Abstract
Nocturnal water consumption (NWC), known as including stem refilling (SR) and nocturnal transpiration (NT), has been documented in many plant species, but we do not yet have a clear understanding of species differences and the biotic and abiotic regulation of this phenomenon, especially for subtropical and tropical plantations. In this study, we examine the magnitude, seasonality, and biotic and abiotic regulation of NWC, SR, and NT in three widely planted subtropical and tropical species, Eucalyptus spp., Hevea brasiliensis, and Castanopsis hystrix, through the measured sap and meteorological variables. Stand-level NWC and SR differ significantly among the three plantations, where the mean daily NWC and SR of Eucalyptus spp. (2022–2023), Hevea brasiliensis (2014), and Castanopsis hystrix (2022–2023) are 0.08 mm and 0.05 mm, 0.36 mm and 0.28 mm, and 0.14 mm and 0.12 mm, respectively. Their stand-level NT values are 0.03 mm, 0.08 mm, and 0.02 mm, respectively. Additionally, distinct differences in the seasonality of NWC, SR, and NT are observed among the three plantations, with higher values during spring and autumn and lower values in summer and winter. SR is identified as the predominant role in NWC for all the plantations. VPD is identified as the primary meteorological factor driving NWC, SR, and NR in Eucalyptus spp. and Hevea brasiliensis plantations, while no prominent abiotic variables show the main driver in Castanopsis hystrix. Our findings reveal important implications for the NWC of tropical plantations related to soil–plant–atmosphere equilibrium and hydrology modeling.
1. Introduction
Plant nocturnal water consumption (NWC) is an important physiological process in hydrating the stem and phloem and maintaining adequate water in living tissues, thus preventing wilting due to nocturnal dehydration [1,2]. Additionally, it accelerates nutrient uptake by plants during the night [3,4]. Although considerable efforts have been made to improve our understanding of NWC of trees in temperate and cold regions, including its inter-annual and seasonal variability and responses to environmental factors [4,5,6,7], these factors have not been investigated much for trees in subtropical and tropical regions, especially for planted trees.
NWC generally involves two ecohydrological processes: stem refilling (SR) and nocturnal transpiration (NT) [6,7,8,9,10,11,12]. SR is the process of replenishing water in the stem that is lost due to transpiration during the day [13]. NT is the process by which plants lose water at night driven by environmental factors. For a long time, there has been academic controversy regarding the primary reason for nocturnal water consumption in plants: whether it is due to SR or NT. Lots of studies suggest that SR is the primary reason for NWC in plants [6,7,14]. For instances, Wu et al. demonstrate that the nocturnal sap flow in Acer truncatum is primarily attributed to the SR, as it accounted for approximately 90% of NWC [7]. Yu et al. demonstrate that nocturnal SR is also the main cause of nocturnal sap flow in Populus euphratica Oliv., accounting for approximately 80% of NWC [6]. In contrast, there are also studies that suggest that NT is the primary cause of NWC in plants [1,8,9]. For instance, Zeppel et al. demonstrate that NT accounts for approximately 50–70% of NWC in Angophora bakeri and Eucalyptus parramattensis in the Sierra Nevada mountains of California in the United States [9]. Phillips et al. demonstrate that NT accounts for approximately 67% of nocturnal sap flow among eight species of Eucalyptus spp. at Hawkesbury Forest Experiment site, Richmond, New South Wales, Australia [8]. Given the ongoing debate on the primary cause of NWC, further research is needed to better understand the NWC of different plantations, especially in the tropical and subtropical regions.
The thermal diffusion method has been widely used for measuring plant water consumption due to the strengths of simplicity, high accuracy, low cost, and the ability for automated and continuous measurement [15,16]. It calculates plant water consumption through monitoring sap flow in real time by measuring the temperature difference between two ends of a thermal diffusion probe [17]. However, this method cannot directly separate SR and NT from NWC. Therefore, numerous studies have been dedicated to developing various methods for distinguishing them (Table 1). One is the double sap flow curve separation method developed by Zeppel et al. [9]. This method assumes the positive area of sap flow difference between the canopy and the base position as NT, while the negative area is assumed to be SR. This method has a solid physiological mechanism. However, installing the canopy probe is challenging, as the position of the “canopy” is difficult to define, leading to a biased estimate of NT. Another method is an empirical method based on VPD division developed by Phillips et al. [8]. This method first identifies two consecutive days with similar daytime VPD but contrasting nighttime values (i.e., high VPD on the first day and low VPD on the second day). Then, it assumes that the sap flow of the first night with high VPD is the sum of both NT and SR, while the sap flow of the second night with low VPD is assumed to be SR. The NT of the first night is the difference between the sap flow of the first and the second night. The method seems to overestimate both NT and SR. Moreover, its application is limited, as it necessitates two consecutive days with contrasting nighttime VPD. A third method is a resistance network model separation method developed by Buckley et al. [10]. This study proposes a flow model based on a resistance network to separate SR and NT. The method quantifies the time constant for refilling and not only infers integrals but also tracks the time courses of water loss. However, it requires two important model parameters that are difficult to obtain. A fourth method is a forecasted refilling method developed by Fisher et al. [5], which suggests that SR and NT can occur simultaneously. Based on the fitting curve of the declining part in the early nocturnal sap flow, it assumes that the area below the curve is SR, while the area above is NT. Owing to its reasonable mechanism and simplicity for operation, this method has been widely used to calculate stem refilling and nocturnal transpiration [4,5,6,7,18,19].
In tropical and subtropical regions, numerous studies have been conducted to investigate daytime plant water consumption (namely daytime transpiration) [20,21,22,23,24,25,26]. For example, Wang et al. analyze the differences in daytime transpiration characteristics and their influencing factors among three different Eucalyptus spp. plantations on the Leizhou Peninsula [26]. They demonstrate significant differences in daytime transpiration among the three eucalyptus spp. trees, ranging from 311.52 mm to 743.41 mm. Pérez et al. analyze the transpiration rates of eucalyptus on temperate grasslands in NE Argentina. Their study finds that the annual transpiration of Eucalyptus grandis plantations is approximately 800 mm, accounting for about 90% and 40% of the annual precipitation in dry and wet years, respectively [27]. Giambelluca et al. measure the evapotranspiration of Hevea brasiliensis cultivated at at two plantation sites in Southeast Asia. The results reveal that the average monthly ET for the two plantation sites is 4.1 and 4.8 mm/d, respectively [28]. However, there are limited studies on NWC [1,7,29]. The differences in nighttime water use among different subtropical plantations are not fully understood, especially for plantations. This study focuses on three commonly subtropical and tropical plantations, Eucalyptus spp., Hevea brasiliensis, and Castanopsis hystrix, and investigates their NWC and corresponding SR and NT using the forecasted refilling method. We hypothesize that the nocturnal water consumption of these three tree species will exhibit significant differences across various temporal scales and be influenced by distinct driving factors. The objectives of this study are as follows: (1) to analyze the variations and differences of NWC, NT, and SR among the three plantations; (2) to investigate whether the SR or the NT mainly contributes to NWC in the three plantations; and (3) to explore major driving factors of NWC, SR, and NT among the three plantations. The framework of this study includes six parts: Section 2 mainly describes the studied plantations, data sources, and the methods for calculating NWC, SR, and NT, as well as the approaches for analyzing the driving factors. Section 3 mainly illustrates the results of NWC, SR, and NT and their primary driving factors. Section 4 mainly discusses reasons and comparisons, as well as implications and future work. Section 5 summarizes the remarkable results.

