Effects of Bamboo-Sourced Organic Fertilizer on the Soil Microbial Necromass Carbon and Its Contribution to Soil Organic Carbon in Moso Bamboo (Phyllostachys edulis) Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Material Preparation, Experimental Design, and Soil Sampling
2.3. Basic Soil Physicochemical Properties
2.4. Soil Microbial Necromass Carbon
2.5. Data Analysis
3. Results
3.1. Effects of BSOF on Soil Physicochemical Properties of Moso Bamboo Forests
3.2. Effects of BSOF on Soil Microbial Necromass C and Its Contribution to SOC of Moso Bamboo Forests
3.3. Factors Affecting Soil Microbial Necromass C Under BSOF Application
4. Discussion
4.1. Effects of BSOF on Soil Microbial Necromass C of Moso Bamboo Forests
4.2. Factors Affecting Soil Microbial Necromass C Under BSOF Application
4.3. Effects of Soil Microbial Necromass C on Soil Carbon Sequestration Under BSOF Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOC | Soil organic carbon |
| BSOF | Bamboo-sourced organic fertilizers |
| MNC | Microbial necromass carbon |
| BNC | Bacterial necromass carbon |
| FNC | Fungal necromass carbon |
| AS | Amino sugar |
| GluN | Glucosamine |
| GalN | Galactosamine |
| MurA | Muramic acid |
| BD | Bulk density |
| NH4+-N | Ammonium-nitrogen |
| NO3−-N | Nitrate-nitrogen |
| AP | Available phosphorus |
| AK | Available potassium |
| RDA | Redundancy analysis |
| PLS-PM | Partial least squares path modeling |
| GOF | Goodness of fit |
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
References
- Feng, P.; Li, Y. China’s Bamboo Resources in 2021. World Bamboo Ratt. 2023, 21, 100–103. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Cai, Z.; Zhou, X.; Peng, C. The Response of the Net Primary Production of Moso Bamboo Forest to the On and Off-Year Management: A Case Study in Anji County, Zhejiang, China. For. Ecol. Manag. 2018, 409, 1–7. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Zhou, G.; Xu, X.; Liu, E.; Zhou, Y.; Zhang, F.; Li, C.; Fang, H.; Chen, L. Structural Development and Carbon Dynamics of Moso Bamboo Forests in Zhejiang Province, China. For. Ecol. Manag. 2018, 409, 479–488. [Google Scholar] [CrossRef]
- Xu, L.; Fang, H.; Deng, X.; Ying, J.; Lv, W.; Shi, Y.; Zhou, G.; Zhou, Y. Biochar Application Increased Ecosystem Carbon Sequestration Capacity in a Moso Bamboo Forest. For. Ecol. Manag. 2020, 475, 118447. [Google Scholar] [CrossRef]
- Chiti, T.; Blasi, E.; Chiriacò, M.V. Carbon Sequestration in a Bamboo Plantation: A Case Study in a Mediterranean Area. J. For. Res. 2024, 35, 51. [Google Scholar] [CrossRef]
