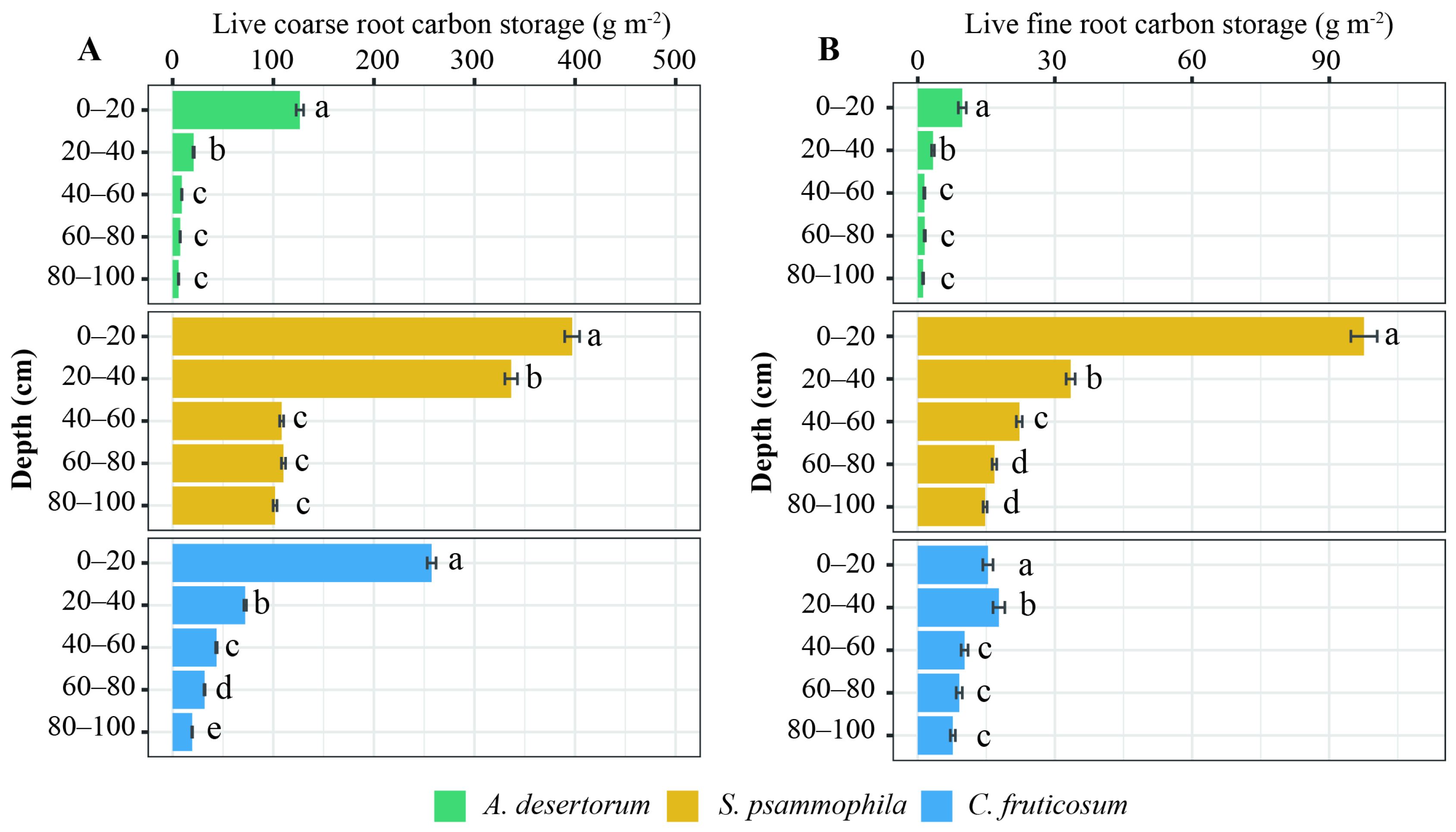
3.1. Biomass C Storage
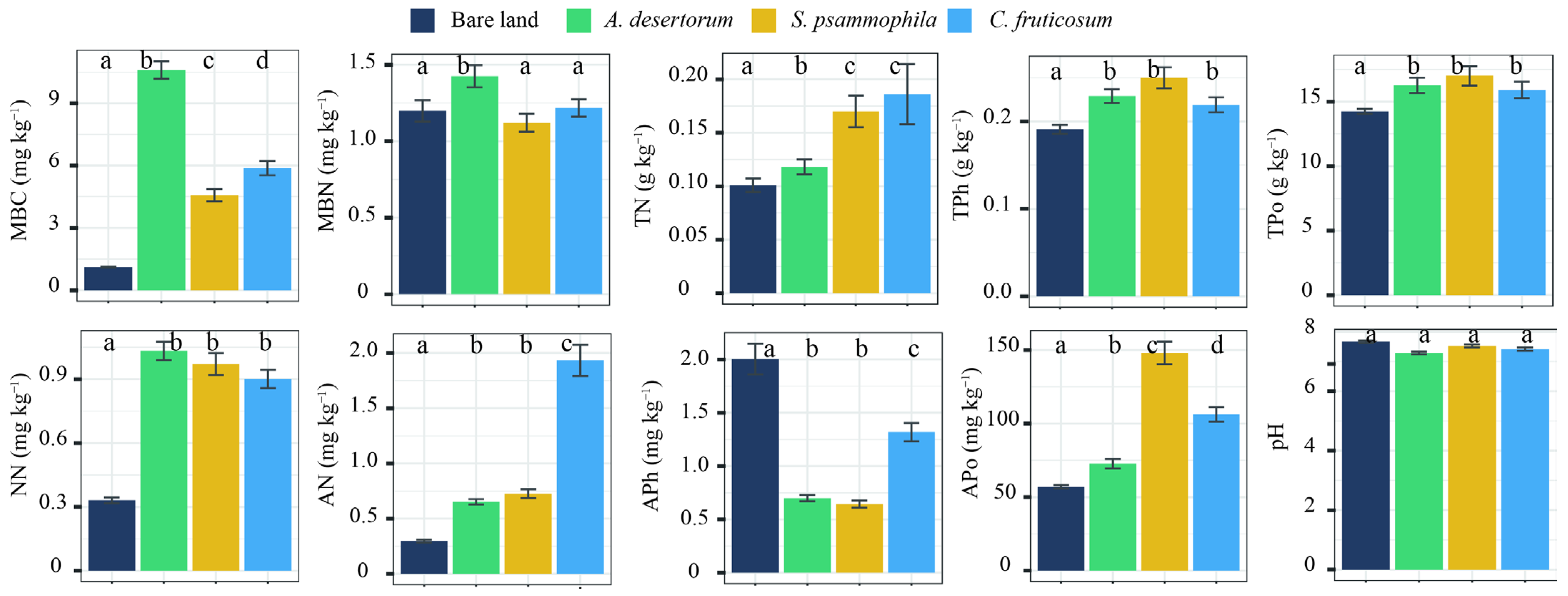
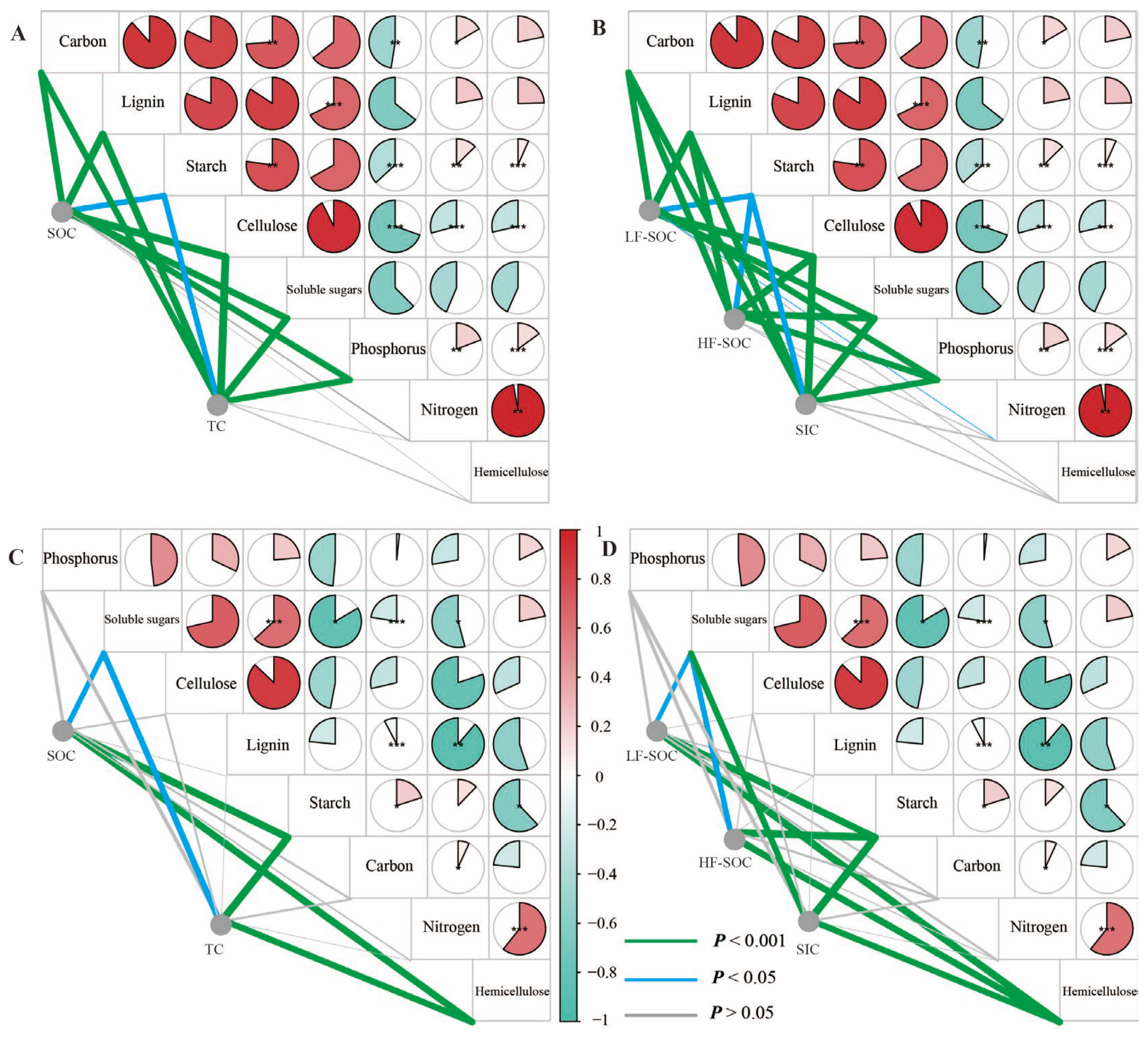
3.2. Soil C Storage and Distribution of Three Revegetated Shrubs
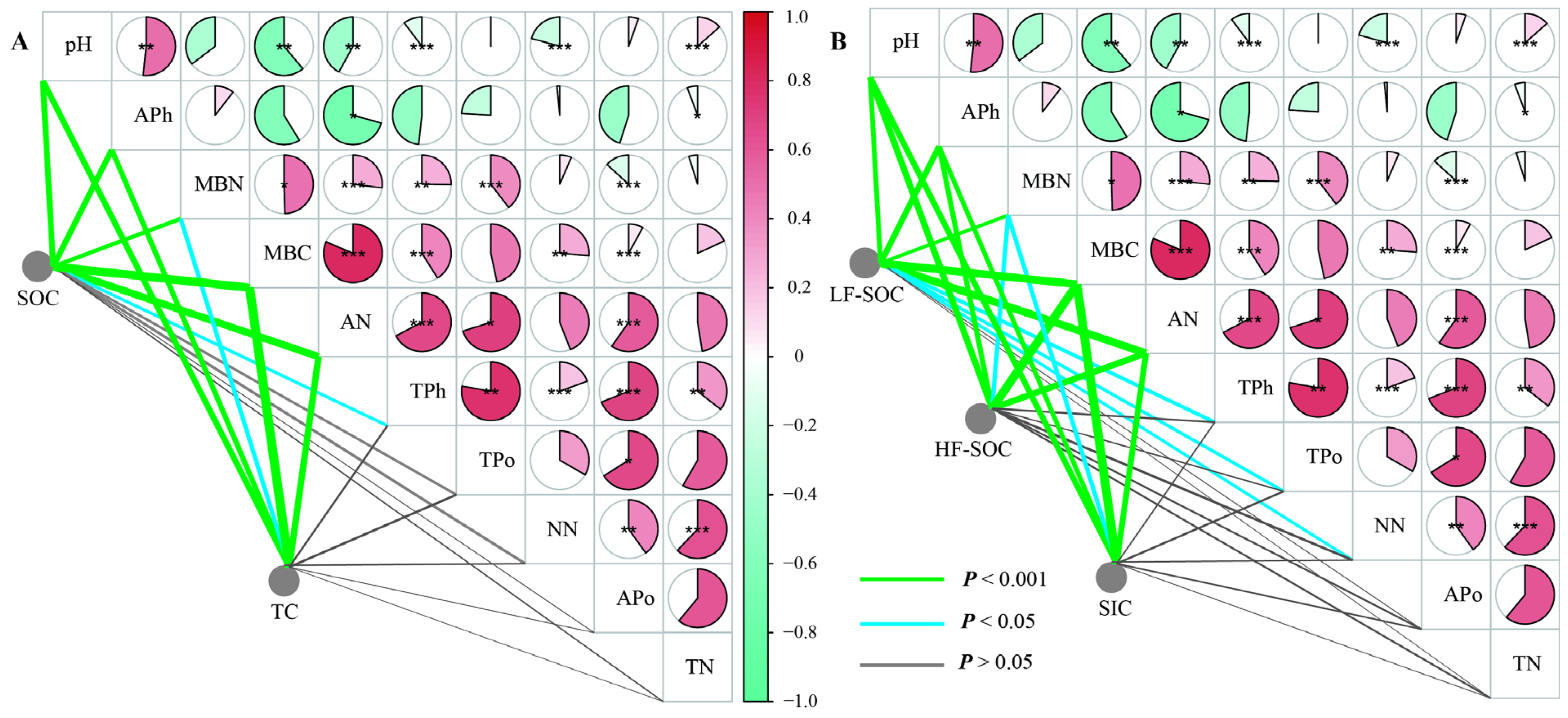
3.3. Correlation Between Carbon Storage and Soil Properties
4. Discussion
4.1. Biomass and Soil C Storage in Revegetated Shrublands
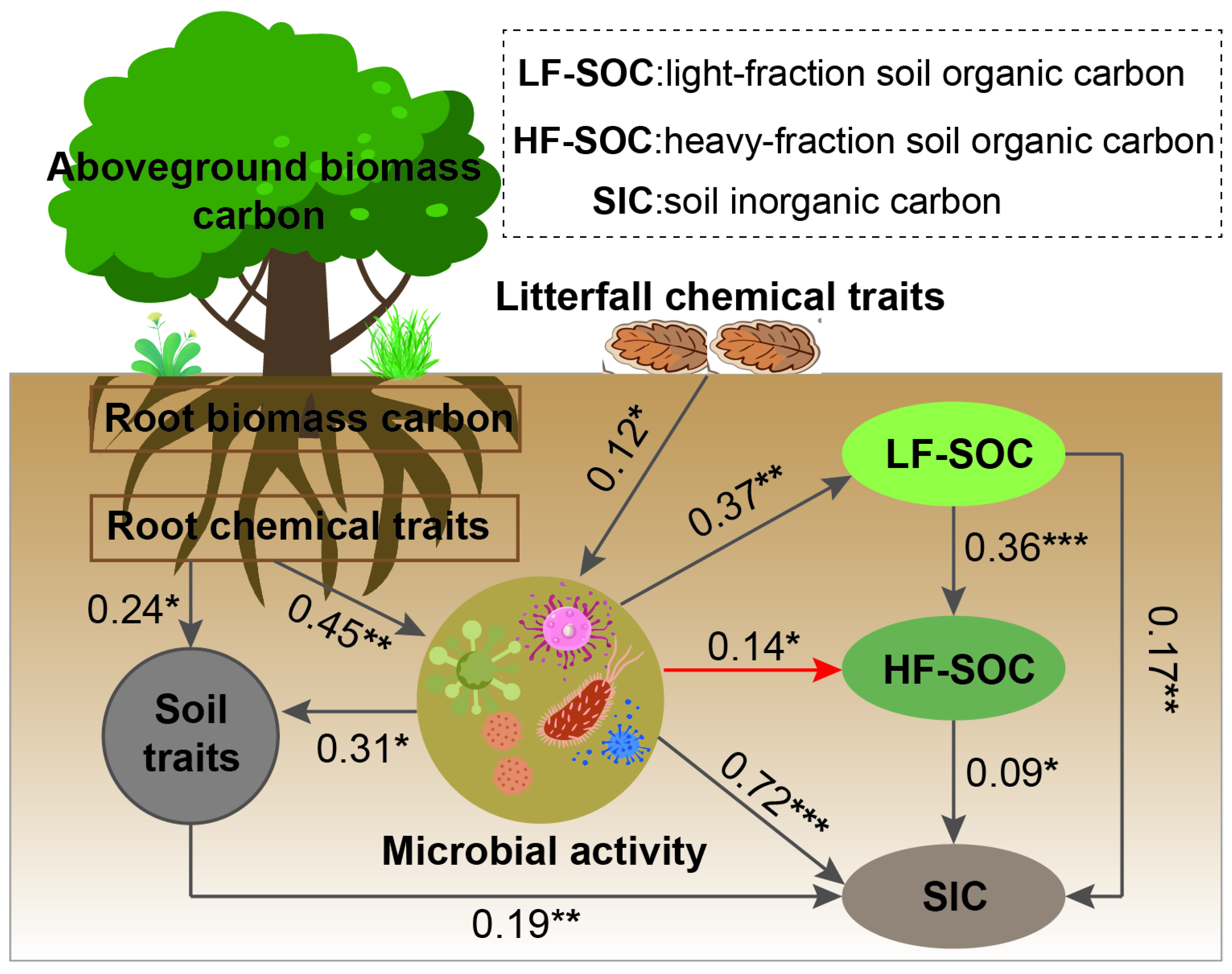
4.2. Factors Influencing Soil C in Revegetated Shrublands
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Carbon cycling in global drylands. Curr. Clim. Change Rep. 2019, 5, 221–232. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; Core Writing Team, IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Liang, S.; Liang, L.; Wang, D.; Zeng, Z. Dryland forestation: Uncovering the carbon sequestration potential. Innov. Geosci. 2024, 2, 100058. [Google Scholar] [CrossRef]
- Ren, Z.; Li, C.; Fu, B.; Wang, S.; Stringer, L.C. Effects of aridification on soil total carbon pools in China’s drylands. Glob. Change Biol. 2023, 30, e17091. [Google Scholar] [CrossRef]
- Vojdani, A.; Baur, L.E.; Rudgers, J.A.; Collins, S.L. Sensitivity of root production to long-term aridity under environmental perturbations in Chihuahuan Desert ecosystems. J. Ecol. 2024, 112, 1487–1500. [Google Scholar] [CrossRef]
- Rohatyn, S.; Yakir, D.; Rotenberg, E.; Carmel, Y. Limited climate change mitigation potential through forestation of the vast dryland regions. Science 2022, 377, 1436–1439. [Google Scholar] [CrossRef]
- Song, J.; Wan, S.; Zhang, K.; Hong, S.; Xia, J.; Piao, S.; Wang, Y.-P.; Chen, J.; Hui, D.; Luo, Y.; et al. Ecological restoration enhances dryland carbon stock by reducing surface soil carbon loss due to wind erosion. Proc. Natl. Acad. Sci. USA 2024, 121, e2416281121. [Google Scholar] [CrossRef]