Table 1.
Summary of methods for distinguishing stem refilling and nocturnal transpiration.
Table 1.
Summary of methods for distinguishing stem refilling and nocturnal transpiration.
| Method | Theoretical Assumptions | Pros and Cons | Key References |
|---|---|---|---|
| Double sap flow curve separation | This method assumes the positive area of sap flow difference between the canopy and the base position to be nocturnal transpiration, while the negative area as assumed to be stem refilling. | This method has a solid physiological mechanism. However, installing the canopy probe is challenging as the position of the “canopy” is difficult to define, leading to a biased estimate of NT. | [9] |
| Empirical method based on VPD division | This method first identifies two consecutive days with similar daytime VPD but contrasting nighttime values (i.e., high VPD on the first day with low VPD on the second day). It then assumes the sap flow of the first night with high VPD to be the sum of both nocturnal transpiration and stem refilling, while the sap flow of the second night with low VPD is assumed to be stem refilling. | The method seems to overestimate both NT and SR. Moreover, its application is limited, as its necessitates two consecutive days with contrasting nighttime VPD. | [8] |
| Resistance network model separation | This method proposes a flow model that makes the ratio of storage to stem resistance a parameter based on a resistance network to separate stem refilling and nocturnal transpiration. | The method quantifies the time constant for refilling and not only infers integrals but also tracks the time courses of water loss. However, it requires two important model parameters that are difficult to obtain | [10] |
| Forecasted refilling | This method suggests that stem refilling and nocturnal transpiration can occur simultaneously. Based on the fitting curve of the declining part in the early nocturnal sap flow, it assumes the area below the curve to be stem refilling, while the area above is assumed to be nocturnal transpiration. | Owing to its reasonable mechanism and simplicity for operation, this method has been widely used to calculate stem refilling and nocturnal transpiration | [5,6,7,18,19,30] |
2. Materials and Methods
2.1. Study Sites and Plantations
The Hevea brasiliensis plantation site (21°55′ N, 101°15′ E, the elevation is 580 m) is carried out in the Xishuangbanna Tropical Botanical Garden, south of Yunnan Province, in southwestern China (Figure 1). The region has a tropical seasonal climate with a mean annual temperature of 22.48 °C (2013–2015). The coldest month is January (15.60 °C), and the hottest month is June (26.80 °C). The Hevea brasiliensis plantation was established in 1982 using a wide-narrow row planting mode 29 (Figure 1c). The average diameter at breast height (DBH) of Hevea brasiliensis trees is 32.30 ± 4.67 cm, tree height ranges from 20 m to 30 m, and stand density is 370 trees per hectare (Table 2) [31].
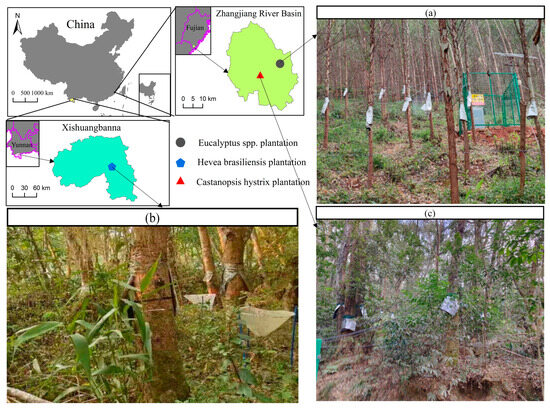
Figure 1.
Location of three plantation sites and display of the sap flow instruments: (a) Eucalyptus spp. plantation, (b) Hevea brasiliensis plantation, and (c) Castanopsis hystrix plantation.

Table 2.
Information for the three sites.
The Eucalyptus spp. plantation site (24°30′ N, 117°24′ E, the elevation is 106 m) and Castanopsis hystrix plantation site (24°00′ N, 117°18′ E, elevation 74 m) are carried out in Yunxiao County, Zhangzhou, Fujian Province, southeast China (Figure 1). The region has a subtropical monsoon humid climate, with a mean annual temperature of 22.03 °C (2020–2022). The average DBH of Eucalyptus spp. trees is 9.02 ± 0.92 cm, and the stand density is 2050 trees per hectare (Figure 1a) (Table 2). The average DBH of Castanopsis hystrix trees is 30.12 ± 11.20 cm, and the stand density is 755.56 trees per hectare (Figure 1b) (Table 2). The distance between the Eucalyptus spp. plantation site and the Castanopsis hystrix plantation site is around 10 km. It is noteworthy that the selection of these sampling sites was not random.
2.2. Meteorological Data
Meteorological data are continuously measured by automatic weather stations located in an open field about 200 m away from the Eucalyptus spp. site and the Castanopsis hystrix site beginning in June 2022. The meteorological data in the Hevea brasiliensis site are continuously measured by a flux tower located in Xishuangbanna Tropical Botanical Garden [32]. Meteorological variables are recorded every 30 min, including air temperature (Ta, °C), relative air humidity (RH, %), atmospheric pressure (atm, mbar), solar radiation (Rs, W/m2), wind speed (WS, m/s), and precipitation (P, mm).
The saturation vapor pressure deficit (VPD) is calculated as follows with Equations (1)–(3) [33,34], using the difference between the saturation vapor pressure (Es) and the actual vapor pressure (E).
2.3. Sap Flow Measurements
The sap flow (g/m2/s) is measured by a thermal dissipation probe (Made in Fuzhou, China) at the breast height of 1.30 m above the ground. For each plantation, we select 6–20 sample trees representing the DBH classes distribution. The 15 mm long probes used in the Eucalyptus spp. plantation and 25 mm long probes used in the Hevea brasiliensis and Castanopsis hystrix plantations are installed at 90° angles to the main branch [35]. After being sealed with rubber putty, the sensor units are covered with aluminum foil to shied from solar radiation and rainwater. Averaged values for each 10 min are recorded by CR300 data loggers.
2.4. Sapwood Area
The sapwood area is estimated by its allometric regression relation with DBH [36,37]. The fitted equations for the Eucalyptus spp. plantation and Hevea brasiliensis plantation are taken from Zhao et al. (2014) [38] (Equation (4), R2 = 0.9872) and Lin et al. (2016) [39] (Equation (5), R2 = 0.89), respectively. A total of 20 Castanopsis hystrix trees are used to establish the regression relation between DBH and AC. We use an increment borer to extract the tree core at 1.30 m at four different directions. The heartwood area is identified by observing the dark color often associated with heartwood [40]. Based on the measured sapwood area and DBH of 20 Castanopsis hystrix trees, the regression is established and used for the sapwood area prediction of probe-equipped trees (Equation (6), R2 = 0.87).
where AE (m2), AH (m2), and AC (m2) refer to the sapwood area of Eucalyptus spp., Hevea brasiliensis, and Castanopsis hystrix, respectively. DBH (m) refers to the diameter at breast height (1.3 m above the ground) of trees.
