Hydraulic Parameters of Pressure–Volume Curves and Their Relationship with the Moisture Content of Live Fuels in Two Woody Species and an Epiphyte
Abstract
1. Introduction
2. Materials and Methods
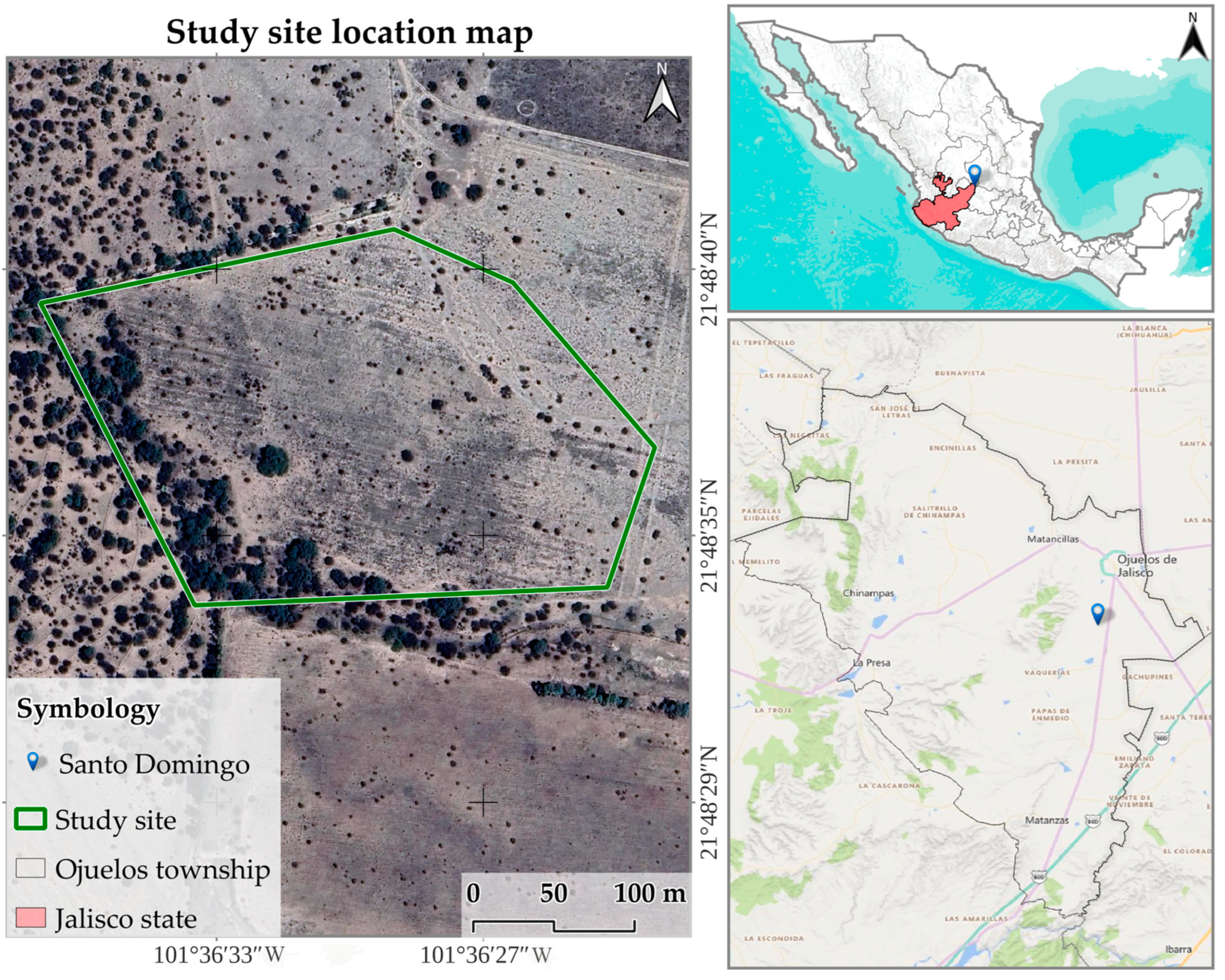
2.1. Study Site
2.2. Precipitarion and Soil Measurements
2.3. Plant Selection
2.4. Plant Water Potential
2.5. Pressure–Volume Curves
2.6. Live Fuel Moisture Content
2.7. Data Analysis
3. Results
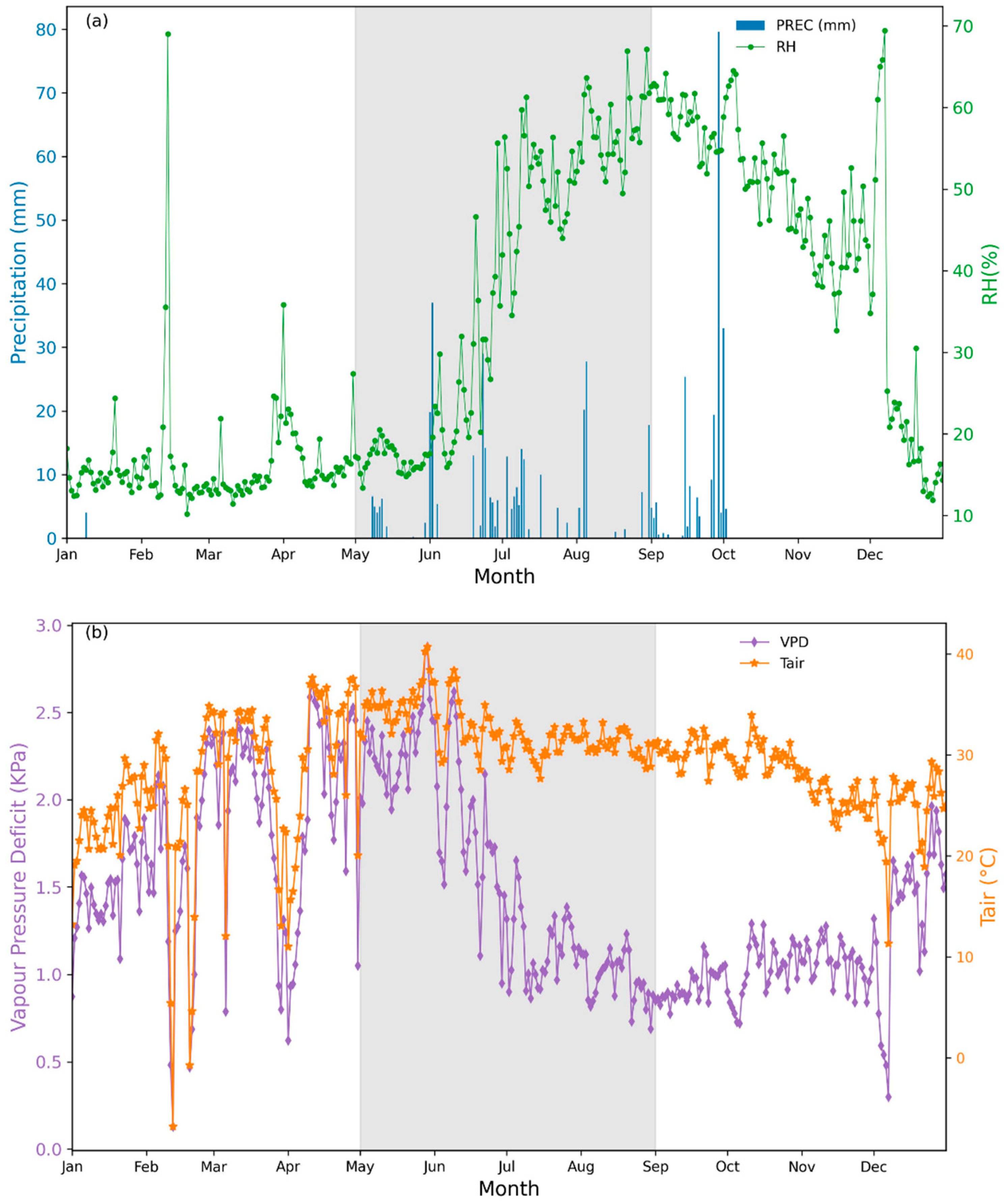
3.1. Environmental Variables and Leaf Water Potentials
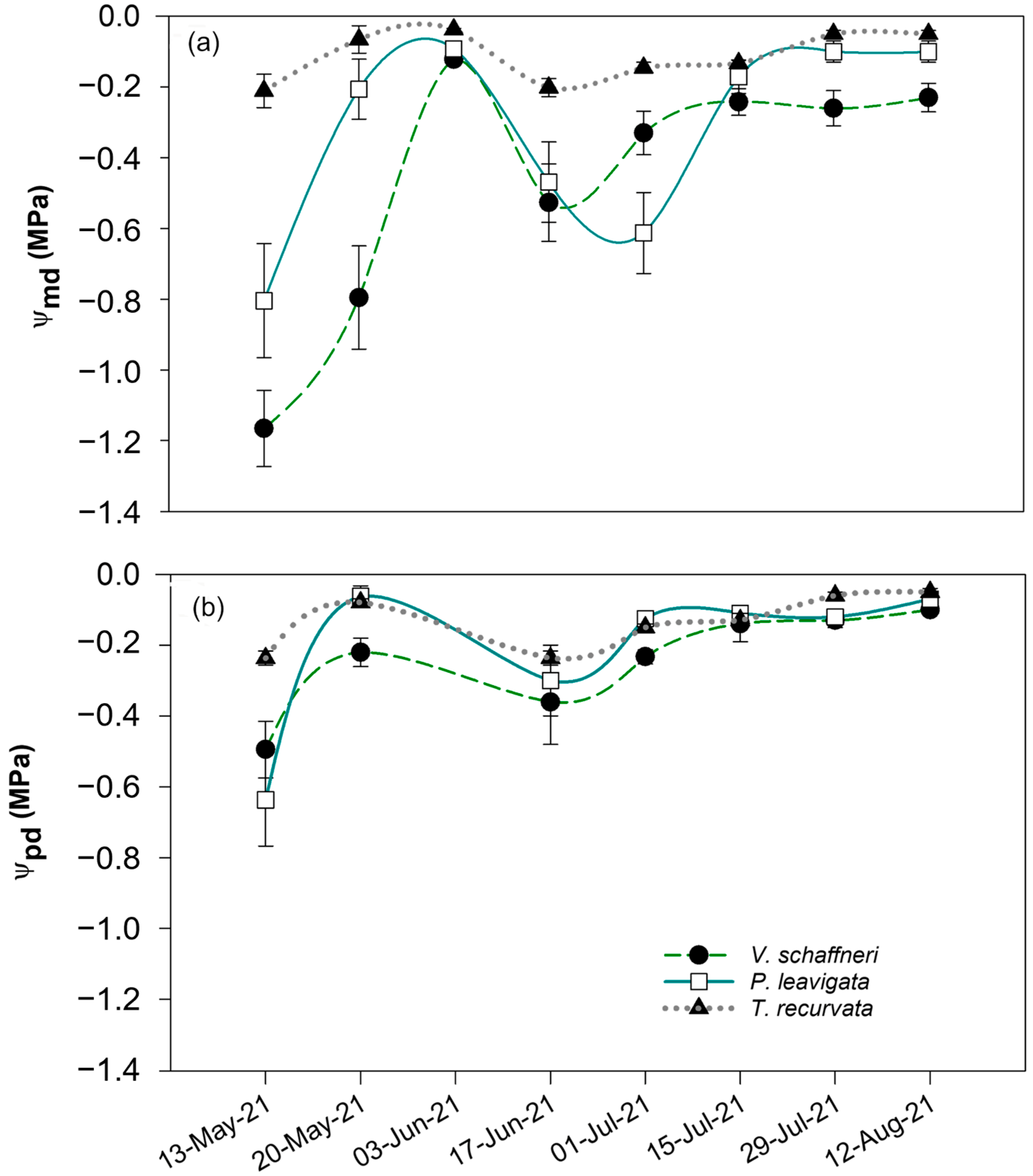