- Yao, L.; Liu, T.; Qin, J.; Jiang, H.; Yang, L.; Smith, P.; Chen, X.; Zhou, C.; Piao, S. Carbon sequestration potential of tree planting in China. Nat. Commun. 2024, 15, 8398. [Google Scholar] [CrossRef]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world–challenges and progress: Introduction to a Virtual Issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Rog, I.; Hilman, B.; Fox, H.; Yalin, D.; Qubaja, R.; Klein, T. Increased belowground tree carbon allocation in a mature mixed forest in a dry versus a wet year. Glob. Change Biol. 2024, 30, e17172. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Gessler, A.; Zweifel, R. Beyond source and sink control–toward an integrated approach to understand the carbon balance in plants. New Phytol. 2024, 242, 858–869. [Google Scholar] [CrossRef]
- Ma, H.; Mo, L.; Crowther, T.W.; Maynard, D.S.; Hoogen, J.V.D.; Stocker, B.D.; Terrer, C.; Zohner, C.M. The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat. Ecol. Evol. 2021, 5, 1110–1122. [Google Scholar] [CrossRef]
- Plaza, C.; Zaccone, C.; Sawicka, K.; Méndez, A.M.; Tarquis, A.; Gascó, G.; Heuvelink, G.B.M.; Schuur, E.A.G.; Maestre, F.T. Soil resources and element stocks in drylands to face global issues. Sci. Rep. 2018, 8, 13788. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Ridgeway, J.R.; Morrissey, E.M.; Brzostek, E.R. Plant litter traits control microbial decomposition and drive soil carbon stabilization. Soil Biol. Biochem. 2022, 175, 108857. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, H.; Zhang, J.; Li, X.; Wang, B.; Miao, H.; Sial, T.A.; Dong, Q.; Fu, G.; Li, L. Long-term vegetation restoration increases carbon sequestration of different soil particles in a semi-arid desert. Ecosphere 2021, 12, e03848. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chen, S.-Y.; Chen, J.-W.; Xue, K.; Chen, S.-L.; Wang, X.-M.; Chen, T.; Kang, S.-C.; Rui, J.-P.; Thies, J.E.; et al. Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025321118. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.B.; Fuentes-Alburquenque, S.; Díez, B.; Vargas, I.; Bonilla, C.A. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 107692. [Google Scholar] [CrossRef]
- Lai, Z.R.; Zhang, Y.Q.; Liu, J.B.; Wu, B.; Qin, S.G.; Fa, K.Y. Fine-root distribution, production, decomposition, and effect on soil organic carbon of three revegetation shrub species in northwest China. For. Ecol. Manag. 2016, 359, 381–388. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, Y.; Lai, Z.; She, W.; Bai, Y.; Feng, W.; Qin, S. Microbial communities and their genetic repertoire mediate the decomposition of soil organic carbon pools in revegetation shrublands in a desert in northern China. Eur. J. Soil Sci. 2020, 71, 93–105. [Google Scholar] [CrossRef]
- Guillemot, J.; Kunz, M.; Schnabel, F.; Fichtner, A.; Madsen, C.P.; Gebauer, T.; Härdtle, W.; von Oheimb, G.; Potvin, C. Neighbourhood-mediated shifts in tree biomass allocation drive overyielding in tropical species mixtures. New Phytol. 2020, 228, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Thomas, B.W.; Powlson, D.; Hu, Y.; Zhang, Y.; Jun, X.; Shi, X.; Zhang, Y. Soil organic carbon fractions in response to soil, environmental and agronomic factors under cover cropping systems: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 355, 108591. [Google Scholar] [CrossRef]