2.5. Calculation of Sap Flow
The sap flow density is calculated based on the temperature difference (ΔT, °C) between the heated and reference probes following Granier’s equation [35]:
where SFD represents the instantaneous sap flow density (g/m2/s), K represents the dimensionless sap flow index, ∆T is the temperature difference between the two probes, and ∆Tmax represents the ∆T when the sap flux density is zero. Considering the sap flux density may not reach zero every night, using the daily maximum values of ∆T could lead to an underestimation of SFD. Therefore, the calculation of ∆Tmax uses the Baseliner Software 38. The software can identify the moments of daily “zero” sap flow density and the times of maximum temperature difference during the day based on two environmental factors: VPD and photosynthetically active radiation (PAR). It then fits a curve to determine the baseline of zero stem sap flow density and ultimately provides the value of K.
2.6. Calculation of Water Consumption
The nocturnal water consumption is calculated based on the sap flow, the sapwood area, and the area of the experimental stand following Equations (9) and (10) proposed by Kumagai et al. (2005) [20]:
where NWC and DWC are night water consumption and day water consumption, respectively; SFDa (cm/s) represents SFDnight or SFDday, namely, the arithmetic mean sap flow density during nighttime or daytime; n is the number of trees with installed probes; and SFDi represents the instantaneous sap flow density of each tree during daytime or nighttime. tnight (s) and tday (s) refer to the duration of night and day, respectively; SA (cm2) is the average sapwood area at the location of probe installation, and AG (cm2) represents the area of the experimental stand. Nocturnal data were filtered following the principle of solar radiation < 10 W/m2. The sapwood area (SA) is calculated based on the DBH and Equations (4)–(6).
2.7. Differentiating Stem Refilling and Nocturnal Transpiration
The forecasted refilling is used to differentiate SR and NT following the method mentioned by Fisher et al. (2007) [5]. The method is based on the following assumptions: (1) SR and NT occur simultaneously; (2) when the nocturnal VPD is small, the corresponding nocturnal sap flow is considered to be SR; and (3) sap flow decreases at night as solar radiation decreases, and if there is no water loss, the sap flow will tail off to zero. Therefore, nocturnal sap flow is first multiplied by sapwood area to obtain the nocturnal transpiration rate. Based on the initial slope of the nocturnal transpiration rate curve, a logarithmic function is used to fit its trend (Equation (12), Figure 2). The area below the fitted curve represents nocturnal SR (Equation (13)), while the area enclosed between the fitted curve and the actual water consumption curve represents NT (Equation (14), Figure 2b).
where f(t) is the fit curve of the initial nocturnal transpiration rate; t refers to time step; a and b are fitting parameters; c is the time when night begins; and d is the time when stem filling is zero.
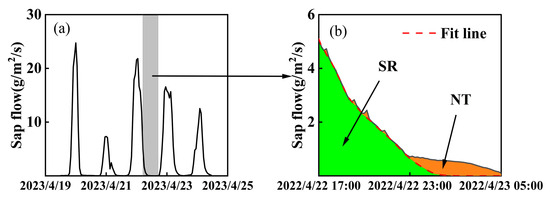
Figure 2.
Schematic diagram of differentiating stem refilling and nocturnal transpiration from night water consumption. Note: (a) represents the diurnal variation curve of sap flow over five consecutive days, (b) represents a schematic diagram depicting the separation of stem refilling and nocturnal transpiration.
2.8. Factor Analysis
Considering the effect of noisy sap flow data on rainy days, all statistical analysis is conducted for non-rainy days. The Pearson correlation analysis and stepwise regression analysis is applied to analyze the main meteorological driving factors of NWC, SR, and NT. The Pearson correlation coefficient (r, Equation (15)) reflects the degree of linear correlation between two random variables. Stepwise regression (Equation (16)) introduces each variable into the regression equation sequentially, including variables that pass the significance test and excluding those that do not. The relevant calculation equations are as follows:
where “xi” and “yi” are the values of the i-th data, respectively, and and are the means of the two data sets, respectively. z is the dependent variable (NWC, SR, or NT in this study), α is the regression coefficient, and vi is the independent variable. m is the number of variables introduced.
In addition, structural equation modeling (SEM) is also applied to quantify the effects of environmental factors on the nocturnal water use of three plantations. SEM is a statistical method that analyzes relationships between variables based on their covariance matrix. The standardized path coefficient () reflects the strength of direct or indirect relationships between variables.
3. Results
3.1. Climatic and Sap Flow Dynamics
The overall trend of sap flow density in the three plantations generally shows higher values during the day and lower values at night (Figure 3a1–a4), which is similar to the daily variation trends of temperature and VPD (Figure 3b1–b4,e1–e4) but opposite to the daily variation trend of humidity (Figure 3c1–c4). The daily mean nocturnal sap flow, Ta, RH, WS, and VPD are 1.99 ± 1.69 (mean + standard deviation) g/m2/s, 19.76 ± 4.45 °C, 86.07 ± 10.28%, 0.31 ± 0.21 m/s, and 282.23 ± 171.06 Pa for the Eucalyptus spp. Plantation; 5.99 ± 3.18 gm2/s, 21.36 ± 3.60 °C, 74.90 ± 9.17%, 0.66 ± 0.31 m/s, and 798.76 ± 330.62 Pa for the Hevea brasiliensis plantation; and 3.05 ± 1.19 g/m2/s, 17.90 ± 5.86 °C, 93.14 ± 4.68%, 0.10 ± 0.09 m/s, and 157.79 ± 114.52 Pa for the Castanopsis hystrix plantation (Figure 3), respectively. The seasonal mean nocturnal sap flow reaches its maximum in autumn for the Eucalyptus spp. Plantation and in spring for the Hevea brasiliensis plantation and the Castanopsis hystrix plantation, and the values are 2.30 ± 0.58 g/m2/s, 8.30 ± 2.56 g/m2/s, and 3.31 ± 1.14 g/m2/s, respectively (Figure 3). The seasonal mean nocturnal VPD is highest in autumn for the Eucalyptus spp. plantation and the Castanopsis hystrix plantation and in spring for the Hevea brasiliensis plantation, and the values are 361.27 ± 190.16 Pa, 218.47 ± 128.11 Pa, and 994.67 ± 295.40 Pa, respectively. The nocturnal sap flow and VPD of the Hevea brasiliensis plantation are much higher in spring than in autumn (Figure 3).
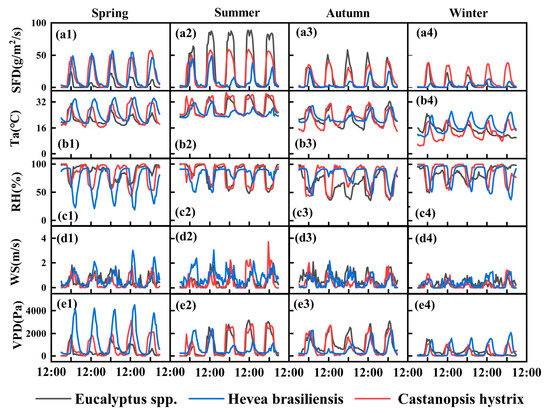
Figure 3.