3.2. Leaf Water Potentials
3.3. Relationship Between Leaf Water Potentials and Environmental Variables
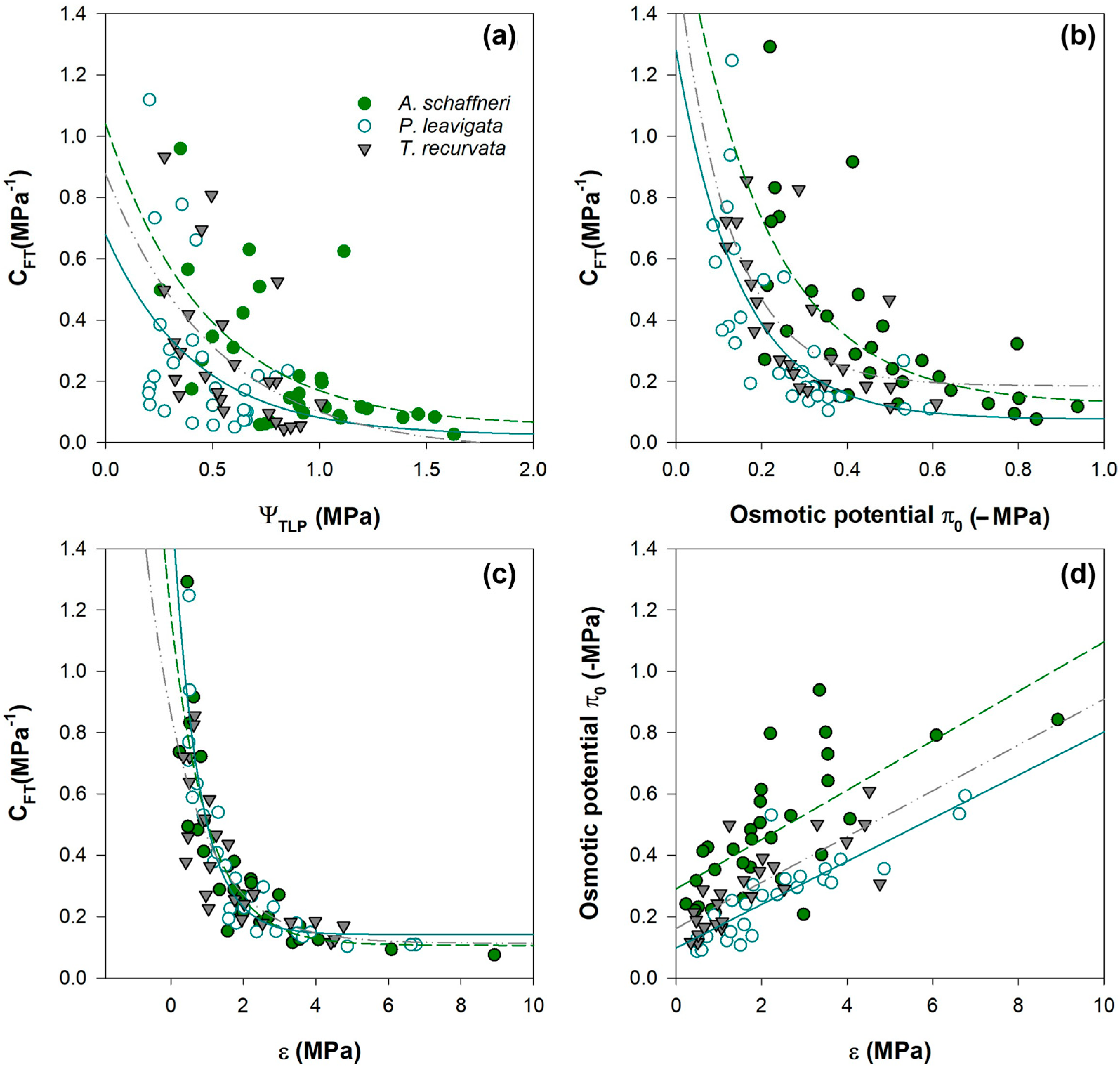
3.4. Pressure–Volume Curves
3.5. Correlation P–V Parameters
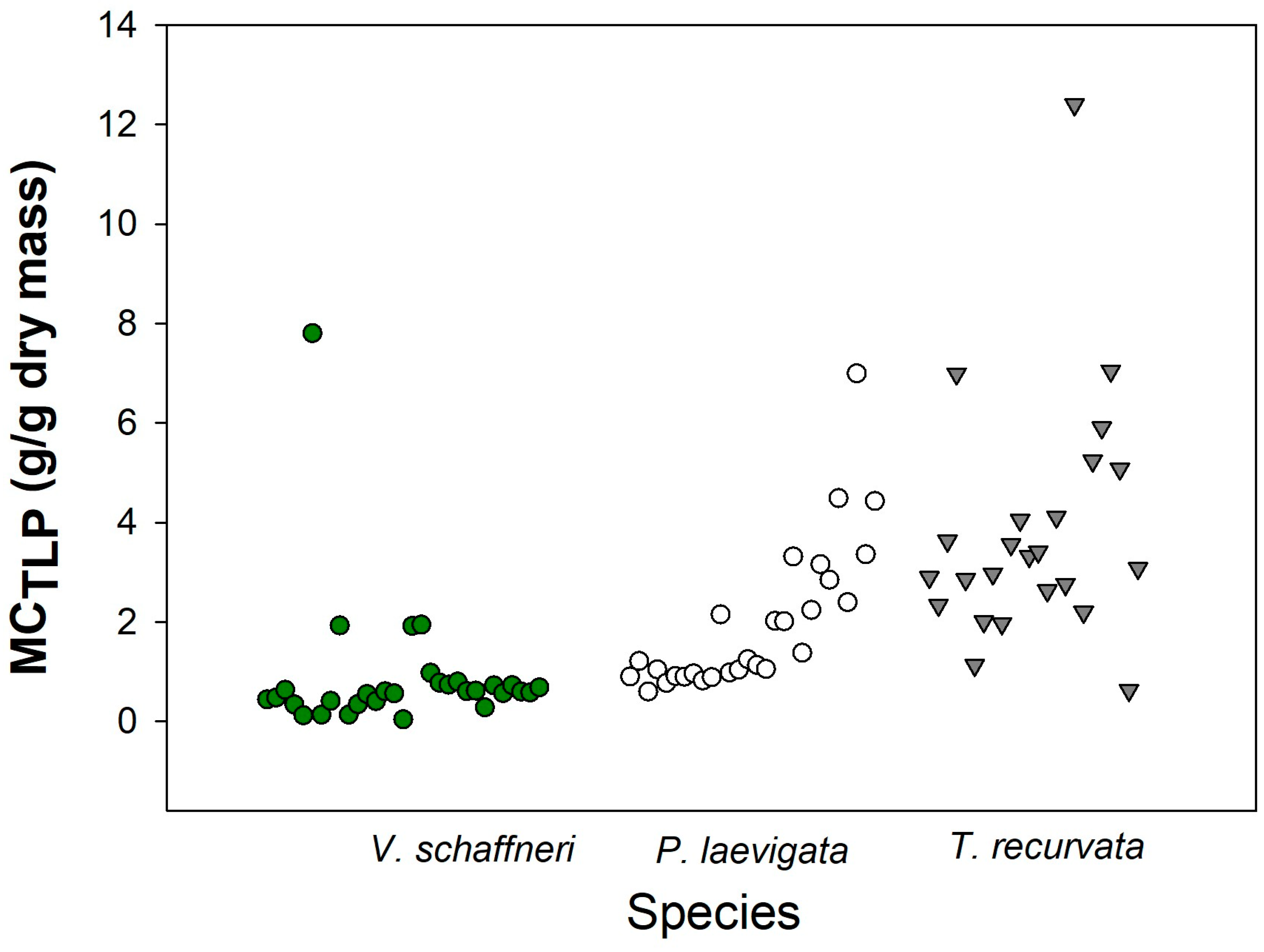
3.6. Fuel Moisture Content
4. Discussion
4.1. Seasonal Patterns in Leaf Water Potentials
4.2. Hydraulic Traits
4.3. Moisture Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; d’Odorico, P.; Evans, J.P.; Eldridge, D.J.; McCabe, M.F.; Caylor, K.K.; King, E.G. Dryland ecohydrology and climate