- Huang, L.; Gao, Y.; Wang, D.; Cui, X.; Zhang, H.; Yuan, J.; Gao, M. Natural grassland restoration exhibits enhanced carbon sequestration and soil improvement potential in northern sandy grasslands of China: An empirical study. Catena 2024, 246, 108396. [Google Scholar] [CrossRef]
- Chai, Y.; Zhong, J.; Zhao, J.; Guo, J.; Yue, M.; Guo, Y.; Wang, M.; Wan, P. Environment and plant traits explain shrub biomass allocation and species composition across ecoregions in North China. J. Veg. Sci. 2021, 32, e13080. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Wiesmeier, M.; Goberna, M.; Martínez-Mena, M.; Albaladejo, J. Carbon dynamics after afforestation of semiarid shrublands: Implications of site preparation techniques. For. Ecol. Manag. 2014, 319, 107–115. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Z.; Feng, L.; He, L.; Yang, K. Dynamics of biomass and carbon sequestration across a chronosequence of Caragana intermedia plantations on alpine sandy land. Sci. Rep. 2018, 8, 12432. [Google Scholar] [CrossRef]
- Asefa, M.; Worthy, S.J.; Cao, M.; Song, X.; Lozano, Y.M.; Yang, J. Above-and below-ground plant traits are not consistent in response to drought and competition treatments. Ann. Bot. 2022, 130, 939–950. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Bach, E.M.; Guan, P.; Sala, O.E.; Wall, D.H. Root herbivory controls the effects of water availability on the partitioning between above-and below-ground grass biomass. Funct. Ecol. 2020, 34, 2403–2410. [Google Scholar] [CrossRef]
- Rytter, R.M. The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef]