Diurnal variation curves of sap flow density and meteorological variables. Note: SFD represents sap flow density, Ta represents temperature, RH represents relative humidity, WS represents wind speed, and VPD represents vapor pressure deficit, (a–e) represents the diurnal variations in sap flow density, temperature, relative humidity, wind speed, and VPD for three tree species, (1–4) representing spring, summer, autumn, and winter, respectively.
3.2. Water Consumption of Different Plantations
3.2.1. Nighttime, Daytime, and Total Daily Water Consumption
Overall, the NWC of the Hevea brasiliensis plantation (0.36 ± 0.22 mm/d) is higher than that of the Castanopsis hystrix plantation (0.14 ± 0.05 mm/d) (Figure 4 and Figure 5) and significantly higher than that of the Eucalyptus spp. plantation (0.08 ± 0.03 mm/d, Figure 5). NWC is generally lower than daytime water consumption, as shown in Figure 4. Unlike daytime water consumption, which exhibits similar daily variation patterns, NWC shows inconsistent daily variation patterns of total daily water consumption for all three plantations (Figure 4). Daily variation of NWC in the Eucalyptus spp. plantation remains relatively stable at a low level (less than 0.10 mm/d) throughout the whole year, while those of both the Castanopsis hystrix plantation and the Hevea brasiliensis plantation exhibit low values during the rainy season and high values during the dry season.
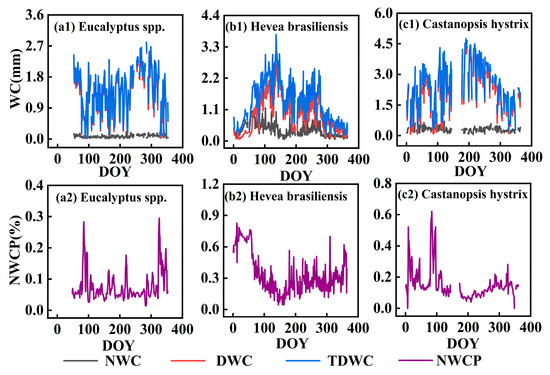
Figure 4.
Daily variations in nighttime, daytime, and total daily water consumption and daily proportion of nocturnal water consumption in the three plantations. Note: NWC represents nocturnal water consumption, DWC represents daytime water consumption, TDWC represents total daily water consumption, and NWCP represents proportion of nocturnal water consumption over the total daily water consumption.
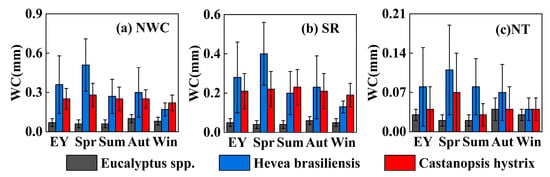
Figure 5.
Daily average of nocturnal water consumption, stem refilling, and nocturnal transpiration for the three plantations. Note: NWC represents nocturnal water consumption, and WC represents water consumption.
The proportion of nocturnal water consumption over total daily water consumption (NWCP) also shows similar seasonal variation with low values in summer and high values in winter for all three plantations (Figure 4). This may be caused by the lower temperature in winter, resulting in weaker photosynthesis and a significant decrease in daytime water consumption. Among the three plantations, the Hevea brasiliensis plantation has the highest average NWCP (33%), with the majority ranging from 20% to 80%. It decreases rapidly in summer and increases quickly in winter, with instances exceeding 50% during the dry season. This implies that the NWC of the Hevea brasiliensis plantation is greater than its daytime water consumption during winter. The NWCP of the Castanopsis hystrix plantation ranges from 5% to 25% (with average of 15.50%), and its variation pattern is similar to that of the Hevea brasiliensis plantation, with lower values in summer and higher values in winter, although the decrease in summer is not significant. The NWCP of the Eucalyptus spp. plantation is relatively low (with an average of 7.50%), ranging from 0 to 10%, and remains stable without significant seasonal increases or decreases.
3.2.2. Nocturnal Transpiration and Stem Refilling
It is clearly observed that SR is greater than NT in all three plantations (Figure 5). Both the SR and NT of the Hevea brasiliensis plantation are highest among the three plantations. The daily averages of SR and NT in the Hevea brasiliensis plantation are 0.28 ± 0.18 mm and 0.08 ± 0.07 mm, respectively, which are 0.23 mm and 0.05 mm higher than those of the Eucalyptus spp. plantation and 0.16 mm and 0.06 mm higher than those of the Castanopsis hystrix plantation (Figure 5). The SR of the Castanopsis hystrix plantation is greater than that of the Eucalyptus spp. plantation, but their NT values are comparable. For the Hevea brasiliensis plantation, both SR and NT exhibit pronounced seasonal variations, with a high level in spring and a low level in summer (Figure 5 and Figure 6). However, SR and NT in the Castanopsis hystrix and Eucalyptus spp. plantations do not exhibit pronounced seasonal variations (Figure 5 and Figure 6). The proportion of NT in the three plantations does not show significant daily variation with small seasonal changes (Figure 6). The Eucalyptus spp. plantation has the highest proportion of NT, with a daily average of 38%, followed by the Hevea brasiliensis plantation and the Castanopsis hystrix plantation, with daily averages of 25% and 19%, respectively. This also implies that the utilization of water is primarily through SR rather than NT during nighttime for all three plantations.
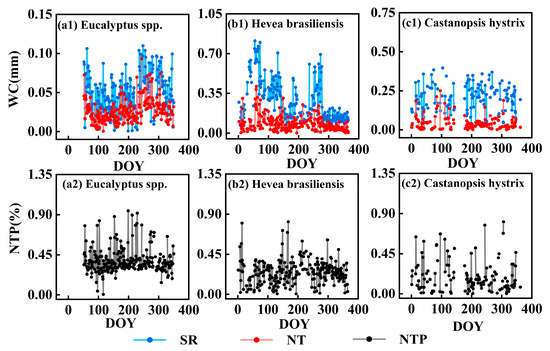
Figure 6.
Daily variation curves of stem refilling, nocturnal transpiration, and daily proportion of nocturnal transpiration in the three plantations. Note: SR represents stem refilling, NT represents nocturnal transpiration, and NTP represents proportion of nocturnal transpiration.
3.2.3. Correlations Between Nocturnal Water Consumption, Nocturnal Transpiration, and Stem Refilling
Strong positive correlations between NWC and SR can be observed in all three plantations, with correlation coefficients (r) all exceeding 0.95 (Figure 7a1,b1,c1). This may indicate that SR is more indicative of a physiological need. Strong positive correlations between NT and NWC can be observed in both the Hevea brasiliensis plantation (r = 0.79) and the Eucalyptus spp. plantation (r = 0.89), while there is no correlation in the Castanopsis hystrix plantation (r = −0.06). Additionally, strong positive correlations between SR and NT can also be found in the Hevea brasiliensis plantation (r = 0.67) and the Eucalyptus spp. plantation (r = 0.78), while there is a weak negative correlation in the Castanopsis hystrix plantation (r = −0.33).