change: Critical issues and technical advances. Hydrol. Earth Syst. Sci. 2012, 16, 2585–2603. [Google Scholar] [CrossRef]
- Achite, M.; Caloiero, T.; Jehanzaib, M.; Wałęga, A.; Kuriqi, A.; Pellicone, G. Geo-Statistical Characterization of Annual Maximum Daily Rainfall Variability in Semi-Arid Regions. Atmosphere 2024, 15, 1519. [Google Scholar] [CrossRef]
- Briones, O.; Búrquez, A.; Martínez-Yrízar, A.; Pavón, N.; Perroni, Y. Biomasa y productividad en las zonas áridas mexicanas. Madera Bosques 2018, 24, 1–19. [Google Scholar] [CrossRef]
- Armenteras, D.; Dávalos, L.M.; Barreto, J.S.; Miranda, A.; Hernández-Moreno, A.; Zamorano-Elgueta, C.; González-Delgado, T.M.; Meza-Elizalde, M.C.; Retana, J. Fire-induced loss of the world’s most biodiverse forests in Latin America. Sci. Adv. 2021, 7, eabd3357. [Google Scholar] [CrossRef]
- Loram-Lourenco, L.; Farnese, F.D.S.; Sousa, L.F.D.; Alves, R.D.F.B.; Andrade, M.C.P.D.; Almeida, S.E.D.S.; Moura, L.M.; Costa, A.C.; Silva, F.G.; Galmés, J.; et al. A structure shaped by fire, but also water: Ecological consequences of the variability in bark properties across 31 species from the Brazilian Cerrado. Front. Plant Sci. 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Graciano, C.; Fernández, M.E. Curvas de presión-volumen para la estimación de parámetros hídricos. In Técnicas en Medición en Ecofisiología Vegetal: Conceptos y Procedimientos; Fernández, M.E., Gyenge, J., Eds.; Ediciones INTA: Buenos Aires, Argentina, 2010; 140p, ISBN 978-987-1623-76-1. Available online: https://openlibrary.org/books/OL27090860M/T%C3%A9cnicas_en_medici%C3%B3n_en_ecofisiolog%C3%ADa_vegetal (accessed on 10 January 2025).