- Yen, T.-M.; Lee, J.-S. Comparing Aboveground Carbon Sequestration between Moso Bamboo (Phyllostachys heterocycla) and China Fir (Cunninghamia lanceolata) Forests Based on the Allometric Model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Li, P.; Zhou, G.; Du, H.; Lu, D.; Mo, L.; Xu, X.; Shi, Y.; Zhou, Y. Current and Potential Carbon Stocks in Moso Bamboo Forests in China. J. Environ. Manag. 2015, 156, 89–96. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, H.; Wang, B.; Yang, Z. Fish Protein Fertilizer Serves as a Sustainable Alternative, Improving Soil Properties, Bamboo Growth and Shoots Yield in Lei Bamboo Forests. Sci. Rep. 2025, 15, 4363. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, C.; Zhang, J.; Li, Z.; Liu, Y.; Song, X.; Gao, Z. Nitrogen Fertilization in Bamboo Forest Accelerates the Shoot Growth and Alters the Lignification Process in Shoots. Ind. Crops Prod. 2022, 187, 115368. [Google Scholar] [CrossRef]
- Toljander, J.F.; Santos-González, J.C.; Tehler, A.; Finlay, R.D. Community Analysis of Arbuscular Mycorrhizal Fungi and Bacteria in the Maize Mycorrhizosphere in a Long-Term Fertilization Trial: Soil Microbial Communities in Arable Fields. FEMS Microbiol. Ecol. 2008, 65, 323–338. [Google Scholar] [CrossRef]
- Divito, G.A.; Rozas, H.R.S.; Echeverría, H.E.; Studdert, G.A.; Wyngaard, N. Long Term Nitrogen Fertilization: Soil Property Changes in an Argentinean Pampas Soil under No Tillage. Soil Tillage Res. 2011, 114, 117–126. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Liu, D.; Fan, J.; Luo, J.; Lindsey, S.; He, J.-S.; Ding, W. Nutrient Addition Reduces Carbon Sequestration in a Tibetan Grassland Soil: Disentangling Microbial and Physical Controls. Soil Boil. Biochem. 2020, 144, 107764. [Google Scholar] [CrossRef]
- Salvo, L.; Hernández, J.; Ernst, O. Distribution of Soil Organic Carbon in Different Size Fractions, under Pasture and Crop Rotations with Conventional Tillage and No-till Systems. Soil Tillage Res. 2010, 109, 116–122. [Google Scholar] [CrossRef]
- Palmer, J.; Thorburn, P.J.; Biggs, J.S.; Dominati, E.J.; Probert, M.E.; Meier, E.A.; Huth, N.I.; Dodd, M.; Snow, V.; Larsen, J.R.; et al. Nitrogen Cycling from Increased Soil Organic Carbon Contributes Both Positively and Negatively to Ecosystem Services in Wheat Agro-Ecosystems. Front. Plant Sci. 2017, 8, 731. [Google Scholar] [CrossRef]