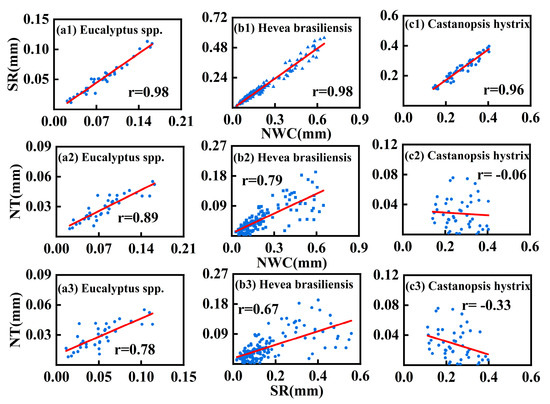
Figure 7.
Correlations between nocturnal water consumption, nocturnal transpiration, and stem refilling in the three plantations. Note: NWC represents nocturnal water consumption, SR represents stem refilling, and NT represents nocturnal transpiration.
3.3. Driving Factors
3.3.1. Driving Factors for Nocturnal Water Consumption
In the Eucalyptus spp. plantation, NWC also has both a strong positive correlation with VPD and a strong negative correlation with relative humidity, but the former is higher than the latter (Table 3), indicating that VPD is also the primary factor. Wind speed has a weak negative correlation (r = −0.34, p < 0.05) with NWC. Temperature and the product of VPD and wind speed (U × D) have no correlation with NWC. Generally, VPD can explain 45.90% of the variability in nocturnal water consumption (Table 4). The SEM also confirms that the NWC of the Eucalyptus spp. plantation is mainly influenced by VPD (Figure 8, = 0.69).

Table 3.
Pearson correlations between nocturnal water consumption, nocturnal transpiration, stem refilling, and meteorological variables for the entire year, dry season, and rainy season.

Table 4.
Stepwise regression between nocturnal water consumption, nocturnal transpiration, stem refilling, and meteorological variables for the entire year, dry season, and rainy season.
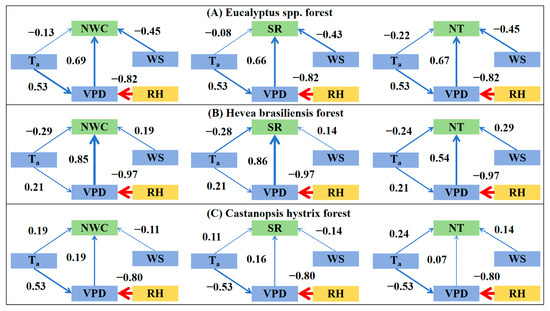
Figure 8.
Direct and indirect effects of meteorological variables on nocturnal water consumption, stem refilling, and nocturnal transpiration for the three plantations.
Significant correlations between NWC and all meteorological factors can be observed in the Hevea brasiliensis plantation in almost all the period stages (except the dry season) (Table 3). Among them, both VPD and RH have strong correlations with NWC in all the period stages (entire year, dry season, and wet season), indicating that atmosphere water demand is the primary driving factor for the NWC of the Hevea brasiliensis plantation. VPD can explain 70.40% of the variability in NWC, which also confirms the dominant role of VPD (Table 4) in the Hevea brasiliensis plantation. The structural equation (SEM) also confirms that NWC is mainly influenced by VPD (Figure 8, the standardized path coefficient = 0.38) in the Hevea brasiliensis plantation.
In the Castanopsis hystrix plantation, NWC shows a weak positive correlation with VPD (r = 0.26, p < 0.1) and Ta (r = 0.28, p < 0.1), and basically no correlation with other factors. During the rainy season, correlations between NWC and meteorological factors are slightly stronger. During the dry season, however, these correlations are very small, almost negligible. It is worth noting that although Ta has the strongest relevance with NWC, it can only explain 9.40% of its variability (Table 4). The SEM also confirms that the meteorological factors have a weak influence on the NWC of the Castanopsis hystrix plantation (Figure 8, < 0.20).
3.3.2. Driving Factors for Nocturnal Transpiration
In the Eucalyptus spp. plantation, water-related factors (VPD and RH) have the strongest correlation with NT, indicating their dominant impact. Generally, VPD can explain 35.50% of the variability in NT (Table 4). Temperature has no correlation with NT at a yearly scale but exhibits a considerable positive correlation in the rainy season and a negative correlation in the dry season. Wind speed and U×D have weak correlations with NT. The SEM also confirms that NT is mainly influenced by VPD (Figure 8, = 0.67) in the Eucalyptus spp. plantation.
Significant correlations between NT and all meteorological factors can be observed in the Hevea brasiliensis plantation in almost all the period stages (except dry season for Ta), with stronger correlations in the rainy season than in the dry season (Table 3). It is worth noting that water-related factors (i.e., VPD and RH) have stronger correlations with NT than other factors. This also implies that atmosphere water demand is the primary driving factor for the NT of the Hevea brasiliensis plantation, with VPD explaining 38.10% of the variability of NT (Table 4). The SEM also confirms that NT is mainly influenced by VPD (Figure 8, = 0.54) in the Hevea brasiliensis plantation.
In the Castanopsis hystrix plantation, NT shows weak positive correlations with all the factors. Factors with the strongest influence on NT vary across the three periods. This implies that the NT of the Castanopsis hystrix plantation is not controlled by a single factor (Table 4). The SEM confirms that the meteorological factors have no significant impact on NT in the Castanopsis hystrix plantation (Figure 8, < 0.25).
3.3.3. Driving Factors for Stem Refilling
In the Eucalyptus spp. plantation, water-related factors have strong correlations with SR, while other factors show weak correlations (Ta and WS) and even no correlation at all (U × D). VPD can explain 43.40% of the variability in SR. SEM also shows that the SR of the Eucalyptus spp. plantation is mainly influenced by VPD (Figure 8, = 0.66). The influence of meteorological factors on the SR of the three plantation is highly similar to their influence on NWC (Table 2). For the Hevea brasiliensis plantation, almost all factors show a strong correlation with SR (except for temperature). Even temperature has a fairly high correlation with SR during the rainy season. The strongest correlations are with water-related factors (VPD and RH). VPD can explain 70.40% of the variability in SR (Table 4). SEM also shows that the SR of the Hevea brasiliensis plantation is mainly influenced by VPD (Figure 8, = 0.86). Notably, U×D also shows a high correlation with SR, with no significant difference from the correlation between VPD and SR. For the Castanopsis hystrix plantation, all factors have weak correlations with SR. SEM also confirms that all factors in Castanopsis hystrix plantations have weak correlations with SR (Figure 8, < 0.20). Interestingly, similar to NWC and NT, correlations of these factors with SR in the dry season are much weaker than those in the rainy season and the entire year.
4. Discussion
4.1. Nocturnal Water Consumption Behaviors Across Different Plantations
In this study, daytime water consumption significantly exceeds nocturnal water consumption across all three plantation types (Figure 4). This is consistent with the results reflected in other studies [1,41]. NWCP varies seasonally across different plantations, showing higher values in spring and autumn and lower values in summer and winter (Figure 4). This pattern aligns with the findings of Li et al. [19], Di et al. [42], and Phillips et al. [8]. Notably, in the Hevea brasiliensis plantation, NWC can exceed daytime water consumption during winter. This anomaly may be attributed to mass defoliation phenomenon during winter, leading to reduced daytime photosynthesis activity and transpiration. Consequently, the decrease in daytime water consumption is greater than the decrease in NWC, resulting in a higher NWCP.