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant Responses to Drought Stress: Physiological, Biochemical and Molecular Basis. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor maintenance by osmotic adjustment, an adaptive mechanism for coping with plant water deficits. Plant Cell Environ. 2017, 40, 1–3. [Google Scholar] [CrossRef]
- Sanders, G.J.; Arndt, S.K. Osmotic Adjustment Under Drought Conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Choat, B.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Drought tolerance traits do not vary across sites differing in water availability in Banksia serrata (Proteaceae). Funct. Plant Biol. 2019, 46, 624–633. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Leuschner, C.; Wedde, P.; Lübbe, T. The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann. For. Sci. 2019, 76, 60. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-bomb Technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Mart, K.B.; Veneklaas, E.J.; Bramley, H. Osmotic potential at full turgor: An easily measurable trait to help breeders select for drought tolerance in wheat. Plant Breed. 2016, 135, 279–285. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Ye, Q.; He, P.; Liu, H.; Li, R.; Fu, P.; Jiang, G.; Cao, K. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Sinclair, G.; Fontanesi, G.; Knipfer, T.; Walker, M.A.; McElrone, A.J. Root pressure–volume curve traits capture rootstock drought tolerance. Ann. Bot. 2022, 129, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.H.; Blackman, C.J.; de Dios, V.R.; Choat, B.; Medlyn, B.E.; Li, X.; Bradstock, R.A.; Boer, M.M. Linking Forest Flammability and Plant Vulnerability to Drought. Forests 2020, 11, 779. [Google Scholar] [CrossRef]
- Nolan, R.H.; Foster, B.; Griebel, A.; Choat, B.; Medlyn, B.E.; Yebra, M.; Younes, N.; Boer, M.M. Drought-related leaf functional traits control spatial and temporal dynamics of live fuel moisture content. Agric. For. Meteorol. 2022, 319, 108941. [Google Scholar] [CrossRef]
- Pivovaroff, A.L.; Emery, N.; Sharifi, M.R.; Witter, M.; Keeley, J.E.; Rundel, P.W. The Effect of Ecophysiological Traits on Live Fuel Moisture Content. Fire 2019, 2, 28. [Google Scholar] [CrossRef]
- Yebra, M.; Dennison, P.E.; Chuvieco, E.; Riaño, D.; Zylstra, P.; Hunt, E.R., Jr.; Danson, F.M.; Qi, Y.; Jurdao, S. A global review of remote sensing of live fuel moisture content for fire danger assessment: Moving towards operational products. Remote Sens. Environ. 2013, 136, 455–468. [Google Scholar] [CrossRef]
- Manzello, S.L. (Ed.) Encyclopedia of Wildfires and Wildland-Urban Interface (WUI) Fires; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Griebel, A.; Boer, M.M.; Blackman, C.; Choat, B.; Ellsworth, D.S.; Madden, P.; Medlyn, B.; de Dios, V.R.; Wujeska-Klause, A.; Yebra, M.; et al. Specific leaf area and vapour pressure deficit control live fuel moisture content. Funct. Ecol. 2023, 37, 719–731. [Google Scholar] [CrossRef]
- Delgado-Balbuena, J.; Arredondo, J.T.; Loescher, H.W.; Huber-Sannwald, E.; Chavez-Aguilar, G.; Luna-Luna, M.; Barretero-Hernandez, R. Differences in plant cover and species composition of semiarid grassland communities of central Mexico and its effects on net ecosystem exchange. Biogeosciences 2013, 10, 4673–4690. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, C.A.; Holwerda, F.; Goldsmith, G.R.; Delgado, J.; Yepez, E.; Carbajal, N.; Escoto-Rodríguez, M.; Arredondo, J.T. The importance of dew in the water balance of a continental semiarid grassland. J. Arid Environ. 2019, 168, 26–35. [Google Scholar] [CrossRef]
- Delgado-Balbuena, J.; Arredondo, J.T.; Loescher, H.W.; Pineda-Martínez, L.F.; Carbajal, J.N.; Vargas, R. Seasonal precipitation legacy effects determine the carbon balance of a semiarid grassland. J. Geophys. Res. Biogeosci. 2019, 124, 987–1000. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Scarff, F.R.; Lenz, T.; Richards, A.E.; Zanne, A.E.; Wright, I.J.; Westoby, M. Effects of plant hydraulic traits on the flammability of live fine canopy fuels. Funct. Ecol. 2021, 35, 835–846. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 July 2024).