- Roach, D.A.; Smith, E.F. Life-history trade-offs and senescence in plants. Funct. Ecol. 2020, 34, 17–25. [Google Scholar] [CrossRef]
- Mahood, A.L.; Jones, R.O.; Board, D.I.; Balch, J.K.; Chambers, J.C. Interannual climate variability mediates changes in carbon and nitrogen pools caused by annual grass invasion in a semiarid shrubland. Glob. Change Biol. 2022, 28, 267–284. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, W.; MacBean, N.; Rulli, M.C.; Manzoni, S.; Vico, G.; D’odorico, P. Dryland productivity under a changing climate. Nat. Clim. Change 2022, 12, 981–994. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, B.; Wu, X.; Wang, Z.; Liu, L.; Yang, H. Revegetation promotes soil mineral-associated organic carbon sequestration and soil carbon stability in the Tengger Desert, northern China. Soil Biol. Biochem. 2023, 185, 109155. [Google Scholar] [CrossRef]
- Maschler, J.; Bialic-Murphy, L.; Wan, J.; Andresen, L.C.; Zohner, C.M.; Reich, P.B.; Lüscher, A.; Schneider, M.K.; Müller, C.; Moser, G.; et al. Links across ecological scales: Plant biomass responses to elevated CO2. Glob. Change Biol. 2022, 28, 6115–6134. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Munson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Xie, T.; Chang, Z.; Li, X. Recovery of soil carbon and nitrogen stocks following afforestation with xerophytic shrubs in the Tengger Desert, North China. Catena 2022, 214, 106277. [Google Scholar] [CrossRef]
- Hong, S.; Yin, G.; Piao, S.; Dybzinski, R.; Cong, N.; Li, X.; Wang, K.; Peñuelas, J.; Zeng, H.; Chen, A. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Ge, J.; Xu, W.; Liu, Q.; Tang, Z.; Xie, Z. Patterns and environmental controls of soil organic carbon density in Chinese shrublands. Geoderma 2020, 363, 114161. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Dong, L.; Sun, Y.; Ran, J.; Hu, W.; Ji, M.; Du, Q.; Xiong, J.; Gong, H.; Yao, S.; Akram, M.A.; et al. Ecosystem organic carbon storage and their drivers across the drylands of China. Catena 2022, 214, 106280. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Shao, M. Spatial variations and influencing factors of soil organic carbon under different land use types in the alpine region of Qinghai-Tibet Plateau. Catena 2023, 220, 106706. [Google Scholar] [CrossRef]
- Li, J.; Awasthi, M.K.; Zhu, Q.; Chen, X.; Wu, F.; Wu, F.; Tong, X. Modified soil physicochemical properties promoted sequestration of organic and inorganic carbon synergistically during revegetation in desertified land. J. Environ. Chem. Eng. 2021, 9, 106331. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Gao, Y. Effects of vegetation rehabilitation on soil inorganic carbon in deserts: A meta-analysis. Catena 2023, 231, 107290. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.; Wang, T.; Liu, T. Soil properties and herbaceous characteristics in an age sequence of Haloxylon ammodendron plantations in an oasis-desert ecotone of northwestern China. J. Arid Land 2016, 8, 960–972. [Google Scholar] [CrossRef]
- Tao, X.; Yang, Z.; Feng, J.; Jian, S.; Yang, Y.; Bates, C.T.; Wang, G.; Guo, X.; Ning, D.; Kempher, M.L.; et al. Experimental warming accelerates positive soil priming in a temperate grassland ecosystem. Nat. Commun. 2024, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kim, D.G.; Peng, C.; Shangguan, Z. Controls of soil and aggregate-associated organic carbon variations following natural vegetation restoration on the Loess Plateau in China. Land Degrad. Dev. 2018, 29, 3974–3984. [Google Scholar] [CrossRef]