The annual average NWCP is approximately 7.50% in the Eucalyptus spp. plantation (Figure 4), which aligns with the study by Phillips et al. [8] (4.9%–7.3%). For the Castanopsis hystrix plantation, the average is approximately 15.4% (Figure 4), similar to the findings of Siddiq et al. [1] (12%–19%). The Hevea brasiliensis plantation has the highest annual average NWCP, at approximately 32.90% (Figure 4). This could be due to higher nocturnal temperatures and VPD, leading to greater nocturnal water loss.
In this study, SR plays a predominant role over NT in NWC (Figure 6). This finding is consistent with previous research by Wu et al. [7], Yu et al. [6], and Phillips et al. [8]. Similar to NWC, the proportions of both SR and NT vary seasonally in different plantations, with higher proportions during spring and autumn, and lower proportions in summer and winter (Figure 6).
In the Eucalyptus spp. plantation, the average ratio of NT to NWC is approximately 38.20% (Figure 6), which is lower than the 47%–67% reported by Phillips et al. [8]. This discrepancy may stem from differences in stand age: this study focuses on a younger Eucalyptus spp. plantation, where higher daytime transpiration necessitates more water for SR overnight. Similarly, in the Castanopsis hystrix plantation, the ratio of NT to NWC averages at 19.1% (Figure 6), lower than the 40.20%–88.62% range reported by Siddiq et al. [1]. This divergence likely arises from distinct plantations and their physiological traits.
It is worth noting that the proportion of NT in the Castanopsis hystrix plantation is higher than in the Hevea brasiliensis plantation, and that of the Eucalyptus spp. plantation is the lowest. The reason may be related to the different ages in the three plantations. The younger Eucalyptus spp. plantation with thinner trunks and smaller sapwood areas requires less water for SR, leading to a higher ratio of NT. However, the Castanopsis hystrix plantation is older and may not need to consume more water for its growth at night, leading to a lower proportion of NT compared to the Hevea brasiliensis plantation.
Both SR and NT demonstrate a strong positive correlation with NWC in the Hevea brasiliensis and Eucalyptus spp. plantations (Figure 7), with the correlation notably stronger for the former. This suggests that SR is more likely a biophysical process of replenishing water at night due to daytime transpiration losses. The significant positive correlations observed between NT and both NWC and SR in Hevea brasiliensis and Eucalyptus spp. plantations suggest a direct response to atmospheric demand, particularly pronounced in younger stands where growth demand necessitates higher nocturnal transpiration rate. Thus, NT appears to be a physiological and ecological water loss behavior driven by environmental factors (such as VPD) in the two plantations. NT does not correlate significantly with NWC in the Castanopsis hystrix plantation (Figure 7), underscoring the complexity of older plantation conditions where NT dynamics may be influenced by multiple factors beyond meteorological variables.
4.2. Driving Factors of Nighttime Water Consumption in Different Plantations
Many studies hypothesize that plants keep their stomata closed at night to reduce water loss, thereby preventing NT [43,44]. However, extensive studies have found that under conditions of high nocturnal vapor pressure deficit (VPD), some stomata may remain open, leading to water consumption during the night [5,29,30,45,46,47,48,49]. We also demonstrate that VPD is the primary meteorological factor driving NWC, SR, and NT, especially significant in Eucalyptus spp. and Hevea brasiliensis plantations (Table 3 and Figure 8), which is consistent with the findings of other studies [1,6,7,30,50]. Additionally, NWC is influenced by wind speed, aligning with Deng, J. and Zhu, H. 48, who also demonstrate that wind speed is significantly correlated with nocturnal sap flow. However, for the Castanopsis hystrix plantation, correlations between water consumption components and meteorological factors are generally low (Table 3). This may be due to the complexity and advanced age of the plantation, suggesting that its NWC and NT are not driven by a single meteorological factor.
4.3. Implication and Future Direction
Our results demonstrate that SR plays a predominant role over NT in NWC. Our results also highlight that VPD and age are two dominant factors that may control NWC and its two components. If climate warming continues, daytime transpiration in tropical regions will increase. Thus, young Eucalyptus spp. plantations and Hevea brasiliensis plantations will consume more nocturnal soil water through SR and NT, which will significantly impact the soil–vegetation–atmosphere water cycle. These findings can also be used to improve the models for the eco-hydrology of tropical plantations, predict the impact of global warming on hydrology, and provide scientific references for these two tree species to cope with extreme climate changes (such as extreme drought) in the future. In addition, our research enables readers to gain a deeper understanding of the physiological and ecological behaviors of tropical plantation tree species. Our findings also indicate that the proportion of nocturnal water consumption for these three tree species is relatively higher during the dry season. Therefore, interventions such as thinning and weeding in these plantations should be minimized during the dry season to reduce additional evapotranspiration water loss.
Our results suggest that SR seems to be a biophysical mechanism replenishing water at night lost in daytime transpiration for all three plantations, while NT appears to be a physiological and ecological water loss behavior driven by VPD for young plantations. However, given that only three tropical plantations were studied, this phenomenon needs to be examined by conducting more experiments on other tropical plantation types in the future. In addition, more attention should be paid to the selection of plantations. Ages and stand conditions of the plantation need to be as similar as possible. This will allow for a more direct comparison of nocturnal water usage among different plantations.
5. Conclusions
We measured nocturnal sap flow of three plantations using the thermal diffusion method and partitioning stem refilling and nocturnal transpiration from nocturnal water consumption using the forecasted refilling method. Overall, our results verify distinct differences in NWC, NSR, and NT among the three plantations. Daytime water consumption is found to be much greater than nocturnal water consumption. Stem refilling plays a predominant role over nocturnal transpiration in nocturnal water consumption in all three plantations. The strong positive correlation between nocturnal water consumption and stem refilling indicates that stem refilling is more indicative of a physiological need. VPD is found to be the primary meteorological factor driving NWC, SR, and NR in Eucalyptus spp. and Hevea brasiliensis plantations. Our findings may be used to improve models for the eco-hydrology of tropical plantations and predict the impact of global warming on hydrology. Our research enriches the understanding of the ecohydrological processes of tropical plantation tree species.
Author Contributions
Conceptualization, Z.S., H.X. and Y.C.; methodology, Z.S., H.X., S.W., Q.S., D.L. and Y.C.; software, Z.S.; validation, Z.S.; formal analysis, Z.S. and Y.C.; investigation, Z.S., S.W., H.L., Y.L. and Y.C.; resources, Y.L. and Y.C.; data curation, Z.S. and Y.C.; writing—original draft preparation, Z.S., H.X., S.W., H.L. and Y.C.; writing—review and editing, Y.C.; visualization, Z.S.; supervision, H.X., Q.S., Y.L., D.L. and Y.C.; project administration, Y.C. and Z.S.; funding acquisition, Y.C. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 42277450, and the Natural Science Foundation of Fujian Province, grant number 2022J01179.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors extend their appreciation to reviewers.