- Garrido, S.M. Estudio de la Arquitectura Hidráulica y Estrategia Hidráulica de Prosopis Tamarugo Como Mecanismo de Aclimatación Bajo Condiones de Descenso de Nivel Freático. Ph.D. Thesis, Universidad de Chile, Santiago, Chile, 2018. [Google Scholar]
- Donovan, L.A.; Richards, J.H.; Linton, M.J. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 2003, 84, 463–470. [Google Scholar] [CrossRef]
- García López, A.; Cun González, R.; Montero San José, L. Effect of day time on leaf water potential in sorghum and their relationship with soil humidity. Rev. Cienc. Técnicas Agropecu. 2010, 19, 7–11. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2071-00542010000300002&lng=es&tlng=en (accessed on 4 December 2024).
- Lange, O.L.; Medina, E. Stomata of the CAM plant Tillandsia recurvata respond directly to humidity. Oecologia 1979, 40, 357–363. [Google Scholar] [CrossRef]
- Nowak, E.J.; Martin, C.E. Physiological and anatomical responses to water deficits in the CAM epiphyte Tillandsia ionantha (Bromeliaceae). Int. J. Plant Sci. 1997, 158, 818–826. [Google Scholar] [CrossRef]
- RodrÍguez, H.G.; Silva, I.C.; Meza, M.V.G.; Jordan, W.R. Seasonal Plant Water Relationships in Acacia berlandieri. Arid Soil Res. Rehabil. 2000, 14, 343–357. [Google Scholar] [CrossRef]
- Merine, A.K.; Rodríguez-García, E.; Alía, R.; Pando, V.; Bravo, F. Effects of water stress and substrate fertility on the early growth of Acacia senegal and Acacia seyal from Ethiopian Savanna woodlands. Trees 2015, 29, 593–604. [Google Scholar] [CrossRef]
- Richards, J.H. Root growth response to defoliation in two Agropyron bunchgrasses: Field observations with an improved root periscope. Oecologia 1984, 64, 21–25. [Google Scholar] [CrossRef]
- Perkins, S.R.; Keith Owens, M. Growth and biomass allocation of shrub and grass seedlings in response to predicted changes in precipitation seasonality. Plant Ecol. 2003, 168, 107–120. [Google Scholar] [CrossRef]
- Villagra, P.E.; Cavagnaro, J.B. Water stress effects on the seedling growth of Prosopis argentina and Prosopis alpataco. J. Arid Environ. 2006, 64, 390–400. [Google Scholar] [CrossRef]
- Johnson, D.M.; Domec, J.-C.; Carter Berry, Z.; Schwantes, A.M.; McCulloh, K.A.; Woodruff, D.R.; Wayne Polley, H.; Wortemann, R.; Swenson, J.J.; Scott Mackay, D.; et al. Co-occurring woody species have diverse hydraulic strategies and mortality rates during an extreme drought. Plant Cell Environ. 2018, 41, 576–588. [Google Scholar] [CrossRef]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Pritzkow, C.; Williamson, V.; Szota, C.; Trouvé, R.; Arndt, S.K. Phenotypic plasticity and genetic adaptation of functional traits influences intra-specific variation in hydraulic efficiency and safety. Tree Physiol. 2020, 40, 215–229. [Google Scholar] [CrossRef]
- Rodriguez-Zaccaro, F.D.; Valdovinos-Ayala, J.; Percolla, M.I.; Venturas, M.D.; Pratt, R.B.; Jacobsen, A.L. Wood structure and function change with maturity: Age of the vascular cambium is associated with xylem changes in current-year growth. Plant Cell Environ. 2019, 42, 1816–1831. [Google Scholar] [CrossRef]
- Otieno, D.O.; Schmidt, M.W.T.; Adiku, S.; Tenhunen, J. Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol. 2005, 25, 361–371. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Protein synthesis by plants under stressful conditions. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 405–449. [Google Scholar] [CrossRef]
- Stiles, K.C.; Martin, C.E. Effects of drought stress on CO2 exchange and water relations in the CAM epiphyte Tillandsia utriculata (Bromeliaceae). J. Plant Physiol. 1996, 149, 721–728. [Google Scholar] [CrossRef]
- Saito, T.; Soga, K.; Hoson, T.; Terashima, I. The Bulk Elastic Modulus and the Reversible Properties of Cell Walls in Developing Quercus Leaves. Plant Cell Physiol. 2006, 47, 715–725. [Google Scholar] [CrossRef]
- Fan, S.; Blake, T.J.; Blumwald, E. The relative contribution of elastic and osmotic adjustments to turgor maintenance of woody species. Physiol. Plant. 1994, 90, 408–413. [Google Scholar] [CrossRef]
- Passera, C.B. Fisiología de Prosopis spp. Multequina 2000, 9, 53–80. [Google Scholar]
- Geydan, T.D.; Melgarejo, L.M. Metabolismo ácido de las crasuláceas. Acta Biológica Colomb. 2005, 10, 3–16. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-548X2005000200001&lng=en&tlng=es (accessed on 4 December 2024).