- Wade, A.M.; Richter, D.D.; Medjibe, V.P.; Bacon, A.R.; Heine, P.R.; White, L.J.; Poulsen, J.R. Estimates and determinants of stocks of deep soil carbon in Gabon, Central Africa. Geoderma 2019, 341, 236–248. [Google Scholar] [CrossRef]
- Ge, J.; Xu, W.; Xiong, G.; Zhao, C.; Li, J.; Liu, Q.; Tang, Z.; Xie, Z. Depth-dependent controls over soil organic carbon stock across Chinese shrublands. Ecosystems 2023, 26, 277–289. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Dong, Y.; Yang, H.; He, P.; Yu, B.; Ye, H.; Li, S.; Zhou, L. Precipitation drives the accumulation of soil organic carbon in the sandy desert of the Junggar Basin, Northwest China. Ecol. Indic. 2022, 142, 109224. [Google Scholar] [CrossRef]
- Smith, K.R.; Waring, B.G. Broad-scale patterns of soil carbon (C) pools and fluxes across semiarid ecosystems are linked to climate and soil texture. Ecosystems 2019, 22, 742–753. [Google Scholar] [CrossRef]
- Yu, T.; Fu, Y.; Hou, Q.; Xia, X.; Yan, B.; Yang, Z. Soil organic carbon increase in semi-arid regions of China from 1980s to 2010s. Appl. Geochem. 2020, 116, 104575. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Hicks, L.C.; Hu, B.; Liu, X.; Wei, D.; Wang, Z.; Bao, W. Latitudinal patterns of light and heavy organic matter fractions in arid and semi-arid soils. Catena 2022, 215, 106293. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Ishii, S.; Reich, P.B.; Wei, G.; Jiao, S.; et al. Afforestation can lower microbial diversity and functionality in deep soil layers in a semiarid region. Glob. Change Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, D.; Wei, B.; Yang, Y. Dual roles of microbes in mediating soil carbon dynamics in response to warming. Nat. Commun. 2024, 15, 6439. [Google Scholar] [CrossRef]