Conflicts of Interest
The authors declare no conflicts of interest. Yun Li is employed by Mengla Tianye Rubber Sales Co., Ltd.; his employer’s company was not involved in this study, and there is no relevance between this research and their company.
References
- Siddiq, Z.; Cao, K. Nocturnal Transpiration in 18 Broadleaf Timber Species under a Tropical Seasonal Climate. For. Ecol. Manag. 2018, 418, 47–54. [Google Scholar] [CrossRef]
- Goldstein, G.; Bucci, S.; Scholz, F. Why Do Trees Adjust Water Relations and Hydraulic Architecture in Response to Nutrient Availability? Tree Physiol. 2013, 33, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Christman, M.; Donovan, L.; Richards, J. Magnitude of Nighttime Transpiration Does Not Affect Plant Growth or Nutrition in Well-Watered Arabidopsis. Physiol. Plant. 2009, 136, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Si, J.; Feng, Q.; Yu, T.; Du Li, P. Comparative Study of Daytime and Nighttime Sap Flow of Populus Euphratica. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Fisher, J.; Baldocchi, D.; Misson, L.; Dawson, T.; Goldstein, A. What the Towers Don’t See at Night: Nocturnal Sap Flow in Trees and Shrubs at Two AmeriFlux Sites in California. Tree Physiol. 2007, 27, 597–610. [Google Scholar] [CrossRef]
- Yu, T.; Feng, Q.; Si, J.; Mitchell, P.; Forster, M.; Zhang, X.; Zhao, C. Depressed Hydraulic Redistribution of Roots More by Stem Refilling than by Nocturnal Transpiration for Populus Euphratica Oliv. in Situ Measurement. Ecol. Evol. 2018, 8, 2607–2616. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Zhu, J.; Gong, L.; Xu, L.; Jin, G.; Li, J.; Hauer, R.; Xu, C. Nocturnal Sap Flow Is Mainly Caused by Stem Refilling Rather than Nocturnal Transpiration for Acer Truncatum in Urban Environment. Urban For. Urban Green. 2020, 56, 126800. [Google Scholar] [CrossRef]
- Phillips, N.; Lewis, J.; Logan, B.; Tissue, D. Inter- and Intra-Specific Variation in Nocturnal Water Transport in Eucalyptus. Tree Physiol. 2010, 30, 586–596. [Google Scholar] [CrossRef]
- Zeppel, M.; Tissue, D.; Taylor, D.; Macinnis-Ng, C.; Eamus, D. Rates of Nocturnal Transpiration in Two Evergreen Temperate Woodland Species with Differing Water-Use Strategies. Tree Physiol. 2010, 30, 988–1000. [Google Scholar] [CrossRef]
- Buckley, T.; Turnbull, T.; Pfautsch, S.; Adams, M. Nocturnal Water Loss in Mature Subalpine Eucalyptus Delegatensis Tall Open Forests and Adjacent E. Pauciflora Woodlands. Ecol. Evol. 2011, 1, 435–450. [Google Scholar] [CrossRef]
- Pfautsch, S.; Keitel, C.; Turnbull, T.; Braimbridge, M.; Wright, T.; Simpson, R.; O’Brien, J.; Adams, M. Diurnal Patterns of Water Use in Eucalyptus Victrix Indicate Pronounced Desiccation-Rehydration Cycles despite Unlimited Water Supply. Tree Physiol. 2011, 31, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Katul, G.; Wang, J.; Xu, J.; Kang, S.; Liu, C.; Zhang, B.; Li, L.; Cajucom, E. Differential Response of Rice Evapotranspiration to Varying Patterns of Warming. Agric. For. Meteorol. 2021, 298–299, 108293. [Google Scholar] [CrossRef]
- Snyder, K.A.; Richards, J.H.; Donovan, L.A. Night-time Conductance in C3 and C4 Species: Do Plants Lose Water at Night? J. Exp. Bot. 2003, 54, 861–865. [Google Scholar] [CrossRef]
- Caird, M.; Richards, J.; Donovan, L. Nighttime Stomatal Conductance and Transpiration in C3 and C4 Plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef]
- Matyssek, R.; Wieser, G.; Patzner, K.; Blaschke, H.; Häberle, K.H. Transpiration of Forest Trees and Stands at Different Altitude: Consistencies Rather than Contrasts? Eur. J. For. Res. 2009, 128, 579–596. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Hölscher, D.; Wang, Q.; Lu, P.; Cai, X.; Zeng, X. Nighttime Sap Flow of Acacia Mangium and Its Implications for Nighttime Transpiration and Stem Water Storage. J. Plant Ecol. 2012, 5, 294–304. [Google Scholar] [CrossRef]
- Vandegehuchte, M.; Steppe, K. Sap-Flux Density Measurement Methods: Working Principles and Applicability. Funct. Plant Biol. 2013, 40, 213–223. [Google Scholar] [CrossRef]
- Kangur, O.; Tullus, A.; Sellin, A. Night-Time Transpiration, Predawn Hydraulic Conductance and Water Potential Disequilibrium in Hybrid Aspen Coppice. Trees Struct. Funct. 2020, 34, 133–141. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wang, N.; Liang, C.; Xie, B.; Qin, Z.; Yuan, Y.; Cao, J. Nocturnal Water Use Partitioning and Its Environmental and Stomatal Control Mechanism in Caragana Korshinskii Kom in a Semi-Arid Region of Northern China. Forests 2023, 14, 2154. [Google Scholar] [CrossRef]
- Kumagai, T.; Aoki, S.; Nagasawa, H.; Mabuchi, T.; Kubota, K.; Inoue, S.; Utsumi, Y.; Otsuki, K. Effects of Tree-to-Tree and Radial Variations on Sap Flow Estimates of Transpiration in Japanese Cedar. Agric. For. Meteorol. 2005, 135, 110–116. [Google Scholar] [CrossRef]
- Niu, F.; Röll, A.; Meijide, A.; Hendrayanto; Hölscher, D. Rubber Tree Transpiration in the Lowlands of Sumatra. Ecohydrology 2017, 10, e1882. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, P.; Hu, Y.; Ouyang, L.; Zhu, L.; Ni, G. Canopy Transpiration and Its Cooling Effect of Three Urban Tree Species in a Subtropical City- Guangzhou, China. Urban For. Urban Green. 2019, 43, 126368. [Google Scholar] [CrossRef]
- Abreu, M.; Soares, A.; de Freitas, C.; Martins, F. Transpiration and Growth Responses by Eucalyptus Species to Progressive Soil Drying. J. For. Res. 2022, 33, 1529–1543. [Google Scholar] [CrossRef]
- Ouyang, L.; He, W.; Huang, K.; Zhou, C.; Gu, D.; Huang, Y.; Zhao, P. Seasonal Water Use Strategy of Canopy Tree Species and Possible Implication for Their Coexistence in a Subtropical Secondary Forest. Ecohydrology 2019, 12, e2129. [Google Scholar] [CrossRef]