- Zavala Alcaña, J.C. Manejo Integral del Heno Motita (Tillandsia recurvata L.). Bachelor’s Thesis, Universidad Autónoma Agraria Antonio Narro, Saltillo, Mexico, 2019. Available online: http://repositorio.uaaan.mx:8080/xmlui/handle/123456789/46173 (accessed on 15 July 2024).
- Scholz, F.G.; Phillips, N.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G. Hydraulic Capacitance: Biophysics and Functional Significance of Internal Water Sources in Relation to Tree Size. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F., Lachenbruch, B., Dawson, T., Eds.; Tree Physiology; Springer: Dordrecht, The Netherlands, 2011; Volume 4. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Cary, G.J.; Bradstock, R.A.; Gill, A.M.; Williams, R.J. Global change and fire regimes in Australia. In Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World; CSIRO Publishing: Victoria, Australia, 2012; pp. 149–169. [Google Scholar]
- Flannigan, M.D.; Wotton, B.M.; Marshall, G.A.; De Groot, W.J.; Johnston, J.; Jurko, N.; Cantin, A.S. Fuel moisture sensitivity to temperature and precipitation: Climate change implications. Clim. Change 2016, 134, 59–71. [Google Scholar] [CrossRef]
- Trenberth, K.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Change 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Pimont, F.; Ruffault, J.; Martin-StPaul, N.K.; Dupuy, J.L. Why is the effect of live fuel moisture content on fire rate of spread underestimated in field experiments in shrublands? Int. J. Wildland Fire 2019, 28, 127–137. [Google Scholar] [CrossRef]



| Hydraulic Trait | ||||||||
|---|---|---|---|---|---|---|---|---|
| Specie | MCTLP (g/g) | SWC (%) | IIo (MPa) | ΨTLP (MPa) | RWCTLP (%) | ε * (MPa) | CFT * (MPa−1) | CTLP (MPa−1) |
| V. schaffneri | 0.89 ± 0.35 | 1.12 ± 0.39 | −0.47 ± 0.07 | −0.91 ± 0.12 | 76.17 ± 3.87 | 2.25 ± 0.60 | 12.94 ± 0.08 | 0.24 ± 0.06 |
| P. laevigata | 1.99 ± 0.29 | 2.28 ± 0.31 | −0.25 ± 0.04 | −0.45 ± 0.06 | 87.63 ± 2.11 | 2.20 ± 0.57 | 28.03 ± 0.11 | 0.31 ± 0.08 |
| T. recurvata | 3.84 ± 0.71 | 3.67 ±0.82 | −0.33 ± 0.05 | −0.63 ± 0.08 | 81.53 ± 2.41 | 1.91 ± 0.51 | 21.48 ± 0.08 | 0.27 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felipe, F.G.; Reyna, T.A.; Balbuena, J.D.; Aguilar, F.F.C.; Gutierrez, C.A.A. Hydraulic Parameters of Pressure–Volume Curves and Their Relationship with the Moisture Content of Live Fuels in Two Woody Species and an Epiphyte. Forests 2025, 16, 568. https://doi.org/10.3390/f16040568
Felipe FG, Reyna TA, Balbuena JD, Aguilar FFC, Gutierrez CAA. Hydraulic Parameters of Pressure–Volume Curves and Their Relationship with the Moisture Content of Live Fuels in Two Woody Species and an Epiphyte. Forests. 2025; 16(4):568. https://doi.org/10.3390/f16040568
Chicago/Turabian StyleFelipe, Fabiola Guerrero, Teresa Alfaro Reyna, Josué Delgado Balbuena, Francisco Fábian Calvillo Aguilar, and Carlos Alberto Aguirre Gutierrez. 2025. "Hydraulic Parameters of Pressure–Volume Curves and Their Relationship with the Moisture Content of Live Fuels in Two Woody Species and an Epiphyte" Forests 16, no. 4: 568. https://doi.org/10.3390/f16040568
APA StyleFelipe, F. G., Reyna, T. A., Balbuena, J. D., Aguilar, F. F. C., & Gutierrez, C. A. A. (2025). Hydraulic Parameters of Pressure–Volume Curves and Their Relationship with the Moisture Content of Live Fuels in Two Woody Species and an Epiphyte. Forests, 16(4), 568. https://doi.org/10.3390/f16040568