- Kaushal, M.; Tumuhairwe, J.B.; Kaingo, J.; Richard, M.; Nakamanya, F.; Taulya, G.; Coyne, D. Compositional Shifts in Microbial Diversity under Traditional Banana Cropping Systems of Sub-Saharan Africa. Biology 2022, 11, 756. [Google Scholar] [CrossRef]
- Yang, H.; Mo, B.; Zhou, M.; Zhu, T.; Cao, J. Effects of Plum Plantation Ages on Soil Organic Carbon Mineralization in the Karst Rocky Desertification Ecosystem of Southwest China. Forests 2019, 10, 1107. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Wang, H.; Li, F.; Pan, H.; Yang, Q.; Li, J.; Zhang, J. The Effects of Natural Humus Material Amendment on Soil Organic Matter and Integrated Fertility in the Black Soil of Northeast China: Preliminary Results. Agronomy 2023, 13, 794. [Google Scholar] [CrossRef]
- He, Z.; Cao, H.; Qi, C.; Hu, Q.; Liang, J.; Li, Z. Straw Management in Paddy Fields Can Reduce Greenhouse Gas Emissions: A Global Meta-Analysis. Field Crops Res. 2024, 306, 109218. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Cao, W.; Wang, Y.; Chen, J.; Guo, J. Responses of Soil Organic Carbon Stock to Animal Manure Application: A New Global Synthesis Integrating the Impacts of Agricultural Managements and Environmental Conditions. Glob. Chang. Biol. 2021, 27, 5356–5367. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.; Tiemann, L.K.; Zhang, F.; Kuzyakov, Y.; Tian, J. Microbial Necromass in Cropland Soils: A Global Meta-analysis of Management Effects. Glob. Chang. Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef]
- Islam, M.U.; Jiang, F.; Guo, Z.; Liu, S.; Peng, X. Impacts of Straw Return Coupled with Tillage Practices on Soil Organic Carbon Stock in Upland Wheat and Maize Croplands in China: A Meta-Analysis. Soil Tillage Res. 2023, 232, 105786. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, J.; Jin, W.; Liu, Z.; Wang, Q.; Zha, L.; Zhou, Z.; Meng, Y. Responses of SOC, Labile SOC Fractions, and Amino Sugars to Different Organic Amendments in a Coastal Saline-Alkali Soil. Soil Tillage Res. 2024, 239, 106051. [Google Scholar] [CrossRef]
- Zhong, Z.; Bian, F.; Zhang, X. Testing Composted Bamboo Residues with and without Added Effective Microorganisms as a Renewable Alternative to Peat in Horticultural Production. Ind. Crops Prod. 2018, 112, 602–607. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Z.; Bian, F.; Yang, C. Effects of Composted Bamboo Residue Amendments on Soil Microbial Communities in an Intensively Managed Bamboo (Phyllostachys praecox) Plantation. Appl. Soil Ecol. 2019, 136, 178–183. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Chai, Q.; Huang, Y.; Bian, F.; Huang, Z.; Yi, K.; Zhang, R.; Zhang, J. Effect of Mixed Fermentation of Bamboo Processing Waste with Different Proportions on Quality of Organic Fertilizer. World Bamboo Ratt. 2023, 21, 28–33. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Boil. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Liu, J.; Liu, J.; Han, L.; Wang, X.; Liu, H.; Xu, M.; Yang, G.; Ren, C.; et al. The contribution of microbial necromass carbon to soil organic carbon in soil aggregates. Appl. Soil Ecol. 2023, 190, 104985. [Google Scholar] [CrossRef]
- Hu, J.; Du, M.; Chen, J.; Tie, L.; Zhou, S.; Buckeridge, K.M.; Cornelissen, J.H.C.; Huang, C.; Kuzyakov, Y. Microbial Necromass under Global Change and Implications for Soil Organic Matter. Glob. Chang. Biol. 2023, 29, 3503–3515. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Pei, G.; Xia, Z.; Peng, B.; Sun, L.; Wang, J.; Gao, D.; Chen, S.; Liu, D.; et al. Stabilization of Microbial Residues in Soil Organic Matter after Two Years of Decomposition. Soil Boil. Biochem. 2020, 141, 107687. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; McNamara, N.P.; Ostle, N.; Puissant, J.; Goodall, T.; Griffiths, R.I.; Stott, A.W.; Whitaker, J. Environmental and Microbial Controls on Microbial Necromass Recycling, an Important Precursor for Soil Carbon Stabilization. Commun. Earth Environ. 2020, 1, 36. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Heinzle, J.; Salas, E.; Kwatcho-Kengdo, S.; Borken, W.; Schindlbacher, A.; Wanek, W. Long-term Soil Warming Decreases Soil Microbial Necromass Carbon by Adversely Affecting Its Production and Decomposition. Glob. Chang. Biol. 2024, 30, e17379. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jing, X.; Xiao, X.; Zhang, N.; Wang, X.; Sui, Y.; Sun, B.; Liang, Y. Microbial Metabolism and Necromass Mediated Fertilization Effect on Soil Organic Carbon after Long-Term Community Incubation in Different Climates. ISME J. 2021, 15, 2561–2573. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Yang, Y.; Ren, L.; Wang, Z.; Liao, Y.; Yong, T. Global Synthesis on the Response of Soil Microbial Necromass Carbon to Climate-smart Agriculture. Glob. Chang. Biol. 2024, 30, e17302. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative Assessment of Microbial Necromass Contribution to Soil Organic Matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Zhong, A.; Guo, S.; Zhang, H. Variations in Microbial Residue and Its Contribution to SOC between Organic and Mineral Soil Layers along an Altitude Gradient in the Wuyi Mountains. Forests 2023, 14, 1678. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial Biomass, Fungal and Bacterial Residues, and Their Relationships to the Soil Organic Matter C/N/P/S Ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, C.; Duan, X.; Chen, H.; Li, D. Variation of Microbial Residue Contribution to Soil Organic Carbon Sequestration Following Land Use Change in a Subtropical Karst Region. Geoderma 2019, 353, 340–346. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Zhong, Z.; Li, Q.; Bian, F. Forest Management Alters Soil Microbial Necromass and Its Contribution to Soil Organic Carbon in Moso Bamboo Plantations in Subtropical China. Appl. Soil Ecol. 2024, 196, 105320. [Google Scholar] [CrossRef]
- Zhu, X.; Min, K.; Feng, K.; Xie, H.; He, H.; Zhang, X.; Deng, Y.; Liang, C. Microbial Necromass Contribution to Soil Carbon Storage via Community Assembly Processes. Sci. Total Environ. 2024, 951, 175749. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lin, Y.; Kuzyakov, Y.; Liu, D.; Luo, J.; Lindsey, S.; Wang, W.; Fan, J.; Ding, W. Manure over Crop Residues Increases Soil Organic Matter but Decreases Microbial Necromass Relative Contribution in Upland Ultisols: Results of a 27-Year Field Experiment. Soil Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino Sugars as Specific Indices for Fungal and Bacterial Residues in Soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.-B.; Alef, K. Amino Sugars and Muramic Acid—Biomarkers for Soil Microbial Community Structure Analysis. Soil Boil. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Quantitative Assessment of the Fungal Contribution to Microbial Tissue in Soil. Soil Boil. Biochem. 2008, 40, 2977–2991. [Google Scholar] [CrossRef]
- FAO. Soil Map of the World. Revised Legend; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- She, W.; Bai, Y.; Zhang, Y.; Qin, S.; Feng, W.; Sun, Y.; Zheng, J.; Wu, B. Resource Availability Drives Responses of Soil Microbial Communities to Short-Term Precipitation and Nitrogen Addition in a Desert Shrubland. Front. Microbiol. 2018, 9, 186. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Bhandari, A.L.; Ladha, J.K.; Pathak, H.; Padre, A.T.; Dawe, D.; Gupta, R.K. Yield and Soil Nutrient Changes in a Long-Term Rice-Wheat Rotation in India. Soil Sci. Soc. Am. J. 2002, 66, 162–170. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W.; Yuan, Y.; Zech, W. Amino Sugar Signature of Particle-Size Fractions in Soils of the Native Prairie as Affected by Climate. Soil Sci. 1998, 163, 220–229. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.6-4; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Gaston, S.; Laura, T.; Giorgio, R. PLSPM: Partial Least Squares Path Modeling (PLS-PM); R Package Version 0.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Kong, C.; Zhang, S.; Yuan, S.; Wang, W.; Song, X.; Guo, D.; Lawi, A.S. Soil Bacterial Community Characteristics and Its Effect on Organic Carbon under Different Fertilization Treatments. Front. Microbiol. 2024, 15, 1356171. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, B.; Chen, G.; Ni, X.; Zhou, L.; Su, H.; Cai, Q.; Chen, X.; Zhu, J.; Ji, C.; et al. Loss of Soil Microbial Residue Carbon by Converting a Tropical Forest to Tea Plantation. Sci. Total Environ. 2022, 818, 151742. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kuzyakov, Y. Mechanisms and Implications of Bacterial–Fungal Competition for Soil Resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tao, C.; Liu, X.; Cheng, X.; Zhou, C.; Huang, S.; Shou, M.; Zhang, Q.; Huang, B.; Li, C.; et al. Effects of Different Management Measures on Carbon Stocks and Soil Carbon Stocks in Moso Bamboo Forests: Meta-Analysis and Control Experiment. Forests 2024, 15, 496. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, Y.; Morrissey, E.; Liu, Y.; Sun, L.; Qu, L.; Sang, C.; Zhang, H.; Li, G.; et al. Integrating Microbial Community Properties, Biomass and Necromass to Predict Cropland Soil Organic Carbon. ISME Commun. 2023, 3, 86. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Peñuelas, J.; Sardans, J.; et al. Phosphorus Addition Decreases Microbial Residual Contribution to Soil Organic Carbon Pool in a Tropical Coastal Forest. Glob. Chang. Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef]
- Liu, M.; Lin, H.; Li, J. Are There Links between Nutrient Inputs and the Response of Microbial Carbon Use Efficiency or Soil Organic Carbon? A Meta-Analysis. Soil Biol. Biochem. 2025, 201, 109656. [Google Scholar] [CrossRef]