- Song, X.; Lyu, S.; Wen, X. Limitation of Soil Moisture on the Response of Transpiration to Vapor Pressure Deficit in a Subtropical Coniferous Plantation Subjected to Seasonal Drought. J. Hydrol. 2020, 591, 125301. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Xu, Y.; Zhu, W.; Du, A. Differences in Transpiration Characteristics among Eucalyptus Plantations of Three Species on the Leizhou Peninsula, Southern China. Forests 2022, 13, 1544. [Google Scholar] [CrossRef]
- Pérez, C.; Frangi, J.; Tesón, N.; Arturi, M. Transpiration Rates, Climate and Soil Water Balance of Eucalyptus Grandis Afforestation on Temperate Grasslands in Ne Argentina. J. Sustain. For. 2021, 40, 607–621. [Google Scholar] [CrossRef]
- Giambelluca, T.; Mudd, R.; Liu, W.; Ziegler, A.; Kobayashi, N.; Kumagai, T.; Miyazawa, Y.; Lim, T.; Huang, M.; Fox, J.; et al. Evapotranspiration of Rubber (Hevea brasiliensis) Cultivated at Two Plantation Sites in Southeast Asia. Water Resour. Res. 2016, 52, 660–679. [Google Scholar] [CrossRef]
- Kavanagh, K.; Pangle, R.; Schotzko, A. Nocturnal Transpiration Causing Disequilibrium between Soil and Stem Predawn Water Potential in Mixed Conifer Forests of Idaho. Tree Physiol. 2007, 27, 621–629. [Google Scholar] [CrossRef]
- Zhao, C.; Si, J.; Feng, Q.; Yu, T.; Li, P.; Forster, M. Nighttime Transpiration of Populus Euphratica during Different Phenophases. J. For. Res. 2019, 30, 435–444. [Google Scholar] [CrossRef]
- Song, Q. The Carbon Water Vapor Exchange of Rubber Plantation in Xishuangbanna Retrieved from ChinaNational Knowledge Infrastructure. Ph.D. Thesis, Chinese Academy of Sciences University, Xishuangbanna, China, 2013. [Google Scholar]
- Zhao, J.; Zhang, Y.; Song, F.; Xu, Z.; Xiao, L. Phenological Response of Tropical Plants to Regional Climate Change in Xishuangbanna, South-Western China. J. Trop. Ecol. 2013, 29, 161–172. [Google Scholar] [CrossRef]
- Murray, F.W. On the Computation of Saturation Vapor Pressure. J. Appl. Meterology 1967, 6, 203–204. [Google Scholar] [CrossRef]
- Mu, Q.; Zhao, M.; Running, S.W. Improvements to a MODIS Global Terrestrial Evapotranspiration Algorithm. Remote Sens. Environ. 2011, 115, 1781–1800. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of Transpiration in a Douglas-Fir Stand by Means of Sap Flow Measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Liu, S.; Wang, P. Modelling Diurnal and Seasonal Hysteresis Phenomena of Canopy Conductance in an Oasis Forest Ecosystem. Agric. For. Meteorol. 2017, 246, 98–110. [Google Scholar] [CrossRef]
- Jonard, F.; André, F.; Ponette, Q.; Vincke, C.; Jonard, M. Sap Flux Density and Stomatal Conductance of European Beech and Common Oak Trees in Pure and Mixed Stands during the Summer Drought of 2003. J. Hydrol. 2011, 409, 371–381. [Google Scholar] [CrossRef]
- Zhao, P.-Q.; Zhao, P.; Niu, J.-F.; Zhu, L.-W.; Ni, G.-Y.; Gao, J.-G.; Zhang, Z.-Z. Relationship between Vessel Characteristics and Sap Flow of Eight Subtropical Tree Species. J. Trop. Subtrop. Bot. 2014, 22, 537–548. [Google Scholar] [CrossRef]
- Lin, Y.X.; Zhang, Y.P.; Zhao, W.; Zhang, X.; Li, J. Comparison of Transpiration Characteristics in Different Aged Rubber Plantations. Chin. J. Ecol. 2016, 35, 855–863. [Google Scholar]
- Burgess, S.; Adams, M.; Turner, N.; Beverly, C.; Ong, C.; Khan, A.; Bleby, T. An Improved Heat Pulse Method to Measure Low and Reverse Rates of Sap Flow in Woody Plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Hayat, M.; Yan, C.; Xiang, J.; Xiong, B.; Qin, L.; Khan, A.; Wang, B.; Khan, M.; Zou, Z.; Qiu, G. Multiple-Temporal Scale Variations in Nighttime Sap Flow Response to Environmental Factors in Ficus Concinna over a Subtropical Megacity, Southern China. Forests 2022, 13, 1059. [Google Scholar] [CrossRef]
- Di, N.; Yang, S.; Liu, Y.; Fan, Y.; Duan, J.; Nadezhdina, N.; Li, X.; Xi, B. Soil-Moisture-Dependent Nocturnal Water Use Strategy and Its Responses to Meteorological Factors in a Seasonal-Arid Poplar Plantation. Agric. Water Manag. 2022, 274, 107984. [Google Scholar] [CrossRef]
- Barbeta, A.; Ogaya, R.; Peñuelas, J. Comparative Study of Diurnal and Nocturnal Sap Flow of Quercus Ilex and Phillyrea Latifolia in a Mediterranean Holm Oak Forest in Prades (Catalonia, NE Spain). Trees-Struct. Funct. 2012, 26, 1651–1659. [Google Scholar] [CrossRef]
- Zhang, H.; Levia, D.; He, B.; Wu, H.; Liao, A.; Carlyle-Moses, D.; Liu, J.; Wang, N.; Li, J.; Fu, C. Interspecific Variation in Tree- and Stand-Scale Stemflow Funneling Ratios in a Subtropical Deciduous Forest in Eastern China. J. Hydrol. 2020, 590, 125455. [Google Scholar] [CrossRef]
- Daley, M.; Phillips, N. Interspecific Variation in Nighttime Transpiration and Stomatal Conductance in a Mixed New England Deciduous Forest. Tree Physiol. 2006, 26, 411–419. [Google Scholar] [CrossRef]
- Scholz, F.; Bucci, S.; Goldstein, G.; Meinzer, F.; Franco, A.; Miralles-Wilhelm, F. Removal of Nutrient Limitations by Long-Term Fertilization Decreases Nocturnal Water Loss in Savanna Trees. Tree Physiol. 2007, 27, 551–559. [Google Scholar] [CrossRef]
- de Dios, V.; Díaz-Sierra, R.; Goulden, M.; Barton, C.; Boer, M.; Gessler, A.; Ferrio, J.; Pfautsch, S.; Tissue, D. Woody Clockworks: Circadian Regulation of Night-Time Water Use in Eucalyptus Globulus. New Phytol. 2013, 200, 743–752. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.; De Boeck, H.; Menzel, A.; Nijs, I.; Peaucelle, M.; Peñuelas, J.; Piao, S.; Janssens, I. Three Times Greater Weight of Daytime than of Night-Time Temperature on Leaf Unfolding Phenology in Temperate Trees. New Phytol. 2016, 212, 590–597. [Google Scholar] [CrossRef]
- Devi, M.; Reddy, V. Transpiration Response of Cotton to Vapor Pressure Deficit and Its Relationship With Stomatal Traits. Front. Plant Sci. 2018, 9, 1572. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, H. Nocturnal Sap Flow of Hedysarum Scoparium and Its Response to Meteorological Factors in Semiarid Northwest China. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).