- Wu, D.; Du, E.; Eisenhauer, N.; Mathieu, J.; Chu, C. Global engineering effects of soil invertebrates on ecosystem functions. Nature 2025, 1–10. [Google Scholar] [CrossRef]

| Plot Traits and Soil Properties | S. psammophila | A. desertorum | C. fruticosum |
|---|---|---|---|
| Plot area (m × m) | 10 × 10 | 10 × 10 | 10 × 10 |
| Plant density (stem ha−1) | 90,280 (individuals) | 2780 (clusters) | 73,219 (individuals) |
| Mean height (m) | 2.72 ± 0.29 a | 0.50 ± 0.07 b | 0.77 ± 0.06 c |
| Canopy coverage (%) | 95 | 85 | 90 |
| Soil bulk (g cm−3) | 1.48 ± 0.11 a | 1.50 ± 0.10 a | 1.51 ± 0.09 a |
| <0.05 in grain diameter (%) | 8.40 ± 0.51 a | 8.68 ± 0.67 ab | 8.70 ± 0.49 b |
| Silt (%) | 5.99 ± 0.56 a | 5.88 ± 0.54 a | 6.09 ± 0.50 a |
| Clay (%) | 1.45 ± 0.16 a | 1.56 ± 0.10 ab | 1.59 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Z.; Jin, A.; Feng, W.; She, W.; Lang, T.; Liu, Z. Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert. Forests 2025, 16, 586. https://doi.org/10.3390/f16040586
Lai Z, Jin A, Feng W, She W, Lang T, Liu Z. Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert. Forests. 2025; 16(4):586. https://doi.org/10.3390/f16040586
Chicago/Turabian StyleLai, Zongrui, Aliang Jin, Wei Feng, Weiwei She, Tao Lang, and Zhonghua Liu. 2025. "Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert" Forests 16, no. 4: 586. https://doi.org/10.3390/f16040586
APA StyleLai, Z., Jin, A., Feng, W., She, W., Lang, T., & Liu, Z. (2025). Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert. Forests, 16(4), 586. https://doi.org/10.3390/f16040586