- Huang, C.; He, Y.; Zhou, L.; Liu, R.; Chen, H.; Du, Z.; Fu, Y.; Zhu, Y.; Zhou, Y.; Wu, C.; et al. Opposite Effects of Soil pH on Bacteria and Fungi β Diversity in Forests at a Continental Scale. J. Environ. Manag. 2024, 370, 122428. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, R.; Chang, S.; Zheng, Y.; Ma, T.; Xu, S.; Zhang, X.; Shi, X.; Lu, J.; Luo, D.; et al. The contribution of microbial necromass to soil organic carbon and influencing factors along a variation of habitats in alpine ecosystems. Sci. Total Environ. 2024, 921, 171126. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.; Zhang, X.; Bian, F.; Du, X. Responses of Soil Organic Carbon Sequestration Potential and Bacterial Community Structure in Moso Bamboo Plantations to Different Management Strategies in Subtropical China. Forests 2018, 9, 657. [Google Scholar] [CrossRef]
- He, M.; Fang, K.; Chen, L.; Feng, X.; Qin, S.; Kou, D.; He, H.; Liang, C.; Yang, Y. Depth-Dependent Drivers of Soil Microbial Necromass Carbon across Tibetan Alpine Grasslands. Glob. Chang. Biol. 2022, 28, 936–949. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; La Rosa, A.F.; Mason, K.E.; Whitaker, J.; McNamara, N.P.; Grant, H.K.; Ostle, N.J. Sticky Dead Microbes: Rapid Abiotic Retention of Microbial Necromass in Soil. Soil Biol. Biochem. 2020, 149, 107929. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Yang, F.; Dong, W.; Dai, X.; Yang, Y.; Sun, X. Specific Responses of Soil Microbial Residue Carbon to Long-Term Mineral Fertilizer Applications to Reddish Paddy Soils. Pedosphere 2018, 28, 488–496. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, G.; Zhao, B.; Cong, N.; Zheng, Z.; Zhu, J.; Duan, X.; Zhang, Y. Climate Warming Alters the Relative Importance of Plant Root and Microbial Community in Regulating the Accumulation of Soil Microbial Necromass Carbon in a Tibetan Alpine Meadow. Glob. Chang. Biol. 2023, 29, 3193–3204. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Li, L.; Wei, S.; Liu, J.; Zhao, Y. A Network Meta-Analysis on Responses of Forest Soil Carbon Concentration to Interventions. Ecol. Process. 2024, 13, 41. [Google Scholar] [CrossRef]
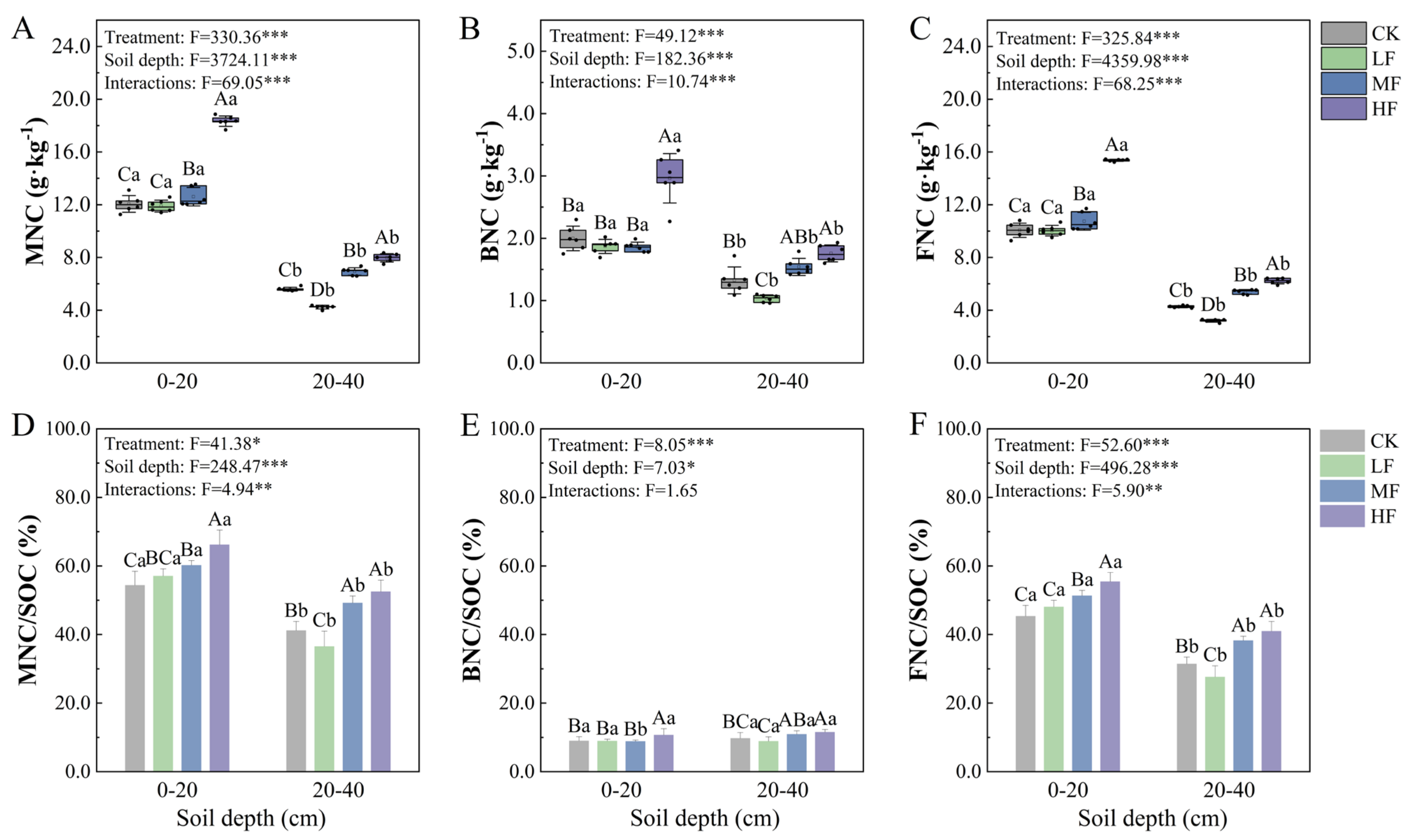
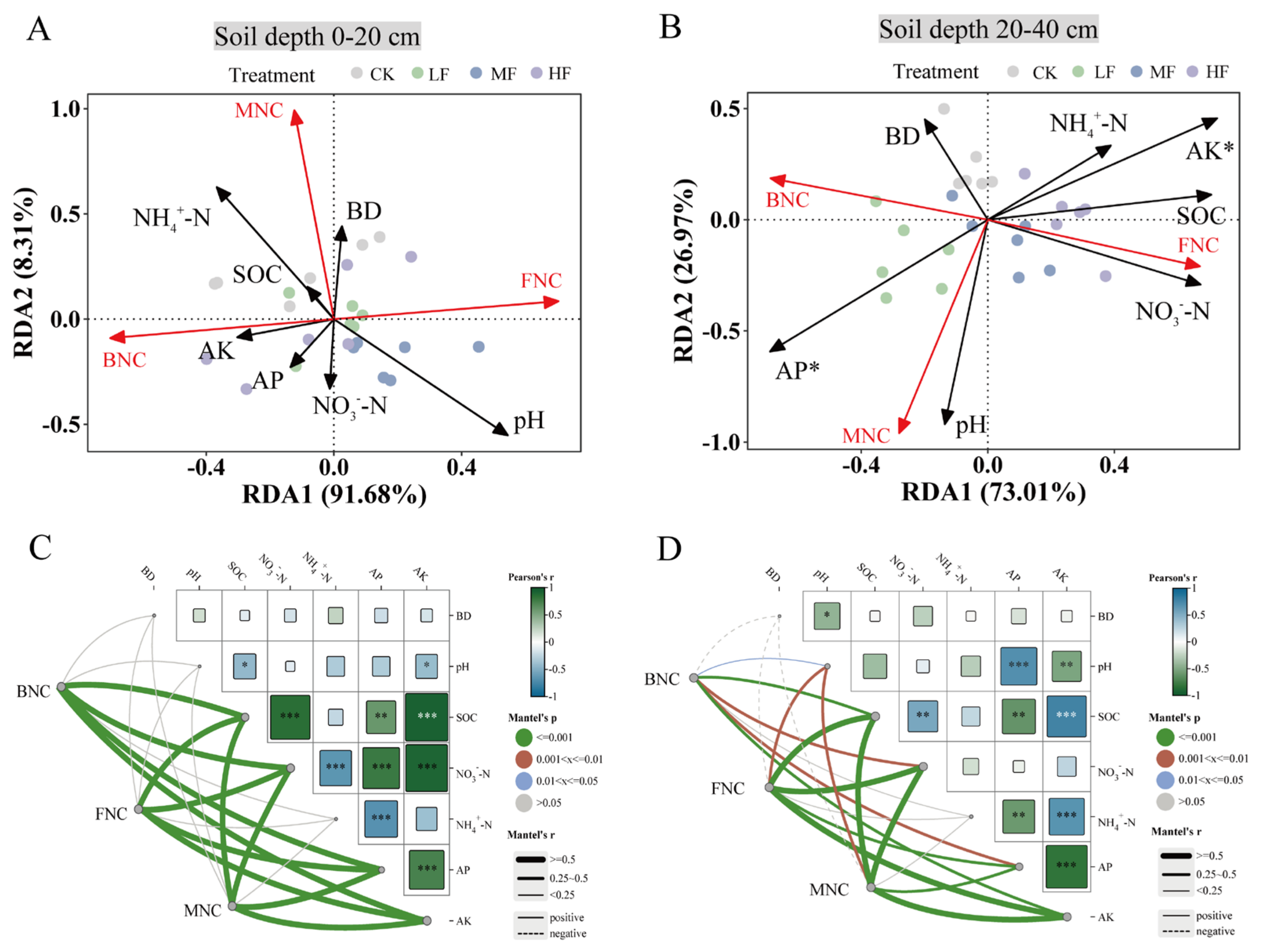

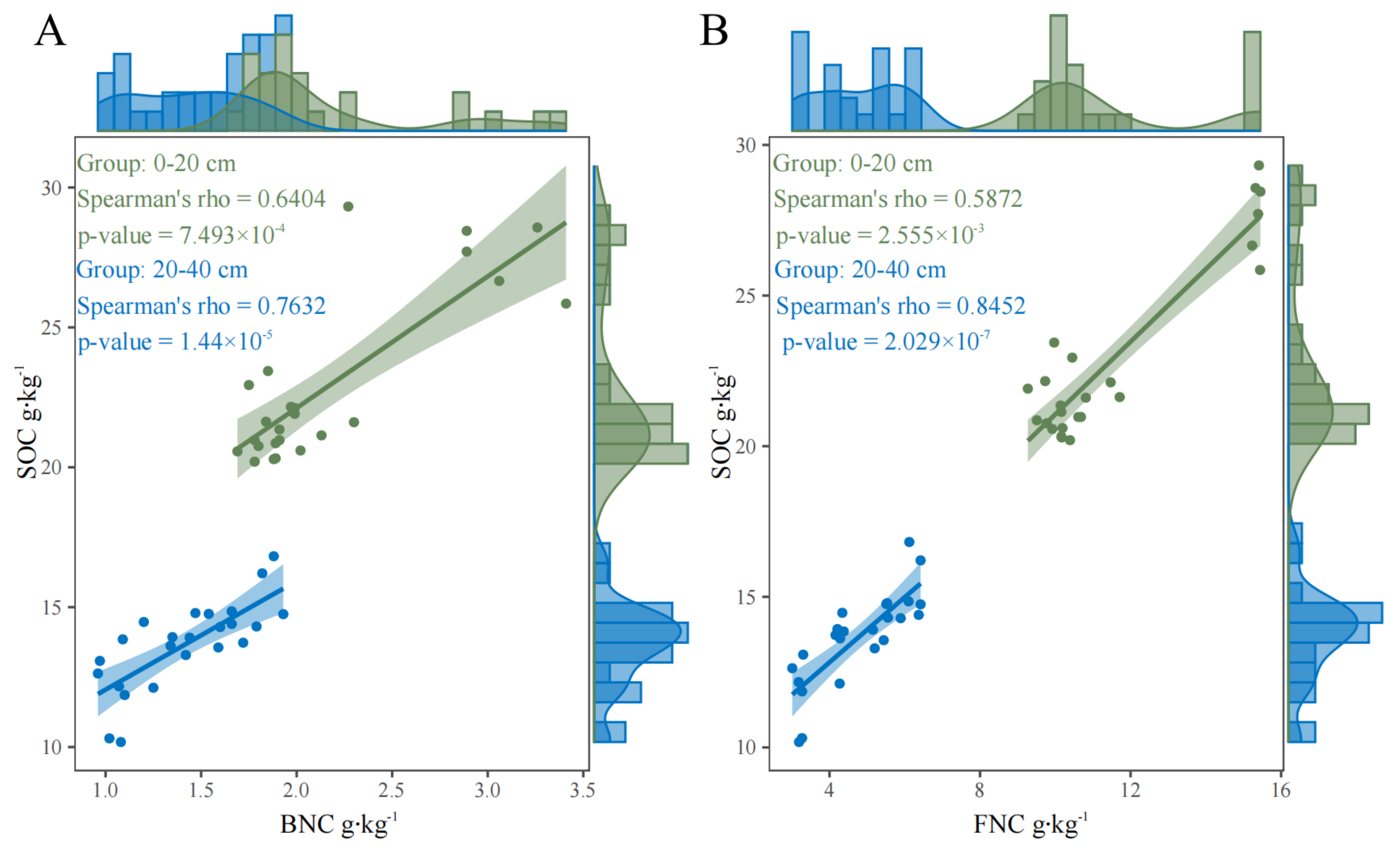
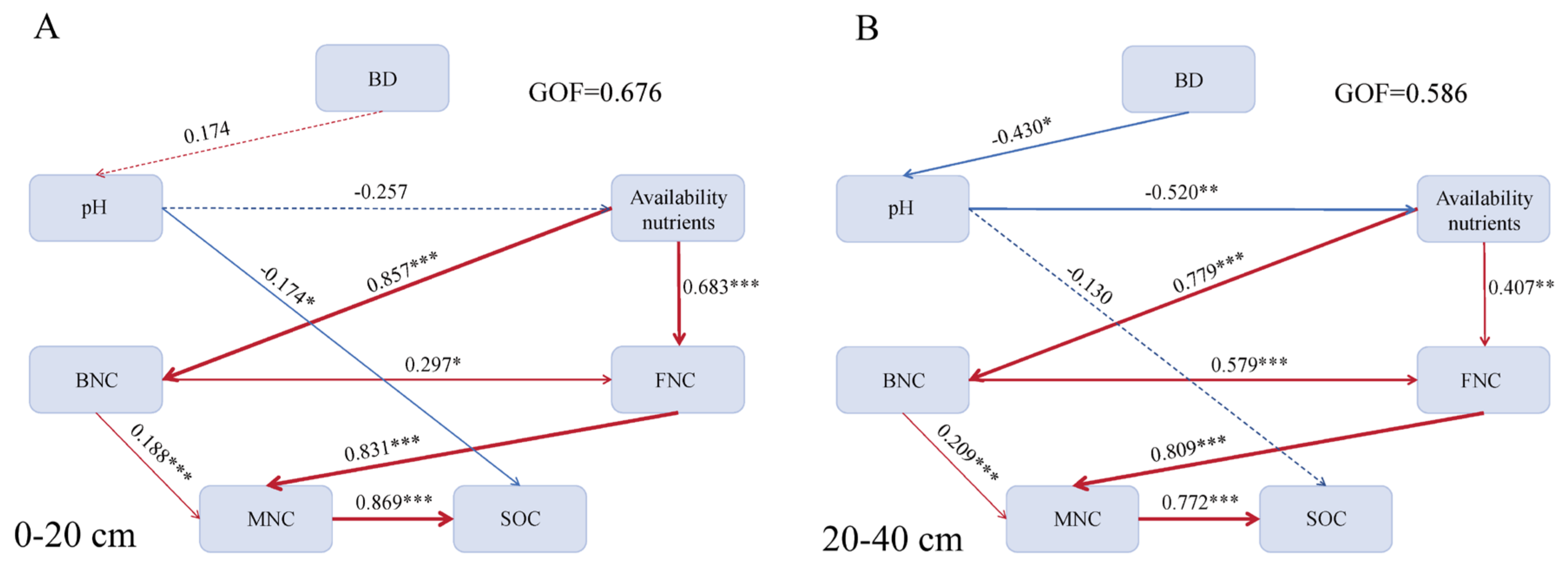
| Soil Layer | Treatment | BD (g·cm−3) | pH | SOC (g·kg−1) | NO3−-N (g·kg−1) | NH4+-N (g·kg−1) | AP (g·kg−1) | AK (g·kg−1) |
|---|---|---|---|---|---|---|---|---|
| 0–20 cm | CK | 1.18 ± 0.07 Ab | 4.48 ± 0.02 Ba | 22.20 ± 0.85 Ba | 3.15 ± 0.65 Da | 6.86 ± 0.59 Aa | 0.53 ± 0.07 Da | 46.97 ± 3.50 Ba |
| LF | 1.13 ± 0.06 Ab | 4.49 ± 0.01 Bb | 20.85 ± 0.29 Ca | 7.52 ± 0.71 Ca | 4.62 ± 0.25 Ba | 1.00 ± 0.19 Bb | 44.35 ± 4.23 Ba | |
| MF | 1.16 ± 0.07 Ab | 4.69 ± 0.05 Aa | 20.92 ± 0.80 Ca | 9.80 ± 1.34 Aa | 4.02 ± 0.11 Ca | 0.76 ± 0.10 Cb | 44.68 ± 2.47 Ba | |
| HF | 1.12 ± 0.08 Ab | 4.47 ± 0.07 Bb | 27.76 ± 1.30 Aa | 24.44 ± 3.28 Ba | 3.90 ± 0.65 Cb | 1.33 ± 0.16 Aa | 88.32 ± 3.32 Aa | |
| 20–40 cm | CK | 1.60 ± 0.04 Aa | 4.67 ± 0.01 Cb | 13.62 ± 0.79 Bb | 1.03 ± 0.31 Bb | 5.65 ± 0.25 Bb | 0.43 ± 0.04 Cb | 34.99 ± 1.30 Bb |
| LF | 1.53 ± 0.06 ABa | 4.77 ± 0.02 Aa | 11.71 ± 1.20 Cb | 1.28 ± 0.33 Bb | 5.11 ± 0.32 Ca | 1.56 ± 0.14 Aa | 26.65 ± 1.97 Db | |
| MF | 1.56 ± 0.05 ABa | 4.73 ± 0.03 Ba | 14.10 ± 0.62 ABb | 3.77 ± 0.47 Ab | 4.57 ± 0.43 Da | 1.07 ± 0.17 Ba | 30.74 ± 1.60 Cb | |
| HF | 1.52 ± 0.09 Ba | 4.72 ± 0.02 Ba | 15.22 ± 1.04 Ab | 3.12 ± 0.46 Ab | 6.38 ± 0.66 Aa | 0.48 ± 0.05 Cb | 42.12 ± 2.41 Ab | |
| Source of variation (F statistic and probability level) | Treatment | 2.63 (p = 0.064) ns | 35.37 (p < 0.001) | 71.87 (p < 0.001) | 173.95 (p < 0.001) | 40.06 (p < 0.001) | 77.82 (p < 0.001) | 297.23 (p < 0.001) |
| Soil depth | 204.72 (p < 0.001) | 381.55 (p < 0.001) | 1232.68 (p < 0.001) | 204.72 (p < 0.001) | 204.72 (p < 0.001) | 0.50 (p = 0.482) ns | 204.72 (p < 0.001) | |
| Treatment × Soil depth | 0.14 (p = 0.933) ns | 29.53 (p < 0.001) | 20.55 (p < 0.001) | 122.37 (p < 0.001) | 33.49 (p < 0.001) | 69.59 (p < 0.001) | 100.49 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Li, Q.; Bian, F.; Zhong, Z.; Zhang, X. Effects of Bamboo-Sourced Organic Fertilizer on the Soil Microbial Necromass Carbon and Its Contribution to Soil Organic Carbon in Moso Bamboo (Phyllostachys edulis) Forest. Forests 2025, 16, 553. https://doi.org/10.3390/f16030553
Huang Z, Li Q, Bian F, Zhong Z, Zhang X. Effects of Bamboo-Sourced Organic Fertilizer on the Soil Microbial Necromass Carbon and Its Contribution to Soil Organic Carbon in Moso Bamboo (Phyllostachys edulis) Forest. Forests. 2025; 16(3):553. https://doi.org/10.3390/f16030553
Chicago/Turabian StyleHuang, Zhiyuan, Qiaoling Li, Fangyuan Bian, Zheke Zhong, and Xiaoping Zhang. 2025. "Effects of Bamboo-Sourced Organic Fertilizer on the Soil Microbial Necromass Carbon and Its Contribution to Soil Organic Carbon in Moso Bamboo (Phyllostachys edulis) Forest" Forests 16, no. 3: 553. https://doi.org/10.3390/f16030553
APA StyleHuang, Z., Li, Q., Bian, F., Zhong, Z., & Zhang, X. (2025). Effects of Bamboo-Sourced Organic Fertilizer on the Soil Microbial Necromass Carbon and Its Contribution to Soil Organic Carbon in Moso Bamboo (Phyllostachys edulis) Forest. Forests, 16(3), 553. https://doi.org/10.3390/f16030553






